The Effect of Bovine Serum Albumin on Benzo[a]pyrene Removal by Lactobacillus Strains
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Media
2.2. Investigating the Effect of BSA on the Binding of BaP by Lactobacillus
2.2.1. BaP Binding Assay
2.2.2. Stability of Lactobacillus–BSA Binding to BaP
2.2.3. Effect of BaP Binding in a Simulated Gastrointestinal Environment
2.2.4. Heat- and Ultrasonically Treated BSA’s Impact on the Binding of BaP by Lactobacillus–BSA
2.3. Exploring the Mechanism of BaP Binding to Lactobacillus–BSA
2.3.1. Effect of BSA on the Properties of Bacterial Cell Surface
Surface Hydrophobicity
Automatic Aggregation
2.3.2. FTIR Analysis
2.3.3. SEM Analysis
2.3.4. Isotherm Model Studies
2.3.5. Adsorption Kinetics Studies
2.4. Statistical Analysis
3. Results
3.1. The Effect of BSA on the Binding of BaP by Lactobacillus
3.1.1. The BaP-Binding Ability of Lactobacillus–BSA
3.1.2. Stability of BaP Binding to Lactobacillus–BSA
3.1.3. Simulated Gastrointestinal Tract Analysis
3.1.4. The Effect of Heat- and Ultrasonically Treated BSA on the Binding of Lactobacillus–BSA to BaP
3.2. The Mechanism of BaP Binding to Lactobacillus–BSA
3.2.1. The Effect of BSA on Bacterial Cell Surface Properties
3.2.2. FTIR Analysis
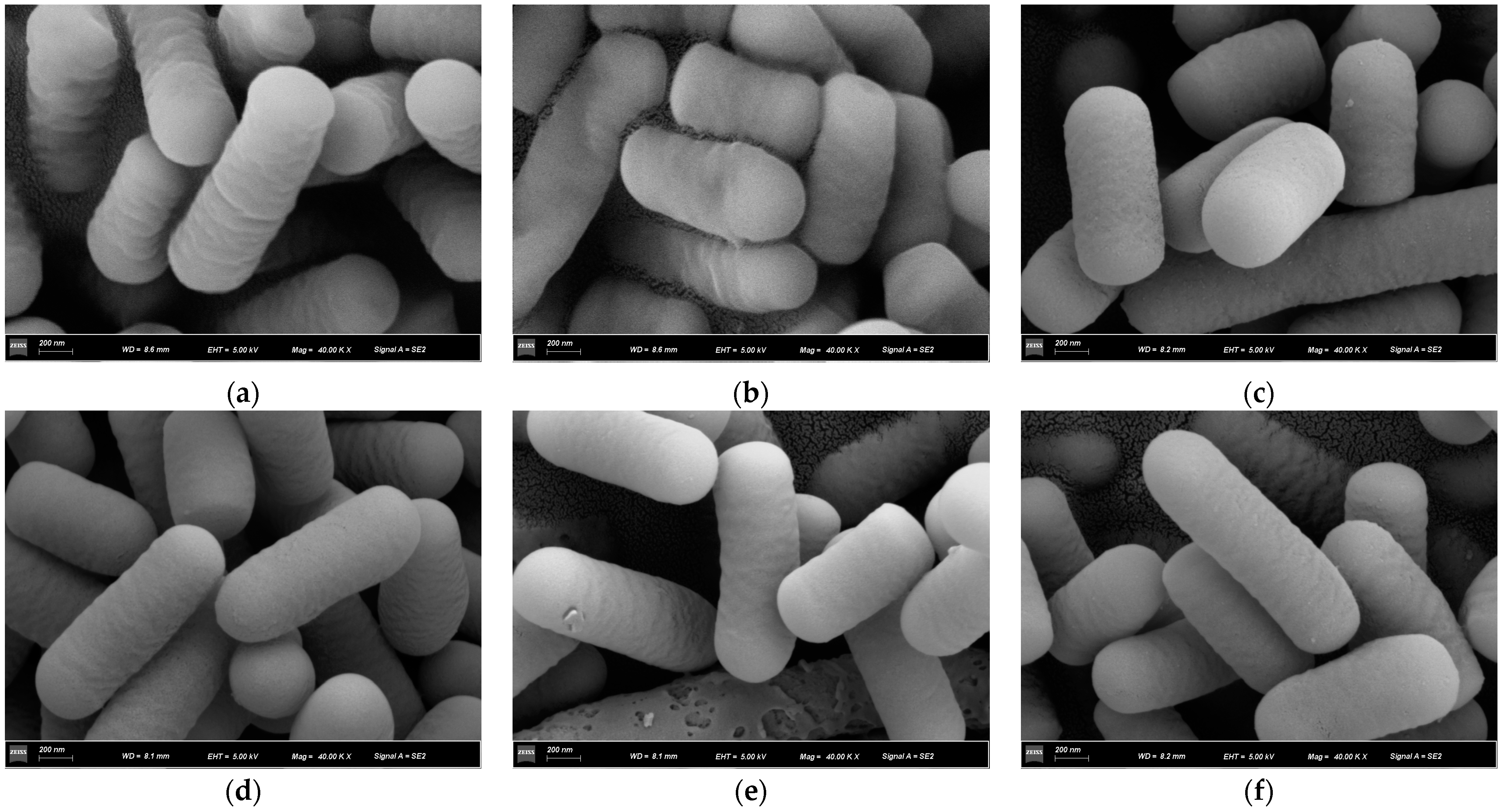
3.2.3. SEM Analysis
3.2.4. Biosorption Isotherm of BaP
3.2.5. Kinetic Model Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ledesma, E.; Rendueles, M.; Díaz, M. Spanish smoked meat products: Benzo(a)pyrene (BaP) contamination and moisture. J. Food Compos. Anal. 2015, 37, 87–94. [Google Scholar] [CrossRef]
- Shoukat, S. Potential anti-carcinogenic effect of probiotic and lactic acid bacteria in detoxification of benzo[a]pyrene: A review. Trends Food Sci. Technol. 2020, 99, 450–459. [Google Scholar] [CrossRef]
- Wu, R.; Jiang, Y.; Qin, R.; Shi, H.; Jia, C.; Rong, J.; Liu, R. Study of the formation of food hazard factors in fried fish nuggets. Food Chem. 2021, 373, 131562. [Google Scholar] [CrossRef]
- Reizer, E.; Csizmadia, I.G.; Palotás, B.; Viskolcz, B.; Fiser, B. Formation Mechanism of Benzo(a)pyrene: One of the Most Carcinogenic Polycyclic Aromatic Hydrocarbons (PAH). Molecules 2019, 24, 1040. [Google Scholar] [CrossRef]
- Moserová, M.; Kotrbová, V.; Aimová, D.; Šulc, M.; Frei, E.; Stiborová, M. Analysis of benzo[a]pyrene metabolites formed by rat hepatic microsomes using high pressure liquid chromatography: Optimization of the method. Interdiscip. Toxicol. 2009, 2, 239–244. [Google Scholar] [CrossRef]
- Yousefi, M.; Khorshidian, N.; Hosseini, H. The Ability of Probiotic Lactobacillus Strains in Removal of Benzo[a]pyrene: A Response Surface Methodology Study. Probiotics Antimicrob. Proteins 2021, 14, 464–475. [Google Scholar] [CrossRef] [PubMed]
- Niu, B.; Zhang, H.; Zhou, G.; Zhang, S.; Yang, Y.; Deng, X.; Chen, Q. Safety risk assessment and early warning of chemical contamination in vegetable oil. Food Control 2021, 125, 107970. [Google Scholar] [CrossRef]
- Yi, Y.-J.; Lim, J.-M.; Gu, S.; Lee, W.-K.; Oh, E. Potential use of lactic acid bacteria Leuconostoc mesenteroides as a probiotic for the removal of Pb(II) toxicity. J. Microbiol. 2017, 55, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wang, X.; Zhang, J.; Zhang, J.; Zhang, B. The mechanism of Lactobacillus strains for their ability to remove fumonisins B1 and B2. Food Chem. Toxicol. 2016, 97, 40–46. [Google Scholar] [CrossRef]
- Sadiq, F.A.; Yan, B.; Tian, F.; Zhao, J.; Zhang, H.; Chen, W. Lactic Acid Bacteria as Antifungal and Anti-Mycotoxigenic Agents: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1403–1436. [Google Scholar] [CrossRef]
- Qi, Y.Q.; Zhang, J.T.; Pan, X.H.; Pei, J.W.; Zhang, B.L. Binding of benzo(a)pyrene by Lactobacilli strains. Wei Sheng Wu Xue Bao 2011, 51, 956–964. [Google Scholar] [CrossRef]
- Zhao, H.F.; Zhou, F.; Qi, Y.Q.; Piotr, D.; Bai, F.L.; Piotr, W.; Zhang, B.L. Screening of Lactobacillus strains for their ability to bind Benzo(a)pyrene and the mechanism of the process. Food Chem. Toxicol. 2013, 59, 67–71. [Google Scholar] [CrossRef]
- Liu, J.X.; He, H.; Xu, M.F.; Wang, T.; Dziugan, P.; Zhao, H.F.; Zhang, B.L. Detoxification of oral exposure to benzo(a)pyrene by Lactobacillus plantarum CICC 23121 in mice. Mol. Nutr. Food Res. 2021, 65, 2001149. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, M.; Wang, S.; Lin, J.; Cai, L.; Song, L. New insight into the stereoselective interactions of quinine and quinidine, with bovine serum albumin. J. Mol. Recognit. 2014, 27, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.S.; Sarkar, A.; Rai, S.R.; Dasgupta, S.; Majumder, I.; Bhattacharya, A.; Das, D.; Bose, D.; Mukhopadhyay, J.; Mukhopadhyay, M. Probing the binding interaction of zinc (II) Schiff bases with bovine serum albumin: A spectroscopic and molecular docking study. Appl. Organomet. Chem. 2021, 35, e6164. [Google Scholar] [CrossRef]
- Papastavros, E.; Remmers, R.A.; Snow, D.D.; Cassada, D.A.; Hage, D.S. Affinity extraction of emerging contaminants from water based on bovine serum albumin as a binding agent. J. Sep. Sci. 2017, 41, 1074–1082. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Li, H.; Deng, Q.; Yuan, Z. Study of nobiletin binding to bovine serum albumin by capillary electrophoresis-frontal analysis and circular dichroism. Biomed. Chromatogr. 2010, 24, 1023–1028. [Google Scholar] [CrossRef]
- Gil, A.; Amiri, M.J.; Abedi-Koupai, J.; Eslamian, S. Adsorption/reduction of Hg(II) and Pb(II) from aqueous solutions by using bone ash/nZVI composite: Effects of aging time, Fe loading quantity and co-existing ions. Environ. Sci. Pollut. Res. 2017, 25, 2814–2829. [Google Scholar] [CrossRef]
- Hu, Q.; Lan, R.; He, L.; Liu, H.; Pei, X. A critical review of adsorption isotherm models for aqueous contaminants: Curve characteristics, site energy distribution and common controversies. J. Environ. Manag. 2023, 329, 117104. [Google Scholar] [CrossRef]
- Chakrapani, C.; Babu, C.S.; Vani, K.N.K.; Rao, K.S. Adsorption Kinetics for the Removal of Fluoride from Aqueous Solution by Activated Carbon Adsorbents Derived from the Peels of Selected Citrus Fruits. J. Chem. 2010, 7, S419–S427. [Google Scholar] [CrossRef]
- Paiva, P.H.C.; Coelho, Y.L.; da Silva, L.H.M.; Pinto, M.S.; Vidigal, M.C.T.; Pires, A.C.D.S. Influence of protein conformation and selected Hofmeister salts on bovine serum albumin/lutein complex formation. Food Chem. 2019, 305, 125463. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Pan, D.-Q.; Qiu, M.-J.; Liu, T.-T.; Jiang, M.; Wang, Q.; Shi, J.-H. Probing into the binding interaction between medroxyprogesterone acetate and bovine serum albumin (BSA): Spectroscopic and molecular docking methods. Luminescence 2016, 31, 1242–1250. [Google Scholar] [CrossRef]
- Fochesato, A.; Cuello, D.; Poloni, V.; Galvagno, M.A.; Dogi, C.A.; Cavaglieri, L.R. Aflatoxin B1adsorption/desorption dynamics in the presence of Lactobacillus rhamnosus RC007 in a gastrointestinal tract-simulated model. J. Appl. Microbiol. 2018, 126, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ming, Y.; Guo, H.; Zhu, Y.; Yang, Y.; Chen, S.; He, L.; Ao, X.; Liu, A.; Zhou, K.; et al. Screening of lactic acid bacteria for their capacity to bind cypermethrin in vitro and the binding characteristics and its application. Food Chem. 2021, 347, 129000. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.B.; Wu, N.; Yang, X.; He, X.T.; Wang, L.J. Improvement of emulsifying properties of Maillard reaction products from β-conglycinin and dextran using controlled enzymatic hydrolysis. Food Hydrocoll. 2012, 28, 301–312. [Google Scholar] [CrossRef]
- Ursache, F.-M.; Aprodu, I.; Nistor, O.-V.; Bratu, M.; Botez, E.; Stănciuc, N. Probing the heat-induced structural changes in bovine serum albumin by fluorescence spectroscopy and molecular modelling. Int. J. Dairy Technol. 2016, 70, 424–431. [Google Scholar] [CrossRef]
- Li, H.J.; Hu, Y.F.; Zhao, X.H.; Wan, W.; Du, X.; Kong, B.H.; Xia, X.F. Effects of different ultrasound powers on the structure and stability of protein from sea cucumber gonad. LWT-Food Sci. Technol. 2020, 137, 110403. [Google Scholar] [CrossRef]
- Haddaji, N.; Khouadja, S.; Fdhila, K.; Krifi, B.; Ben Ismail, M.; Lagha, R.; Bakir, K.; Bakhrouf, A. Acid stress suggests different determinants for polystyrene and HeLa cell adhesion in Lactobacillus casei. J. Dairy Sci. 2015, 98, 4302–4309. [Google Scholar] [CrossRef]
- Pithva, S.P.; Dave, J.M.; Vyas, B.R.M. Binding of acridine orange by probiotic Lactobacillus rhamnosus strains of human origin. Ann. Microbiol. 2014, 65, 1373–1379. [Google Scholar] [CrossRef]
- Zhao, L.; Wei, J.; Pan, X.; Jie, Y.; Zhu, B.; Zhao, H.; Zhang, B. Critical analysis of peptidoglycan structure of Lactobacillus acidophilus for phthalate removal. Chemosphere 2021, 282, 130982. [Google Scholar] [CrossRef]
- Shen, Y.; Zhao, S.; Zhao, X.; Sun, H.; Shao, M.; Xu, H. In vitro adsorption mechanism of acrylamide by lactic acid bacteria. LWT 2018, 100, 119–125. [Google Scholar] [CrossRef]
- Ge, N.; Xu, J.; Peng, B.; Pan, S. Adsorption mechanism of tenuazonic acid using inactivated lactic acid bacteria. Food Control 2017, 82, 274–282. [Google Scholar] [CrossRef]
- Costa-Trigo, I.; Paz, A.; Otero-Penedo, P.; Outeiriño, D.; Oliveira, R.P.D.S.; Domínguez, J.M. Detoxification of chestnut burrs hydrolyzates to produce biomolecules. Biochem. Eng. J. 2020, 159, 107599. [Google Scholar] [CrossRef]
- Le, B.; Yang, S.H. Biosorption of cadmium by potential probiotic Pediococcus pentosaceus using in vitro digestion model. Biotechnol. Appl. Biochem. 2017, 66, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Zhao, Q.; Malkmes, M.J.; Gao, G.; He, J.; Zheng, L.; Jiang, L. Biosorption of lead ions from aqueous solution by Clostridium tyrobutyricum immobilized in macroporous Ca-alginate-lignin beads. J. Appl. Microbiol. 2021, 132, 2080–2092. [Google Scholar] [CrossRef]
- Zoghi, A.; Darani, K.K.; Hekmatdoost, A. Effects of Pretreatments on Patulin Removal from Apple Juices Using Lactobacilli: Binding Stability in Simulated Gastrointestinal Condition and Modeling. Probiotics Antimicrob. Proteins 2020, 13, 135–145. [Google Scholar] [CrossRef]
- Gao, Z.P.; Yu, Z.F.; Yue, T.L.; Quek, S.Y. Adsorption isotherm, thermodynamics and kinetics studies of polyphenols separation from kiwifruit juice using adsorbent resin. J. Food Eng. 2012, 116, 195–201. [Google Scholar] [CrossRef]
 Control,
Control,  121, and
121, and  ML32.
ML32.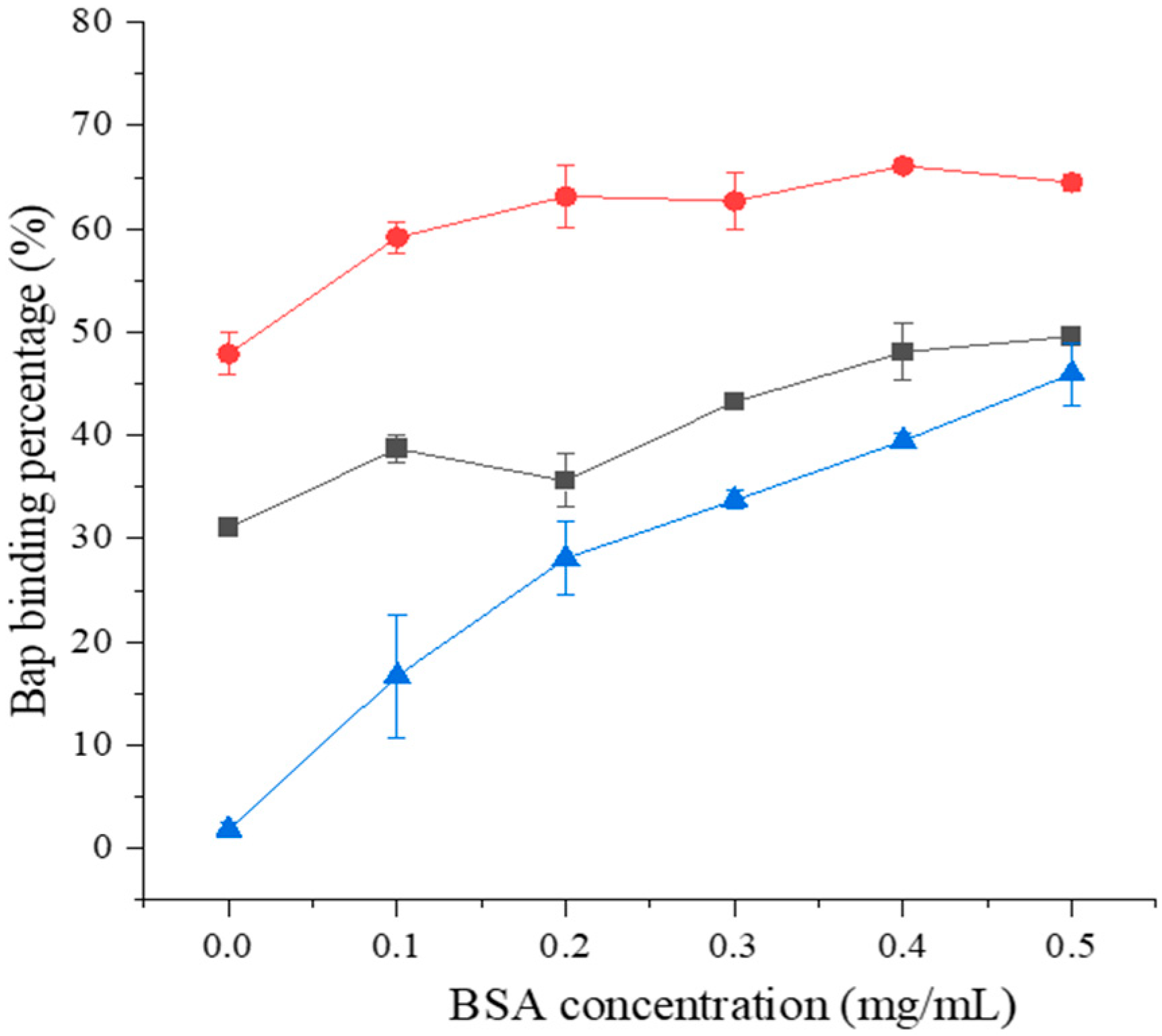
 121 and
121 and  ML32.
ML32.
 121 and
121 and  ML32.
ML32.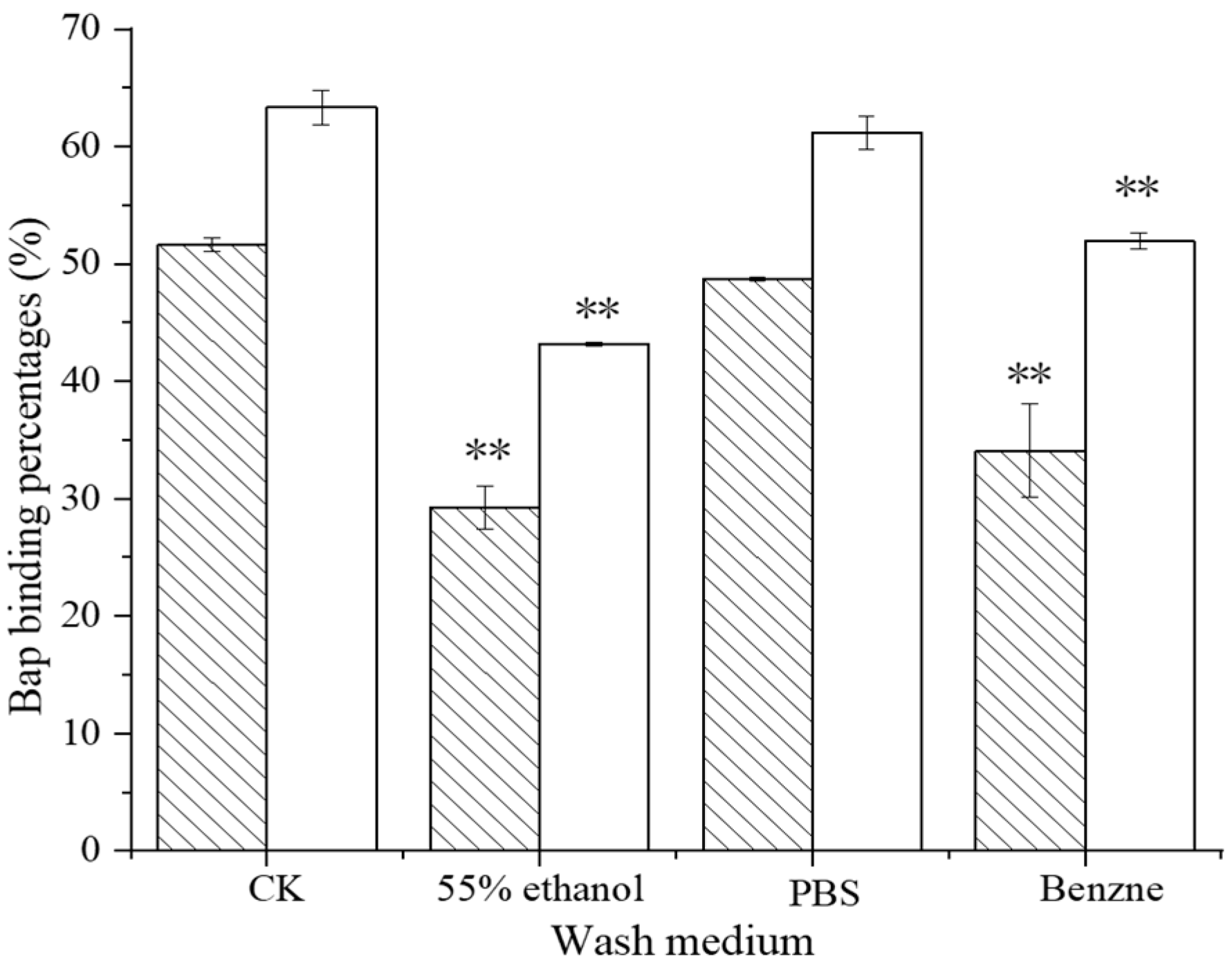
 121;
121;  ML32.
ML32.
 121;
121;  ML32.
ML32.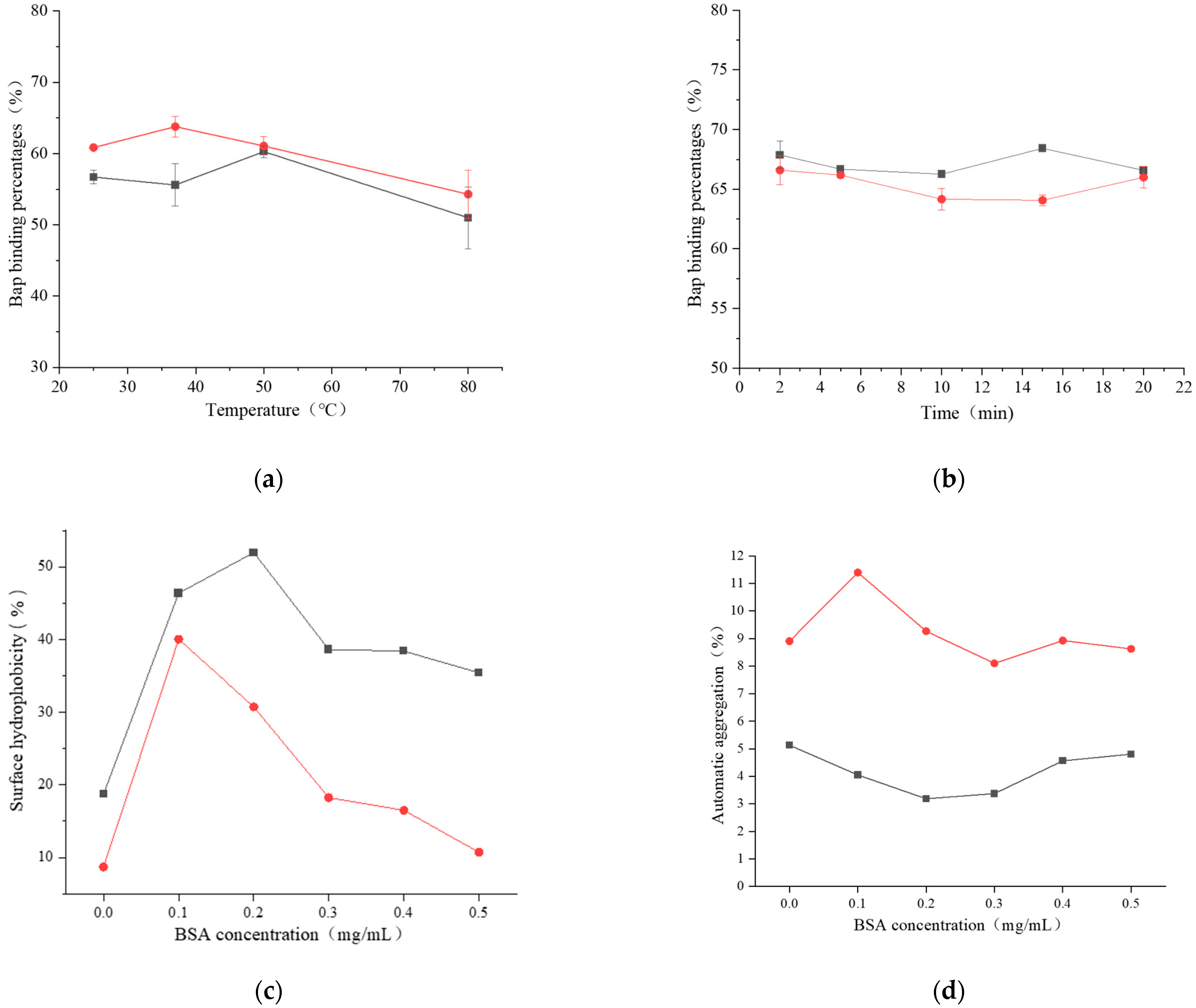
 121,
121,  121 + BaP + BSA,
121 + BaP + BSA,  121 + BaP; (b)
121 + BaP; (b)  ML32,
ML32,  ML32 + BaP + BSA, and
ML32 + BaP + BSA, and  ML32 + BaP.
ML32 + BaP.
 121,
121,  121 + BaP + BSA,
121 + BaP + BSA,  121 + BaP; (b)
121 + BaP; (b)  ML32,
ML32,  ML32 + BaP + BSA, and
ML32 + BaP + BSA, and  ML32 + BaP.
ML32 + BaP.
 121,
121,  121 + BSA,
121 + BSA,  ML32, and
ML32, and  ML32 + BSA. (b) Isotherm models of BaP;
ML32 + BSA. (b) Isotherm models of BaP;  121,
121,  121 + BSA,
121 + BSA,  Langmuir,
Langmuir,  Freundlich,
Freundlich,  Langmuir, and
Langmuir, and  Freundlich. (c) Isotherm models of BaP;
Freundlich. (c) Isotherm models of BaP;  ML32,
ML32,  ML32 + BSA,
ML32 + BSA,  Langmuir,
Langmuir,  Freundlich,
Freundlich,  Langmuir, and
Langmuir, and  Freundlich.
Freundlich.
 121,
121,  121 + BSA,
121 + BSA,  ML32, and
ML32, and  ML32 + BSA. (b) Isotherm models of BaP;
ML32 + BSA. (b) Isotherm models of BaP;  121,
121,  121 + BSA,
121 + BSA,  Langmuir,
Langmuir,  Freundlich,
Freundlich,  Langmuir, and
Langmuir, and  Freundlich. (c) Isotherm models of BaP;
Freundlich. (c) Isotherm models of BaP;  ML32,
ML32,  ML32 + BSA,
ML32 + BSA,  Langmuir,
Langmuir,  Freundlich,
Freundlich,  Langmuir, and
Langmuir, and  Freundlich.
Freundlich.
 121,
121,  121 + BSA,
121 + BSA,  ML32, and
ML32, and  ML32 + BSA.
ML32 + BSA.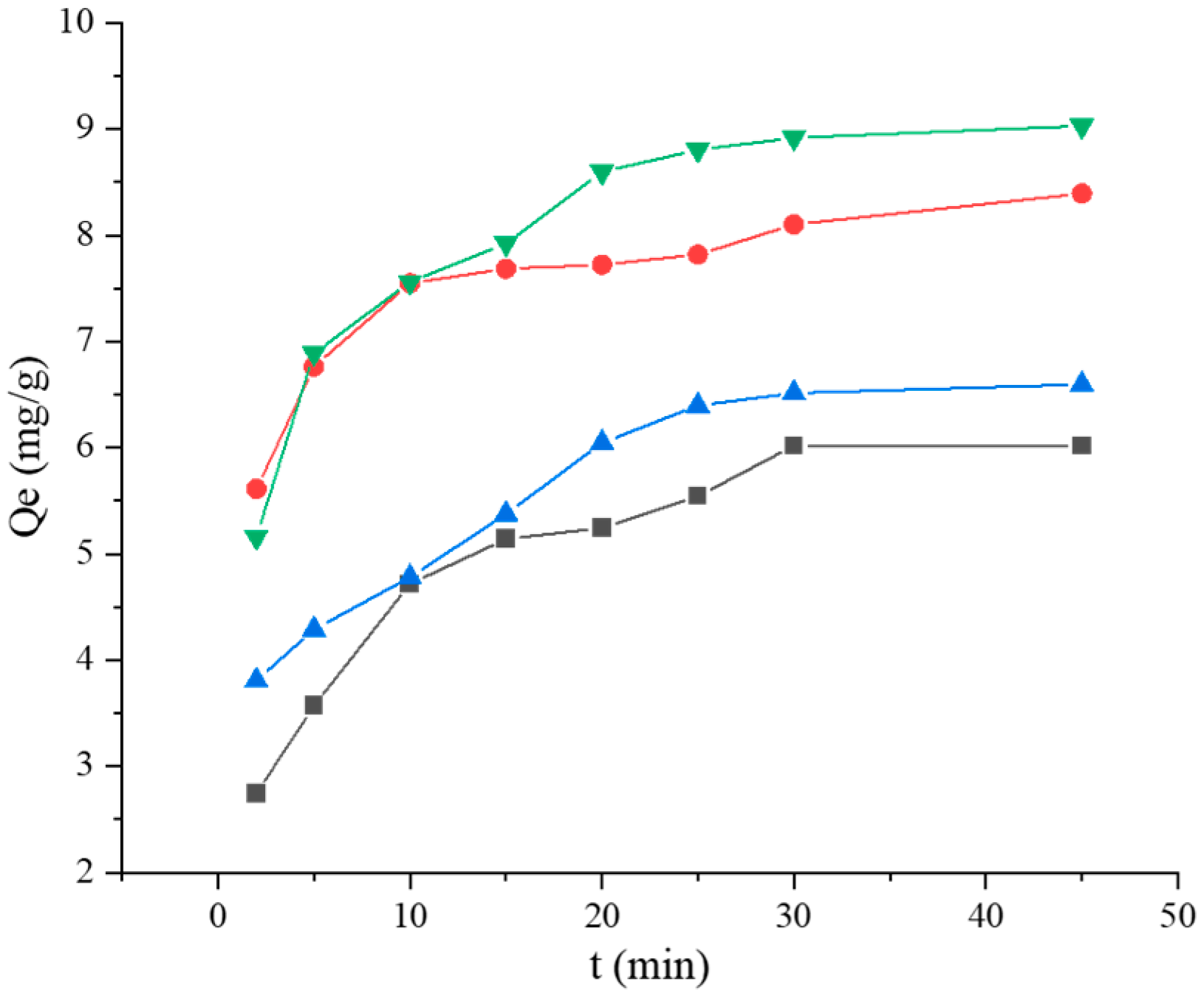
 121,
121,  121 + BSA, (a) pseudo-first-order, (b) pseudo-second-order, (c) Elovich, and (d) Weber–Morris. Adsorption kinetics models fitting of BaP by strain ML32 and its combination with BSA;
121 + BSA, (a) pseudo-first-order, (b) pseudo-second-order, (c) Elovich, and (d) Weber–Morris. Adsorption kinetics models fitting of BaP by strain ML32 and its combination with BSA;  ML32,
ML32,  ML32 + BSA, (e) pseudo-first-order, (f) pseudo-second-order, (g) Elovich, and (h) Weber–Morris.
ML32 + BSA, (e) pseudo-first-order, (f) pseudo-second-order, (g) Elovich, and (h) Weber–Morris.
 121,
121,  121 + BSA, (a) pseudo-first-order, (b) pseudo-second-order, (c) Elovich, and (d) Weber–Morris. Adsorption kinetics models fitting of BaP by strain ML32 and its combination with BSA;
121 + BSA, (a) pseudo-first-order, (b) pseudo-second-order, (c) Elovich, and (d) Weber–Morris. Adsorption kinetics models fitting of BaP by strain ML32 and its combination with BSA;  ML32,
ML32,  ML32 + BSA, (e) pseudo-first-order, (f) pseudo-second-order, (g) Elovich, and (h) Weber–Morris.
ML32 + BSA, (e) pseudo-first-order, (f) pseudo-second-order, (g) Elovich, and (h) Weber–Morris.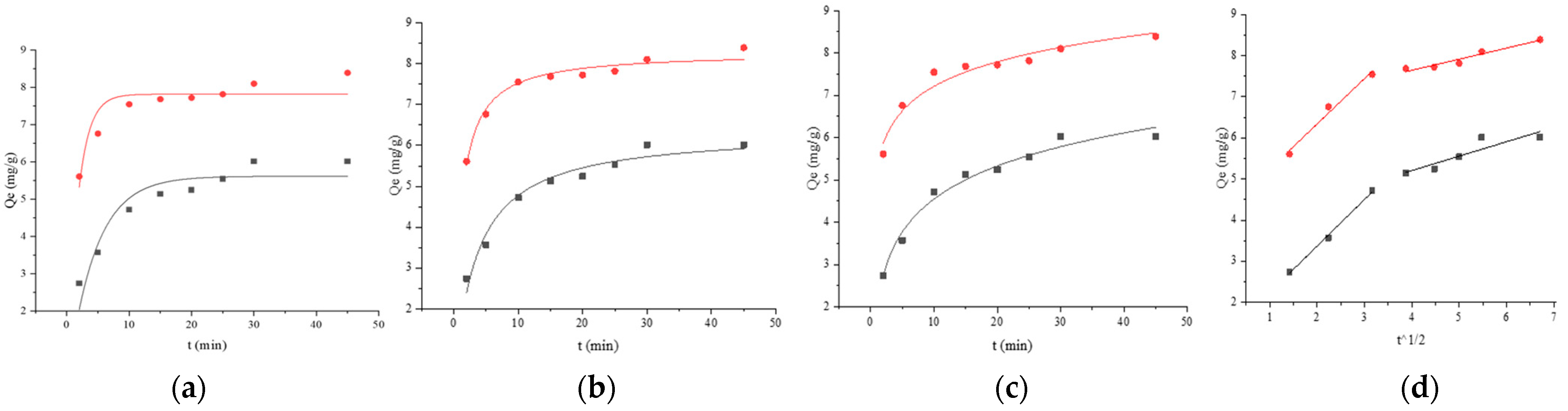

| Strains | Initial Concentration/(CFU/mL) | Simulated Gastric Juice | Simulated Intestinal Juice | ||
|---|---|---|---|---|---|
| Viable Count/(CFU/mL) | Survival Rate/(%) | Viable Count/(CFU/mL) | Survival Rate/(%) | ||
| 121 | 1.14 × 1010 | 9.75 × 109 | 85.53 | 9.7 × 108 | 8.51 |
| 121-BSA | 7.9 × 109 | 69.30 | 1.65 × 109 | 14.47 | |
| ML32 | 1.56 × 1010 | 1.2 × 1010 | 76.92 | 1.9 × 109 | 12.18 |
| ML32-BSA | 1.25 × 1010 | 80.12 | 2.06 × 109 | 13.20 | |
| Strains | Simulated Gastric Juice | Simulated Intestinal Juice | ||
|---|---|---|---|---|
| Cells | Cells Combined with BSA | Cells | Cells Combined with BSA | |
| 121 | 69.64 ± 1.24 | 74.17 ± 0.88 * | 6.56 ± 4.68 | 6.33 ± 0.58 |
| ML32 | 69.71 ± 2.32 | 72.86 ± 0.99 * | 4.64 ± 3.39 | 16.88 ± 2.23 * |
| Strains | Pearson Correlation Coefficient | |
|---|---|---|
| Surface Hydrophobicity | Automatic Aggregation | |
| 121 | 0.19 | 0.09 |
| ML32 | 0.22 | −0.13 |
| Strains | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|
| KL | qmax | R2 | KF | n | R2 | |
| 121 | 4.3167 × 10−6 | 1.0728 × 105 | 0.9654 | 0.0919 | 0.6988 | 0.9975 |
| 121-BSA | 7.6971 × 10−6 | 3.3348 × 105 | 0.9560 | 0.9182 | 0.7320 | 0.9911 |
| ML32 | 2.2721 × 10−5 | 1.5443 × 104 | 0.9656 | 0.4125 | 1.0443 | 0.9661 |
| ML32-BSA | 8.2851 × 10−6 | 2.4599 × 105 | 0.9679 | 0.9074 | 0.7865 | 0.9905 |
| Strains | Qexp | Pseudo-First-Order Kinetic | Pseudo-Second-Order Kinetic | Elovich Model | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| qe | K1 | R2 | qe | K2 | R2 | a | b | R2 | ||
| 121 | 6.0189 | 5.6158 | 0.2255 | 0.8793 | 6.3520 | 0.0477 | 0.9646 | 1.9525 | 1.1272 | 0.9793 |
| 121-BSA | 8.3920 | 7.8194 | 0.5710 | 0.8262 | 8.2810 | 0.1197 | 0.9681 | 5.2774 | 0.8400 | 0.9540 |
| ML32 | 6.5945 | 6.0094 | 0.3248 | 0.6032 | 6.6501 | 0.0684 | 0.8294 | 2.8213 | 1.0239 | 0.9417 |
| ML32-BSA | 9.0297 | 8.4909 | 0.3962 | 0.8432 | 9.2421 | 0.0631 | 0.9708 | 4.5667 | 1.2706 | 0.9686 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Sun, Z.; Liu, J.; Wang, T.; Zhang, B.; Zhao, H. The Effect of Bovine Serum Albumin on Benzo[a]pyrene Removal by Lactobacillus Strains. Foods 2023, 12, 1676. https://doi.org/10.3390/foods12081676
Zhang X, Sun Z, Liu J, Wang T, Zhang B, Zhao H. The Effect of Bovine Serum Albumin on Benzo[a]pyrene Removal by Lactobacillus Strains. Foods. 2023; 12(8):1676. https://doi.org/10.3390/foods12081676
Chicago/Turabian StyleZhang, Xue, Zihan Sun, Jinxia Liu, Tao Wang, Bolin Zhang, and Hongfei Zhao. 2023. "The Effect of Bovine Serum Albumin on Benzo[a]pyrene Removal by Lactobacillus Strains" Foods 12, no. 8: 1676. https://doi.org/10.3390/foods12081676
APA StyleZhang, X., Sun, Z., Liu, J., Wang, T., Zhang, B., & Zhao, H. (2023). The Effect of Bovine Serum Albumin on Benzo[a]pyrene Removal by Lactobacillus Strains. Foods, 12(8), 1676. https://doi.org/10.3390/foods12081676






