Physicochemical Characteristics, Antioxidant Properties and Consumer Acceptance of Greek Yogurt Fortified with Apple Pomace Syrup
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
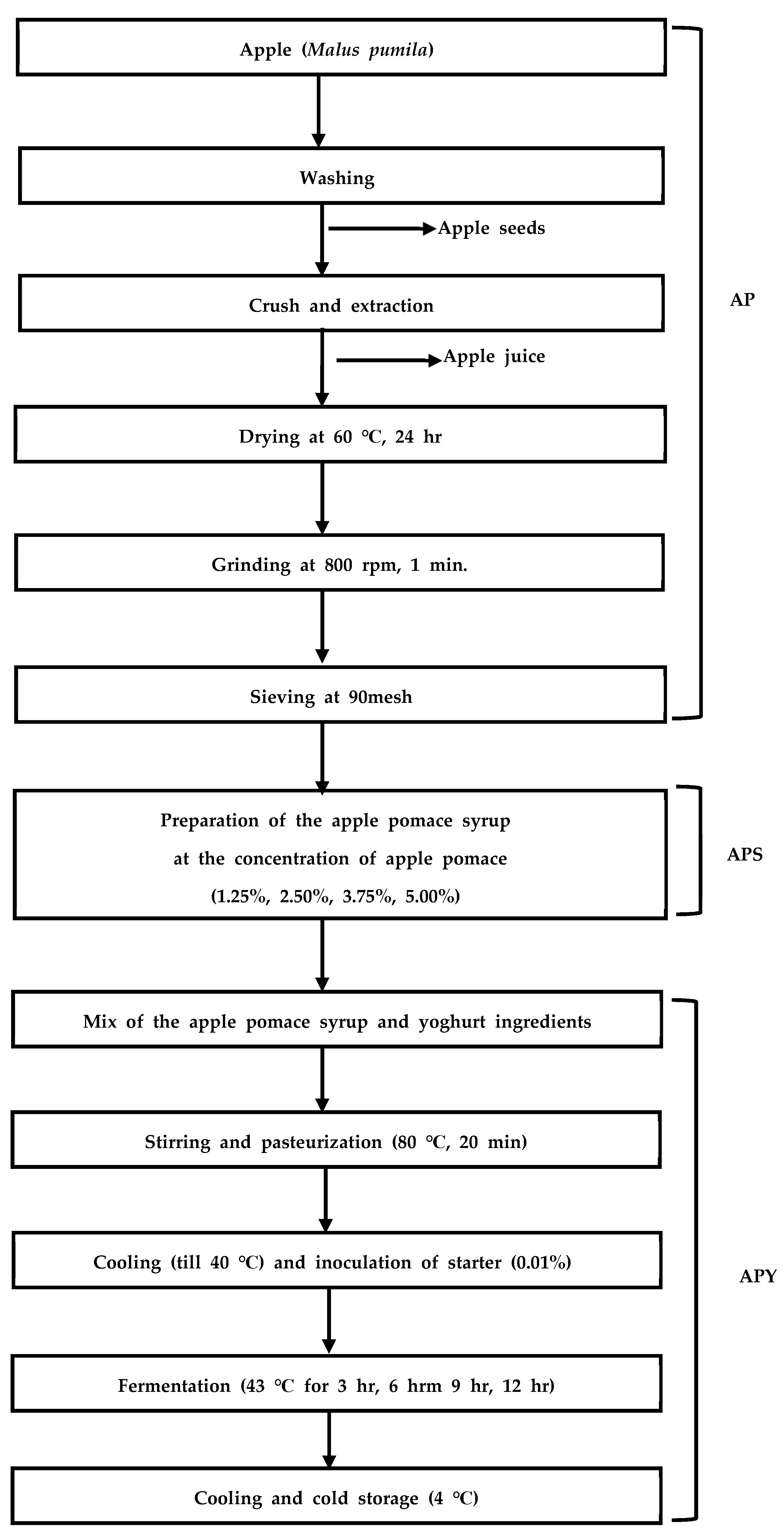
2.2. Preparation of Greek Yogurt Enhanced with Apple Pomace Syrup
2.3. Physicochemical Properties
2.4. Antioxidant Component Analysis and Antioxidant Activity Assay
2.5. Sensory Evaluation
2.6. Statistical Analysis
3. Results and Discussion
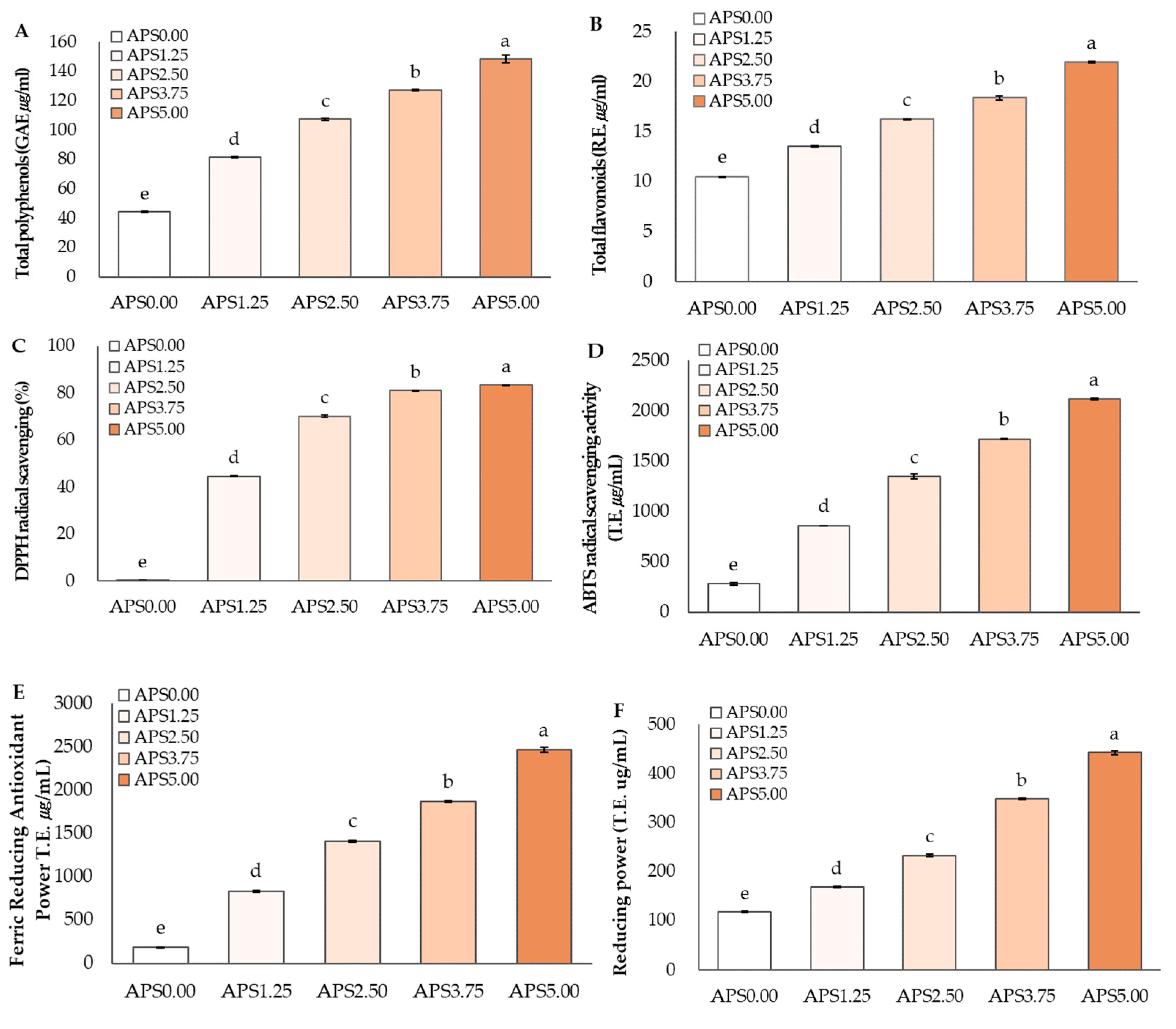
3.1. Physicochemical Characteristics of Apple Pomace Syrup
3.2. Antioxidant Components and Antioxidant Activities of Apple Pomace Syrup
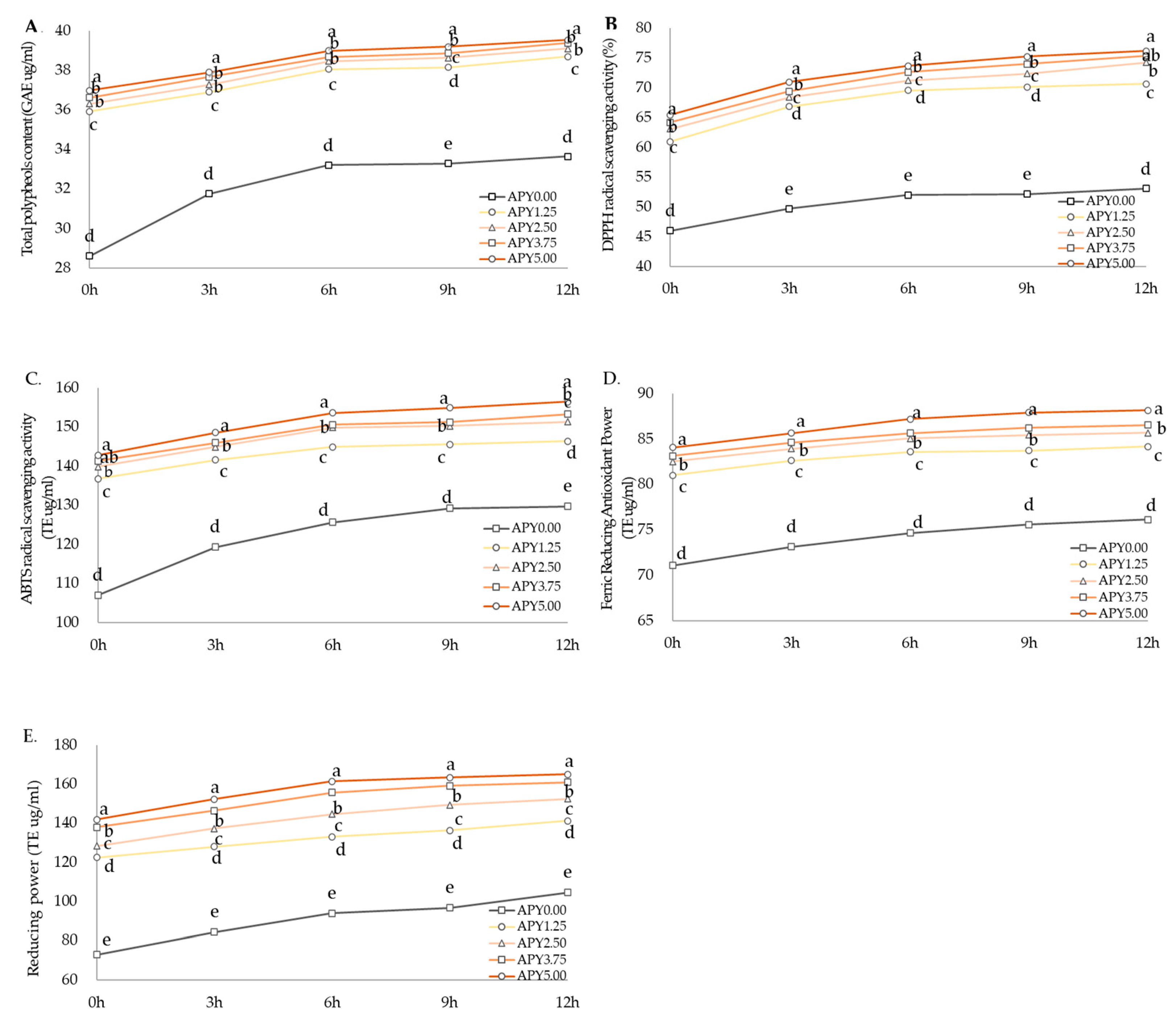
3.3. Physicochemical Characteristics of Greek Yogurt Fortified with Apple Pomace Syrup
3.4. Antioxidant Contents and Antioxidant Activities of Greek Yogurt Fortified with Apple Pomace Syrup
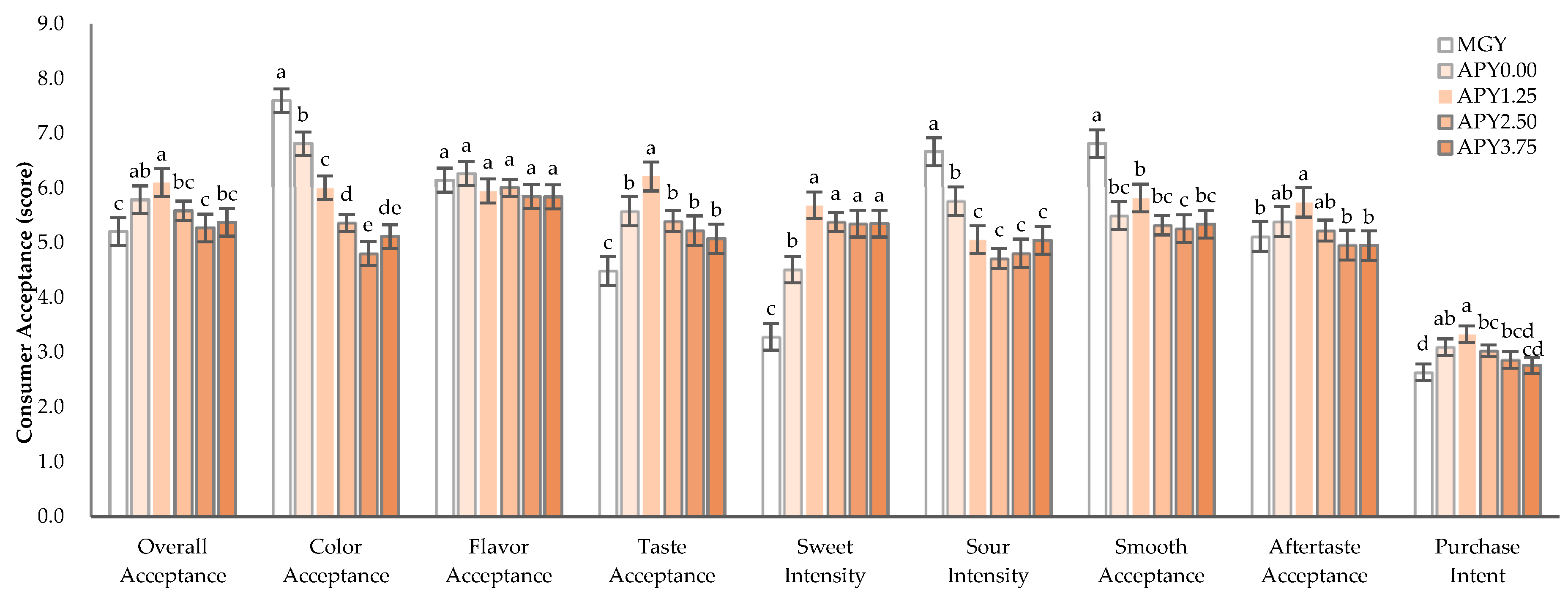
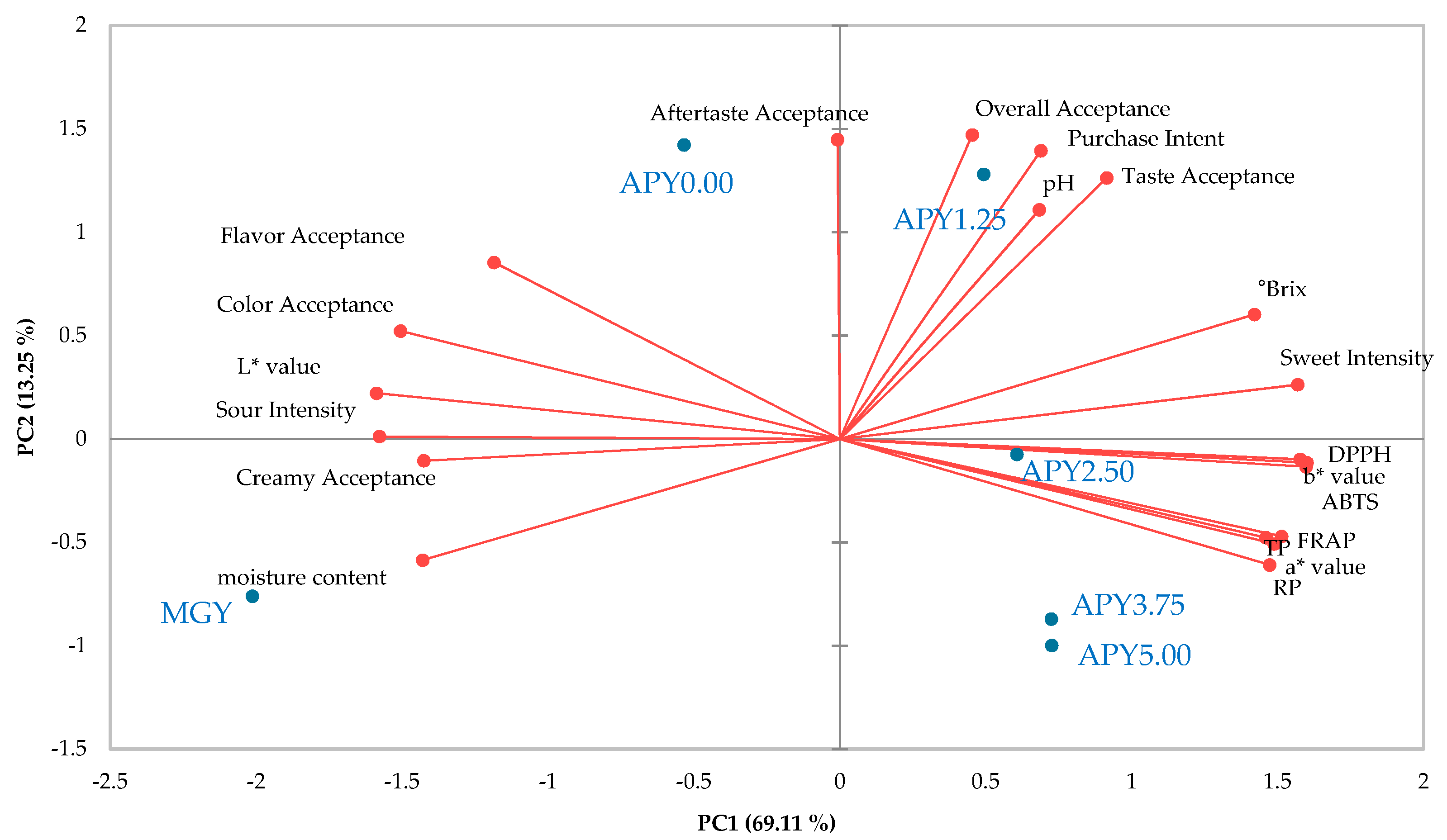
3.5. Consumer Acceptance of Greek Yogurt Fortified with Apple Pomace Syrup
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Vendruscolo, F.; Albuquerque, P.M.; Streit, F.; Esposito, E.; Ninow, J.L. Apple pomace: A versatile substrate for biotechnological applications. Crit. Rev. Biotechnol. 2008, 28, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lyu, F.; Luiz, S.F.; Azeredo, D.R.P.; Cruz, A.G.; Ajlouni, S.; Ranadheera, C.S. Apple pomace as a functional and healthy ingredient in food products: A review. Processes 2020, 8, 319. [Google Scholar] [CrossRef]
- Kim, J.; Shin, J.; Yang, J. Nutritional analyses and antioxidant activity of apple pomace. J. Life Sci. 2021, 31, 617–625. [Google Scholar] [CrossRef]
- Luo, J.; Xu, Y. Comparison of biological and chemical pretreatment on coproduction of pectin and fermentable sugars from apple pomace. Appl. Biochem. Biotechnol. 2020, 190, 129–137. [Google Scholar] [CrossRef]
- Yan, H.; Kerr, W.L. Total phenolics content, anthocyanins, and dietary fiber content of apple pomace powders produced by vacuum-belt drying. J. Sci. Food Agric. 2013, 93, 1499–1504. [Google Scholar] [CrossRef] [PubMed]
- Antonic, B.; Jancikova, S.; Dordevic, D.; Tremlova, B. Apple pomace as food fortification ingredients: A systematic review and meta-analysis. J. Food Sci. 2020, 85, 2977–2985. [Google Scholar] [CrossRef] [PubMed]
- Skinner, R.C.; Gigliotti, J.C.; Ku, K.; Tou, J.C. A comprehensive analysis of the composition, health benefits, and safety of apple pomace. Nutr. Rev. 2018, 76, 893–909. [Google Scholar] [CrossRef]
- Bai, X.; Zhang, H.; Ren, S. Antioxidant activity and HPLC analysis of polyphenol-enriched extracts from industrial apple pomace. J. Sci. Food Agric. 2013, 93, 2502–2506. [Google Scholar] [CrossRef] [PubMed]
- Sinrod, A.J.G.; Avena-Bustillos, R.J.; Olson, D.A.; Crawford, L.M.; Wang, S.C.; McHugh, T.H. Phenolics and antioxidant capacity of pitted olive pomace affected by three drying technologies. J. Food Sci. 2019, 84, 412–420. [Google Scholar] [CrossRef]
- Chang, X.; Chen, X.; Gong, P.; Yang, W.; Wang, L.; Liu, N.; Su, Y.; Zhao, Y. Ant-oxidant and anti-fatigue properties of apple pomace polysaccharides by acid or alkali extraction. Int. J. Food Sci. Technol. 2021, 57, 78–91. [Google Scholar] [CrossRef]
- Kalinowska, M.; Bielawska, A.; Lewandowska-Siwkiewicz, H.; Priebe, W.; Lewandowski, W. Apples: Content of phenolic compounds vs. variety, part of apple and cultivation model, extraction of phenolic compounds, biological properties. Plant Physiol. Biochem. 2014, 84, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Boussetta, N.; Lebovka, N.; Vorobiev, E. Effects of ultrasound treatment and concentration of ethanol on selectivity of phenolic extraction from apple pomace. Int. J. Food Sci. Technol. 2018, 53, 2104–2109. [Google Scholar] [CrossRef]
- Fernandes, P.A.R.; Ferreira, S.S.; Bastos, R.; Ferreira, I.; Cruz, M.T.; Pinto, A.; Coelho, E.; Passos, C.P.; Coimbra, M.A.; Cardoso, S.M.; et al. Apple pomace extract as sustainable food ingredient. Antioxidants 2019, 8, 189. [Google Scholar] [CrossRef]
- Fernandes, P.A.R.; Bourvellec, C.L.; Renard, C.M.G.C.; Nunes, F.M.; Bastos, R.; Coelho, E.; Wessel, D.F.; Coimbra, M.A.; Cardoso, S.M. Revisiting the chemistry of apple pomace polyphenols. Food Chem. 2019, 294, 9–18. [Google Scholar] [CrossRef]
- Skrypnik, L.; Novikova, A. Response surface modeling and optimization of polyphenols extraction from apple pomace based on nonionic emulsifiers. Agronomy 2020, 10, 92. [Google Scholar] [CrossRef]
- Schmid, V.; Karbstein, H.P.; Emin, M.A. The Influence of extrusion processing on the gelation properties of apple pomace dispersions: Involved cell wall components and their gelation kinetics. Foods 2020, 9, 1536. [Google Scholar] [CrossRef]
- Ahmed, M.; Ali, A.; Sarfraz, A.; Hong, Q.; Boran, H. Effect of freeze-drying on apple pomace and pomegranate peel powders used as a source of bioactive ingredients for the development of functional yogurt. J. Food Qual. 2022, 2022, 3327401. [Google Scholar] [CrossRef]
- Pollini, L.; Juan-García, A.; Blasi, F.; Mañes, J.; Cossignani, L.; Juan, C. Assessing bioaccessibility and bioavailability in vitro of phenolic compounds from freeze-dried apple pomace by LC-Q-TOF-MS. Food Biosci. 2022, 48, 101799–101806. [Google Scholar] [CrossRef]
- Tulej, W.; Głowacki, S. Modeling of the drying process of apple pomace. Appl. Sci. 2022, 12, 1434. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Kaur, S.; Brar, S.K. Perspective of apple processing wastes as low-cost substrates for bioproduction of high value products: A review. Renew. Sustain. Energy Rev. 2013, 27, 789–805. [Google Scholar] [CrossRef]
- Golebiewska, E.; Kalinowska, M.; Yildiz, G. Sustainable use of apple pomace (AP) in different industrial sectors. Materials 2022, 15, 1788. [Google Scholar] [CrossRef] [PubMed]
- Issar, K.; Sharma, P.C.; Gupta, A. Utilization of apple pomace in the preparation of fiber-enriched acidophilus yoghurt. J. Food Process. Preserv. 2017, 41, 13098–13104. [Google Scholar] [CrossRef]
- Yan, T.; Liu, R.; Shi, L.; Wang, Y.; Meng, X.; Shen, Y. Superfine grinding improves the physicochemical, sensory and functional characteristics of Hanfu apple pomace. Int. J. Food Sci. Technol. 2023, 58, 2077–2084. [Google Scholar] [CrossRef]
- Oh, C.; Kang, C. Effect of apple pomace on cookie quality. Culin. Sci. Hosp. Res. 2016, 22, 89–98. [Google Scholar] [CrossRef]
- Kim, Y.K.; Jeong, S.; Cha, S.; Yi, J.; Kim, D.; Yoo, D.; Hyun, T.K.; Jang, K. Quality and antioxidant properties of muffin added with ‘Fuji’ apple pomace powder. J. Korean Soc. Food Sci. Nutr. 2019, 48, 319–327. [Google Scholar] [CrossRef]
- Skaltsi, A.; Marinopoulou, A.; Poriazi, A.; Petridis, D.; Papageorgiou, M. Development and optimization of gluten-free biscuits with carob flour and dry apple pomace. J. Food Process. Preserv. 2021, 46, e15938–e15952. [Google Scholar] [CrossRef]
- Reis, S.F.; Rai, D.K.; Abu-Channam, N. Apple pomace as a potential ingredient for the development of new functional foods. J. Food Sci. Technol. 2014, 49, 1743–1750. [Google Scholar] [CrossRef]
- Lu, Q.; Liu, H.; Wang, Q.; Liu, J. Sensory and physical quality characteristics of bread fortified with apple pomace using fuzzy mathematical model. Int. J. Food Sci. Technol. 2017, 52, 1092–1100. [Google Scholar] [CrossRef]
- Ricci, A.; Cirlini, M.; Guido, A.; Liberatore, C.M.; Ganino, T.; Lazzi, C.; Chiancone, B. From byproduct to resource: Fermented apple pomace as beer flavoring. Foods 2019, 8, 309. [Google Scholar] [CrossRef]
- Kim, J.; Jo, S.; Kim, E.; Jeong, D. Development of an apple/pear pomace fermented with Lentinus edodes Mycelia. Korean J. Food Sci. Technol. 2019, 51, 286–294. [Google Scholar] [CrossRef]
- Wang, X.; Kristo, E.; LaPointe, G. Adding apple pomace as a functional ingredient in stirred-type yogurt and yogurt drinks. Food Hydrocoll. 2020, 100, 105453–105462. [Google Scholar] [CrossRef]
- Jovanović, M.; Petrović, M.; Miočinović, J.; Zlatanovic, S.; Petronijević, J.L.; Mitić-Ćulafić, D.; Gorjanović, S. Bioactivity and sensory properties of probiotic yogurt fortified with apple pomace flour. Foods 2020, 9, 763. [Google Scholar] [CrossRef]
- Ahmad, I.; Khalique, A.; Junaid, M.; Shahid, M.Q.; Imran, M.; Rashid, A.A. Effect of polyphenol from apple peel extract on the survival of probiotics in yoghurt ice cream. Int. J. Food Sci. Technol. 2020, 55, 2580–2588. [Google Scholar] [CrossRef]
- Ahmad, I.; Khalique, A.; Shahid, M.Q.; Rashid, A.A.; Faiz, F.; Ikram, M.A.; Ahmed, S.; Imran, M.; Khan, M.A.; Nadeem, M.; et al. Studying the influence of apple peel polyphenol extract fortification on the characteristics of probiotic yoghurt. Plants 2020, 9, 77. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Zhou, S.; Ye, F.; Zhou, G.; Gao, R.; Qin, D.; Zhao, G. A novel cholesterol-free mayonnaise made from Pickering emulsion stabilized by apple pomace particles. Food Chem. 2021, 353, 129418–129426. [Google Scholar] [CrossRef]
- Kok, C.R.; Hutkins, R. Yogurt and other fermented foods as sources of health-promoting bacteria. Nutr. Rev. 2018, 76 (Suppl. 1), 4–15. [Google Scholar] [CrossRef]
- Sun, J.; Song, J.; Yang, J.; Chen, L.; Wang, Z.; Duan, M.; Yang, S.; Hu, C.; Bi, Q. Higher yogurt consumption is associated with lower risk of colorectal cancer: A systematic review and meta-analysis of observational studies. Front. Nutr. 2022, 8, 789006. [Google Scholar] [CrossRef] [PubMed]
- Pourrajab, B.; Fatahi, S.; Dehnad, A.; Varkaneh, H.K.; Shidfar, F. The impact of probiotic yogurt consumption on lipid profiles in subjects with mild to moderate hypercholesterolemia: A systematic review and meta-analysis of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Cornes, R.; Sintes, C.; Peña, A.; Albin, S.; O’Brien, K.O.; Abrams, S.A.; Donangelo, C.M. Daily intake of a functional synbiotic yogurt increases calcium absorption in young adult women. J. Nutr. 2022, 152, 1647–1654. [Google Scholar] [CrossRef]
- Savaiano, D.A. Lactose digestion from yogurt: Mechanism and relevance. Am. J. Clin. Nutr. 2014, 99, 1251S–1255S. [Google Scholar] [CrossRef] [PubMed]
- Illikoud, N.; Mantel, M.; Rolli-Derkinderen, M.; Gagnaire, V.; Jan, G. Dairy starters and fermented dairy products modulate gut mucosal immunity. Immunol. Lett. 2022, 251–252, 91–102. [Google Scholar] [CrossRef]
- Salas-Salvadó, J.; Guasch-Ferré, M.; Díaz-López, A.; Babio, N. Yogurt and diabetes: Overview of recent observational studies. J. Nutr. 2017, 147, 1452S–1461S. [Google Scholar] [CrossRef] [PubMed]
- Codex Alimentarius. Standard for Fermented Milks—Codex Stan 243-2003; CODEX: Rome, Italy, 2018; pp. 1–12. [Google Scholar]
- Food & Drug Administration. CFR—Code of Federal Regulations Title 21; FDA: Washington, DC, USA, 2023. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=131.200 (accessed on 24 April 2023).
- Nyanzi, R.; Jooste, P.J.; Buys, E.M. Invited Review: Probiotic yogurt quality criteria, regulatory framework, clinical evidence, and analytical aspects. J. Dairy Sci. 2021, 104, 1–19. [Google Scholar] [CrossRef]
- Korea Ministry of Education. Guidelines for the School Cafeteria Sanitation 11-1340000-000185-14; Korea Ministry of Education: Sejong, Republic of Korea, 2021; pp. 1–294.
- AOAC. Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2000; pp. 33–36. [Google Scholar]
- Arnous, A.; Makris, D.P.; Kefalas, P. Effect of principal polyphenolic components in relation to antioxidant characteristics of aged red wines. J. Agric. Food Chem. 2001, 49, 5736–5742. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Jin, L.; Xiao, P.; Lu, Y.; Bao, J. Total phenolics, flavonoids, antioxidant capacity in rice grain and their relations to grain color, size, and weight. J. Cereal Sci. 2009, 49, 106–111. [Google Scholar] [CrossRef]
- Garavand, F.; Daly, D.F.; Gomez-Mascaraque, L.G. Biofunctional, structural, and tribological attributes of GABA-enriched probiotic yoghurts containing Lacticaseibacillus paracasei alone or in combination with prebiotics. Int. Dairy J. 2022, 129, 105348. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. J. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reaction: Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Bouhlali, E.; Derouich, M.; Meziani, R.; Bourkhis, B.; Filali-Zegzouti, Y.; Alem, C. Nutritional, mineral and organic acid composition of syrups produced from six Moroccan date fruit (Phoenix dactylifera L.) varieties. J. Food Compos. Anal. 2020, 93, 103591–103598. [Google Scholar] [CrossRef]
- Wang, X.; Kristo, E.; LaPointe, G. The effect of apple pomace on the texture, rheology and microstructure of set type yogurt. Food Hydrocoll. 2019, 91, 83–91. [Google Scholar] [CrossRef]
- Korea Ministry of Food and Drug Safety. Standard for Sugar—Korean Food Standards Codex 2022-84; Korea Ministry of Food and Drug Safety: Cheongju, Republic of Korea, 2022; pp. 1–7.
- Codex Alimentarius. Standard for Sugar—Codex Stan 212-1999; CODEX: Rome, Italy, 2019; pp. 1–6. [Google Scholar]
- Kim, S.; Park, J.; Hwang, I.K. Quality attributes of various varieties of Korean red pepper powders (Capsicum annuum L.) and color stability during sunlight exposure. J. Food Sci. 2002, 67, 2957–2961. [Google Scholar] [CrossRef]
- Rana, A.; Samtiya, M.; Dhewa, T.; Mishra, V.; Aluko, R.E. Health benefits of polyphenols: A concise review. J. Food Biochem. 2022, 45, e14264. [Google Scholar] [CrossRef]
- Lee, K.; Yoon, Y.; Kwon, H.; Lee, E. Antioxidant component and activity of different part extracts in apple (Malus domestica cv. Fuji). Korean J. Food Nutr. 2018, 31, 858–864. [Google Scholar] [CrossRef]
- Szôllôsi, R.; Varga, S. Total antioxidant power in some species of Labiatae (adaptation of FRAP method). Acta Biol. Szeged. 2002, 46, 125–127. [Google Scholar]
- Sung, J.M.; Choi, H.Y. Effect of mulberry powder on antioxidant activities and quality characteristics of yogurt. J. Korean Soc. Food Sci. Nutr. 2014, 43, 690–697. [Google Scholar] [CrossRef]
- Kim, D.S.; Oh, H.B.; Kim, S.Y.; Lee, P.R.; Kim, Y.S. Quality characteristics and antioxidant activity of fermented milk added with Cacao nibs powder. Culin. Sci. Hosp. Res. 2020, 26, 55–65. [Google Scholar]
- Yang, J.; Choi, I.S. Comparisons of the physicochemical characteristics, antioxidant properties, and consumer acceptance of Greek-style yogurt enhanced with black tea syrup instead of sugar syrup. J. East Asian Soc. Diet. Life 2021, 31, 36–49. [Google Scholar] [CrossRef]
- Fanyuk, M.; Patel, M.K.; Ovadia, R.; Maurer, D.; Feygenberg, O.; Oren-Shamir, M.; Alkan, N. Preharvest application of phenylalanine induces red color in mango and apple fruit’s skin. Antioxidants 2022, 11, 491. [Google Scholar] [CrossRef] [PubMed]
- Santos, K.M.; Oliveira, I.C.; Lopes, M.A.; Cruz, A.P.G.; Buriti, F.C.; Cabral, L.M. Addition of grape pomace extract to probiotic fermented goat milk: The effect on phenolic content, probiotic viability and sensory acceptability. J. Sci. Food Agric. 2017, 97, 1108–1115. [Google Scholar] [CrossRef]
- Yeo, S.; Yeo, S.; Park, H. Quality characteristics, antioxidant activity and storage properties of fermented milk added with green tea powder. Korean J. Food Preserv. 2017, 24, 576–584. [Google Scholar] [CrossRef]
- Ćetković, G.; Čanadanović-Brunet, J.; Djilas, S.; Savatović, S.; Mandić, A.; Tumbas, V. Assessment of polyphenolic content and in vitro antiradical characteristics of apple pomace. Food Chem. 2008, 109, 340–347. [Google Scholar] [CrossRef] [PubMed]
| APS0.00 | APS1.25 | APS2.50 | APS3.75 | APS5.00 | ||
|---|---|---|---|---|---|---|
| Moisture content (%) | 30.58 ± 0.28 b | 35.41 ± 0.28 a | 35.59 ± 0.16 a | 38.20 ± 0.60 a | 39.64 ± 0.58 a | |
| Crude ash content (%) | 0.02 ± 0.02 c | 0.03 ± 0.03 c | 0.14 ± 0.01 b | 0.16 ± 0.02 b | 0.21 ± 0.03 a | |
| Total soluble solids (°Brix) | 61.37 ± 0.06 d | 62.03 ± 0.06 c | 62.90 ± 0.00 a | 60.20 ± 0.01 e | 62.23 ± 0.06 b | |
| pH | 4.77 ± 0.01 a | 4.61 ± 0.00 b | 4.46 ± 0.01 c | 4.32 ± 0.01 d | 4.28 ± 0.01 e | |
| Titratable acidity (%) | 0.01 ± 0.00 e | 0.03 ± 0.00 d | 0.06 ± 0.00 c | 0.08 ± 0.00 b | 0.12 ± 0.00 a | |
| Viscosity (cP) | ND | 169.37 ± 3.06 b | 1213.00 ± 6.93 b | 2323.33 ± 2.08 b | 33606.33 ± 25.40 a | |
| Color | L* value | 60.10 ± 0.69 a | 54.10 ± 0.17 b | 50.23 ± 0.32 c | 48.67 ± 0.32 d | 44.27 ± 0.84 e |
| a* value | −1.00 ± 0.00 d | 0.67 ± 0.06 c | 1.97 ± 0.15 b | 2.53 ± 0.29 a | 2.37 ± 0.12 a | |
| b* value | −2.03 ± 0.31 d | 6.03 ± 0.40 b | 7.27 ± 0.06 a | 6.80 ± 0.35 a | 2.77 ± 0.35 e | |
| ΔE* | - | 9.10 ± 0.42 d | 12.50 ± 0.22 c | 13.30 ± 0.23 b | 14.9 ± 0.70 a | |
| Fermentation Time (hr) | APY0.00 | APY1.25 | APY2.50 | APY3.75 | APY5.00 | ||
|---|---|---|---|---|---|---|---|
| Moisture content (%) | 0 | 79.90 ± 0.06 aA | 79.91 ± 0.01 aA | 79.92 ± 0.02 aA | 79.96 ± 0.05 aA | 80.06 ± 0.06 aA | |
| 3 | 79.81 ± 0.12 aB | 79.83 ± 0.03 aB | 79.89 ± 0.05 aAB | 79.93 ± 0.05 aAB | 80.02 ± 0.24 aA | ||
| 6 | 79.55 ± 0.15 bB | 79.61 ± 0.09bAB | 79.65 ± 0.04 bAB | 79.67 ± 0.02 bAB | 79.70 ± 0.03 bA | ||
| 9 | 79.52 ± 0.07 bB | 79.54 ± 0.08 bB | 79.58 ± 0.06 cAB | 79.61 ± 0.03 cAB | 79.66 ± 0.13 cA | ||
| 12 | 79.51 ± 0.07 bC | 79.53 ± 0.08 bBC | 79.58 ± 0.05 cABC | 79.61 ± 0.02 cAB | 79.66 ± 0.03 cA | ||
| Crude ash content (%) | 0 | 1.50 ± 0.04 bB | 1.52 ±0.02 cAB | 1.53 ± 0.02 cAB | 1.53 ± 0.03 cAB | 1.54 ± 0.00 cA | |
| 3 | 1.51 ± 0.03 bB | 1.53 ± 0.01 cAB | 1.54 ± 0.01 cA | 1.55 ± 0.01 cA | 1.55 ± 0.01 cA | ||
| 6 | 1.55 ± 0.01 abC | 1.57 ± 0.01 bBC | 1.57 ± 0.01 bAB | 1.58 ± 0.02 bAB | 1.59 ± 0.01 bA | ||
| 9 | 1.59 ± 0.05 aA | 1.60 ± 0.03 aA | 1.61 ± 0.02 aA | 1.62 ± 0.01 aA | 1.63 ± 0.02 aA | ||
| 12 | 1.60 ± 0.01 aD | 1.61 ± 0.02 aCD | 1.62 ± 0.01 aBC | 1.63 ± 0.01 aAB | 1.64 ± 0.01 aA | ||
| Total soluble solids (°Brix) | 0 | 22.20 ± 0.00 aA | 21.70 ± 0.00 aB | 21.70 ± 0.00 aB | 21.70 ± 0.00 aB | 21.23 ± 0.05 aC | |
| 3 | 20.70 ± 0.20 bA | 20.65 ± 0.10 bA | 20.65 ± 0.06 bA | 20.60 ± 0.00 bA | 20.60 ± 0.00 bA | ||
| 6 | 20.40 ± 0.00 cA | 20.00 ± 0.00 cB | 20.00 ± 0.00 cB | 20.00 ± 0.00 cB | 19.90 ± 0.08 cC | ||
| 9 | 20.35 ± 0.06 cA | 20.00 ± 0.00 cB | 20.00 ± 0.00 cB | 20.00 ± 0.00 cB | 19.88 ± 0.10 cC | ||
| 12 | 19.58 ± 0.26 dA | 19.40 ± 0.14 dAB | 19.30 ± 0.12 dB | 19.88 ± 0.10 dBC | 19.00 ± 0.00 dC | ||
| Reducing sugar (mg/mL) | 0 | 0.55 ± 0.00 dC | 0.66 ± 0.01 dB | 0.66 ± 0.01 cB | 0.68 ± 0.01 dA | 0.68 ± 0.01 dA | |
| 3 | 0.56 ± 0.00 cC | 0.68 ± 0.00 cB | 0.68 ± 0.00 bB | 0.69 ± 0.01 cB | 0.70 ± 0.01 cA | ||
| 6 | 0.57 ± 0.00 bcD | 0.69 ± 0.01 bcC | 0.69 ± 0.00 bD | 0.70 ± 0.00 bcB | 0.71 ± 0.00 bcA | ||
| 9 | 0.57 ± 0.00 abE | 0.69 ± 0.00 abD | 0.70 ± 0.00 aC | 0.70 ± 0.00 abB | 0.71 ± 0.00 abA | ||
| 12 | 0.58 ± 0.00 aD | 0.70 ± 0.00 aC | 0.70 ± 0.00 aB | 0.71 ± 0.00 aAB | 0.71 ± 0.00aA | ||
| pH | 0 | 6.85 ± 0.01 aA | 6.74 ± 0.01 aB | 6.71 ± 0.02 aB | 6.67 ± 0.03 aC | 6.66 ± 0.03 aC | |
| 3 | 5.38 ± 0.06 bA | 5.12 ± 0.04 bB | 5.12 ± 0.06 bB | 5.10 ± 0.05 bB | 5.08 ± 0.03 bB | ||
| 6 | 4.77 ± 0.64 cA | 4.56 ± 0.01 cB | 4.56 ± 0.02 cB | 4.56 ± 0.02 cB | 4.56 ± 0.01 cB | ||
| 9 | 4.46 ± 0.06 dA | 4.38 ± 0.01 dB | 4.37 ± 0.00 dB | 4.37 ± 0.02 dB | 4.36 ± 0.01 dB | ||
| 12 | 4.41 ± 0.03 eA | 4.30 ± 0.02 eB | 4.28 ± 0.01 eB | 4.28 ± 0.01 eB | 4.28 ± 0.02 eB | ||
| Titratable acidity (%) | 0 | 0.07 ± 0.00 eC | 0.07 ± 0.00 eC | 0.07 ± 0.00 eC | 0.07 ± 0.00 eB | 0.09 ± 0.00 eA | |
| 3 | 0.39 ± 0.01 dC | 0.40 ± 0.00 dC | 0.41 ± 0.01 dB | 0.42 ± 0.01 dB | 0.43 ± 0.00 dA | ||
| 6 | 0.41 ± 0.00 cE | 0.45 ± 0.00 cD | 0.46 ± 0.00 cC | 0.46 ± 0.00 cB | 0.47 ± 0.00 cA | ||
| 9 | 0.45 ± 0.00 bD | 0.48 ± 0.00 bC | 0.49 ± 0.00 bB | 0.49 ± 0.00 bB | 0.51 ± 0.00 bA | ||
| 12 | 0.47 ± 0.00 aD | 0.50 ± 0.00 aC | 0.51 ± 0.00 aB | 0.52 ± 0.00 aB | 0.52 ± 0.00 aA | ||
| Color | L* value | 0 | 81.65 ± 0.10 cA | 74.48 ± 0.49 cB | 69.60 ± 0.08 dC | 68.58 ± 0.10 cD | 67.48 ± 0.53 cE |
| 3 | 85.03 ± 1.15 bA | 81.40 ± 0.39 bB | 79.40 ± 0.35 cC | 78.18 ± 0.78 cD | 77.40 ± 0.44 bD | ||
| 6 | 87.83 ± 0.29 aA | 84.70 ± 0.12 aB | 83.80 ± 0.14 bC | 82.85 ± 0.24 bD | 82.53 ± 0.05 aE | ||
| 9 | 87.90 ± 0.18 aA | 84.73 ± 0.13 aB | 83.75 ± 0.06 aC | 82.70 ± 0.28 aD | 82.68 ± 0.05 aD | ||
| 12 | 87.95 ± 0.13 aA | 84.98 ± 0.10 aB | 84.10 ± 0.14 aC | 83.15 ± 0.13 aD | 82.88 ± 0.01 aE | ||
| a* value | 0 | −4.70 ± 0.00 dC | 1.15 ± 0.10 dB | 1.20 ± 0.18 dB | 1.75 ± 0.17 cA | 1.93 ± 0.54 bA | |
| 3 | −2.43 ± 0.21 cC | 1.40 ± 0.08 cB | 1.50 ± 0.08 cB | 1.78 ± 0.10 cA | 1.80 ± 0.08 bA | ||
| 6 | −1.48 ± 0.17 bD | 1.45 ± 0.06 cC | 1.80 ± 0.08 bB | 2.00 ± 0.08 bA | 2.08 ± 0.10 bA | ||
| 9 | −1.33 ± 0.10 bE | 1.68 ± 0.05 bD | 2.25 ± 0.06 aC | 2.53 ± 0.10 aB | 2.80 ± 0.08 aA | ||
| 12 | −1.10 ± 0.08 aE | 1.83 ± 0.10 aD | 2.38 ± 0.05 aC | 2.60 ± 0.08 aB | 2.90 ± 0.08 aA | ||
| b* value | 0 | 7.35 ± 0.13 dE | 15.88 ± 0.10 aD | 16.85 ± 0.17 aC | 17.60 ± 0.36 aB | 18.13 ± 0.22 aA | |
| 3 | 10.35 ± 0.17 cE | 14.28 ± 0.13 bD | 14.70 ± 0.29 bC | 15.18 ± 0.26 bB | 15.53 ± 0.15 bA | ||
| 6 | 11.15 ± 0.19 bD | 14.00 ± 0.00 cC | 14.15 ± 0.06 cC | 14.75 ± 0.17 cB | 15.03 ± 0.10 cA | ||
| 9 | 11.25 ± 0.17 bB | 13.08 ± 0.15 dC | 14.15 ± 0.10 cB | 14.23 ± 0.05 dB | 14.63 ± 0.05 dA | ||
| 12 | 11.73 ± 0.10 aD | 12.75 ± 0.19 eC | 13.98 ± 0.05 cB | 14.15 ± 0.13 dB | 14.50 ± 0.08 dA | ||
| ΔE* | 0 | - | 12.6 ± 0.32 dA | 16.4 ± 0.18 cA | 17.8 ± 0.29 bA | 19.1 ± 0.59 aA | |
| 3 | 5.0 ± 0.92 cB | 9.2 ± 0.14 bB | 9.9 ± 0.30 bBC | 10.7 ± 0.29 aB | 11.3 ± 0.26 aB | ||
| 6 | 7.9 ± 0.21 dA | 9.5 ± 0.05 cC | 9.6 ± 0.06 cC | 10.0 ± 0.12 bC | 10.2 ± 0.13 aC | ||
| 9 | 8.0 ± 0.11 dA | 9.1 ± 0.14 cC | 9.9 ± 0.10 bB | 10.0 ± 0.10 bC | 10,5 ± 0.07 aC | ||
| 12 | 8.4 ± 0.11 dA | 9.1 ± 0.17 cC | 10.0 ± 0.04 bB | 10.0 ± 0.05 bC | 10.5 ± 0.09 aC | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Kim, M.; Choi, I. Physicochemical Characteristics, Antioxidant Properties and Consumer Acceptance of Greek Yogurt Fortified with Apple Pomace Syrup. Foods 2023, 12, 1856. https://doi.org/10.3390/foods12091856
Kim J, Kim M, Choi I. Physicochemical Characteristics, Antioxidant Properties and Consumer Acceptance of Greek Yogurt Fortified with Apple Pomace Syrup. Foods. 2023; 12(9):1856. https://doi.org/10.3390/foods12091856
Chicago/Turabian StyleKim, Jisoo, Moonsook Kim, and Ilsook Choi. 2023. "Physicochemical Characteristics, Antioxidant Properties and Consumer Acceptance of Greek Yogurt Fortified with Apple Pomace Syrup" Foods 12, no. 9: 1856. https://doi.org/10.3390/foods12091856
APA StyleKim, J., Kim, M., & Choi, I. (2023). Physicochemical Characteristics, Antioxidant Properties and Consumer Acceptance of Greek Yogurt Fortified with Apple Pomace Syrup. Foods, 12(9), 1856. https://doi.org/10.3390/foods12091856





