Utilization of Dairy By-Products as a Source of Functional and Health Compounds—The Role of Ovine Colostrum and Milk Whey on Chronic Myeloid Leukemia Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Milk and Colostrum Collection and Whey Preparation
2.2. Cell Culture and Cell Treatment with Ovine Colostrum or Milk Whey
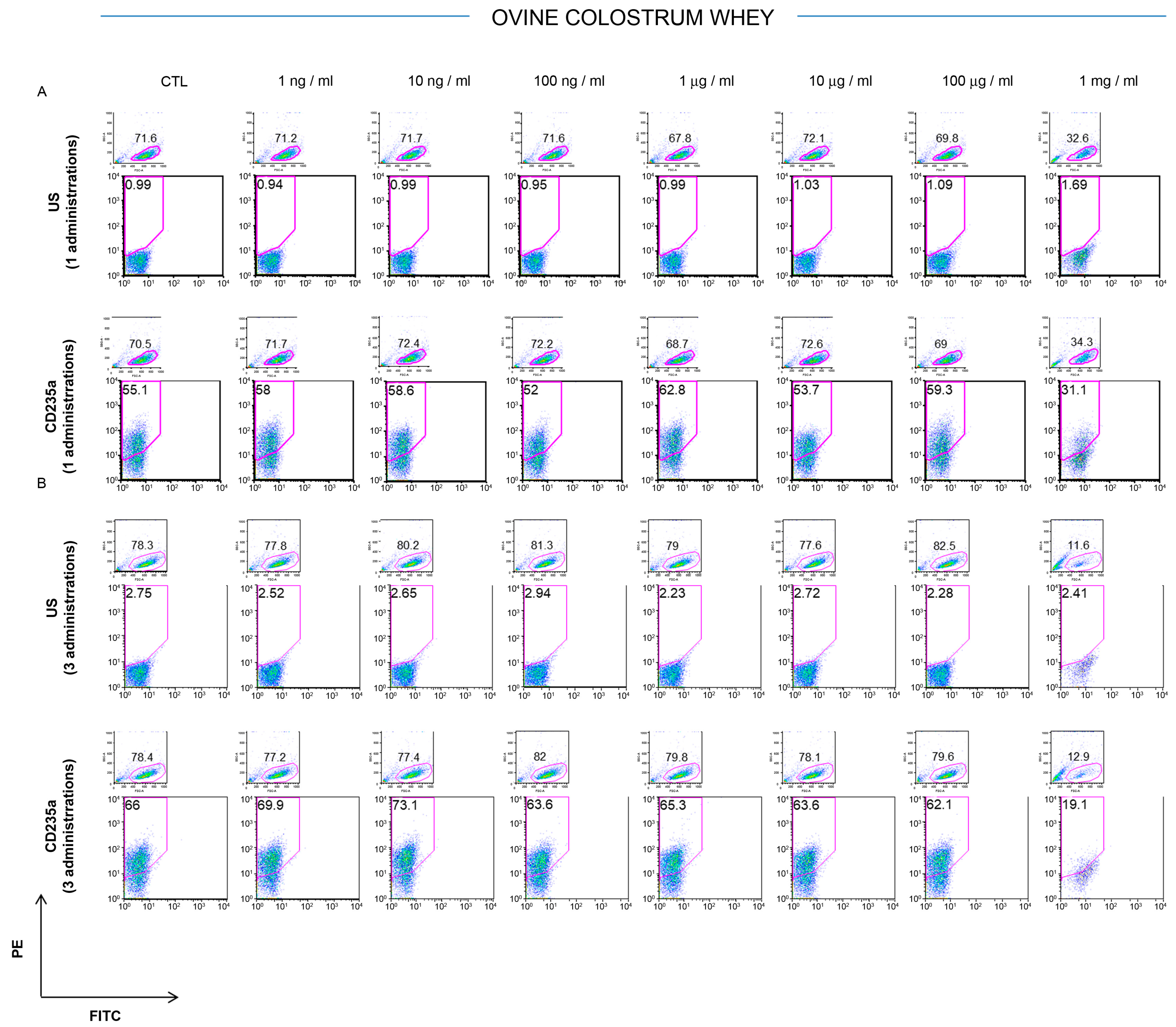
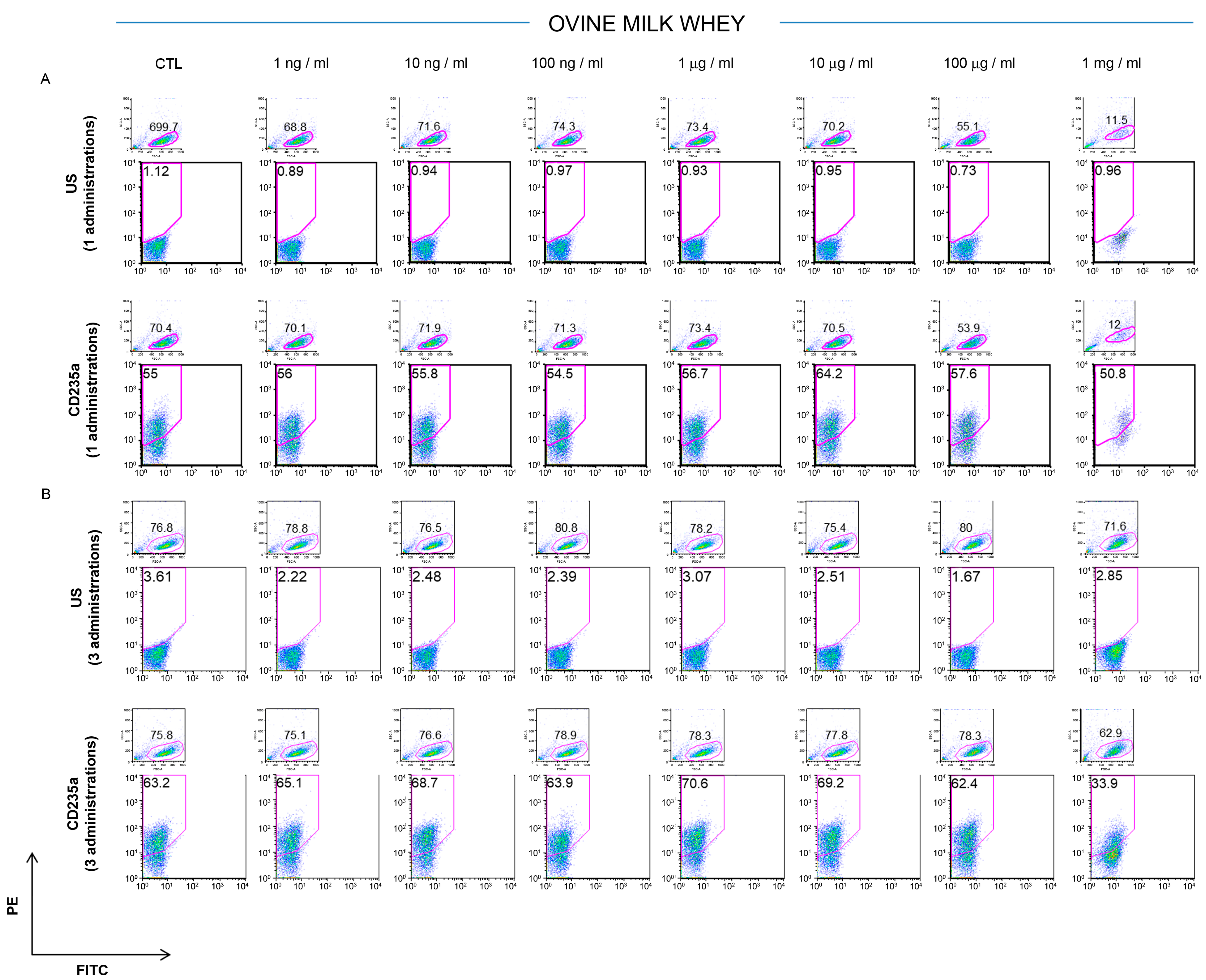
2.3. Effect of Milk Whey and Colostrum Whey on K562 Cell Surface Antigen
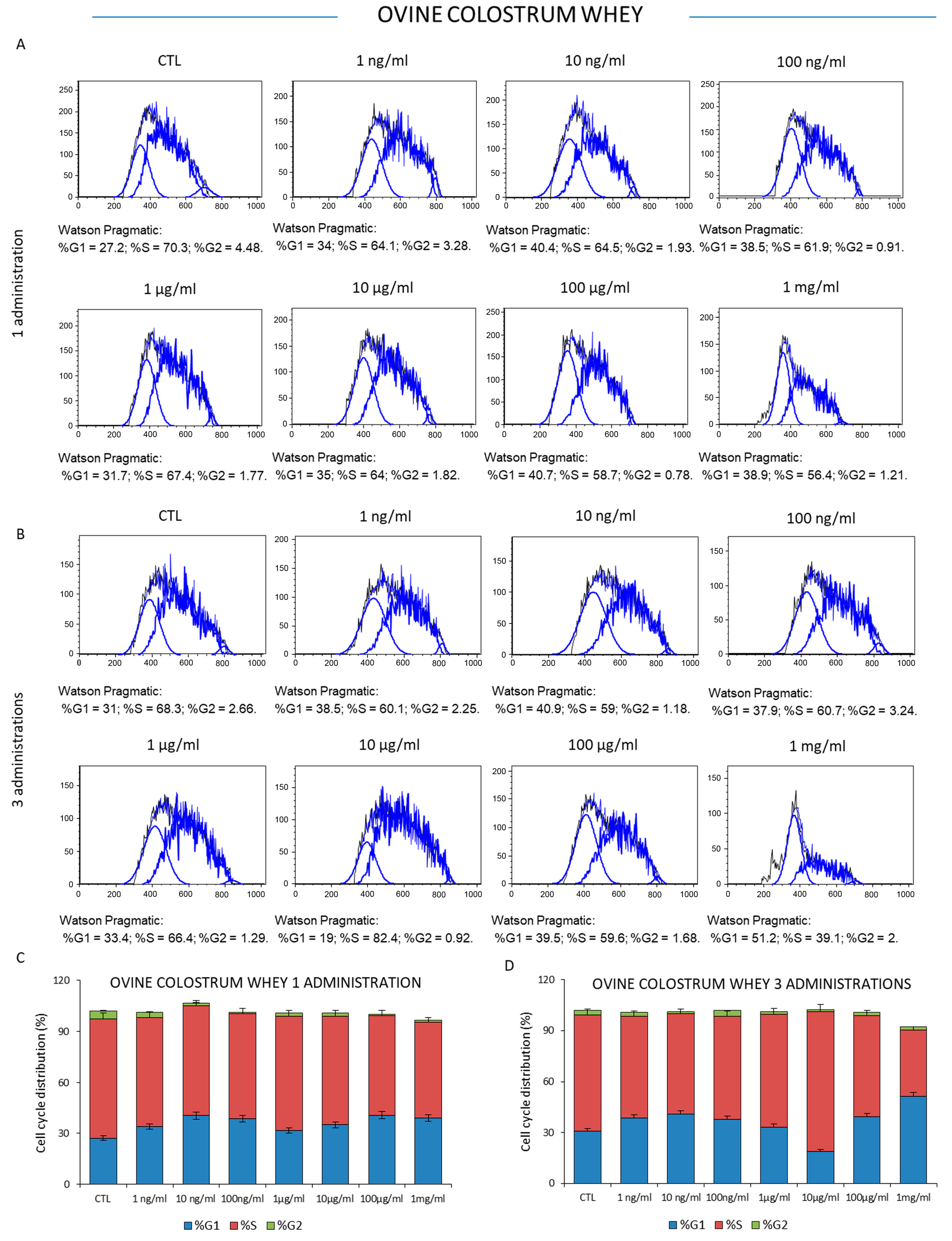
2.4. Analysis of the Cell Cycle
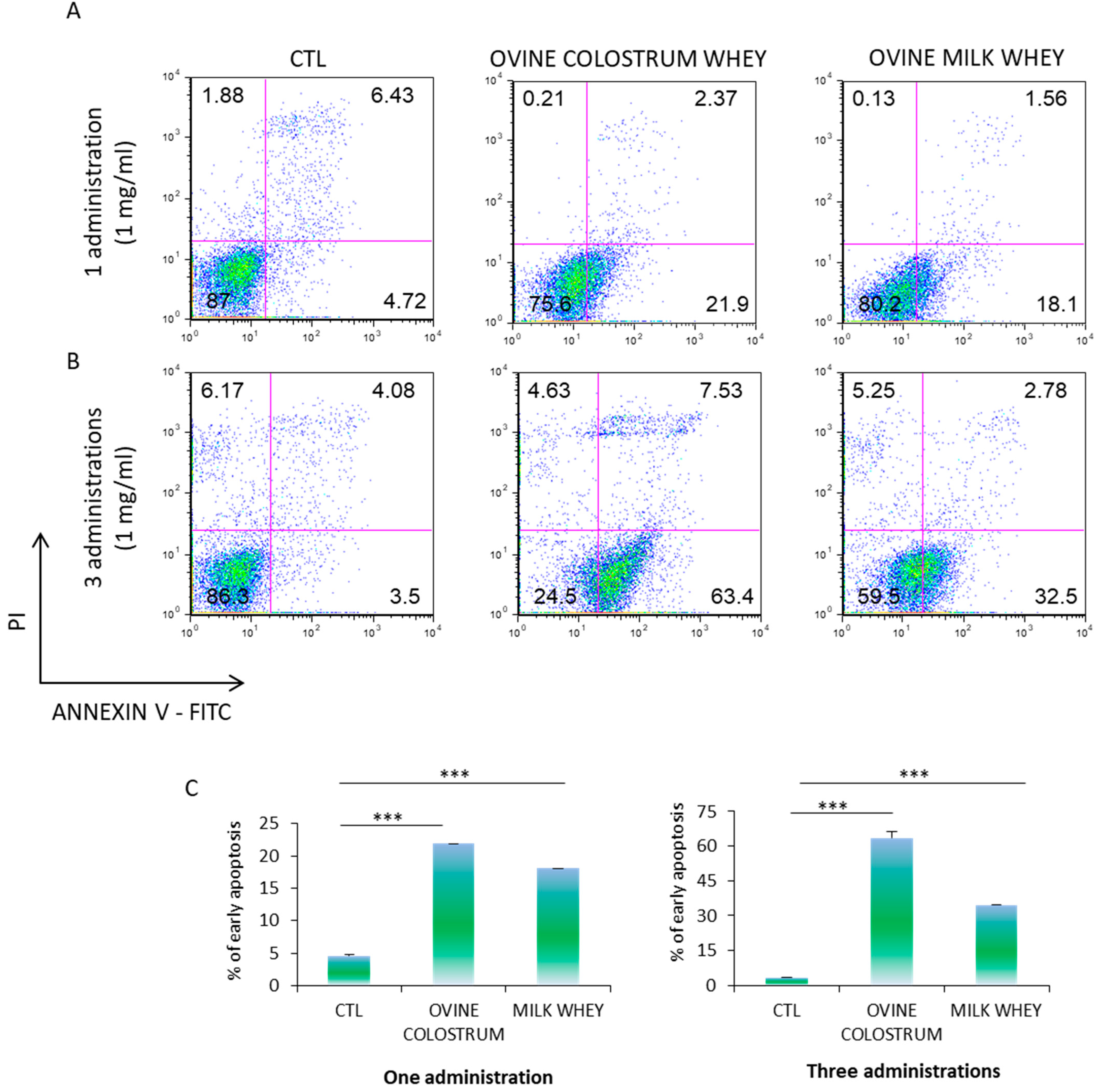
2.5. Annexin V/Propidium Iodide (PI) Staining Assay
2.6. Statistical Analysis
3. Results
3.1. Effect of Ovine Colostrum on K562 Cell Surface Profiling
3.2. Effect of Ovine Colostrum and Ovine Milk Whey on Leukemia K562 Cell Proliferation
3.3. Cytotoxicity of Ovine Colostrum and Ovine Serum
4. Discussion and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fu, Y.; Therkildsen, M.; Aluko, R.E.; Lametsch, R. Exploration of collagen recovered from animal by-products as a precursor of bioactive peptides: Successes and challenges. Crit. Rev. Food Sci. Nutr. 2019, 59, 2011–2027. [Google Scholar] [CrossRef]
- Sar, T.; Harirchi, S.; Ramezani, M.; Bulkan, G.; Akbas, M.Y.; Pandey, A.; Taherzadeh, M.J. Potential utilization of dairy industries by-products and wastes through microbial processes: A critical review. Sci. Total Environ. 2022, 810, 152253. [Google Scholar] [CrossRef]
- Costa, C.; Azoia, N.G.; Coelho, L.; Freixo, R.; Batista, P.; Pintado, M. Proteins derived from the dairy losses and by-products as raw materials for non-food applications. Foods 2021, 10, 135. [Google Scholar] [CrossRef] [PubMed]
- Agenbag, B.; Swinbourne, A.M.; Petrovski, K.; van Wettere, W.H.E.J. Lambs need colostrum: A review. Livest. Sci. 2021, 251, 104624. [Google Scholar] [CrossRef]
- Lopez, A.J.; Heinrichs, A.J. Invited review: The importance of colostrum in the newborn dairy calf. J. Dairy Sci. 2022, 105, 2733–2749. [Google Scholar] [CrossRef] [PubMed]
- Arslan, A.; Kaplan, M.; Duman, H.; Bayraktar, A.; Ertürk, M.; Henrick, B.M.; Frese, S.A.; Karav, S. Bovine Colostrum and Its Potential for Human Health and Nutrition. Front. Nutr. 2021, 8, 651721. [Google Scholar] [CrossRef]
- Playford, R.J.; Weiser, M.J. Bovine colostrum: Its constituents and uses. Nutrients 2021, 13, 265. [Google Scholar] [CrossRef]
- Regolamento CE REGOLAMENTO (CE) N. 853/2004. Gazzetta Ufficiale dell’Unione Europea, 2004.
- Bagwe, S.; Tharappel, L.J.; Kaur, G.; Buttar, H.S. Bovine colostrum: An emerging nutraceutical. J. Complement. Integr. Med. 2015, 12, 175–185. [Google Scholar] [CrossRef]
- Ceniti, C.; Costanzo, N.; Morittu, V.M.; Tilocca, B.; Roncada, P.; Britti, D. Review: Colostrum as an Emerging food: Nutraceutical Properties and Food Supplement. Food Rev. Int. 2022, 1–29. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Z.; Song, Y.; Zhang, Y.; Zhao, L.; Zhang, L.; Lü, X.; Wang, H.; Zhang, X.; Zhang, J.; et al. Characterization and comparison of lipids from human and ewe colostrum based on lipidomics analysis. Food Chem. 2023, 400, 133998. [Google Scholar] [CrossRef]
- Felice, V.D.; Owens, R.A.; Kennedy, D.; Hogan, S.A.; Lane, J.A. Comparative structural and compositional analyses of cow, buffalo, goat and sheep cream. Foods 2021, 10, 2643. [Google Scholar] [CrossRef] [PubMed]
- El-Salam, M.H.A.; El-Shibiny, S. Bioactive Peptides of Buffalo, Camel, Goat, Sheep, Mare, and Yak Milks and Milk Products. Food Rev. Int. 2013, 29, 1–23. [Google Scholar] [CrossRef]
- Carvalho, F.; Prazeres, A.R.; Rivas, J. Cheese whey wastewater: Characterization and treatment. Sci. Total Environ. 2013, 445, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Pires, A.F.; Marnotes, N.G.; Rubio, O.D.; Garcia, A.C.; Pereira, C.D. Dairy By-Products: A Review on the Valorization of Whey and Second Cheese Whey. Foods 2021, 10, 1067. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, A.P.F.; Bertolini, D.; Lopes, N.A.; Veras, F.F.; Gregory, G.; Brandelli, A. Characterization of nanoliposomes containing bioactive peptides obtained from sheep whey hydrolysates. LWT 2019, 101, 107–112. [Google Scholar] [CrossRef]
- Cereda, E.; Turri, A.; Klersy, C.; Cappello, S.; Ferrari, A.; Filippi, A.R.; Brugnatelli, S.; Caraccia, M.; Chiellino, S.; Borioli, V.; et al. Whey protein isolate supplementation improves body composition, muscle strength, and treatment tolerance in malnourished advanced cancer patients undergoing chemotherapy. Cancer Med. 2019, 8, 6923–6932. [Google Scholar] [CrossRef]
- Cacciola, N.A.; Salzano, A.; D’Onofrio, N.; Venneri, T.; De Cicco, P.; Vinale, F.; Petillo, O.; Martano, M.; Maiolino, P.; Neglia, G. Buffalo milk whey activates necroptosis and apoptosis in a xenograft model of colorectal cancer. Int. J. Mol. Sci. 2022, 23, 8464. [Google Scholar] [CrossRef]
- Osman, A.E.G.; Deininger, M.W. Chronic Myeloid Leukemia: Modern therapies, current challenges and future directions. Blood Rev. 2021, 49, 100825. [Google Scholar] [CrossRef]
- Minciacchi, V.R.; Kumar, R.; Krause, D.S. Chronic myeloid leukemia: A model disease of the past, present and future. Cells 2021, 10, 117. [Google Scholar] [CrossRef]
- Lopreiato, V.; Ceniti, C.; Trimboli, F.; Fratto, E.; Marotta, M.; Britti, D.; Morittu, V.M. Evaluation of the capillary electrophoresis method for measurement of immunoglobulin concentration in ewe colostrum. J. Dairy Sci. 2017, 100, 6465–6469. [Google Scholar] [CrossRef] [PubMed]
- Trimboli, F.; Costanzo, N.; Lopreiato, V.; Ceniti, C.; Morittu, V.M.; Spina, A.; Britti, D. Detection of buffalo milk adulteration with cow milk by capillary electrophoresis analysis. J. Dairy Sci. 2019, 102, 5962–5970. [Google Scholar] [CrossRef]
- Chiarella, E.; Carrá, G.; Scicchitano, S.; Codispoti, B.; Mega, T.; Lupia, M.; Pelaggi, D.; Marafioti, M.G.; Aloisio, A.; Giordano, M.; et al. Umg lenti: Novel lentiviral vectors for efficient transgene-and reporter gene expression in human early hematopoietic progenitors. PLoS ONE 2014, 9, e114795. [Google Scholar] [CrossRef] [PubMed]
- Codispoti, B.; Rinaldo, N.; Chiarella, E.; Lupia, M.; Spoleti, C.B.; Marafioti, M.G.; Aloisio, A.; Scicchitano, S.; Giordano, M.; Nappo, G.; et al. Recombinant TAT-BMI-1 fusion protein induces ex vivo expansion of human umbilical cord blood-derived hematopoietic stem cells. Oncotarget 2017, 8, 43782–43798. [Google Scholar] [CrossRef] [PubMed]
- Mesuraca, M.; Nisticò, C.; Lombardo, N.; Piazzetta, G.L.; Lobello, N.; Chiarella, E. Cellular and Biochemical Characterization of Mesenchymal Stem Cells from Killian Nasal Polyp. Int. J. Mol. Sci. 2022, 23, 13214. [Google Scholar] [CrossRef] [PubMed]
- Nisticò, C.; Pagliari, F.; Chiarella, E.; Guerreiro, J.F.; Marafioti, M.G.; Aversa, I.; Genard, G.; Hanley, R.; Garcia-Calderón, D.; Bond, H.M.; et al. Lipid droplet biosynthesis impairment through dgat2 inhibition sensitizes mcf7 breast cancer cells to radiation. Int. J. Mol. Sci. 2021, 22, 10102. [Google Scholar] [CrossRef]
- Chiarella, E.; Lombardo, N.; Lobello, N.; Piazzetta, G.L.; Morrone, H.L.; Mesuraca, M.; Bond, H.M. Deficit in adipose differentiation in mesenchymal stem cells derived from chronic rhinosinusitis nasal polyps compared to nasal mucosal tissue. Int. J. Mol. Sci. 2020, 21, 9214. [Google Scholar] [CrossRef]
- Ogino, T.; Kobuchi, H.; Fujita, H.; Matsukawa, A.; Utsumi, K. Erythroid and megakaryocytic differentiation of K562 erythroleukemic cells by monochloramine. Free Radic. Res. 2014, 48, 292–302. [Google Scholar] [CrossRef]
- Buttar, H.S.; Bagwe, S.M.; Bhullar, S.K.; Kaur, G. Health Benefits of Bovine Colostrum in Children and Adults. In Dairy in Human Health and Disease across the Lifespan; Academic Press: Cambridge, MA, USA, 2017; ISBN 9780128098691. [Google Scholar] [CrossRef]
- Kaplan, M.; Arslan, A.; Duman, H.; Karyelioğlu, M.; Baydemir, B.; Günar, B.B.; Alkan, M.; Bayraktar, A.; Tosun, H.İ.; Ertürk, M.; et al. Production of Bovine Colostrum for Human Consumption to Improve Health. Front. Pharmacol. 2022, 12, 796824. [Google Scholar] [CrossRef]
- Playford, R.J. The use of bovine colostrum in medical practice and human health: Current evidence and areas requiring further examination. Nutrients 2022, 14, 92. [Google Scholar] [CrossRef]
- Playford, R.J.; Macdonald, C.E.; Johnson, W.S. Colostrum and milk-derived peptide growth factors for the treatment of gastrointestinal disorders. Am. J. Clin. Nutr. 2000, 72, 5–14. [Google Scholar] [CrossRef]
- Bagwe-Parab, S.; Yadav, P.; Kaur, G.; Tuli, H.S.; Buttar, H.S. Therapeutic Applications of Human and Bovine Colostrum in the Treatment of Gastrointestinal Diseases and Distinctive Cancer Types: The Current Evidence. Front. Pharmacol. 2020, 11, 01100. [Google Scholar] [CrossRef]
- do Carmo França-Botelho, A.; Yan, B.; Gamal Abd El-Aziz Nasr, H. Beneficial Components of Colostrum for Cancer Patients: A Mini-review Focused on Oxidative Aspects and Properties of Colostrinin. Asian Oncol. Res. J. 2019, 2, 1–6. [Google Scholar]
- Agarwal, P.; Gupta, R. A Review on Anticancer Property of Colostrum. Res. Rev.-J. Med. Health Sci. 2016, 5, 1–9. [Google Scholar]
- Kim, Y.; Kim, M.J.; Han, K.S.; Imm, J.Y.; Oh, S.; Kim, S.H. Anticancer activity of lactoferrin isolated from caprine colostrum on human cancer cell lines. Int. J. Dairy Technol. 2009, 62, 277–281. [Google Scholar] [CrossRef]
- Sharma, A.; Shandilya, U.K.; Sodhi, M.; Mohanty, A.K.; Jain, P.; Mukesh, M. Evaluation of milk colostrum derived lactoferrin of sahiwal (Bos indicus) and karan fries (Cross-bred) cows for its anti-cancerous potential. Int. J. Mol. Sci. 2019, 20, 6318. [Google Scholar] [CrossRef]
- Gibbons, J.A.; Kanwar, J.R.; Kanwar, R.K. Iron-free and iron-saturated bovine lactoferrin inhibit survivin expression and differentially modulate apoptosis in breast cancer. BMC Cancer 2015, 15, 425. [Google Scholar] [CrossRef]
- Alsayed, A.R.; Hasoun, L.Z.; Khader, H.A.; Basheti, I.A.; Permana, A.D. Bovine Colostrum Treatment of Specific Cancer Types: Current Evidence and Future Opportunities. Molecules 2022, 27, 8641. [Google Scholar] [CrossRef]
- Chang, G.; Zhang, H.; Wang, J.; Zhang, Y.; Xu, H.; Wang, C.; Zhang, H.; Ma, L.; Li, Q.; Pang, T. CD44 targets Wnt/β-catenin pathway to mediate the proliferation of K562 cells. Cancer Cell Int. 2013, 13, 117. [Google Scholar] [CrossRef]
- Zolea, F.; Battaglia, A.M.; Chiarella, E.; Malanga, D.; De Marco, C.; Bond, H.M.; Morrone, G.; Costanzo, F.; Biamonte, F. Ferritin heavy subunit silencing blocks the erythroid commitment of K562 cells via miR-150 up-regulation and GATA-1 repression. Int. J. Mol. Sci. 2017, 18, 2167. [Google Scholar] [CrossRef]
- Ji, X.; Xu, W.; Cui, J.; Ma, Y.; Zhou, S. Goat and buffalo milk fat globule membranes exhibit better effects at inducing apoptosis and reduction the viability of HT-29 cells. Sci. Rep. 2019, 9, 2577. [Google Scholar] [CrossRef]
- D’onofrio, N.; Martino, E.; Balestrieri, A.; Mele, L.; Neglia, G.; Balestrieri, M.L.; Campanile, G. Sirt3 and metabolic reprogramming mediate the antiproliferative effects of whey in human colon cancer cells. Cancers 2021, 13, 5196. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceniti, C.; Ambrosio, R.L.; Bria, J.; Di Vito, A.; Tilocca, B.; Anastasio, A.; Britti, D.; Morittu, V.M.; Chiarella, E. Utilization of Dairy By-Products as a Source of Functional and Health Compounds—The Role of Ovine Colostrum and Milk Whey on Chronic Myeloid Leukemia Cells. Foods 2023, 12, 1752. https://doi.org/10.3390/foods12091752
Ceniti C, Ambrosio RL, Bria J, Di Vito A, Tilocca B, Anastasio A, Britti D, Morittu VM, Chiarella E. Utilization of Dairy By-Products as a Source of Functional and Health Compounds—The Role of Ovine Colostrum and Milk Whey on Chronic Myeloid Leukemia Cells. Foods. 2023; 12(9):1752. https://doi.org/10.3390/foods12091752
Chicago/Turabian StyleCeniti, Carlotta, Rosa Luisa Ambrosio, Jessica Bria, Anna Di Vito, Bruno Tilocca, Aniello Anastasio, Domenico Britti, Valeria Maria Morittu, and Emanuela Chiarella. 2023. "Utilization of Dairy By-Products as a Source of Functional and Health Compounds—The Role of Ovine Colostrum and Milk Whey on Chronic Myeloid Leukemia Cells" Foods 12, no. 9: 1752. https://doi.org/10.3390/foods12091752
APA StyleCeniti, C., Ambrosio, R. L., Bria, J., Di Vito, A., Tilocca, B., Anastasio, A., Britti, D., Morittu, V. M., & Chiarella, E. (2023). Utilization of Dairy By-Products as a Source of Functional and Health Compounds—The Role of Ovine Colostrum and Milk Whey on Chronic Myeloid Leukemia Cells. Foods, 12(9), 1752. https://doi.org/10.3390/foods12091752







