Black Bean Hulls as a Byproduct of an Extraction Process to Enhance Nutraceutical and Glycemic-Related Properties of Nixtamalized Maize Tostadas
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Physicochemical Analysis of Raw Materials
- (a)
- Digestible and resistant starch and total dietary fiber.
- (b)
- Total anthocyanins
- (c)
- Trypsin inhibitor
- (d)
- Granulometry analysis
- (e)
- In vitro protein digestibility
2.3. Tostada Production
2.4. Physicochemical Analysis of Corn Tostadas
- (a)
- Proximal analysis of corn tostadas.
- (b)
- Digestible and resistant starch, and total dietary fiber.
- (c)
- Predicted Glycemic Index
- (d)
- Thermo-mechanical behavior of doughs
- (e)
- Qualitative characterization of anthocyanin profile
- (f)
- Trypsin inhibitor
- (g)
- In vitro protein digestibility
- (h)
- Color
- (i)
- Texture
- (j)
- Sensory analysis
- (k)
- Statistical analysis
3. Results and Discussion
3.1. Raw Material Characterization
3.1.1. Proximal Composition of the Raw Material
3.1.2. Starch and Protein Content of the Raw Material
3.1.3. Thermo-Mechanical Behavior of Doughs
3.2. Corn Tostadas Characterization
3.2.1. Proximal Composition of Corn Tostadas
3.2.2. Starch and Protein Information for Corn Tostadas
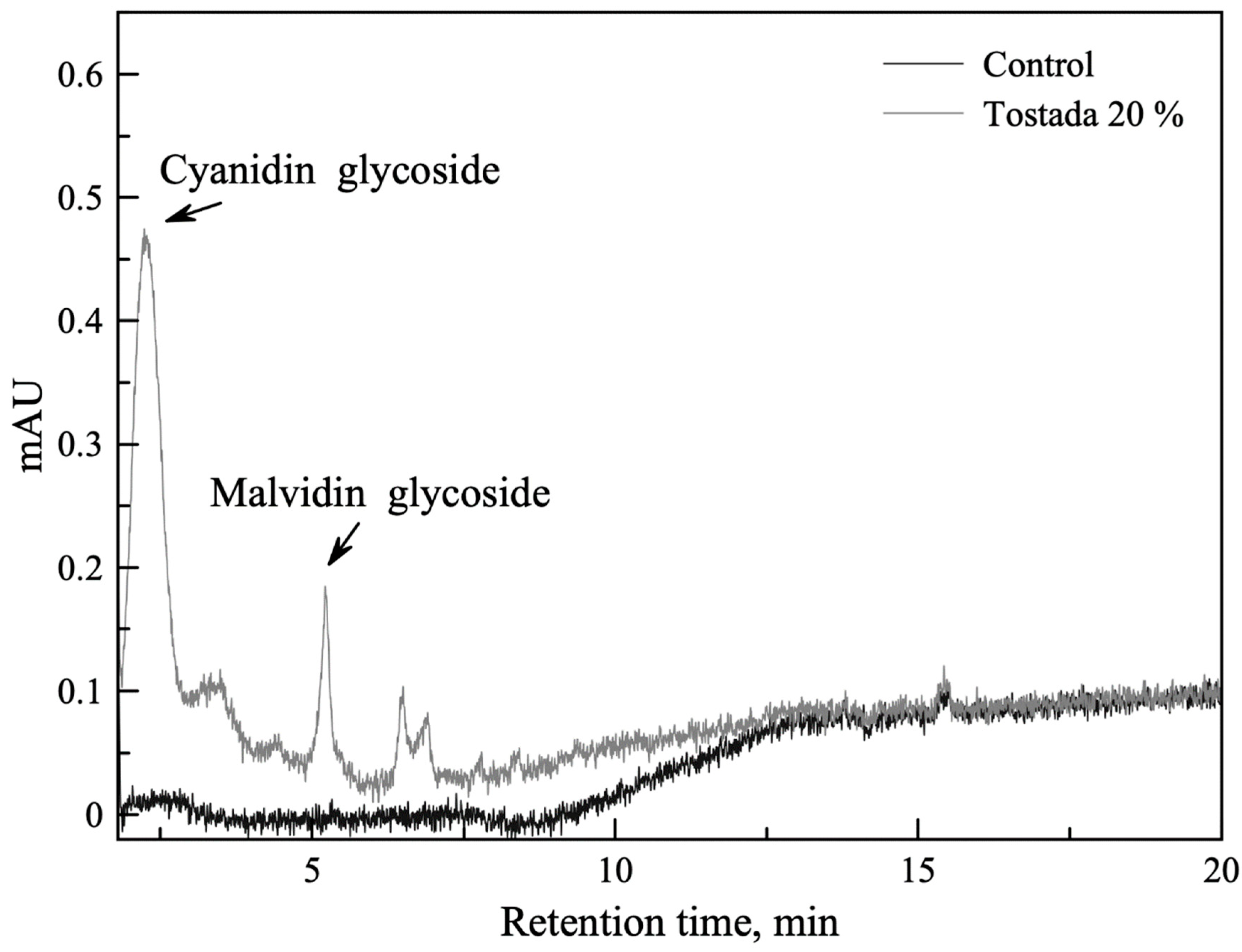
3.2.3. Qualitative Characterization of Anthocyanin Profile by HPLC for Corn Tostadas
3.2.4. Color Analysis of Corn Tostadas
3.2.5. Hardness Analysis of Corn Tostadas
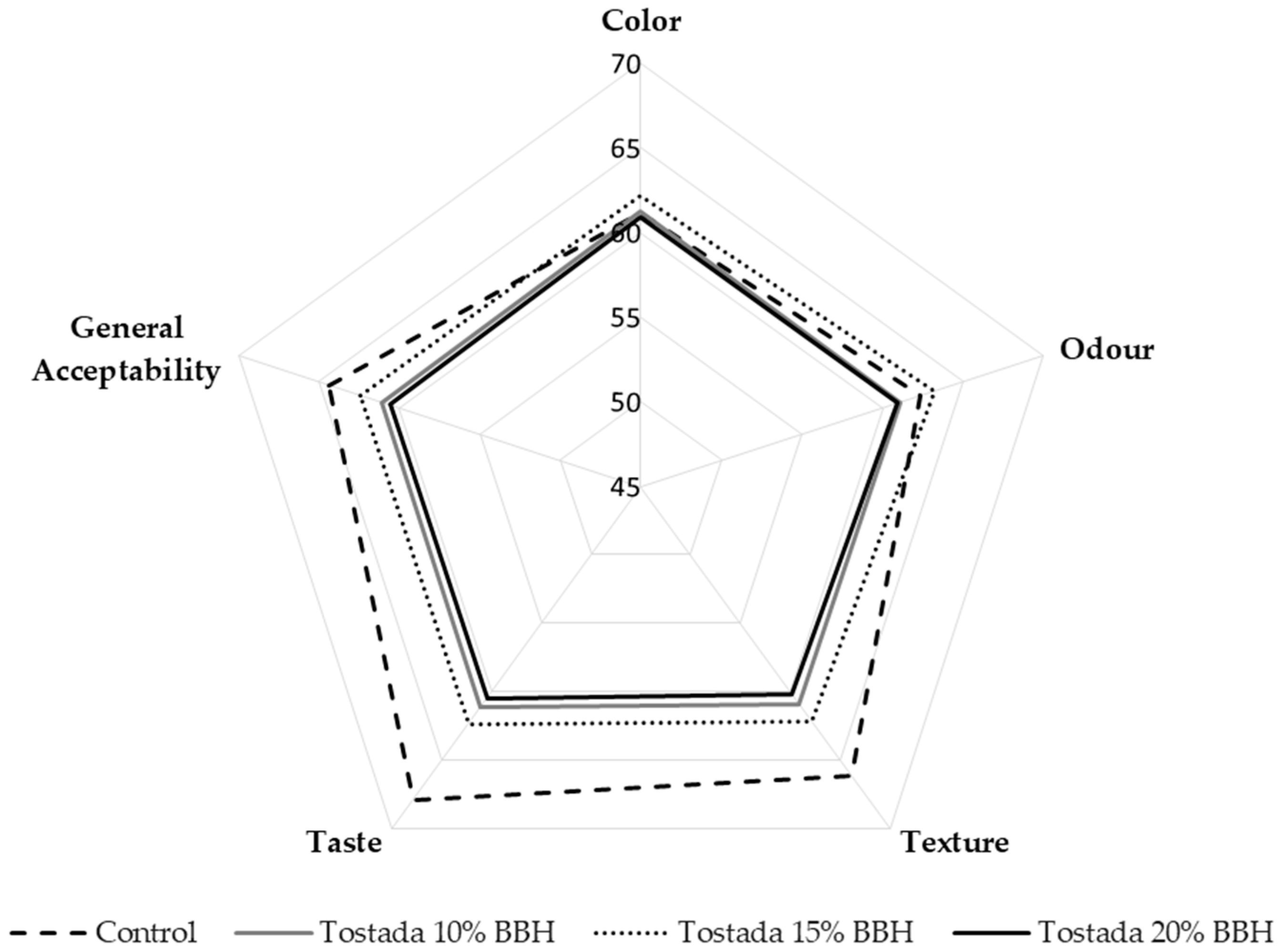
3.2.6. Sensory Analysis of Corn Tostadas
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghisellini, P.; Cialani, C.; Ulgiati, S. A review on circular economy: The expected transition to a balanced interplay of environmental and economic systems. J. Clean. Prod. 2016, 114, 11–32. [Google Scholar] [CrossRef]
- FAO. Food Waste Report 2021. Available online: https://www.fao.org/platform-food-loss-waste/resources/detail/en/c/1378978/ (accessed on 5 March 2023).
- Pojic, M.; Misan, A.; Tiwari, B. Eco-innovative technologies for extraction of proteins for human consumption from renewable protein sources of plant origin. Trends Food Sci. Technol. 2018, 75, 93–104. [Google Scholar] [CrossRef]
- FAOSTAT. Crops and Livestock Products. 2023. Available online: https://www.fao.org/faostat/en (accessed on 19 February 2023).
- Vieira, N.M.; Peghinelli, V.V.; Monte, M.G.; Costa, N.A.; Pereira, A.G.; Seki, M.M.; Minicucci, M.F. Beans comsumption can contribute to the prevention of cardiovascular disease. Clin. Nutr. ESPEN 2023, in press. [CrossRef] [PubMed]
- De la Rosa-Millán, J.; Pérez-Carrillo, E.; Guajardo-Flores, S. Effect of germinated black bean cotyledons (Phaseolus vulgaris L.) as an extruded flour ingredient on physicochemical characteristics, in vitro digestibility starch, and protein of nixtamalized blue maize cookies. Starch 2017, 69, 1600085. [Google Scholar] [CrossRef]
- López-Barrios, L.; Heredia-Olea, E.; Guajardo-Flores, D.; Pérez-Carrillo, E.; Gutiérrez-Uribe, J.A. Bioactive peptides by in vitro digestion of germinated bean cotyledons extrudates. J. Food Res. 2018, 7, 76–85. [Google Scholar] [CrossRef]
- De la Rosa-Millán, J.; Heredia-Olea, E.; Perez-Carrillo, E.; Guajardo-Flores, D.; Serna-Saldívar, S.O. Effect of decortication, germination and extrusion on physicochemical and in vitro protein and starch digestion characteristics of black beans (Phaseolus vulgaris L.). LWT 2019, 102, 330–337. [Google Scholar] [CrossRef]
- Guajardo-Flores, D.; García-Patiño, M.; Serna-Guerrero, D.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Characterization and quantification of saponins and flavonoids in sprouts, seed coats and cotyledons of germinated black beans. Food Chem. 2012, 134, 1312–1319. [Google Scholar] [CrossRef]
- Guajardo-Flores, D.; Rempel, C.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Influence of Excipients and Spray Drying on the Physical and Chemical Properties of Nutraceutical Capsules Containing Phytochemicals from Black Bean Extract. Molecules 2015, 20, 21626–21635. [Google Scholar] [CrossRef]
- Abdulrahman, B.O.; Muntari, B.; Oluwasesan, M.B. Bioactive compounds of black bean (Phaseolus vulgaris L.). In Bioactive Compounds in Underutilized Vegetables and Legumes; Reference Series in Phytochemistry; Murthy, H.N., Paek, K.Y., Eds.; Springer: Cham, Switzerland, 2020; pp. 1–20. [Google Scholar]
- Moreno-García, K.L.; Antunes-Ricardo, M.; Martínez-Ávila, M.; Milán-Carrillo, J.; Guajardo-Flores, D. Evaluation of the antioxidant, anti-inflammatory and antihyperglycemic activities of black bean (Phaseolus vulgaris L.) byproduct extracts obtained by supercritical CO2. J. Supercrit. Fluids 2022, 183, 105560. [Google Scholar] [CrossRef]
- Acosta-Estrada, B.; Serna-Saldívar, S.; Chuck-Hernández, C. Quality assessment of maize tortillas produced from landraces and high yield hybrids and varieties. Front. Nutr. 2023, 10, 1105619. [Google Scholar] [CrossRef]
- Chávez-Santoscoy, R.A.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Effect of Flavonoids and Saponins Extracted from Black Bean (Phaseolus vulgaris L.) Seed Coats as Cholesterol Micelle Disruptors. Plant Foods Hum. Nutr. 2013, 68, 416–423. [Google Scholar] [CrossRef] [PubMed]
- AOAC (Association of Official Agricultural Chemists). Official Methods and Recommended Practices of the AOAC: Method 925.10 Moisture, 17th ed.; AOAC Press: Urbana, IL, USA, 2000. [Google Scholar]
- AOAC (Association of Official Agricultural Chemists). Official Methods and Recommended Practices of the AOAC: Method 960.52 Microchemical Determination of Nitrogen (Micro-Kjeldahl), 17th ed.; AOAC Press: Urbana, IL, USA, 2000. [Google Scholar]
- Pomeranz, Y.; Meloan, C.E. Food Analysis Theory and Practice, 3rd ed.; Chapman & Hall: New York, NY, USA, 1994; pp. 684–692. [Google Scholar]
- AOAC (Association of Official Agricultural Chemists). Official Methods and Recommended Practices of the AOAC: Method 923.03 Total Ash, 17th ed.; AOAC Press: Urbana, IL, USA, 2000. [Google Scholar]
- Aquino, E.N.; García, Y.D.; Chavez, J.L.; Carrillo, J.C.; Vera, A.M.; Heredia, E. Anthocyanin, polyphenol, and flavonoid contents and antioxidant activity in common Mexican bean (Phaseolus vulgaris L.) landraces. EJFA 2016, 28, 581–588. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization and Measurement of Anthocyanins by UV-Visible Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001. [Google Scholar] [CrossRef]
- AOCS (American Oil Chemists’ Society). Official Methods and Recommended Practices of the AOCS: Method Ba 12-75. Trypsin Inhibitor Activity, 17th ed.; AOCS Press: Urbana, IL, USA, 2000. [Google Scholar]
- Hsu, H.W.; Vavak, D.L.; Satterlee, L.; Miller, G.A. A multienzyme technique for estimating protein digestibility. J. Food Sci. 1977, 42, 1269–1273. [Google Scholar] [CrossRef]
- Goñi, I.; Garcia, A.; Saura, F. A starch hydrolysis procedure to estimate glycemic index. Nutr. Res. 1997, 17, 427–437. [Google Scholar] [CrossRef]
- Espinosa-Ramírez, J.; Rosell, C.M.; Serna, S.O.; Pérez, E. Evaluation of the quality of nixtamalized maize flours for tortilla production with a new Mixolab protocol. Cereal Chem. 2020, 97, 527–539. [Google Scholar] [CrossRef]
- Espinosa-Ramírez, J.; De la Rosa, J.; Pérez, E.; Serna, S.O. Assessment of the quality of fresh nixtamalized maize doughs with different degrees of cooking and milling: A comparison of Mixolab and RVA analyses. J. Cereal Sci. 2021, 102, 103321. [Google Scholar] [CrossRef]
- Urias, D.A.; Heredia, J.B.; Muy, M.D.; Valdez, J.B.; Serna, S.O.; Gutiérrez, J.A. Anthocyanins and Phenolic Acids of Hybrid and Native Blue Maize (Zea mays L.) Extracts and Their Antiproliferative Activity in Mammary (MCF7), Liver (HepG2), Colon (Caco2 and HT29) and Prostate (PC3) Cancer Cells. Plant Food Hum. Nutr. 2015, 70, 193–199. [Google Scholar] [CrossRef]
- Lin, L.-Z.; Harnly, J.M.; Pastor, M.S.; Luthria, D.L. The polyphenolic profiles of common bean (Phaseolus vulgaris L.). Food Chem. 2008, 107, 399–410. [Google Scholar] [CrossRef]
- Macz, G.A.; Rivas, J.C.; Pérez, J.J.; González, A.M. Natural occurrence of free anthocyanin aglycones in beans (Phaseolus vulgaris L.). Food Chem. 2006, 94, 448–456. [Google Scholar] [CrossRef]
- Vignoni, L.; Césari, R.; Forte, M.; Mirábile, M. Determinación de Indice de Color en Ajo Picado. Inf. Tecnol. 2006, 17, 63–67. [Google Scholar] [CrossRef]
- Mojica, L.; Berhow, M.; Gonzalez de Mejia, E. Black bean anthocyanin-rich extracts as food colorants: Physicochemical stability and antidiabetes potential. Food Chem. 2017, 229, 628–639. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Hettiararchchy, N.S.; Horax, R. Quality and estimated glycemic profile of baked protein-enriched corn chips. J. Food Sci. Technol. 2019, 56, 2855–2862. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, C.E.; Zepeda, R.; Ramírez, M.E.; Corzo, L.J. Nixtamalized tortillas supplemented with proteins isolated from Phaseolus coccineus and huauzontle (Chenopodium berlandieri subsp. Nuttalliae) flour: Rheological, textural, and sensorial properties. Int. J. Gastron. Food Sci. 2020, 22, 100274. [Google Scholar] [CrossRef]
- Anton, A.A.; Lukow, O.M.; Fulcher, R.G.; Arntfield, S.D. Shelf stability and sensory properties of flour tortillas fortified with pinto bean (Phaseolus vulgaris L.) flour: Effects of hydrocolloid addition. Food Sci. Technol. 2009, 42, 23–29. [Google Scholar] [CrossRef]
- Gutiérrez, E.; Rojas, J.I.; Zambrano, M.L.; Quintanar, D.; González, R.M.; Rojas, A.; Espinosa, D.G. Effect of processing conditions on the production of nixtamalized corn flours by the traditional method. CyTA J. Food 2013, 11, 46–53. [Google Scholar] [CrossRef]
- Palacios, A.J.; Vazquez, C.; Rodríguez, M.E. Physicochemical characterizing of industrial and traditional nixtamalized corn flours. J. Food Eng. 2009, 93, 45–51. [Google Scholar] [CrossRef]
- Abbo, S.; Gopher, A.; Bar-Gal, G. Plant Domestication and the Origins of Agriculture in the Ancient Near East; Cambridge University Press: Cambridge, UK, 2022. [Google Scholar] [CrossRef]
- Arockianathan, P.M.; Rajalakshmi, K.; Nagappan, P. Proximate composition, phytochemicals, minerals and antioxidant activities of Vigna mungo L. seed coat. Bioinformation 2019, 15, 579–585. [Google Scholar] [CrossRef]
- Akinjayeju, A.; Ajayi, O. Effects of Dehulling on Functional and Sensory Properties of Flours from Black Beans (Phaseolus vulgaris). Food Nutr. Sci. 2011, 2, 344–349. [Google Scholar] [CrossRef]
- Grajales, E.M.; Osorio, P.; Goñi, I.; Hervert, D.; Guzmán, S.H.; Bello, L.A. Chemical composition, starch digestibility and antioxidant capacity of tortilla made with a blend of quality protein maize and black bean. Int. J. Mol. Sci. 2012, 13, 286–301. [Google Scholar] [CrossRef]
- Hernandez, J.R. Efecto de las Condiciones de Procesamiento en la Calidad Nixtamalera, Química y Nutracéutica de Harinas Nixtamalizadas por un Proceso de Calentamiento Óhmico Continuo. Master’s Thesis, Universidad Autónoma de Querétaro, Querétaro, México, 2017. [Google Scholar]
- Silva, L.; Osorio, P.; Tovar, J.; Bello, L.A. Chemical composition, carbohydrate digestibility, and antioxidant capacity of cooked black bean, chickpea, and lentil Mexican varieties. CyTA J. Food 2010, 8, 7–14. [Google Scholar] [CrossRef]
- Takeoka, G.R.; Dao, L.T.; Full, G.H.; Wong, R.Y.; Harden, L.A.; Edwards, R.H.; Berrios, J.D.J. Characterization of black bean (Phaseolus vulgaris L.) anthocyanins. J. Agric. Food Chem. 1997, 45, 3395–3400. [Google Scholar] [CrossRef]
- Mojica, L.; Meyer, A.; Berhow, M.A.; González de Mejía, E. Bean cultivars (Phaseolus vulgaris L.) have similar high antioxidant capacity, in vitro inhibition of α-amylase and α-glucosidase while diverse phenolic composition and concentration. J. Food Res. 2015, 69, 38–48. [Google Scholar] [CrossRef]
- Agama, E.; Rendon, R.; Tovar, J.; Paredes, O.; Islas, J.J.; Bello, L.A. In vitro starch digestibility changes during storage of maize flour tortillas. Nahrung 2004, 48, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Mora, A.; Lemus, B.; Miranda, R.; Hernández, D.; Pons, J.L.; Acosta, J.A.; Guzmán, S.H. Effects of common bean enrichment on nutritional quality of tortillas produced from nixtamalized regular and quality protein maize flours. J. Sci. Food Agric. 2007, 87, 880–886. [Google Scholar] [CrossRef]
- Oliveira de Lima, C.V.; Piuvezam, G.; Leal Lima Maciel, B.; Heloneida de Araújo Morais, A. Trypsin inhibitors: Promising candidate satietogenic proteins as complementary treatment for obesity and metabolic disorders? J. Enzym. Inhib. Med. Chem. 2019, 34, 405–419. [Google Scholar] [CrossRef]
- Serna-Saldivar, S.O.; Chuck-Hernandez, C. Food uses of lime-cooked corn with emphasis in tortillas and snacks. In Corn: Chemistry and Technology, 3rd ed.; Serna Saldivar, S.O., Ed.; AACC-International: Cambridge, MA, USA, 2018; pp. 469–500. [Google Scholar]
- Rosell, C.M.; Rojas, J.A.; Benedito de Barber, C. Influence of hydrocolloids on dough rheology and bread quality. Food Hydrocoll. 2001, 15, 75–81. [Google Scholar] [CrossRef]
- Mariscal, R.M.; Chuck-Hernandez, C.; Figueroa, J.d.D.; Serna, S.O. Physicochemical and nutritional evaluation of bread incorporated with ayocote bean (Phaseolus coccineus) and black bean (Phaseolus vulgaris). Processes 2021, 9, 1782. [Google Scholar] [CrossRef]
- Hoehnel, A.; Axel, C.; Bez, J.; Arendt, E.K.; Zannini, E. Comparative analysis of plant-based high-protein ingredients and their impact on quality of high-protein bread. J. Cereal Sci. 2019, 89, 102816. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 139–146. [Google Scholar] [CrossRef]
- Santiago, D.; Figueroa, J.D.D.; Véles, J.J.; Mariscal, R.M.; Reynoso, R.; Ramos, M.; Gaytán, M.; Morales, E. Resistant starch formation in tortillas from an ecological nixtamalization process. Cereal Chem. 2015, 92, 185–192. [Google Scholar] [CrossRef]
- Hsieh-Lo, M.; Castillo-Herrera, G.; Mojica, L. Black bean anthocyanin-rich extract from supercritical and pressurized extraction increased in vitro antidiabetic potential, while having similar storage stability. Foods 2020, 9, 655. [Google Scholar] [CrossRef] [PubMed]
- Inaguma, T.; Han, J.; Isoda, H. Improvement of insulin resistance by Cyanidin 3-glucoside, anthocyanin from black beans through the up-regulation of GLUT4 gene expression. In Proceedings of the 22nd European Society for Animal Cell Technology (ESACT) Meeting on Cell Based Technologies, Vienna, Austria, 15–18 May 2011. [Google Scholar] [CrossRef]
- Chávez-Santoscoy, R.A.; Gutiérrez, J.A.; Serna, S.O.; Perez, E. Production of maize tortillas and cookies from nixtamalized flour enriched with anthocyanins, flavonoids and saponins extracted from black bean (Phaseolus vulgaris) seed coats. Food Chem. 2016, 192, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Kayacier, A.; Singh, R.K. Textural properties of baked tortilla chips. LWT Food Sci. Technol. 2003, 36, 463–466. [Google Scholar] [CrossRef]


| Flour | Moisture (%) | Crude Protein (%) | Fat (%) | Ash (%) | Crude Fiber (%) | Carbohydrates by Difference (%) | Dietary Fiber (%) | Anthocyanins (µg/g) |
|---|---|---|---|---|---|---|---|---|
| Nixtamalized corn flour (NCF) | 10.81 ± 0.12 a | 8.34 ± 0.19 b | 1.87 ± 0.40 a | 1.50 ± 0.01 b | 4.76 ± 1.00 b | 72.73 ± 1.58 a | 6.26 ± 0.10 b | - |
| Black bean hulls (BBH) | 6.78 ± 0.19 b | 14.66 ± 1.07 a | 0.46 ± 0.02 b | 4.59 ± 0.05 a | 16.96 ± 0.50 a | 56.56 ± 1.17 b | 10.55 ± 0.15 a | 282.03 ± 25.15 |
| Flour | Total Digestible Starch (TDS, %) | Slowly Digestible Starch (SDS, %) | Rapidly Digestible Starch (RDS, %) | Resistant Starch (RS, %) | In Vitro Protein Digestibility | Trypsin Inhibitor (TIU/mg) | Average Particle Size (µm) | ||
|---|---|---|---|---|---|---|---|---|---|
| Percentile | |||||||||
| 50th | 90th | 98th | |||||||
| Nixtamalized corn flour (NCF) | 53.7 ± 3.2 a | 21.8 ± 0.3 a | 20.8 ± 1.1 a | 1.2 ± 0.1 b | 83.5 ± 1.3 a | 2.9 ± 0.2 b | 281.3 ± 4.7 a | 698.5 ± 7.5 a | 876.4 ± 3.1 a |
| Black bean hulls (BBH) | 16.8 ± 0.9 b | 1.5 ± 0.1 b | 3.5 ± 0.2 b | 7.6 ± 0.6 a | 76.6 ± 4.1 a | 7.4 ± 0.2 a | 78.9 ± 1.0 b | 309.5 ± 1.6 b | 501.9 ± 5.6 b |
| Parameter | Control | Tostada 10% BBH | Tostada 15% BBH | Tostada 20% BBH |
|---|---|---|---|---|
| C1 (Nm) | 1.95 ± 0.02 d | 2.14 ± 0.01 c | 2.22 ± 0.01 b | 2.37 ± 0.01 a |
| Stability (min) | 12.95 ±0.49 a | 10.55 ± 0.21 b | 10.85 ± 0.21 b | 11.5 ± 0.57 ab |
| C2 (Nm) | 1.16 ± 0.06 a | 1.24 ± 0.03 a | 1.27 ± 0.07 a | 1.3 ± 0.01 a |
| C3 (Nm) | 2.08 ± 0.05 a | 1.95 ± 0.01 b | 1.85 ± 0.01 b | 1.83 ± 0.04 b |
| Gelatinization rate (β) | 0.35 ± 0.05 a | 0.25 ± 0.02 ab | 0.17 ± 0.01 b | 0.17 ± 0.06 b |
| C4 (Nm) | 1.96 ± 0.08 a | 1.83 ± 0.02 ab | 1.75 ± 0 b | 1.72 ± 0.01 b |
| C5 (Nm) | 2.98 ± 0.19 a | 2.79 ± 0.01 a | 2.82 ± 0.02 a | 2.84 ± 0.02 a |
| Sample | Nixtamalized Corn Flour (%) | Black Bean Hulls (%) | Moisture (%) | Crude Protein (%) | Fat (%) | Ash (%) | Crude Fiber (%) | Carbohydrates by Difference (%) | Dietary Fiber (%) | Total Anthocyanin (µg/g Sample) Time: 2 Months |
|---|---|---|---|---|---|---|---|---|---|---|
| Control | 100 | - | 3.77 ± 0.20 c | 8.65 ± 0.20 b | 1.06 ± 0.05 a,b | 1.49 ± 0.00 d | 3.30 ± 0.90 b | 81.72 ± 1.08 a | 3.15 ± 0.17 d | - |
| Tostada 10% BBH | 90 | 10 | 4.93 ± 0.04 a | 9.26 ± 0.50 b | 1.55 ± 0.37 a | 1.82 ± 0.02 c | 4.66 ± 0.90 a,b | 77.78 ± 1.37 b | 4.02 ± 0.2 c | 28.20 ± 2.51 b |
| Tostada 15% BBH | 85 | 15 | 4.37 ± 0.04 b | 9.37 ± 0.71 b | 0.71 ± 0.30 b | 2.00 ± 0.01 b | 5.12 ± 1.10 a,b | 78.43 ± 1.77 b | 4.79 ± 0.33 b | 55.48 ± 5.17 a |
| Tostada 20% BBH | 80 | 20 | 4.40 ± 0.02 b | 10.93 ± 0.58 a | 0.44 ± 0.32 b | 2.15 ± 0.01 a | 6.00 ± 0.30 a | 76.08 ± 0.25 b | 5.65 ± 0.40 a | 66.61 ± 10.34 a |
| Sample | Nixtamalized Corn Flour (%) | Black Bean Hulls (%) | Rapidly Digestible Starch (RDS, %) | Slowly Digestible Starch (SDS, %) | Total Digestible Starch (TDS, %) | Resistant Starch (RS, %) | Predicted Glycemic Index | In Vitro Protein Digestibility | Trypsin Inhibitor (TIU/mg) |
|---|---|---|---|---|---|---|---|---|---|
| Control | 100 | - | 37.47 ± 0.96 a | 22.21 ± 0.58 a | 61.97 ± 1.91 a,b | 0.46 ± 0.01 c | 52.024 ± 0.14 a | 85.57 ± 2.70 a | 6.76 ± 0.87 a |
| Tostada 10% BBH | 90 | 10 | 36.17 ± 3.30 a | 13.10 ± 0.79 b | 65.52 ± 4.52 a | 1.25 ± 0.08 b | 50.96 ± 0.07 b | 85.33 ± 2.89 a | 8.86 ± 1.90 a |
| Tostada 15% BBH | 85 | 15 | 36.04 ± 0.94 a | 13.65 ± 0.43 b | 56.27 ± 3.66 b | 1.14 ± 0.08 b | 50.62 ± 0.07 b | 85.51 ± 1.38 a | 6.10 ± 0.25 a |
| Tostada 20% BBH | 80 | 20 | 35.73 ± 1.16 a | 20.94 ± 2.21 a | 59.07 ± 2.79 a,b | 2.30 ± 0.20 a | 49.17 ± 0.13 c | 84.22 ± 0.57 a | 7.98 ± 0.88 a |
| Parameter | Nixtamalized Corn Flour (100%) | Nixtamalized Corn Flour (90%): Black Bean Hulls (10%) | Nixtamalized Corn Flour (85%): Black Bean Hulls (15%) | Nixtamalized Corn Flour (80%): Black Bean Hulls (20%) |
|---|---|---|---|---|
| L* | 85.10 ± 3.99 a | 47.00 ± 5.34 b | 41.37 ± 3.94 b | 29.87 ± 2.67 c |
| a* | 8.61 ± 0.81 a | −0.25 ± 0.12 b | −0.25 ± 0.21 b | −0.13 ± 0.14 b |
| b* | 3.05 ± 0.41 a | 3.67 ± 1.28 a | 1.72 ± 0.76 b | 1.43 ± 0.25 b |
| Color Index | 33.39 ± 2.37 a | −1.82 ± 1.51 b | −3.72 ± 3.10 b | −2.97 ± 2.51 b |
| Delta E | - | 39.21 ± 8.23 b | 44.65 ± 3.1 b | 55.96 ± 4.92 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machado-Velarde, L.X.; Dávila-Vega, J.P.; Gutiérrez-Uribe, J.; Espinosa-Ramírez, J.; Martínez-Ávila, M.; Guajardo-Flores, D.; Chuck-Hernández, C. Black Bean Hulls as a Byproduct of an Extraction Process to Enhance Nutraceutical and Glycemic-Related Properties of Nixtamalized Maize Tostadas. Foods 2023, 12, 1915. https://doi.org/10.3390/foods12091915
Machado-Velarde LX, Dávila-Vega JP, Gutiérrez-Uribe J, Espinosa-Ramírez J, Martínez-Ávila M, Guajardo-Flores D, Chuck-Hernández C. Black Bean Hulls as a Byproduct of an Extraction Process to Enhance Nutraceutical and Glycemic-Related Properties of Nixtamalized Maize Tostadas. Foods. 2023; 12(9):1915. https://doi.org/10.3390/foods12091915
Chicago/Turabian StyleMachado-Velarde, Lesly Xiomara, Juan Pablo Dávila-Vega, Janet Gutiérrez-Uribe, Johanan Espinosa-Ramírez, Mariana Martínez-Ávila, Daniel Guajardo-Flores, and Cristina Chuck-Hernández. 2023. "Black Bean Hulls as a Byproduct of an Extraction Process to Enhance Nutraceutical and Glycemic-Related Properties of Nixtamalized Maize Tostadas" Foods 12, no. 9: 1915. https://doi.org/10.3390/foods12091915
APA StyleMachado-Velarde, L. X., Dávila-Vega, J. P., Gutiérrez-Uribe, J., Espinosa-Ramírez, J., Martínez-Ávila, M., Guajardo-Flores, D., & Chuck-Hernández, C. (2023). Black Bean Hulls as a Byproduct of an Extraction Process to Enhance Nutraceutical and Glycemic-Related Properties of Nixtamalized Maize Tostadas. Foods, 12(9), 1915. https://doi.org/10.3390/foods12091915







