Study on the Interaction Mechanism of Theaflavin with Whey Protein: Multi-Spectroscopy Analysis and Molecular Docking
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Preparation of Stock Solution
2.3. Fluorescence Spectroscopy Measurements
2.4. Ultraviolet-Visible (UV-Vis) Absorption Spectra Analysis
2.5. Circular Dichroism (CD) Analysis
2.6. Molecular Docking Study
2.7. Statistical Analysis
3. Results and Discussion
3.1. Fluorescence Emission Spectra
3.2. UV–Vis Spectroscopy Analysis
3.3. Influence of TF1 Binding on the Structure of BSA/β-Lg/α-La
3.3.1. Synchronous Fluorescence Spectra
3.3.2. Fluorescence Excitation-Emission Matrix (EEM) Spectra
3.4. Circular Dichroism (CD) Spectroscopy Analysis
3.5. Molecular Docking Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, J.; Wei, Y.; Li, F.; Weng, X.; Wei, X. Regulation of Fungal Community and the Quality Formation and Safety Control of Pu-Erh Tea. Compr. Rev. Food Sci. Food Saf. 2022, 21, 4546–4572. [Google Scholar] [CrossRef] [PubMed]
- Saikia, D.; Boruah, P.K.; Sarma, U. A Sensor Network to Monitor Process Parameters of Fermentation and Drying in Black Tea Production. Mapan 2015, 30, 211–219. [Google Scholar] [CrossRef]
- He, H.F. Research Progress on Theaflavins: Efficacy, Formation, and Preparation. Nutr. Res. 2017, 61, 1344521. [Google Scholar] [CrossRef]
- Wu, Y.H.; Kuraji, R.; Taya, Y.; Ito, H.; Numabe, Y. Effects of Theaflavins on Tissue Inflammation and Bone Resorption on Experimental Periodontitis in Rats. J. Periodontal Res. 2018, 53, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wei, Y.; Huang, Y.; Wei, X. Regulatory Effects and Molecular Mechanisms of Tea and Its Active Compounds on Nonalcoholic Fatty Liver Disease. J. Agric. Food Chem. 2023, 71, 3103–3124. [Google Scholar] [CrossRef]
- Takemoto, M.; Takemoto, H.; Sakurada, A. Synthesis of Theaflavins with Camellia sinensis Cell Culture and Inhibition of Increase in Blood Sugar Values in High-Fat Diet Mice Subjected to Sucrose or Glucose Loading. Tetrahedron Lett. 2014, 55, 5038–5040. [Google Scholar] [CrossRef]
- Tanaka, Y.; Kirita, M.; Miyata, S.; Abe, Y.; Tagashira, M.; Kanda, T.; Maeda-Yamamoto, M. O-Methylated Theaflavins Suppress the Intracellular Accumulation of Triglycerides from Terminally Differentiated Human Visceral Adipocytes. J. Agric. Food Chem. 2013, 61, 12634–12639. [Google Scholar] [CrossRef]
- Braud, L.; Battault, S.; Meyer, G.; Nascimento, A.; Gaillard, S.; De Sousa, G.; Rahmani, R.; Riva, C.; Armand, M.; Reboul, C.; et al. Antioxidant Molecules of Tea (Camellia sinensis) Decrease Hepatic Lipogenesis and Steatosis in a High Fat-Sucrose Diet NAFLD Rat Model. Arch. Cardiovasc. Dis. Suppl. 2016, 8, 213. [Google Scholar] [CrossRef]
- Fatima, M.; Kesharwani, R.K.; Misra, K.; Rizvi, S.I. Protective Effect of Theaflavin on Erythrocytes Subjected to In Vitro Oxidative Stress. Biochem. Res. Int. 2013, 2013, 649759. [Google Scholar] [CrossRef]
- Tan, Q.; Peng, L.; Huang, Y.; Huang, W.; Bai, W.; Shi, L.; Li, X.; Chen, T. Structure–Activity Relationship Analysis on Antioxidant and Anticancer Actions of Theaflavins on Human Colon Cancer Cells. J. Agric. Food Chem. 2019, 67, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Takino, Y.; Imagawa, H. Studies on the Mechanism of the Oxidation of Tea Leaf Catechins: Part III. Formation of a Reddish Orange Pigment and Its Spectral Relationship to Some Benzotropolone Derivatives. Biosci. Biotechnol. Biochem. 1964, 28, 125–130. [Google Scholar] [CrossRef]
- Xu, J.; Wei, Y.; Huang, Y.; Weng, X.; Wei, X. Current Understanding and Future Perspectives on the Extraction, Structures, and Regulation of Muscle Function of Tea Pigments. Crit. Rev. Food Sci. Nutr. 2022, 1–23. [Google Scholar] [CrossRef]
- Lun Su, Y.; Leung, L.K.; Huang, Y.; Chen, Z.-Y. Stability of Tea Theaflavins and Catechins. Food Chem. 2003, 83, 189–195. [Google Scholar] [CrossRef]
- Qu, F.; Ai, Z.; Liu, S.; Zhang, H.; Chen, Y.; Wang, Y.; Ni, D. Study on Mechanism of Low Bioavailability of Black Tea Theaflavins by Using Caco-2 Cell Monolayer. Drug Deliv. 2021, 28, 1737–1747. [Google Scholar] [CrossRef]
- Ali, M.; Keppler, J.K.; Coenye, T.; Schwarz, K. Covalent Whey Protein–Rosmarinic Acid Interactions: A Comparison of Alkaline and Enzymatic Modifications on Physicochemical, Antioxidative, and Antibacterial Properties. J. Food Sci. 2018, 83, 2092–2100. [Google Scholar] [CrossRef]
- Tavares, G.M.; Croguennec, T.; Carvalho, A.F.; Bouhallab, S. Milk Proteins as Encapsulation Devices and Delivery Vehicles: Applications and Trends. Trends Food Sci. Technol. 2014, 37, 5–20. [Google Scholar] [CrossRef]
- Gong, S.; Yang, C.; Zhang, J.; Yu, Y.; Gu, X.; Li, W.; Wang, Z. Study on the Interaction Mechanism of Purple Potato Anthocyanins with Casein and Whey Protein. Food Hydrocoll. 2021, 111, 106223. [Google Scholar] [CrossRef]
- Oancea, A.-M.; Hasan, M.; Vasile, A.M.; Barbu, V.; Enachi, E.; Bahrim, G.; Râpeanu, G.; Silvi, S.; Stănciuc, N. Functional Evaluation of Microencapsulated Anthocyanins from Sour Cherries Skins Extract in Whey Proteins Isolate. LWT-Food Sci Technol. 2018, 95, 129–134. [Google Scholar] [CrossRef]
- Zhu, J.; Sun, X.; Wang, S.; Xu, Y.; Wang, D. Formation of Nanocomplexes Comprising Whey Proteins and Fucoxanthin: Characterization, Spectroscopic Analysis, and Molecular Docking. Food Hydrocoll. 2017, 63, 391–403. [Google Scholar] [CrossRef]
- Sułkowska, A. Interaction of Drugs with Bovine and Human Serum Albumin. J. Mol. Struct. 2002, 614, 227–232. [Google Scholar] [CrossRef]
- Mishra, V.; Mahor, S.; Rawat, A.; Gupta, P.N.; Dubey, P.; Khatri, K.; Vyas, S.P. Targeted Brain Delivery of AZT via Transferrin Anchored Pegylated Albumin Nanoparticles. J. Drug Target. 2006, 14, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wei, Y.; Xu, J.; Wei, X. A Comprehensive Review on the Prevention and Regulation of Alzheimer’s Disease by Tea and Its Active Ingredients. Crit. Rev. Food Sci. Nutr. 2022, 1–25. [Google Scholar] [CrossRef]
- Xie, H.; Luo, Z.C.; Xi-Can, L.I. Chemical Mechanism of Antioxidation of Theaflavin. Food Mach. 2018, 34, 23–26. [Google Scholar] [CrossRef]
- Patel, B.K.; Sepay, N.; Mahapatra, A. Curious Results in the Prospective Binding Interactions of the Food Additive Tartrazine with β-Lactoglobulin. Langmuir 2019, 35, 11579–11589. [Google Scholar] [CrossRef] [PubMed]
- Divsalar, A.; Saboury, A.A.; Mansoori-Torshizi, H.; Moghaddam, M.I.; Ahmad, F.; Hakimelahi, G.H. Comparative Studies on the Interaction Between Bovine β-Lacto-Globulin Type A and B and a New Designed Pd (II) Complex with Anti-Tumor Activity at Different Temperatures. J. Biomol. Struct. Dyn. 2009, 26, 587–597. [Google Scholar] [CrossRef]
- Kanakis, C.D.; Hasni, I.; Bourassa, P.; Tarantilis, P.A.; Polissiou, M.G.; Tajmir-Riahi, H.-A. Milk β-Lactoglobulin Complexes with Tea Polyphenols. Food Chem. 2011, 127, 1046–1055. [Google Scholar] [CrossRef]
- Kamau, S.M.; Cheison, S.C.; Chen, W.; Liu, X.-M.; Lu, R.-R. Alpha-Lactalbumin: Its Production Technologies and Bioactive Peptides. Compr. Rev. Food Sci. Food Saf. 2010, 9, 197–212. [Google Scholar] [CrossRef]
- Permyakov, E.A.; Berliner, L.J. α-Lactalbumin: Structure and Function. FEBS Lett. 2000, 473, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Kang, S.; Zhang, J.; Jiang, L.; Liu, Y.; Yang, M.; Cao, X.; Zheng, Y.; Shao, J.; Yue, X. The Non-Covalent Interactions between Whey Protein and Various Food Functional Ingredients. Food Chem. 2022, 394, 133455. [Google Scholar] [CrossRef] [PubMed]
- Al-Shabib, N.A.; Khan, J.M.; Malik, A.; Tabish Rehman, M.; AlAjmi, M.F.; Husain, F.M.; Hisamuddin, M.; Altwaijry, N. Molecular Interaction of Tea Catechin with Bovine β-Lactoglobulin: A Spectroscopic and in Silico Studies. Saudi Pharm. J. 2020, 28, 238–245. [Google Scholar] [CrossRef]
- Wang, M.; Xu, J.; Han, T.; Tang, L. Effects of Theaflavins on the Structure and Function of Bovine Lactoferrin. Food Chem. 2021, 338, 128048. [Google Scholar] [CrossRef]
- He, Z.; Xu, M.; Zeng, M.; Qin, F.; Chen, J. Interactions of Milk α- and β-Casein with Malvidin-3-O-Glucoside and Their Effects on the Stability of Grape Skin Anthocyanin Extracts. Food Chem. 2016, 199, 314–322. [Google Scholar] [CrossRef]
- Xu, J.; Wang, M.; Zheng, Y.; Tang, L. Spectroscopic Technique-Based Comparative Investigation on the Interaction of Theaflavins with Native and Glycated Human Serum Albumin. Molecules 2019, 24, 3171. [Google Scholar] [CrossRef]
- Alanazi, M.M.; Almehizia, A.A.; Bakheit, A.H.; Alsaif, N.A.; Alkahtani, H.M.; Wani, T.A. Mechanistic Interaction Study of 5,6-Dichloro-2-[2-(Pyridin-2-Yl)Ethyl]Isoindoline-1,3-Dione with Bovine Serum Albumin by Spectroscopic and Molecular Docking Approaches. Saudi Pharm. J. 2019, 27, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.; Chen, L.; Qian, L.; Lin, X.; Fan, X.; Teng, H.; Cao, H. Fabrication of Caseins Nanoparticles to Improve the Stability of Cyanidin 3-O-Glucoside. Food Chem. 2020, 317, 126418. [Google Scholar] [CrossRef] [PubMed]
- Zhan, F.; Ding, S.; Xie, W.; Zhu, X.; Hu, J.; Gao, J.; Li, B.; Chen, Y. Towards Understanding the Interaction of β-Lactoglobulin with Capsaicin: Multi-Spectroscopic, Thermodynamic, Molecular Docking and Molecular Dynamics Simulation Approaches. Food Hydrocoll. 2020, 105, 105767. [Google Scholar] [CrossRef]
- Yu, X.; Cai, X.; Luo, L.; Wang, J.; Ma, M.; Wang, M.; Zeng, L. Influence of Tea Polyphenol and Bovine Serum Albumin on Tea Cream Formation by Multiple Spectroscopy Methods and Molecular Docking. Food Chem. 2020, 333, 127432. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Deng, H.; Wang, Y. Comprehensive Investigations about the Binding Interaction of Acesulfame with Human Serum Albumin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 237, 118410. [Google Scholar] [CrossRef]
- Bi, S.; Yan, L.; Pang, B.; Wang, Y. Investigation of Three Flavonoids Binding to Bovine Serum Albumin Using Molecular Fluorescence Technique. J. Lumin. 2012, 132, 132–140. [Google Scholar] [CrossRef]
- Jia, J.; Gao, X.; Hao, M.; Tang, L. Comparison of Binding Interaction between β-Lactoglobulin and Three Common Polyphenols Using Multi-Spectroscopy and Modeling Methods. Food Chem. 2017, 228, 143–151. [Google Scholar] [CrossRef]
- Diao, M.; Liang, Y.; Zhao, J.; Zhao, C.; Zhang, J.; Zhang, T. Enhanced Cytotoxicity and Antioxidant Capacity of Kaempferol Complexed with α-Lactalbumin. Food Chem. Toxicol. 2021, 153, 112265. [Google Scholar] [CrossRef]
- Li, T.; Hu, P.; Dai, T.; Li, P.; Ye, X.; Chen, J.; Liu, C. Comparing the Binding Interaction between β-Lactoglobulin and Flavonoids with Different Structure by Multi-Spectroscopy Analysis and Molecular Docking. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 201, 197–206. [Google Scholar] [CrossRef]
- Condict, L.; Kaur, J.; Hung, A.; Ashton, J.; Kasapis, S. Combined Spectroscopic, Molecular Docking and Quantum Mechanics Study of β-Casein and Ferulic Acid Interactions Following UHT-like Treatment. Food Hydrocoll. 2019, 89, 351–359. [Google Scholar] [CrossRef]
- Shpigelman, A.; Shoham, Y.; Israeli-Lev, G.; Livney, Y.D. β-Lactoglobulin–Naringenin Complexes: Nano-Vehicles for the Delivery of a Hydrophobic Nutraceutical. Food Hydrocoll. 2014, 40, 214–224. [Google Scholar] [CrossRef]
- Li, X.R.; Wang, S. Binding of Glutathione and Melatonin to Human Serum Albumin: A Comparative Study. Colloids Surf. B Biointerfaces 2015, 125, 86–103. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, X.; Jiang, Y.; Yi, L.; Wu, Q.; Chang, H.; Diao, X.; Sun, Y.; Pan, X.; Zhou, N. A Spectroscopic Study on the Interaction between P-Nitrophenol and Bovine Serum Albumin. J. Lumin. 2014, 149, 353–360. [Google Scholar] [CrossRef]
- Kaur, J.; Katopo, L.; Hung, A.; Ashton, J.; Kasapis, S. Combined Spectroscopic, Molecular Docking and Quantum Mechanics Study of β-Casein and p-Coumaric Acid Interactions Following Thermal Treatment. Food Chem. 2018, 252, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Lu, Y.; Xu, H.; Lin, D.; He, Z.; Wu, H.; Liu, L.; Wang, Z. Reducing the Allergenic Capacity of β-Lactoglobulin by Covalent Conjugation with Dietary Polyphenols. Food Chem. 2018, 256, 427–434. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhong, Q. Probing the Binding between Norbixin and Dairy Proteins by Spectroscopy Methods. Food Chem. 2013, 139, 611–616. [Google Scholar] [CrossRef]
- Diao, M.; Liang, Y.; Zhao, J.; Zhang, J.; Zhang, T. Complexation of Ellagic Acid with α-Lactalbumin and Its Antioxidant Property. Food Chem. 2022, 372, 131307. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhao, R.; Wang, C.; Zhang, X.; Wang, C. Deciphering the Non-Covalent Binding Patterns of Three Whey Proteins with Rosmarinic Acid by Multi-Spectroscopic, Molecular Docking and Molecular Dynamics Simulation Approaches. Food Hydrocoll. 2022, 132, 107895. [Google Scholar] [CrossRef]
- Bobone, S.; van de Weert, M.; Stella, L. A Reassessment of Synchronous Fluorescence in the Separation of Trp and Tyr Contributions in Protein Emission and in the Determination of Conformational Changes. J. Mol. Struct. 2014, 1077, 68–76. [Google Scholar] [CrossRef]
- Bi, H.; Tang, L.; Gao, X.; Jia, J.; Lv, H. Spectroscopic Analysis on the Binding Interaction between Tetracycline Hydrochloride and Bovine Proteins β-Casein, α-Lactalbumin. J. Lumin. 2016, 178, 72–83. [Google Scholar] [CrossRef]
- Peng, X.; Zhang, G.; Liao, Y.; Gong, D. Inhibitory Kinetics and Mechanism of Kaempferol on α-Glucosidase. Food Chem. 2016, 190, 207–2158. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Ding, F.; Peng, Y.-K.; Sun, Y. Molecular Recognition of Malachite Green by Hemoglobin and Their Specific Interactions: Insights from in Silico Docking and Molecular Spectroscopy. Mol. Biosyst. 2014, 10, 138–148. [Google Scholar] [CrossRef]
- Wu, D.; Yan, J.; Wang, J.; Wang, Q.; Li, H. Characterisation of Interaction between Food Colourant Allura Red AC and Human Serum Albumin: Multispectroscopic Analyses and Docking Simulations. Food Chem. 2015, 170, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Mahanta, S.; Paul, S. Bovine α-Lactalbumin Functionalized Graphene Oxide Nano-Sheet Exhibits Enhanced Biocompatibility: A Rational Strategy for Graphene-Based Targeted Cancer Therapy. Colloids Surf. B Biointerfaces 2015, 134, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Sun, Y.; Cheng, J.; Zhang, X.; Guo, M. Changes in Conformation and Functionality of Whey Proteins Induced by the Interactions with Soy Isoflavones. LWT 2022, 163, 113555. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Zhang, L. Study on the Mechanism of Non-Covalent Interaction between Rose Anthocyanin Extracts and Whey Protein Isolate under Different PH Conditions. Food Chem. 2022, 384, 132492. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.; Wang, F.; Han, J.; Liu, B.; Shen, J.; Zhang, L.; Okamoto, Y. Influence of the Substituents on Phenyl Groups on Enantioseparation Property of Amylose Phenylcarbamates. Carbohydr. Polym. 2020, 241, 116372. [Google Scholar] [CrossRef]
- Lakshmana Senthil, S.; Raghu, C.; Arjun, H.A.; Anantharaman, P. In Vitro and in Silico Inhibition Properties of Fucoidan against α-Amylase and α-D-Glucosidase with Relevance to Type 2 Diabetes Mellitus. Carbohydr. Polym. 2019, 209, 350–355. [Google Scholar] [CrossRef]
- Harvey, B.J.; Bell, E.; Brancaleon, L. A Tryptophan Rotamer Located in a Polar Environment Probes PH-Dependent Conformational Changes in Bovine β-Lactoglobulin A. J. Phys. Chem. B 2007, 111, 2610–2620. [Google Scholar] [CrossRef] [PubMed]
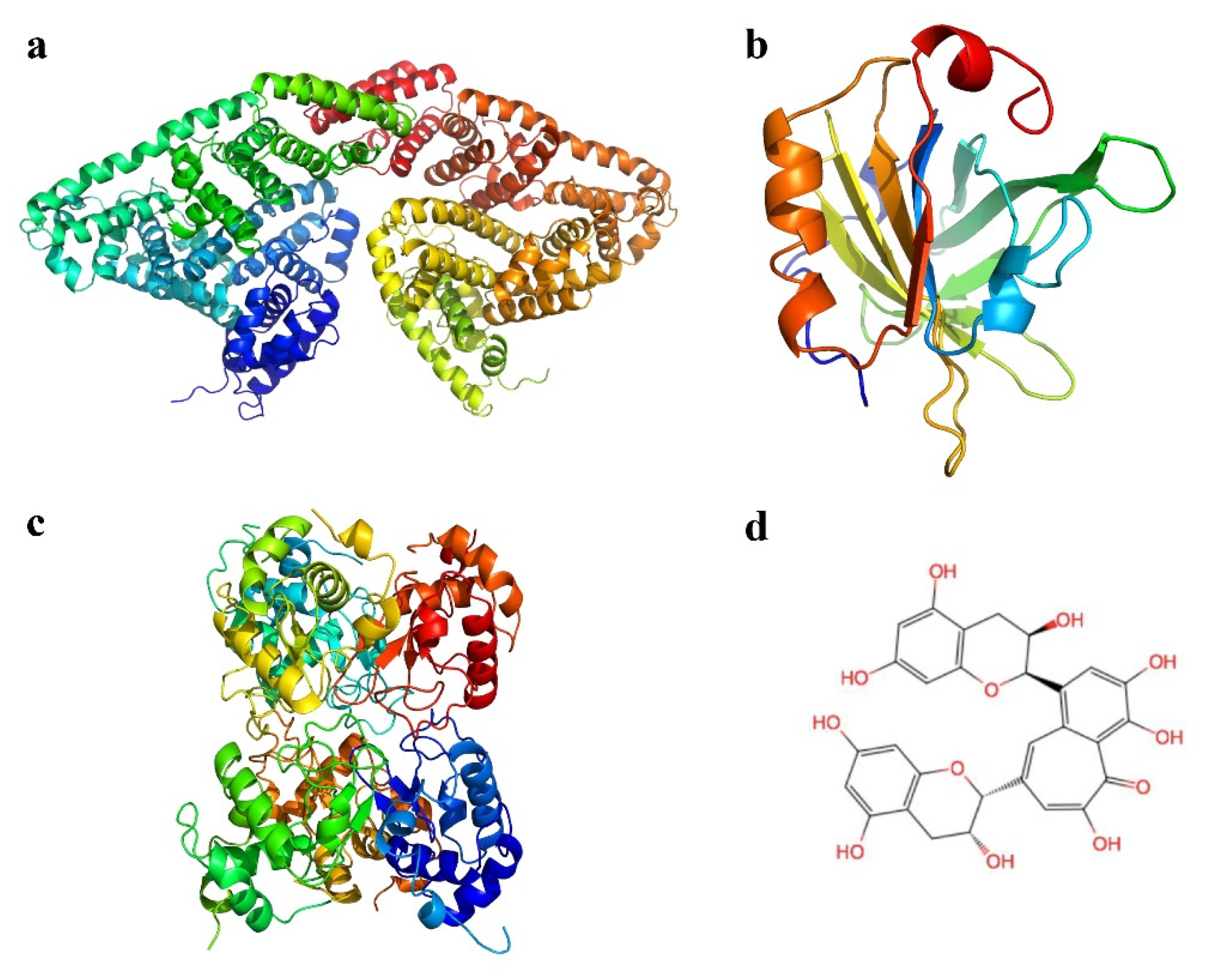

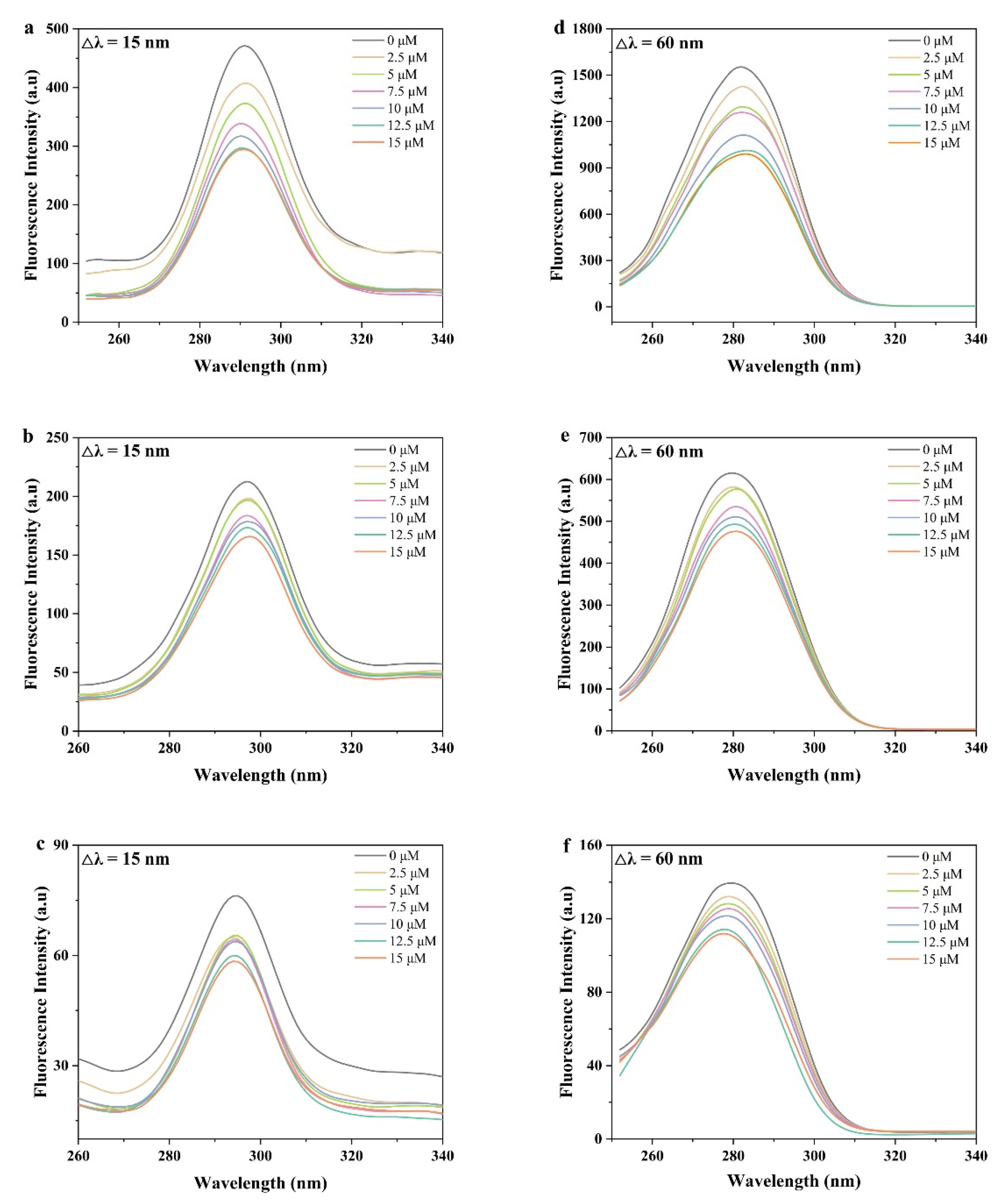
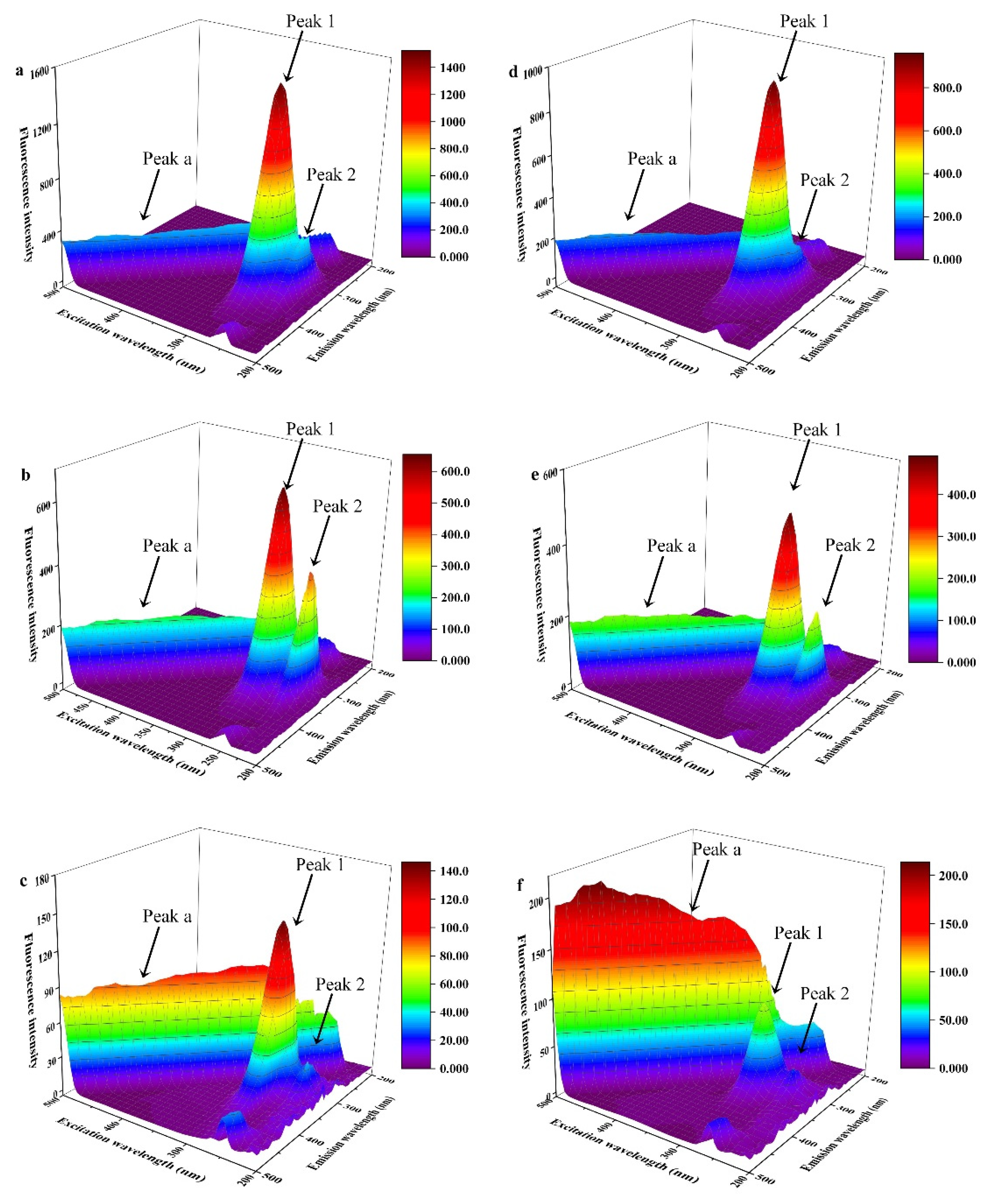
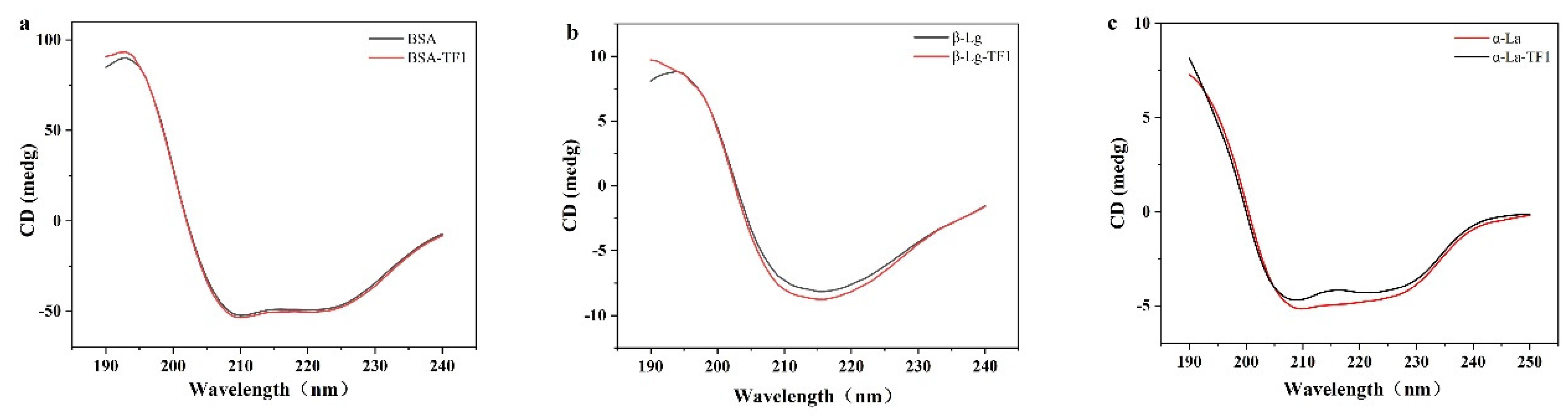


| Compound | Peak 1 (λex/λem) | Intensity | Peak 2 (λex/λem) | Intensity |
|---|---|---|---|---|
| BSA | 280.0/340.0 | 1521.0 | 235.0/340.0 | 447.4 |
| BSA-TF1 | 280.0/340.0 | 958.8 | 235.0/340.0 | 215.8 |
| β-Lg | 280.0/335.0 | 653.0 | 235.0/335.0 | 410.0 |
| β-Lg-TF1 | 280.0/335.0 | 491.5 | 235.0/330.0 | 239.3 |
| α-La | 280.0/330.0 | 147.3 | 230.0/335.0 | 36.7 |
| α-La-TF1 | 285.0/330.0 | 91.76 | 230.0/335.0 | 23.6 |
| Sample | α-Helix (%) | β-Sheet (%) | β-Turn (%) | Random Coil (%) |
|---|---|---|---|---|
| BSA | 48.3 ± 0.2 | 9.2 ± 0.0 | 16.8 ± 0.2 | 25.6 ± 0.1 |
| BSA-TF1 | 50.7 ± 0.4 | 7.5 ± 0.0 | 15.8 ± 0.2 | 26.0 ± 0.2 |
| β-Lg | 39.7 ± 0.1 | 7.6 ± 0.1 | 25.5 ± 0.2 | 27.2 ± 0.0 |
| β-Lg-TF1 | 37.8 ± 0.2 | 10.8 ± 0.1 | 23.2 ± 0.1 | 28.2 ± 0.2 |
| α-La | 38.5 ± 0.1 | 6.4 ± 0.1 | 21.5 ± 0.1 | 33.7 ± 0.1 |
| α-La-TF1 | 40.7 ± 0.2 | 5.2 ± 0.0 | 19.8± 0.1 | 34.3 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Huang, Y.; Wei, Y.; Weng, X.; Wei, X. Study on the Interaction Mechanism of Theaflavin with Whey Protein: Multi-Spectroscopy Analysis and Molecular Docking. Foods 2023, 12, 1637. https://doi.org/10.3390/foods12081637
Xu J, Huang Y, Wei Y, Weng X, Wei X. Study on the Interaction Mechanism of Theaflavin with Whey Protein: Multi-Spectroscopy Analysis and Molecular Docking. Foods. 2023; 12(8):1637. https://doi.org/10.3390/foods12081637
Chicago/Turabian StyleXu, Jia, Yi Huang, Yang Wei, Xinchu Weng, and Xinlin Wei. 2023. "Study on the Interaction Mechanism of Theaflavin with Whey Protein: Multi-Spectroscopy Analysis and Molecular Docking" Foods 12, no. 8: 1637. https://doi.org/10.3390/foods12081637
APA StyleXu, J., Huang, Y., Wei, Y., Weng, X., & Wei, X. (2023). Study on the Interaction Mechanism of Theaflavin with Whey Protein: Multi-Spectroscopy Analysis and Molecular Docking. Foods, 12(8), 1637. https://doi.org/10.3390/foods12081637






