Changes in Structures and Properties of Collagen Fibers during Collagen Casing Film Manufacturing
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
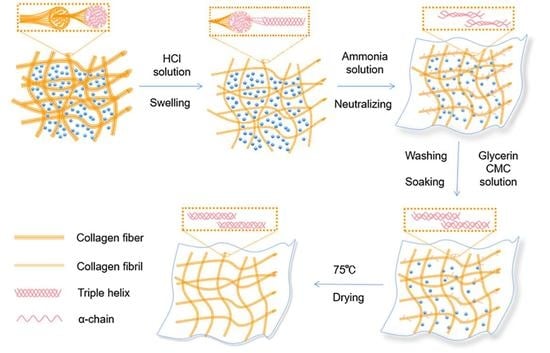
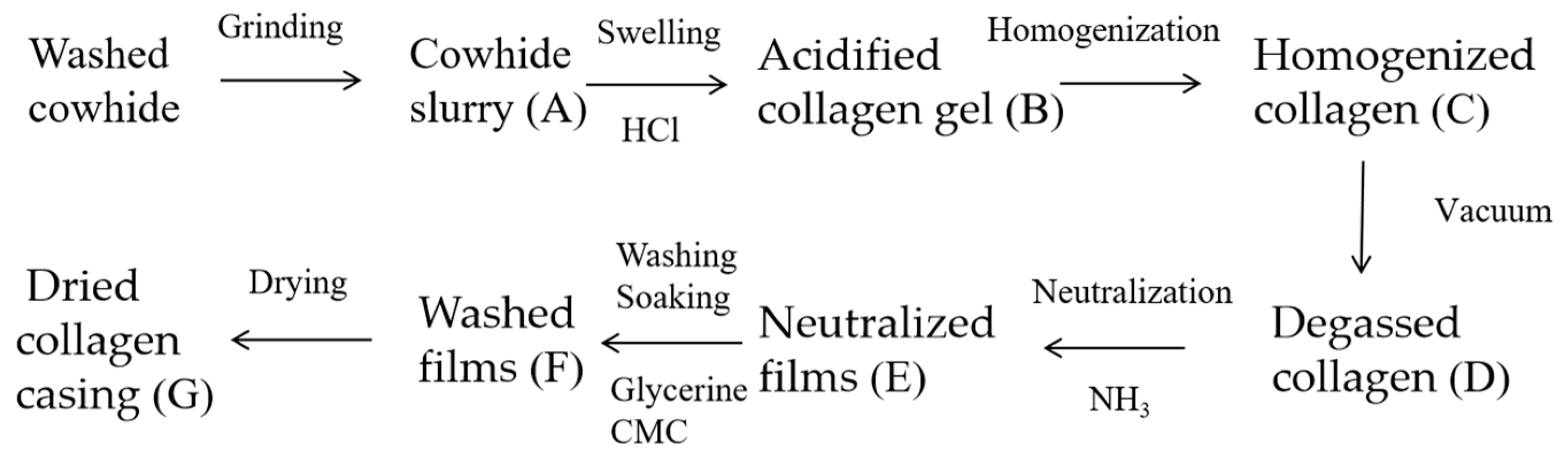
2.2. Preparation Process of Collagen Casing Films and Sampling
2.3. Characterization of Collagen Fibers and Films
2.3.1. Moisture Content Test
2.3.2. Mechanical Property Test
2.3.3. Scanning Electron Microscopy (SEM) Analysis
2.3.4. Fourier-Transform Infrared (FTIR) Spectroscopy Analysis
2.3.5. Differential Scanning Calorimetry (DSC) Analysis
2.3.6. Thermogravimetric (TGA) Analysis
2.3.7. X-ray Diffraction (XRD) Analysis
2.4. Data Analysis
3. Results and Discussion
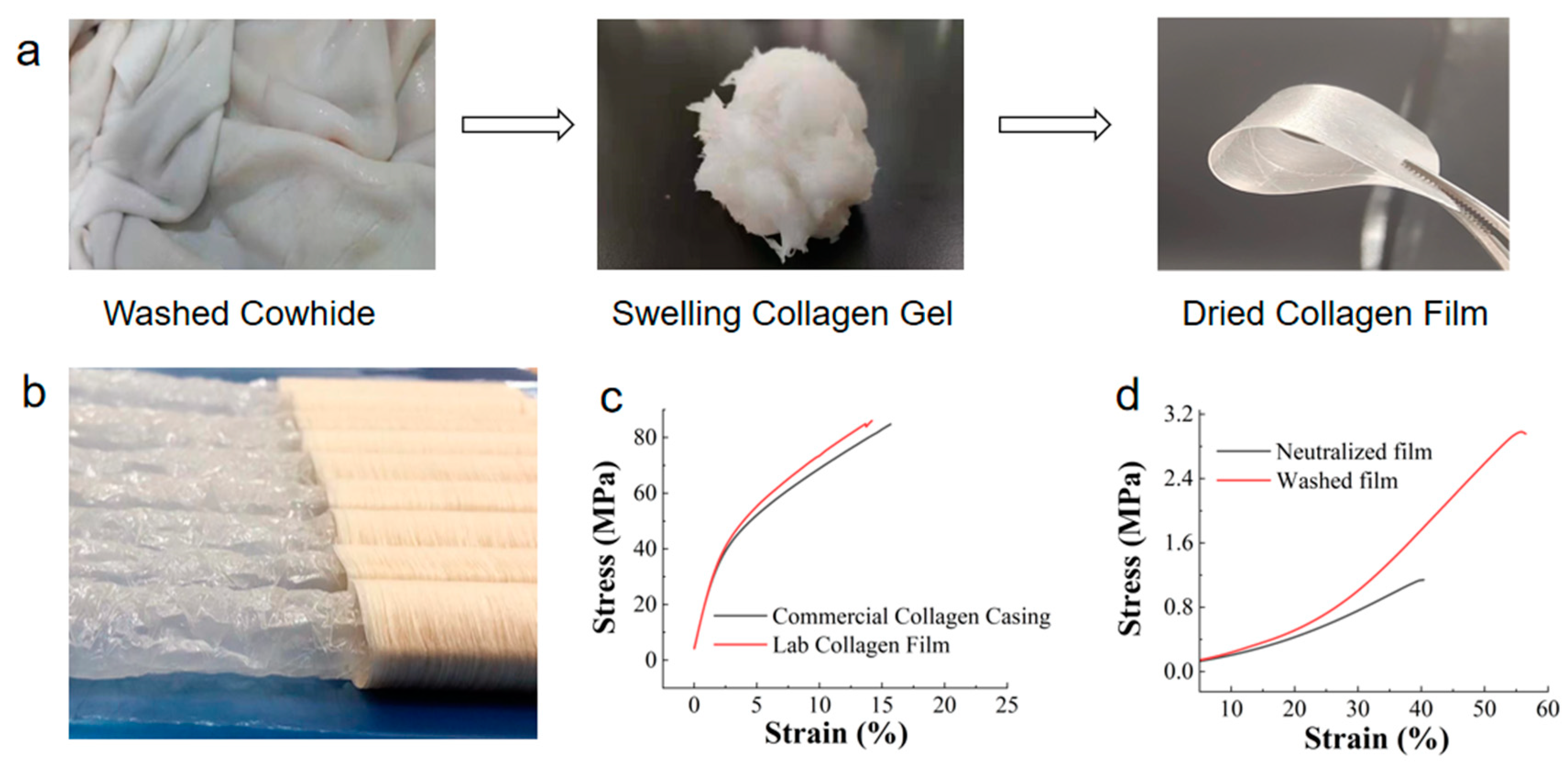
3.1. Moisture Content and Mechanical Properties
3.2. Microstructure Characterization of Collagen Fibers
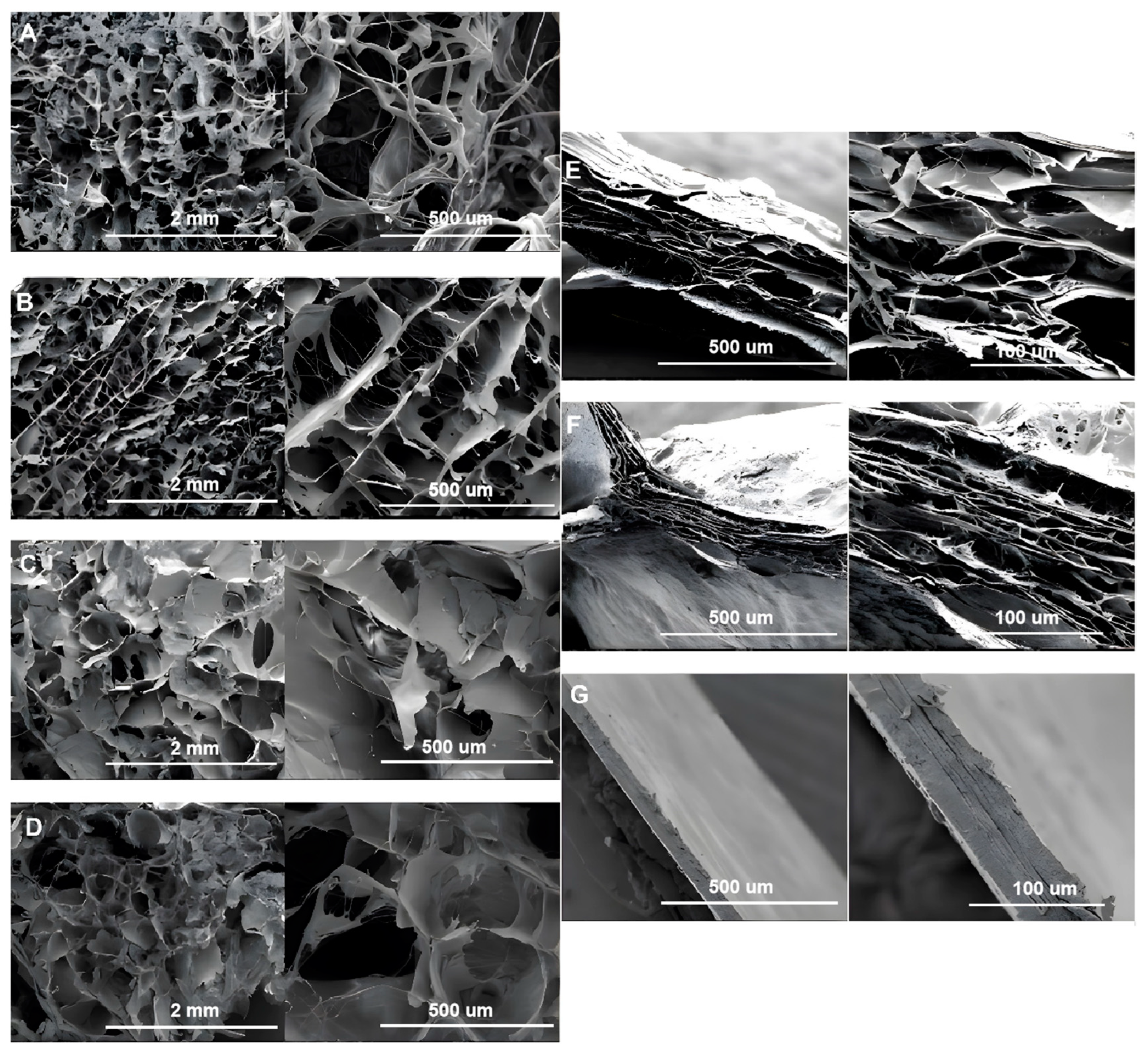
3.2.1. SEM Analysis of Collagen Fibers
3.2.2. Fourier-Transform Infrared (FTIR) Spectroscopy Analysis of Collagen Fibers
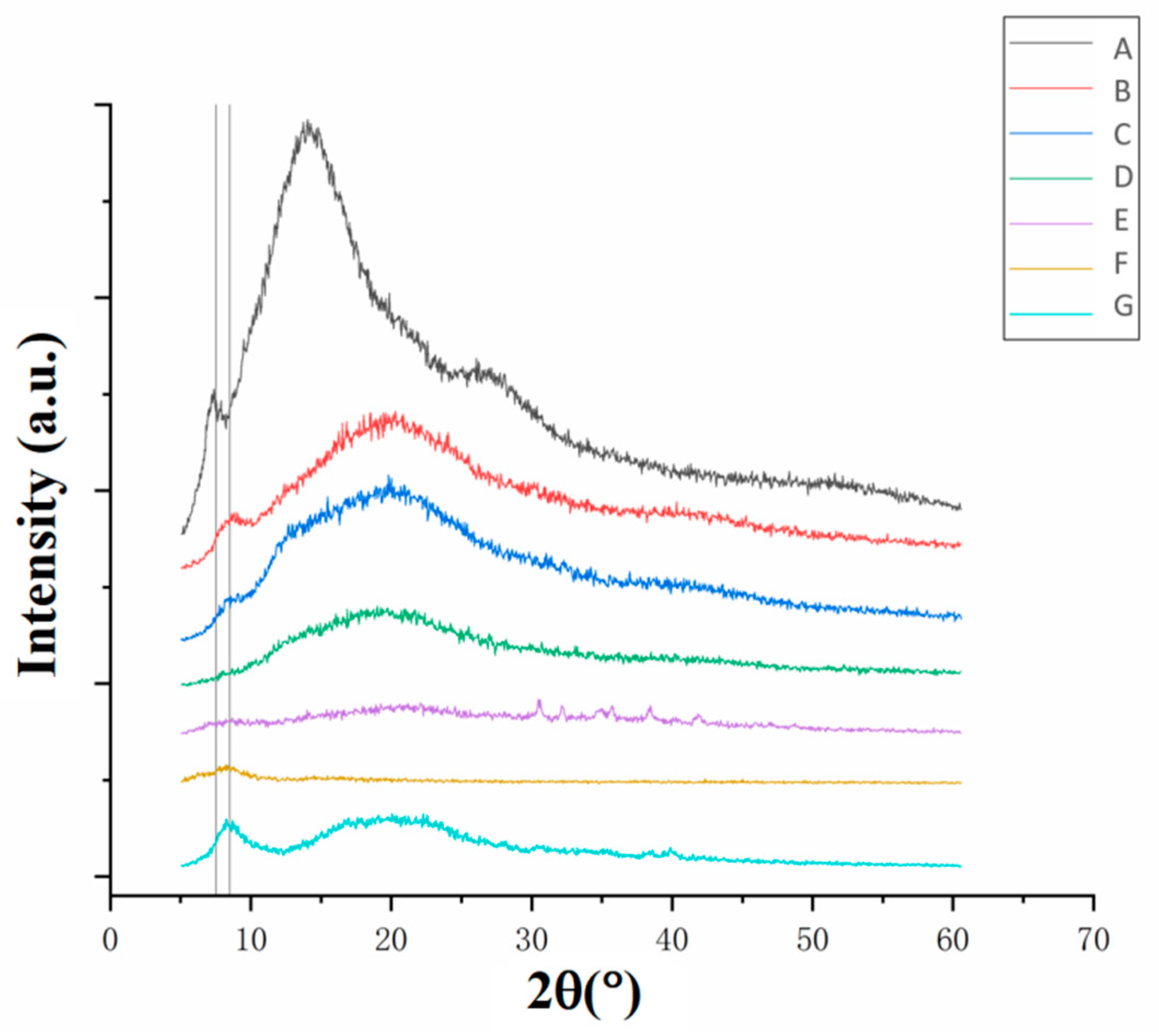
3.2.3. Crystal Structure Analysis of Collagen Fibers
3.3. Thermal Stability Analysis of Collagen Fibers
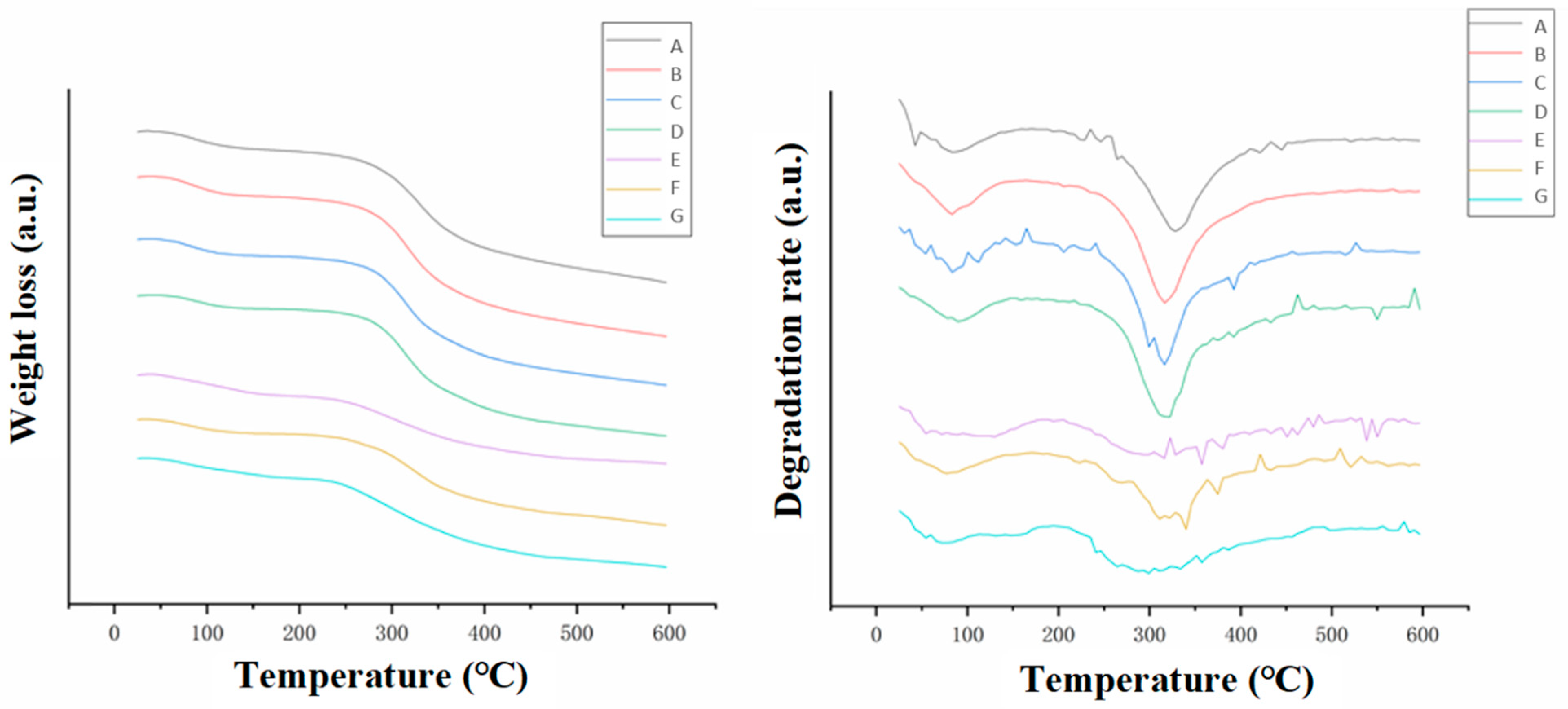
3.3.1. TGA Analysis
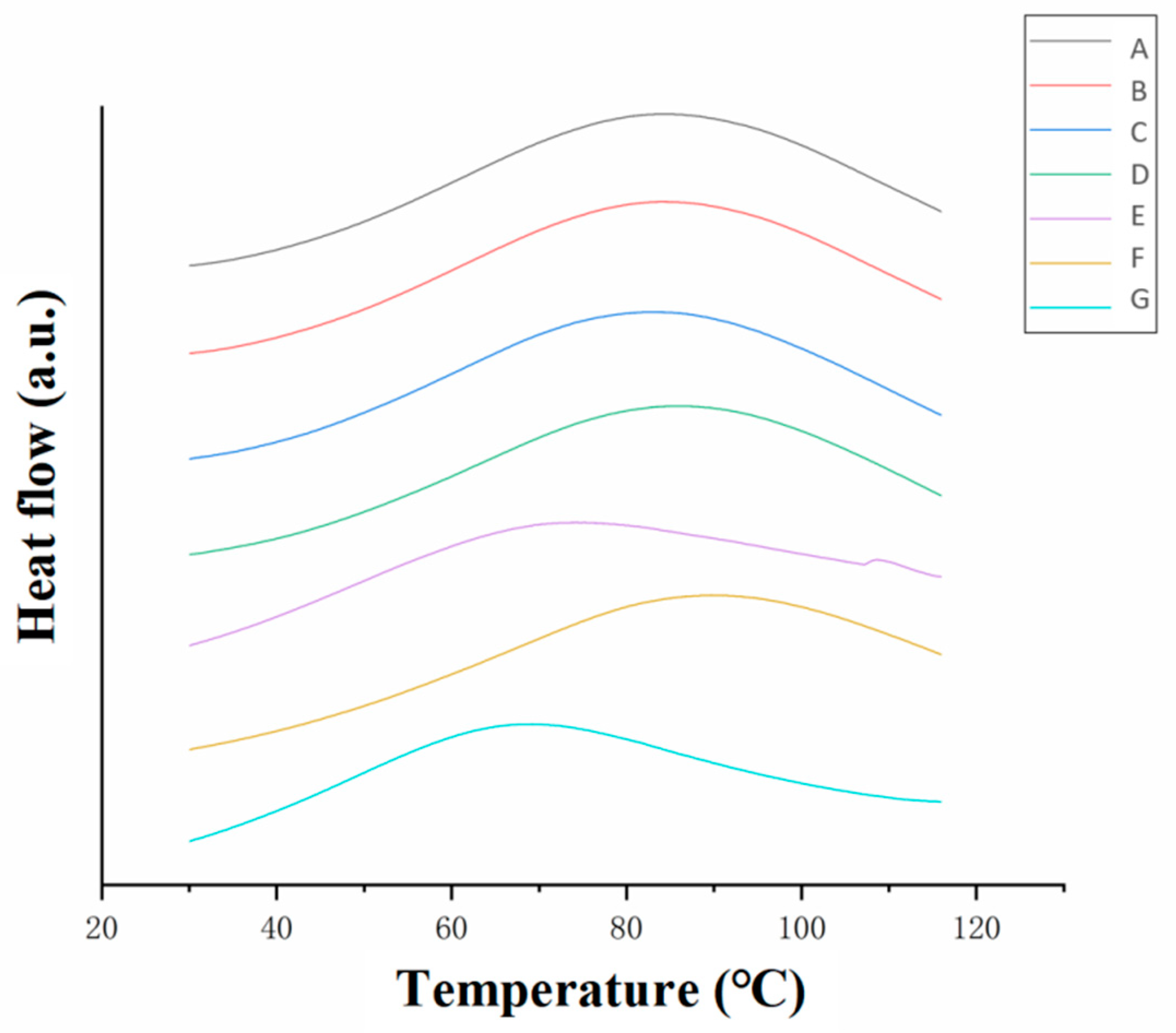
3.3.2. Differential Scanning Calorimetry (DSC) Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- MarketLine Company Profile: Viscofan SA. Available online: https://www.viscofan.com/investor-relations/financial-information-and-annual-report (accessed on 28 April 2023).
- Steinke, P.K.W.; Foster, E.M. Effect of different artificial casings on the microbial changes in refrigerated liver sausage. J. Food Sci. 2019, 16, 289–293. [Google Scholar] [CrossRef]
- Zajac, M.; Pajak, P.; Skowyra, G. Characterization of edible collagen casings in comparison with the ovine casing and their effect on sausage quality. J. Sci. Food Agric. 2021, 101, 6001–6009. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.J.; Zhao, K.X.; Teng, A.G.; Li, Y.; Wang, W.H. A rethinking of collagen as tough biomaterials in meat packaging: Assembly from native to synthetic. Crit. Rev. Food Sci. Nutr. 2022, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Yang, L.; Zhang, Y.; Han, W.; Duan, Y. Effect of collagen casing on the quality characteristics of fermented sausage. PLoS ONE 2022, 17, e0263389. [Google Scholar] [CrossRef] [PubMed]
- Tantala, J.; Vangnai, K.; Rachtanapun, P.; Rachtanapun, C. Active antimicrobial collagen casing. Ital. J. Food Sci. 2019, 31, 171–175. [Google Scholar]
- Chen, C.; Liu, F.; Yu, Z.; Ma, Y.; Goff, H.D.; Zhong, F. Improvement in physicochemical properties of collagen casings by glutaraldehyde cross-linking and drying temperature regulating. Food Chem. 2020, 318, 126404. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Yue, C.; Su, J.; Wang, H.; Yang, N.; Cheng, B. Effects of oxidized cellulose nanocrystals on the structure and mechanical properties of regenerated collagen fibers. Cellulose 2022, 29, 7677–7690. [Google Scholar] [CrossRef]
- Sobanwa, M. Redesign of Collagen Casings for High Quality Performance Using Food Grade Polysaccharides. Ph.D. Dissertation, University of Nottingham, Nottingham, UK, 2008. [Google Scholar]
- Adzaly, N.Z.; Jackson, A.; Kang, I.; Almenar, E. Performance of a novel casing made of chitosan under traditional sausage manufacturing conditions. Meat Sci. 2016, 113, 116–123. [Google Scholar] [CrossRef]
- Barbut, S.; Ioi, M. An investigation of the mechanical, microstructural and thermo-mechanical properties of collagen films cross-linked with smoke condensate and glutaraldehyde. Ital. J. Food Sci. 2019, 31, 644–660. [Google Scholar]
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The Collagen Suprafamily: From Biosynthesis to Advanced Biomaterial Development. Adv. Mater. 2019, 31, 1801651. [Google Scholar] [CrossRef]
- Gentleman, E.; Lay, A.N.; Dickerson, D.A.; Nauman, E.A.; Livesay, G.A.; Dee, K.C. Mechanical characterization of collagen fibers and scaffolds for tissue engineering. Biomaterials 2003, 24, 3805–3813. [Google Scholar] [CrossRef] [PubMed]
- Hoogenkamp, H.R.; Bakker, G.J.; Wolf, L.; Suurs, P.; Dunnewind, B.; Barbut, S.; Friedl, P.; Van Kuppevelt, T.H.; Daamen, W.F. Directing collagen fibers using counter-rotating cone extrusion. Acta Biomater. 2015, 12, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Suárez, S.; López-Campos, J.A.; Segade, A.; Veiga, C.G.; Jiménez, V.A. An study on the influence of collagen fiber directions in TAVs performance using FEM. J. Mech. Behav. Biomed. Mater. 2022, 126, 104969. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, L.; Xu, H.; Yamamoto, M.; Shinoda, M.; Kishimoto, M.; Tanaka, T.; Yamane, H. Effect of the Application of a Dehydrothermal Treatment on the Structure and the Mechanical Properties of Collagen Film. Materials 2020, 13, 377. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liu, F.; Goff, H.D.; Zhong, F. Effect of pre-treatment temperatures on the film-forming properties of collagen fiber dispersions. Food Hydrocolloid. 2020, 107, 105326. [Google Scholar] [CrossRef]
- Xu, J.; Liu, F.; Wang, T.; Goff, H.D.; Zhong, F. Fabrication of films with tailored properties by regulating the swelling of collagen fiber through pH adjustment. Food Hydrocoll. 2020, 108, 106016. [Google Scholar] [CrossRef]
- US2005031741 A1 Collagen Casing. Available online: https://www.freepatentsonline.com/y2005/0031741.html (accessed on 28 April 2023).
- Jingmin, W.; Fei, L.; Zhe, Y.; Yun, M.; H Douglas, G.; Jianguo, M.; Fang, Z. Facile preparation of collagen fiber-glycerol-carboxymethyl cellulose composite film by immersing method. Carbohydr. Polym. 2020, 229, 115429. [Google Scholar] [CrossRef]
- Shi, D.; Liu, F.; Yu, Z.; Chang, B.; Goff, H.; Zhong, F. Effect of aging treatment on the physicochemical properties of collagen films. Food Hydrocolloid. 2019, 87, 436–447. [Google Scholar] [CrossRef]
- Nilsuwan, K.; Fusang, K.; Pripatnanont, P.; Benjakul, S. Properties and Characteristics of Acid-Soluble Collagen from Salmon Skin Defatted with the Aid of Ultrasonication. Fishes 2022, 7, 51. [Google Scholar] [CrossRef]
- Badii, F.; MacNaughtan, W.; Mitchell, J.R.; Farhat, I.A. The Effect of Drying Temperature on Physical Properties of Thin Gelatin Films. Dry Technol. 2014, 32, 30–38. [Google Scholar] [CrossRef]
- Cheng, S.; Wang, W.; Li, Y.; Gao, G.; Zhang, K.; Zhou, J.; Wu, Z. Cross-linking and film-forming properties of transglutaminase-modified collagen fibers tailored by denaturation temperature. Food Chem. 2019, 271, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Persikov, A.V.; Xu, Y.; Brodsky, B. Equilibrium thermal transitions of collagen model peptides. Protein Sci. 2004, 13, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.F.; Xiao, H.Z.; Bi, S. The improvement of dispersity, thermal stability and mechanical properties of collagen fibers by silane modification: An exploration for developing new leather making technology. J. Leather Sci. Eng. 2022, 4, 26. [Google Scholar] [CrossRef]

| Stages of Samples | Moisture Content/% | |
|---|---|---|
| A | Cowhide Slurry | 73.5 ± 1.2 a |
| B | Acidified Collagen Gel | 93.3 ± 0.3 b |
| C | Homogenized Collagen Gel | 93.5 ± 0.2 b |
| D | Degassed Collagen Gel | 93.5 ± 0.3 b |
| E | Neutralized Films | 84.5 ± 0.9 c |
| F | Washed Films | 83.0 ± 0.9 d |
| G | Dried Collagen Films | 19.5 ± 0.8 e |
| Control | Commercial Collagen Casing | 19.7 ± 2.2 e |
| Stages of Samples | A1240 | A1450 | A1240/A1450 | |
|---|---|---|---|---|
| A | Cowhide Slurry | 0.1006 | 0.1005 | 1.000 |
| B | Acidified Collagen Gel | 0.1798 | 0.1627 | 1.105 |
| C | Homogenized Collagen Gel | 0.1571 | 0.1432 | 1.097 |
| D | Degassed Collagen Gel | 0.08385 | 0.07801 | 1.075 |
| E | Neutralized Films | 0.08896 | 0.1268 | 0.7017 |
| F | Washed Films | 0.1682 | 0.1676 | 1.004 |
| G | Dried Collagen Films | 0.1779 | 0.1459 | 1.220 |
| Stages of Samples | Thermal Decomposition Temperature/°C | |
|---|---|---|
| A | Cowhide Slurry | 330 |
| B | Acidified Collagen Gel | 320 |
| C | Homogenized Collagen Gel | 318 |
| D | Degassed Collagen Gel | 315 |
| E | Neutralized Films | 318 |
| F | Washed Films | 320 |
| G | Dried Collagen Films | 300 |
| Stages of Samples | Enthalpy Value/J/g | |
|---|---|---|
| A | Cowhide Slurry | 392.4 ± 15.3 a |
| B | Acidified Collagen Gel | 302.4 ± 18.5 b |
| C | Homogenized Collagen Gel | 302.8 ± 13.5 b |
| D | Degassed Collagen Gel | 310.8 ± 14.7 b |
| E | Neutralized Films | 288.4 ± 12.1 b |
| F | Washed Films | 329.6 ± 18.1 b |
| G | Dried Collagen Films | 208.7 ± 12.4 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.; Yu, Z.; Wang, B.; Chiou, B.-S. Changes in Structures and Properties of Collagen Fibers during Collagen Casing Film Manufacturing. Foods 2023, 12, 1847. https://doi.org/10.3390/foods12091847
Liu F, Yu Z, Wang B, Chiou B-S. Changes in Structures and Properties of Collagen Fibers during Collagen Casing Film Manufacturing. Foods. 2023; 12(9):1847. https://doi.org/10.3390/foods12091847
Chicago/Turabian StyleLiu, Fei, Zhe Yu, Beibei Wang, and Bor-Sen Chiou. 2023. "Changes in Structures and Properties of Collagen Fibers during Collagen Casing Film Manufacturing" Foods 12, no. 9: 1847. https://doi.org/10.3390/foods12091847
APA StyleLiu, F., Yu, Z., Wang, B., & Chiou, B.-S. (2023). Changes in Structures and Properties of Collagen Fibers during Collagen Casing Film Manufacturing. Foods, 12(9), 1847. https://doi.org/10.3390/foods12091847