Complexation with Polysaccharides Enhances the Stability of Isolated Anthocyanins
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Polyphenol Extraction and Anthocyanin Fractionation
2.3. Determination of Total Phenolic Content
2.4. Determination of Total Anthocyanin Content
2.5. Complexation
2.6. Characterization of Complexes
2.6.1. Zeta Potential and Dynamic Light Scattering (DLS) Analyses
2.6.2. Scanning Electron Microscopy (SEM)
2.6.3. FTIR Spectroscopy
2.7. Stability of Anthocyanins
2.7.1. In Vitro Digestion
2.7.2. Complexation Efficiency
2.7.3. Effect of pH on the Stability of Anthocyanins
2.8. Cell Culture
2.8.1. Anthocyanin Colonic Concentration of Complexes
2.8.2. Effects of Complexes in LPS-Induced High Cell Permeability
2.9. Statistical Analysis
3. Results
3.1. Characterization of Complexes
3.1.1. Zeta Potential
3.1.2. Particle Size and Polydispersity Index through Dynamic Light Scattering
3.1.3. Scanning Electron Microscopy (SEM)
3.1.4. Structural Analysis Using Fourier-Transform Infrared Spectroscopy (FTIR)
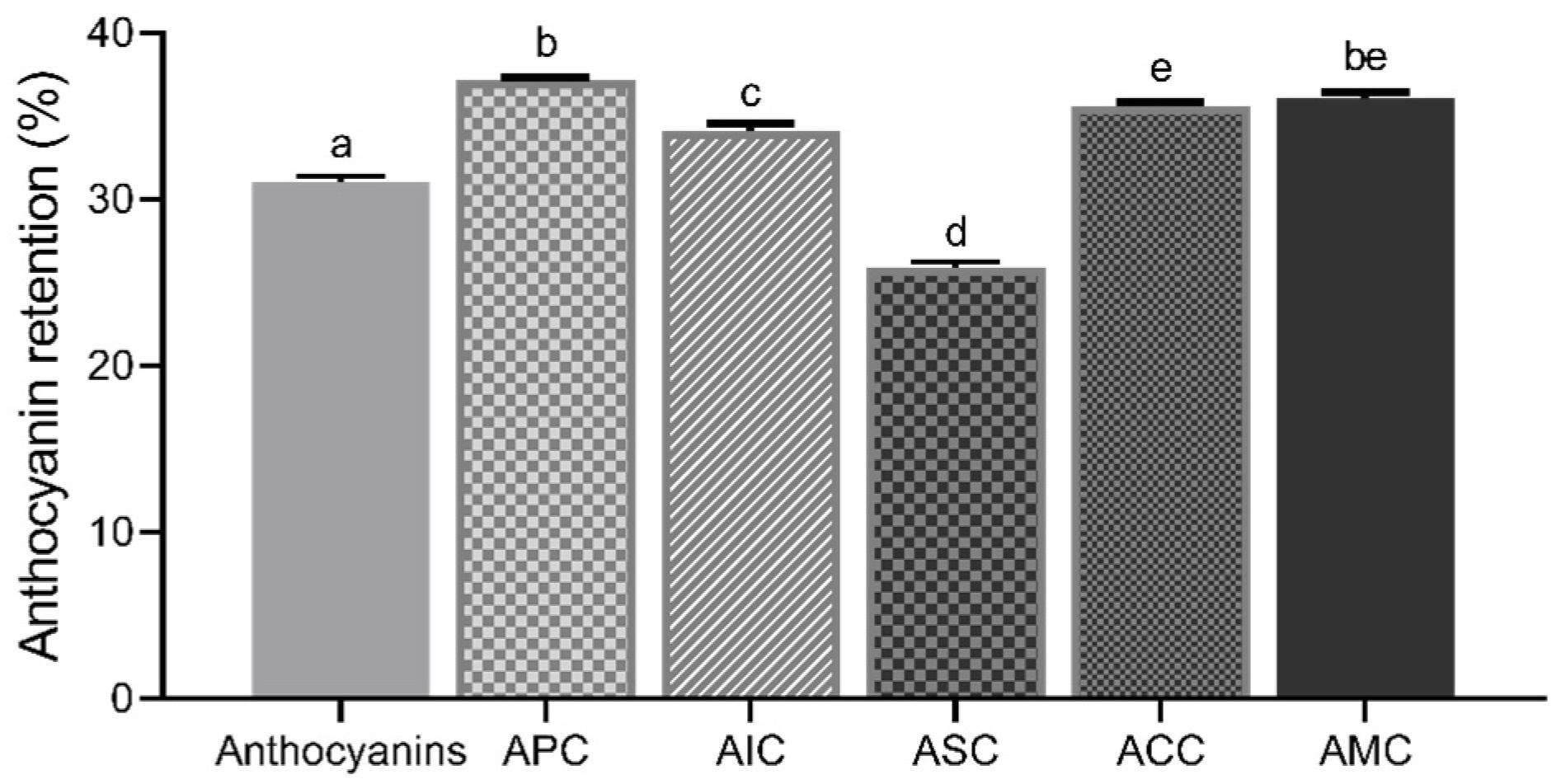
3.2. Stability of Anthocyanins in Complexes
3.2.1. Effect of Simulated Digestions on Anthocyanin Stability in Complexes
3.2.2. Stability of Anthocyanins with Changes in pH and Temperature
3.2.3. The Colonic Concentration of Anthocyanins In Vitro
3.3. Epithelial Cell Permeability In Vitro
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Collings, D.A. Anthocyanin in the vacuole of red onion epidermal cells quenches other fluorescent molecules. Plants 2019, 8, 596. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wu, B.; Fu, W.; Reddivari, L. The anti-inflammatory effects of dietary anthocyanins against ulcerative colitis. Int. J. Mol. Sci. 2019, 20, 2588. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A comprehensive review of their chemical properties and health effects on cardiovascular and neurodegenerative diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef]
- Kubow, S.; Iskandar, M.M.; Melgar-Bermudez, E.; Sleno, L.; Sabally, K.; Azadi, B.; How, E.; Prakash, S.; Burgos, G.; Zum Felde, T. Effects of simulated human gastrointestinal digestion of two purple-fleshed potato cultivars on anthocyanin composition and cytotoxicity in colonic cancer and non-tumorigenic cells. Nutrients 2017, 9, 953. [Google Scholar] [CrossRef] [PubMed]
- Hair, R.; Sakaki, J.R.; Chun, O.K. Anthocyanins, microbiome and health benefits in aging. Molecules 2021, 26, 537. [Google Scholar] [CrossRef]
- Song, J.; Yu, Y.; Chen, M.; Ren, Z.; Chen, L.; Fu, C.; feei Ma, Z.; Li, Z. Advancement of protein-and polysaccharide-based biopolymers for anthocyanin encapsulation. Front. Nutr. 2022, 9, 938829. [Google Scholar] [CrossRef]
- Podsędek, A.; Redzynia, M.; Klewicka, E.; Koziołkiewicz, M. Matrix effects on the stability and antioxidant activity of red cabbage anthocyanins under simulated gastrointestinal digestion. BioMed Res. Int. 2014, 2014, 365738. [Google Scholar] [CrossRef]
- Tan, C.; Wang, J.; Sun, B. Polysaccharide dual coating of yeast capsules for stabilization of anthocyanins. Food Chem. 2021, 357, 129652. [Google Scholar] [CrossRef]
- Fernandes, A.; Rocha, M.A.; Santos, L.M.; Brás, J.; Oliveira, J.; Mateus, N.; de Freitas, V. Blackberry anthocyanins: β-Cyclodextrin fortification for thermal and gastrointestinal stabilization. Food Chem. 2018, 245, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Koh, J.; Xu, Z.; Wicker, L. Blueberry pectin and increased anthocyanins stability under in vitro digestion. Food Chem. 2020, 302, 125343. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.; Serrano, C.; Oliveira, C.; Dias, A.; Moldao-Martins, M. Thermal and light stability of anthocyanins from strawberry by-products non-encapsulated and encapsulated with inulin. Acta Sci. Pol. 2021, 20, 79–92. [Google Scholar]
- Myhrstad, M.C.; Tunsjø, H.; Charnock, C.; Telle-Hansen, V.H. Dietary fiber, gut microbiota, and metabolic regulation—Current status in human randomized trials. Nutrients 2020, 12, 859. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Wang, J.; Guo, Y. Polysaccharides influence human health via microbiota-dependent and-independent pathways. Front. Nutr. 2022, 9, 1030063. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.; Yang, J.; Guo, X.; He, Q.; Wang, Z.; You, J. Dietary seaweed-derived polysaccharides improve growth performance of weaned pigs through maintaining intestinal barrier function and modulating gut microbial populations. J. Anim. Sci. Biotechnol. 2021, 12, 28. [Google Scholar] [CrossRef] [PubMed]
- Reddivari, L.; Wang, T.; Wu, B.; Li, S. Potato: An anti-inflammatory food. Am. J. Potato Res. 2019, 96, 164–169. [Google Scholar] [CrossRef]
- Li, S.; Wang, T.; Wu, B.; Fu, W.; Xu, B.; Pamuru, R.R.; Kennett, M.; Vanamala, J.K.; Reddivari, L. Anthocyanin-containing purple potatoes ameliorate DSS-induced colitis in mice. J. Nutr. Biochem. 2021, 93, 108616. [Google Scholar] [CrossRef]
- Petruskevicius, A.; Viskelis, J.; Urbonaviciene, D.; Viskelis, P. Anthocyanin Accumulation in Berry Fruits and Their Antimicrobial and Antiviral Properties: An Overview. Horticulturae 2023, 9, 288. [Google Scholar] [CrossRef]
- Zhao, R.; Shen, G.X. Impact of anthocyanin component and metabolite of Saskatoon berry on gut microbiome and relationship with fecal short chain fatty acids in diet-induced insulin resistant mice. J. Nutr. Biochem. 2023, 111, 109201. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, T.; Fu, W.; Kennett, M.; Cox, A.D.; Lee, D.; Vanamala, J.K.; Reddivari, L. Role of Gut Microbiota in the Anti-Colitic Effects of Anthocyanin-Containing Potatoes. Mol. Nutr. Food Res. 2021, 65, 2100152. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Y.; Liu, G.; Hao, S.; Wang, C.; Wang, Y. Black rice anthocyanin-rich extract and rosmarinic acid, alone and in combination, protect against DSS-induced colitis in mice. Food Funct. 2018, 9, 2796–2808. [Google Scholar] [CrossRef]
- Nayak, B.; Berrios, J.D.J.; Powers, J.R.; Tang, J. Thermal degradation of anthocyanins from purple potato (Cv. Purple Majesty) and impact on antioxidant capacity. J. Agric. Food Chem. 2011, 59, 11040–11049. [Google Scholar] [CrossRef] [PubMed]
- Moshari Nasirkandi, A.; Alirezalu, A.; Bahadori, S. Phenolic compounds and antioxidant activity of Nepeta fissa-first report from Iran. Nat. Prod. Res. 2021, 35, 4596–4599. [Google Scholar] [CrossRef] [PubMed]
- Taghavi, T.; Patel, H.; Rafie, R. Comparing pH differential and methanol-based methods for anthocyanin assessments of strawberries. Food Sci. Nutr. 2022, 10, 2123–2131. [Google Scholar] [CrossRef]
- Li, M.; Pernell, C.; Ferruzzi, M.G. Complexation with phenolic acids affect rheological properties and digestibility of potato starch and maize amylopectin. Food Hydrocoll. 2018, 77, 843–852. [Google Scholar] [CrossRef]
- Helmick, H.; Hartanto, C.; Bhunia, A.; Liceaga, A.; Kokini, J.L. Validation of bioinformatic modeling for the zeta potential of vicilin, legumin, and commercial pea protein isolate. Food Biophys. 2021, 16, 474–483. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Zarroug, Y.O.; Abdelkarim, A.; Dorra, S.T.; Hamdaoui, G.; Felah, M.E.; Hassouna, M. Biochemical characterization of tunisian Cichorium intybus L. roots and optimization of ultrasonic inulin extraction. Mediterr. J. Chem. 2016, 6, 674–685. [Google Scholar] [CrossRef]
- Hong, T.; Yin, J.-Y.; Nie, S.-P.; Xie, M.-Y. Applications of infrared spectroscopy in polysaccharide structural analysis: Progress, challenge and perspective. Food Chem. X 2021, 12, 100168. [Google Scholar] [CrossRef] [PubMed]
- Padayachee, A.; Netzel, G.; Netzel, M.; Day, L.; Zabaras, D.; Mikkelsen, D.; Gidley, M. Binding of polyphenols to plant cell wall analogues–Part 1: Anthocyanins. Food Chem. 2012, 134, 155–161. [Google Scholar] [CrossRef]
- Apolinário, A.C.; de Carvalho, E.M.; de Lima Damasceno, B.P.G.; da Silva, P.C.D.; Converti, A.; Pessoa, A., Jr.; da Silva, J.A. Extraction, isolation and characterization of inulin from Agave sisalana boles. Ind. Crops Prod. 2017, 108, 355–362. [Google Scholar] [CrossRef]
- García-Tejeda, Y.V.; Salinas-Moreno, Y.; Hernández-Martínez, Á.R.; Martínez-Bustos, F. Encapsulation of purple maize anthocyanins in phosphorylated starch by spray drying. Cereal Chem. 2016, 93, 130–137. [Google Scholar] [CrossRef]
- Valand, R.; Tanna, S.; Lawson, G.; Bengtström, L. A review of Fourier Transform Infrared (FTIR) spectroscopy used in food adulteration and authenticity investigations. Food Addit. Contam. Part A 2020, 37, 19–38. [Google Scholar] [CrossRef]
- Lara-Espinoza, C.; Carvajal-Millán, E.; Balandrán-Quintana, R.; López-Franco, Y.; Rascón-Chu, A. Pectin and pectin-based composite materials: Beyond food texture. Molecules 2018, 23, 942. [Google Scholar] [CrossRef]
- Umoren, S.A.; Obot, I.B.; Madhankumar, A.; Gasem, Z.M. Performance evaluation of pectin as ecofriendly corrosion inhibitor for X60 pipeline steel in acid medium: Experimental and theoretical approaches. Carbohydr. Polym. 2015, 124, 280–291. [Google Scholar] [CrossRef]
- Pismenskaya, N.; Sarapulova, V.; Klevtsova, A.; Mikhaylin, S.; Bazinet, L. Adsorption of anthocyanins by cation and anion exchange resins with aromatic and aliphatic polymer matrices. Int. J. Mol. Sci. 2020, 21, 7874. [Google Scholar] [CrossRef]
- Uzunović, A.; Vranić, E. Stability of anthocyanins from commercial black currant juice under simulated gastrointestinal digestion. Bosn. J. Basic Med. Sci. 2008, 8, 254. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Yuan, C.; Wang, H.; Han, F.; Liu, Y.; Wang, L.; Liu, Y. Stability of anthocyanins and their degradation products from cabernet sauvignon red wine under gastrointestinal pH and temperature conditions. Molecules 2018, 23, 354. [Google Scholar] [CrossRef] [PubMed]
- Renard, C.M.; Watrelot, A.A.; Le Bourvellec, C. Interactions between polyphenols and polysaccharides: Mechanisms and consequences in food processing and digestion. Trends Food Sci. Technol. 2017, 60, 43–51. [Google Scholar] [CrossRef]
- Calvete-Torre, I.; Muñoz-Almagro, N.; Pacheco, M.T.; Antón, M.J.; Dapena, E.; Ruiz, L.; Margolles, A.; Villamiel, M.; Moreno, F.J. Apple pomaces derived from mono-varietal Asturian ciders production are potential source of pectins with appealing functional properties. Carbohydr. Polym. 2021, 264, 117980. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Le Bourvellec, C.; Renard, C.M. Interactions between cell wall polysaccharides and polyphenols: Effect of molecular internal structure. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3574–3617. [Google Scholar] [CrossRef]
- Lin, Z.; Fischer, J.; Wicker, L. Intermolecular binding of blueberry pectin-rich fractions and anthocyanin. Food Chem. 2016, 194, 986–993. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, C.; Gao, X.; Chen, Y.; Santhanam, R.K.; Wang, C.; Xu, L.; Chen, H. Interaction characterization of preheated soy protein isolate with cyanidin-3-O-glucoside and their effects on the stability of black soybean seed coat anthocyanins extracts. Food Chem. 2019, 271, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Enaru, B.; Drețcanu, G.; Pop, T.D.; Stǎnilǎ, A.; Diaconeasa, Z. Anthocyanins: Factors affecting their stability and degradation. Antioxidants 2021, 10, 1967. [Google Scholar] [CrossRef]
- Levy, R.; Okun, Z.; Shpigelman, A. The influence of chemical structure and the presence of ascorbic acid on anthocyanins stability and spectral properties in purified model systems. Foods 2019, 8, 207. [Google Scholar] [CrossRef] [PubMed]
- Kuswandi, B.; Asih, N.P.; Pratoko, D.K.; Kristiningrum, N.; Moradi, M. Edible pH sensor based on immobilized red cabbage anthocyanins into bacterial cellulose membrane for intelligent food packaging. Packag. Technol. Sci. 2020, 33, 321–332. [Google Scholar] [CrossRef]
- Buchweitz, M.; Speth, M.; Kammerer, D.; Carle, R. Impact of pectin type on the storage stability of black currant (Ribes nigrum L.) anthocyanins in pectic model solutions. Food Chem. 2013, 139, 1168–1178. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, Y.; Xie, J.; Xie, L.; Mo, J.; Chen, W. Bioavailability, Absorption, and Metabolism of Pelargonidin-Based Anthocyanins Using Sprague–Dawley Rats and Caco-2 Cell Monolayers. J. Agric. Food Chem. 2021, 69, 7841–7850. [Google Scholar] [CrossRef]
- Cheng, M.; Zhang, X.; Cao, J.; Zheng, X.; Zhang, Z. Caco-2 cell transport of purple sweet potato anthocyanins-phospholipids complex. J. Food Sci. Technol. 2018, 55, 304–312. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, J.Y. Intestinal anti-inflammatory effects of cinnamon extracts in a co-culture model of intestinal epithelial Caco-2 cells and RAW264. 7 macrophages. Appl. Biol. Chem. 2017, 60, 553–561. [Google Scholar] [CrossRef]
- Valdez, J.C.; Cho, J.; Bolling, B.W. Aronia berry inhibits disruption of Caco-2 intestinal barrier function. Arch. Biochem. Biophys. 2020, 688, 108409. [Google Scholar] [CrossRef]
- Cremonini, E.; Mastaloudis, A.; Hester, S.N.; Verstraeten, S.V.; Anderson, M.; Wood, S.M.; Waterhouse, A.L.; Fraga, C.G.; Oteiza, P.I. Anthocyanins inhibit tumor necrosis alpha-induced loss of Caco-2 cell barrier integrity. Food Funct. 2017, 8, 2915–2923. [Google Scholar] [CrossRef] [PubMed]







| Samples | Z-Average (nm) | Polydispersity Index |
|---|---|---|
| Pectin | 1299 ± 14 a | 0.44 ± 0.002 a |
| Inulin | 685.0 ± 24 b | 0.49 ± 0.06 a |
| Starch | 724.8 ± 23 b | 0.50 ± 0.02 a |
| Cellulose | 21080 ± 1200 c | 1 b |
| Mixture | 556.2 ± 20 d | 0.52 ± 0.04 a |
| Anthocyanins | 296.6 ± 7 e | 0.45 ± 0.03 a |
| APC | 1327 ± 12 a | 0.39 ± 0.03 a |
| AIC | 469.2 ± 29 f | 0.67 ± 0.06 c |
| ASC | 434.8 ± 29 f | 0.50 ± 0.02 a |
| ACC | 1545 ± 119 a | 0.99 ± 0.005 b |
| AMC | 658.6 ± 40 bd | 0.44 ± 0.04 a |
| Digestion Phase | Total Phenolics 1 | Anthocyanins 2 |
|---|---|---|
| Anthocyanin fraction (mg/mL dry extract) | ||
| Oral digestion | 5.34 ± 0.09 a | 6.77 ± 0.09 a |
| Pepsin digestion | 5.29 ± 0.08 a | 6.66 ± 0.2 a |
| Pancreatin-bile digestion | 4.82 ± 0.07 b | 0.72 ± 0.02 b |
| Pectin (mg/g) | ||
| Oral digestion | 0.05 ± 0.004 a | ND |
| Pepsin digestion | 0.12 ± 0.02 ab | ND |
| Pancreatin-bile digestion | 0.25 ± 0.01 b | ND |
| APC (mg/mL dry complex) | ||
| Oral digestion | 2.92 ± 0.1 a | 1.46 ± 0.04 a |
| Pepsin digestion | 3.53 ± 0.1 b | 1.87 ± 0.05 b |
| Pancreatin-bile digestion | 4.81 ± 0.2 b | 1.33 ± 0.01 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, W.; Li, S.; Helmick, H.; Hamaker, B.R.; Kokini, J.L.; Reddivari, L. Complexation with Polysaccharides Enhances the Stability of Isolated Anthocyanins. Foods 2023, 12, 1846. https://doi.org/10.3390/foods12091846
Fu W, Li S, Helmick H, Hamaker BR, Kokini JL, Reddivari L. Complexation with Polysaccharides Enhances the Stability of Isolated Anthocyanins. Foods. 2023; 12(9):1846. https://doi.org/10.3390/foods12091846
Chicago/Turabian StyleFu, Wenyi, Shiyu Li, Harrison Helmick, Bruce R. Hamaker, Jozef L. Kokini, and Lavanya Reddivari. 2023. "Complexation with Polysaccharides Enhances the Stability of Isolated Anthocyanins" Foods 12, no. 9: 1846. https://doi.org/10.3390/foods12091846
APA StyleFu, W., Li, S., Helmick, H., Hamaker, B. R., Kokini, J. L., & Reddivari, L. (2023). Complexation with Polysaccharides Enhances the Stability of Isolated Anthocyanins. Foods, 12(9), 1846. https://doi.org/10.3390/foods12091846






