The Impact of Storage Temperature on the Development of Microbial Communities on the Surface of Blueberry Fruit
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Preservation Quality Indices
2.3. Microbiological Analyses
2.3.1. Microbial Collection on Blueberry Surfaces
2.3.2. DNA Extraction, High-Throughput Sequencing, and Data Control
2.4. Bioinformatics Analysis
3. Results
3.1. Storage Temperature Affects Microbial α-Diversity on Blueberry Surfaces
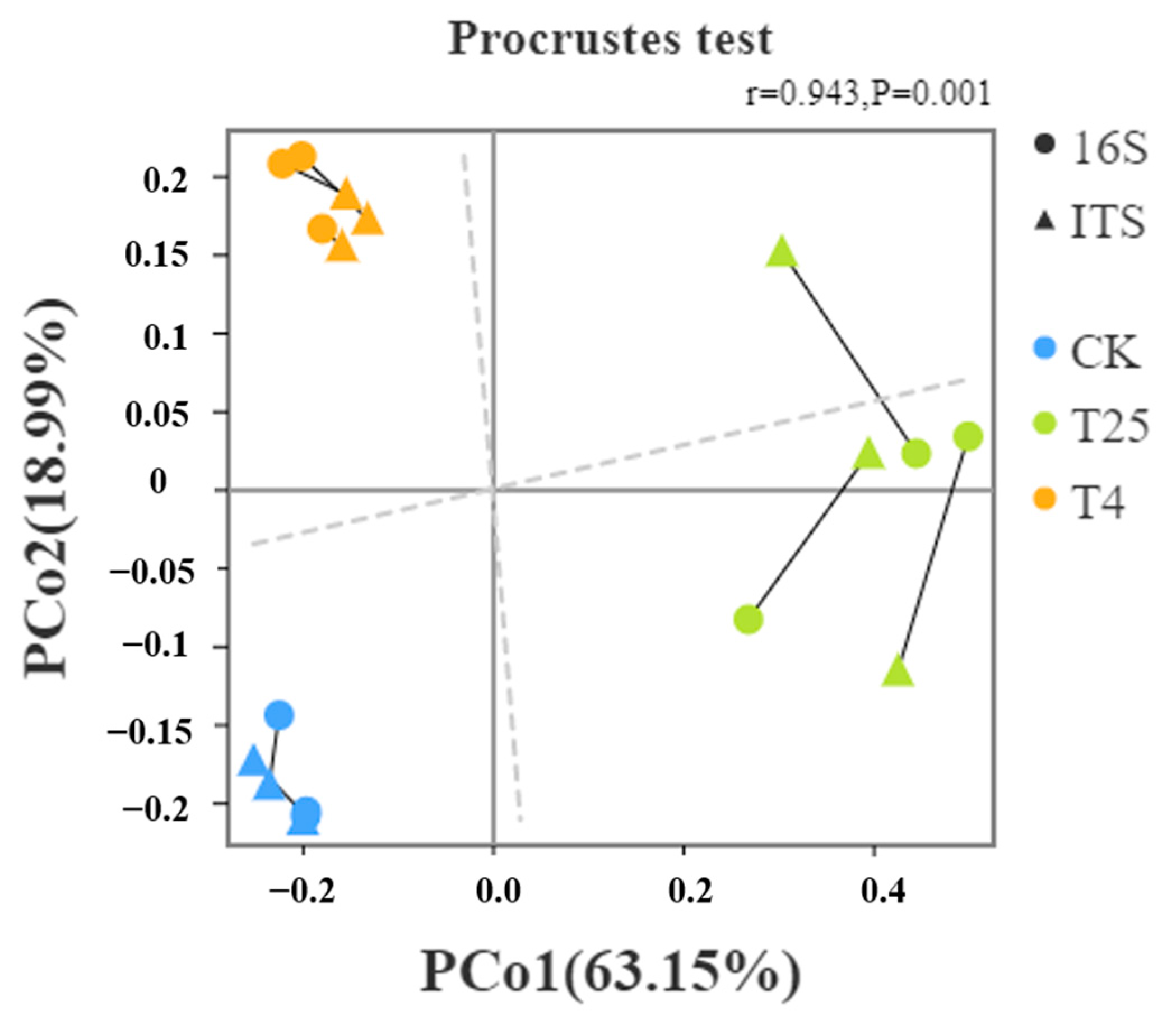
3.2. Storage Temperature Affects Microbial Community Composition and Changes on Blueberry Surfaces
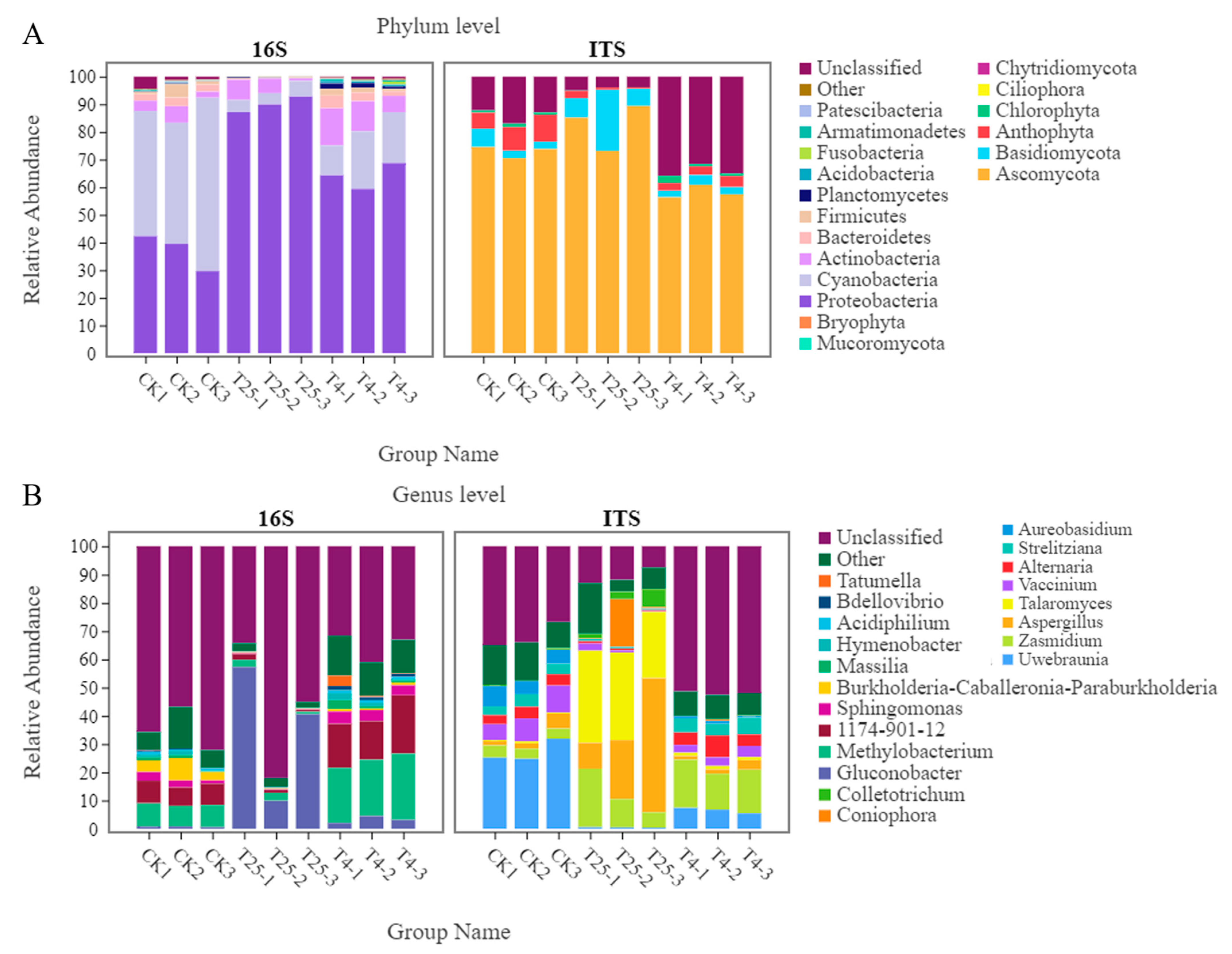
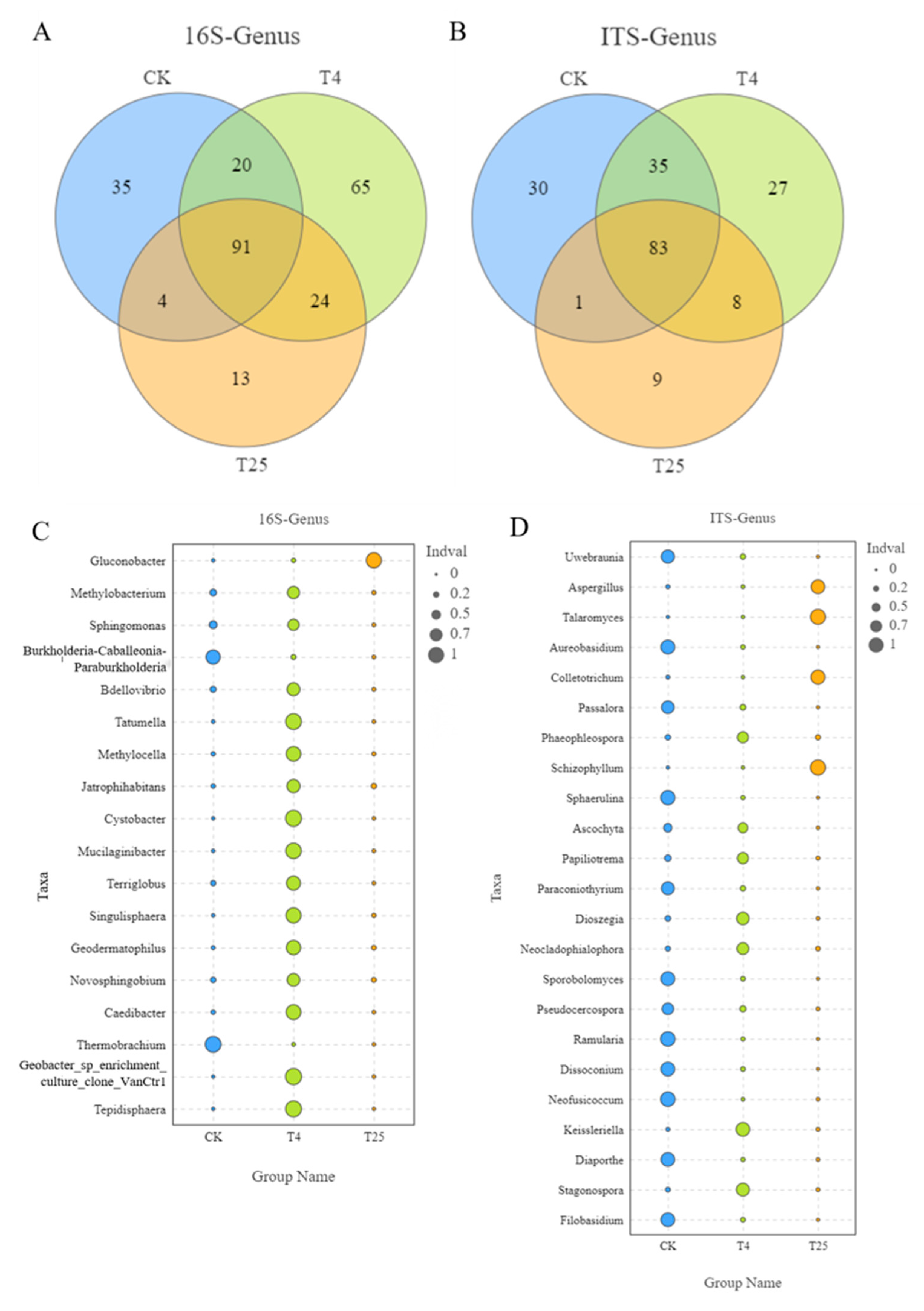
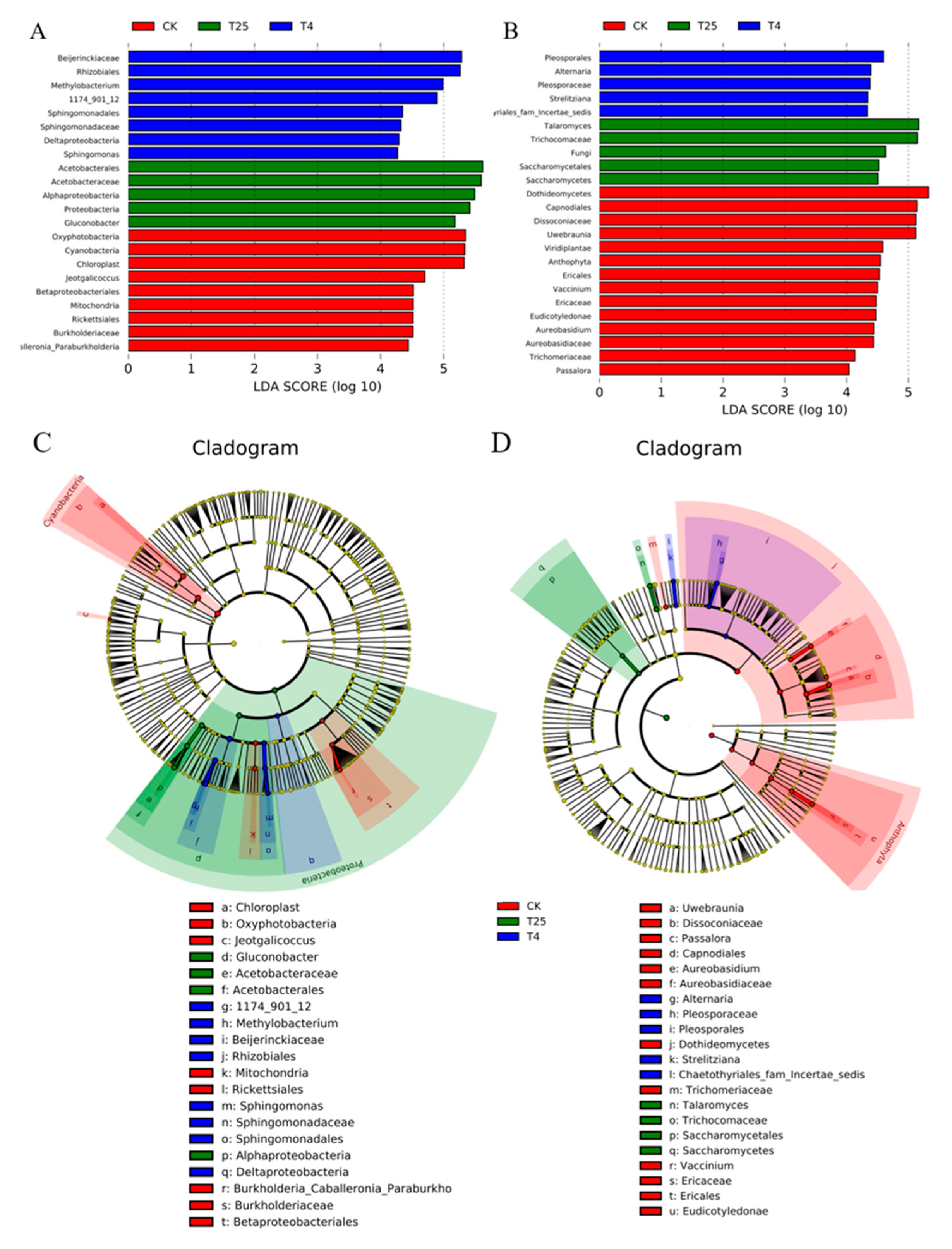
3.3. Major Members of the Microbiota on Blueberry Surfaces
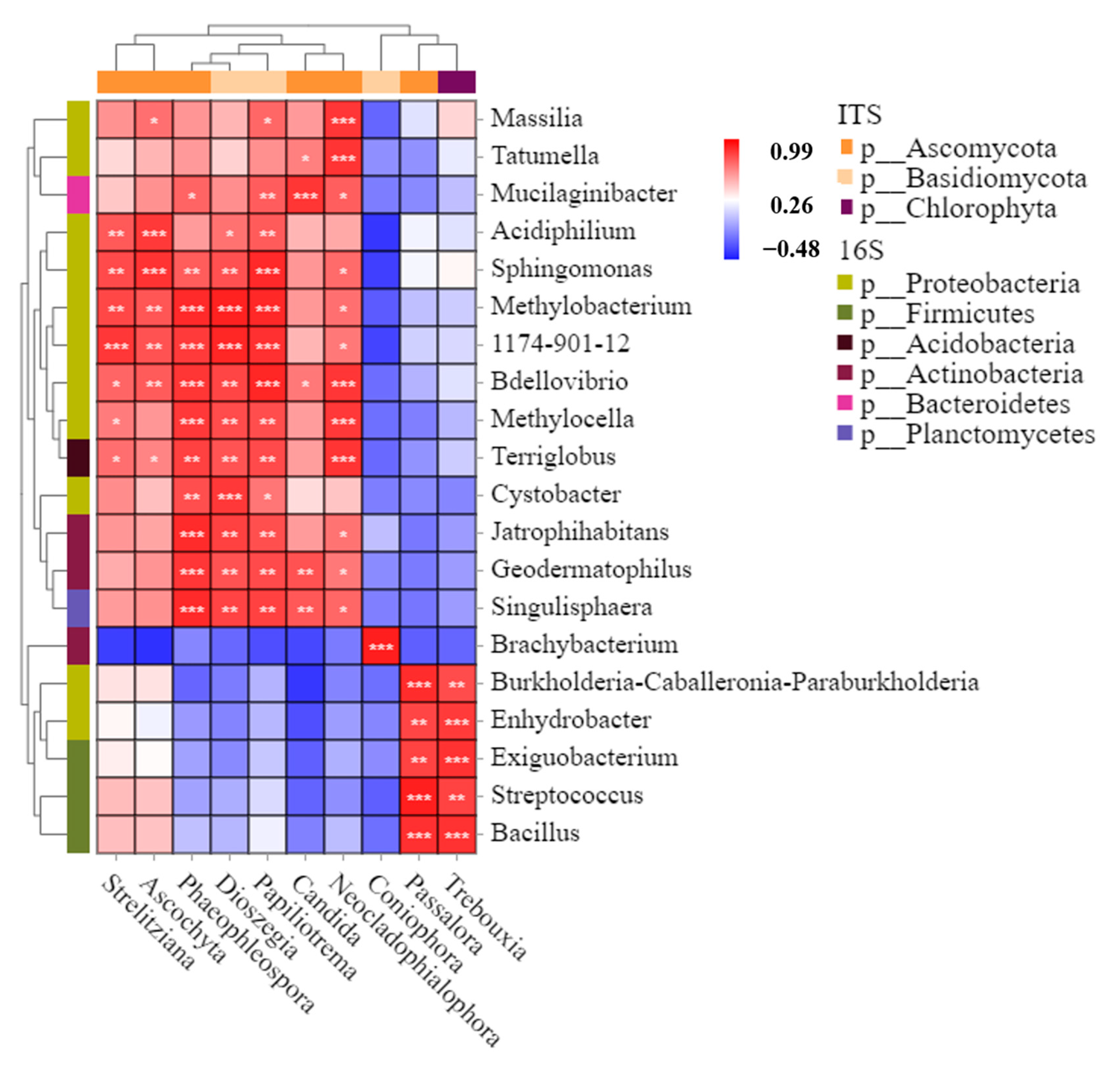
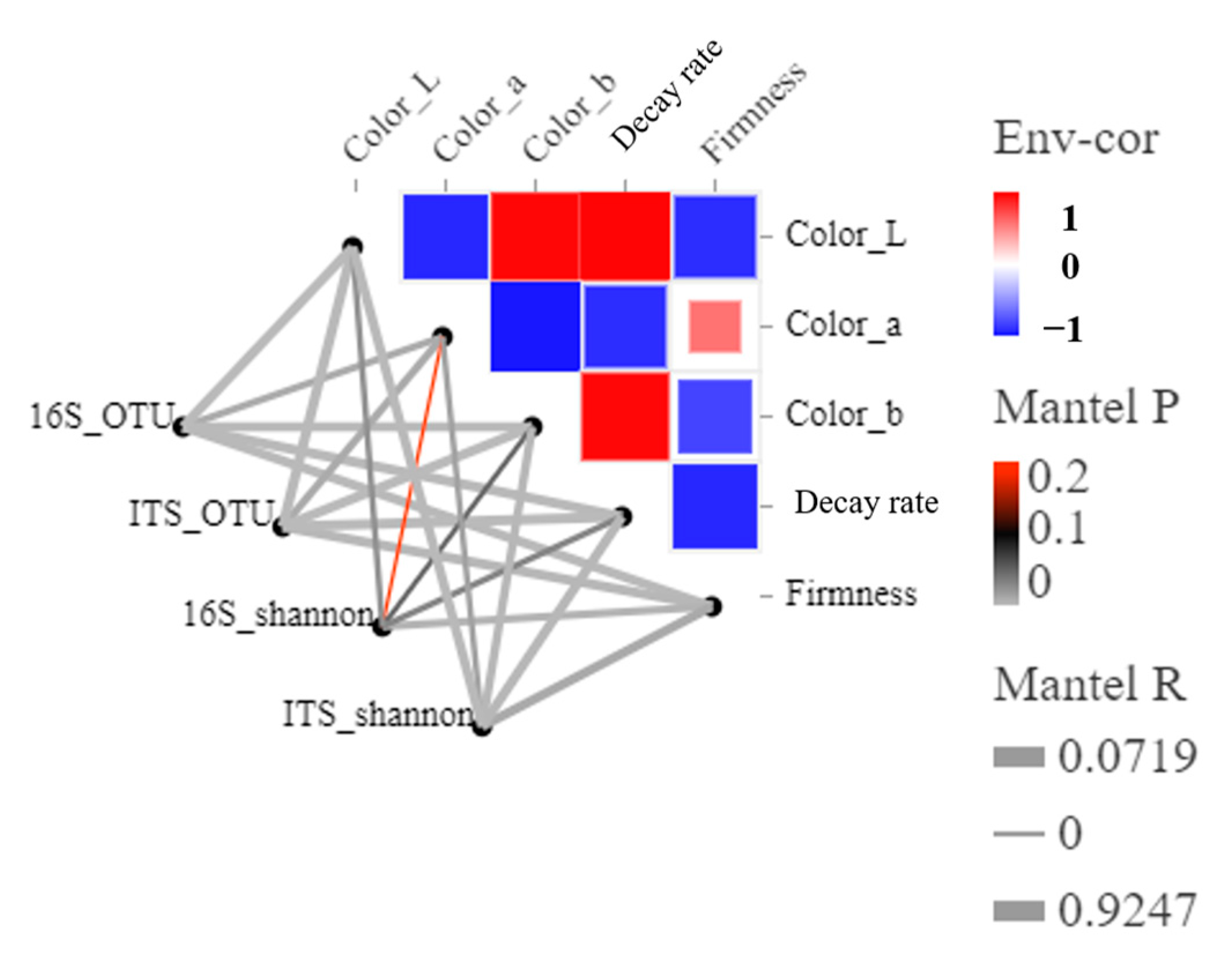
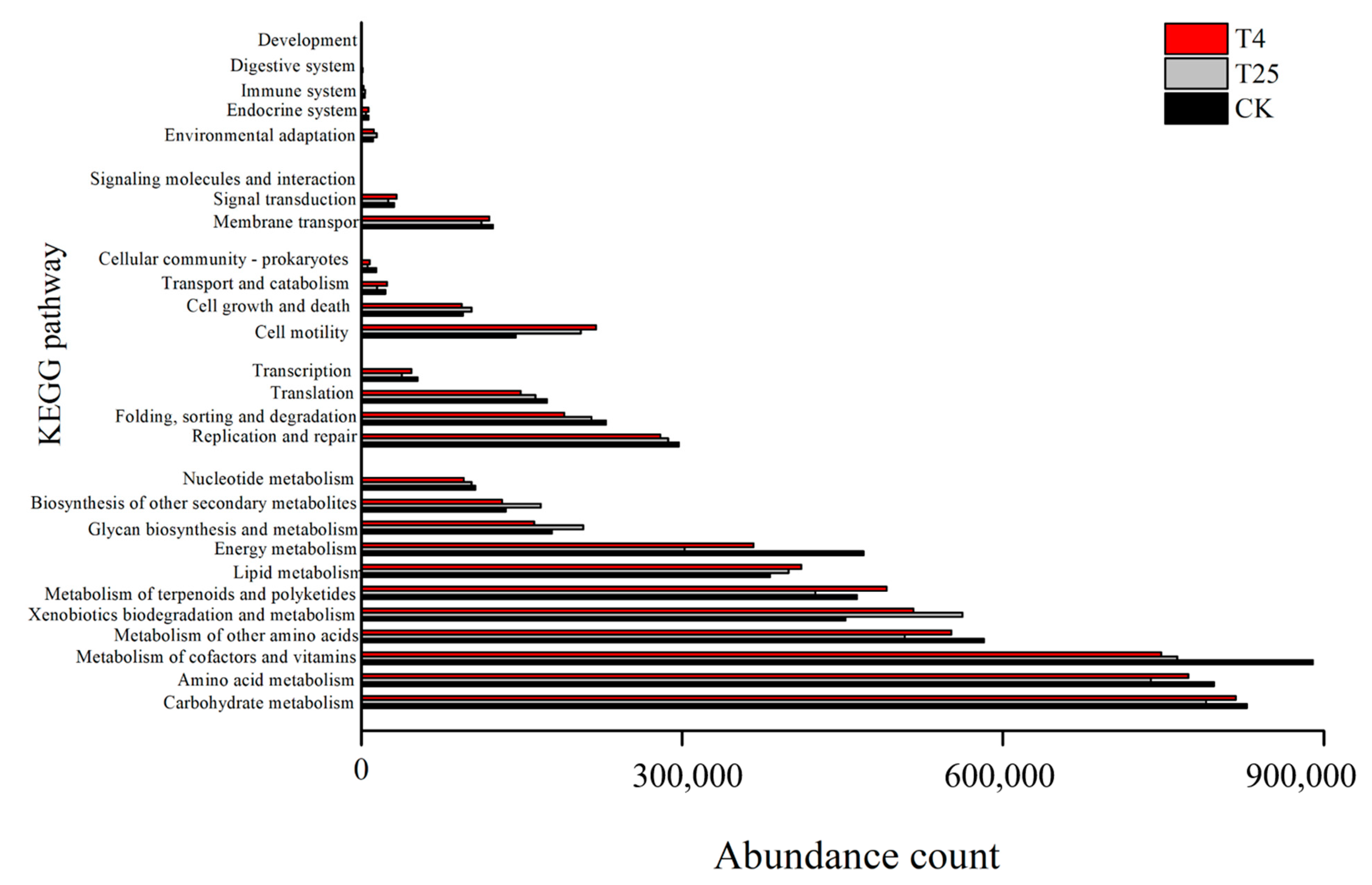
3.4. Function Prediction and Correlation between Blueberry Quality and the Microbial Community during Storage
4. Discussion
4.1. Microbial Community Diversity and Composition at Different Storage Temperatures
4.2. Microbial Community Biomarker Dynamics at Different Storage Temperatures
4.3. Microorganism-Blueberry Fruit Quality Interrelationships
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bell, S.R.; Montiel, L.G.H.; Estrada, R.R.G.; Martínez, P.G. Main diseases in postharvest blueberries, conventional and eco-friendly control methods: A review. LWT 2021, 149, 112046. [Google Scholar] [CrossRef]
- Silva, S.; Costa, E.M.; Veiga, M.; Morais, R.M.; Calhau, C.; Pintado, M. Health promoting properties of blueberries: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 181–200. [Google Scholar] [CrossRef] [PubMed]
- Giongo, L.; Poncetta, P.; Loretti, P.; Costa, F. Texture profiling of blueberries (Vaccinium spp.) during fruit development, ripening and storage. Postharvest Biol. Technol. 2013, 76, 34–39. [Google Scholar] [CrossRef]
- Song, Y.; Kim, H.K.; Yam, K.L. Respiration rate of blueberry in modified atmosphere at various temperatures. J. Am. Soc. Hortic. Sci. 1992, 117, 925–929. [Google Scholar] [CrossRef]
- Gazula, H.; Quansah, J.; Allen, R.; Scherm, H.; Li, C.; Takeda, F.; Chen, J. Microbial loads on selected fresh blueberry packing lines. Food Control 2019, 100, 315–320. [Google Scholar] [CrossRef]
- Zhang, H.; Boateng, N.A.S.; Ngea, G.L.N.; Shi, Y.; Lin, H.; Yang, Q.; Wang, K.; Zhang, X.; Zhao, L.; Droby, S. Unravelling the fruit microbiome: The key for developing effective biological control strategies for postharvest diseases. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4906–4930. [Google Scholar] [CrossRef]
- Chen, T.; Ji, D.; Zhang, Z.; Li, B.; Qin, G.; Tian, S. Advances and strategies for controlling the quality and safety of postharvest fruit. Engineering 2021, 7, 1177–1184. [Google Scholar] [CrossRef]
- Zhang, Q.; Shi, W.; Zhou, B.; Du, H.; Xi, L.; Zou, M.; Zou, H.; Xin, L.; Gao, Z.; Chen, Y. Variable characteristics of microbial communities on the surface of sweet cherries under different storage conditions. Postharvest Biol. Technol. 2021, 27, R713–R715. [Google Scholar] [CrossRef]
- Cabrera-Díaz, E.; Martínez-Chávez, L.; Gutiérrez-González, P.; Pérez-Montaño, J.A.; Rodríguez-García, M.O.; Martínez-Gonzáles, N.E. Effect of storage temperature and time on the behavior of Salmonella, Listeria monocytogenes, and background microbiota on whole fresh avocados (Persea americana var Hass). Int. J. Food Microbiol. 2022, 369, 109614. [Google Scholar] [CrossRef]
- Sim, H.L.; Hong, Y.-K.; Yoon, W.B.; Yuk, H.-G. Behavior of Salmonella spp. and natural microbiota on fresh-cut dragon fruits at different storage temperatures. Int. J. Food Microbiol. 2013, 160, 239–244. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, P.; Long, Z.; Ding, L.; Zhang, W.; Tang, H.; Liu, J. Research progress of soil microorganism application based on high-throughput sequencing technology. IOP Conf. Ser. Earth Environ. Sci. 2021, 692, 042059. [Google Scholar] [CrossRef]
- Cybulska, J.; Drobek, M.; Panek, J.; Cruz-Rubio, J.M.; Kurzyna-Szklarek, M.; Zdunek, A.; Frac, M. Changes of pectin structure and microbial community composition in strawberry fruit (Fragaria x ananassa Duch.) during cold storage. Food Chem. 2022, 381, 132151. [Google Scholar] [CrossRef]
- Fangqing, Y.; Dan, Y.; Sujuan, L.; Shuangmei, X.; Fu, W.; Hongping, C.; Youping, L. Use of High-Throughput Sequencing to Identify Fungal Communities on the Surface of Citri Reticulatae Pericarpium During the 3-Year Aging Process. Curr. Microbiol. 2021, 78, 3142–3151. [Google Scholar] [CrossRef]
- Yan, X.; Yan, J.; Pan, S.; Yuan, F. Changes of the aroma composition and other quality traits of blueberry ‘garden blue’ during the cold storage and subsequent shelf life. Foods 2020, 9, 1223. [Google Scholar] [CrossRef]
- Zou, J.; Chen, J.; Tang, N.; Gao, Y.; Hong, M.; Wei, W.; Cao, H.; Jian, W.; Li, N.; Deng, W.; et al. Transcriptome analysis of aroma volatile metabolism change in tomato (Solanum lycopersicum) fruit under different storage temperatures and 1-MCP treatment. Postharvest Biol. Technol. 2018, 135, 57–67. [Google Scholar] [CrossRef]
- Fawole, O.A.; Opara, U.L. Effects of storage temperature and duration on physiological responses of pomegranate fruit. Ind. Crops Prod. 2013, 47, 300–309. [Google Scholar] [CrossRef]
- Zhang, Q.; Shan, C.; Song, W.; Cai, W.; Zhou, F.; Ning, M.; Tang, F. Transcriptome analysis of starch and sucrose metabolism change in Gold Queen Hami melons under different storage temperatures. Postharvest Biol. Technol. 2021, 174, 111445. [Google Scholar] [CrossRef]
- Li, Y.; Huang, J.; Wang, Z.; Wang, X. Anthocyanin content in mature/immature blueberry fruits under different storage temperature. IOP Conf. Ser.: Earth Environ. Sci. 2019, 310, 052084. [Google Scholar] [CrossRef]
- Shi, C.; Zhou, A.; Fang, D.; Lu, T.; Wang, J.; Song, Y.; Lyu, L.; Wu, W.; Huang, C.; Li, W. Oregano essential oil/β-cyclodextrin inclusion compound polylactic acid/polycaprolactone electrospun nanofibers for active food packaging. Chem. Eng. J. 2022, 445, 136746. [Google Scholar] [CrossRef]
- Mannozzi, C.; Tylewicz, U.; Chinnici, F.; Siroli, L.; Rocculi, P.; Dalla Rosa, M.; Romani, S. Effects of chitosan based coatings enriched with procyanidin by-product on quality of fresh blueberries during storage. Food Chem. 2018, 125, 72–76. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Rubio, V. ggplot2—Elegant graphics for data analysis. J. Stat. Softw. 2017, 77, 2–5. [Google Scholar] [CrossRef]
- Quansah, J.K.; Gazula, H.; Holland, R.; Scherm, H.; Li, C.; Takeda, F.; Chen, J. Microbial quality of blueberries for the fresh market. Food Control. 2019, 100, 92–96. [Google Scholar] [CrossRef]
- Polashock, J.J.; Saftner, R.A.; Kramer, M. Postharvest highbush blueberry fruit antimicrobial volatile profiles in relation to anthracnose fruit rot resistance. J. Am. Soc. Hortic. Sci. 2007, 132, 859–868. [Google Scholar] [CrossRef]
- Sare, A.R.; Jijakli, M.H.; Massart, S. Microbial ecology to support integrative efficacy improvement of biocontrol agents for postharvest diseases management. Postharvest Biol. Technol. 2021, 179, 111572. [Google Scholar] [CrossRef]
- Droby, S.; Wisniewski, M. The fruit microbiome: A new frontier for postharvest biocontrol and postharvest biology. Postharvest Biol. Technol. 2018, 140, 107–112. [Google Scholar] [CrossRef]
- Zhimo, V.Y.; Kumar, A.; Biasi, A.; Salim, S.; Feygenberg, O.; Toamy, M.A.; Abdelfattaah, A.; Medina, S.; Freilich, S.; Wisniewski, M.; et al. Compositional shifts in the strawberry fruit microbiome in response to near-harvest application of Metschnikowia fructicola, a yeast biocontrol agent. Postharvest Biol. Technol. 2021, 175, 111469. [Google Scholar] [CrossRef]
- Salvatore, M.M.; Alves, A.; Andolfi, A. Secondary metabolites produced by neofusicoccum species associated with plants: A review. Agriculture 2021, 11, 149. [Google Scholar] [CrossRef]
- Angeli, D.; Sare, A.R.; Jijakli, M.H.; Pertot, I.; Massart, S. Insights gained from metagenomic shotgun sequencing of apple fruit epiphytic microbiota. Postharvest Biol. Technol. 2019, 153, 96–106. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, X.; Li, D.; Gu, X.; Godana, E.A.; Dhanasekaran, S.; Zhao, L.; Zhang, H. Transcriptome analysis of postharvest grapes in response to Talaromyces rugulosus O1 infection. Postharvest Biol. Technol. 2021, 178, 111542. [Google Scholar] [CrossRef]
- Mamphogoro, T.P.; Babalola, O.O.; Aiyegoro, O.A. Exploitation of epiphytic bacterial antagonists for the management of post-harvest diseases of sweet pepper and other fresh produce—A viable option. Biocontrol Sci. Technol. 2020, 30, 741–761. [Google Scholar] [CrossRef]
- Elif, T.; Parisa, M.; Merve Senol, K.; Hayrunnisa, N.; Recep, K. Biological control of Sclerotinia sclerotiorum (Lib.) de Bary, the causal agent of white mould disease in red cabbage, by some bacteria. Plant Prot. Sci. 2016, 52, 188–198. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, B.; Zhang, W.; Cao, J.; Jiang, W. Enhancement of quality and antioxidant metabolism of sweet cherry fruit by near-freezing temperature storage. Postharvest Biol. Technol. 2019, 147, 113–122. [Google Scholar] [CrossRef]
- Qi, Y.; Lei, Q.; Zhang, Y.; Liu, X.; Zhou, B.; Liu, C.; Ren, X. Comparative transcripome data for commercial maturity and physiological maturity of ‘Royal Gala’ apple fruit under room temperature storage condition. Sci. Hortic. 2017, 225, 386–393. [Google Scholar] [CrossRef]
- Ruxton, G.D.; Wilkinson, D.M.; Schaefer, H.M.; Sherratt, T.N. Why fruit rots: Theoretical support for Janzen’s theory of microbe-macrobe competition. Proc. Biol. Sci. 2014, 281, 20133320. [Google Scholar] [CrossRef]
- Rivera, S.; Kerckhoffs, H.; Sofkova-Bobcheva, S.; Hutchins, D.; East, A. Influence of harvest maturity and storage technology on mechanical properties of blueberries. Postharvest Biol. Technol. 2022, 191, 111961. [Google Scholar] [CrossRef]
- Ortiz, C.M.; Franceschinis, F.; Grozeff, G.E.G.; Chan, H.; Labavitch, J.M.; Crisosto, C.; Vicente, A.R. Pre-treatment with 1-methylcyclopropene alleviates methyl bromide-induced internal breakdown, softening and wall degradation in blueberry. Postharvest Biol. Technol. 2018, 146, 90–98. [Google Scholar] [CrossRef]
- Yao, D.; Xu, L.; Wu, M.; Wang, X.; Zhu, L.; Wang, C. Effects of microbial community succession on flavor compounds and physicochemical properties during CS sufu fermentation. LWT—Food Sci. Technol. 2021, 152, 112313. [Google Scholar] [CrossRef]
- Wang, F.; Saito, S.; Michailides, T.J.; Xiao, C.L. Fungicide resistance in alternaria alternata from blueberry in california and its impact on control of alternaria rot. Plant Dis. 2022, 106, 1446–1453. [Google Scholar] [CrossRef]
- Miles, T.D.; Schilder, A.C. Host defenses associated with fruit infection by colletotrichum species with an emphasis on anthracnose of blueberries. Plant Health Prog. 2013, 14, 30. [Google Scholar] [CrossRef]
- Bordoh, P.K.; Ali, A.; Dickinson, M.; Siddiqui, Y.; Romanazzi, G. A review on the management of postharvest anthracnose in dragon fruits caused by Colletotrichum spp. Crop Prot. 2020, 130, 105067. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, X.; Khaskheli, M.I.; Sun, X.; Chang, X.; Gong, G. Identification of colletotrichum species associated with blueberry anthracnose in Sichuan, China. Pathogens 2020, 9, 718. [Google Scholar] [CrossRef] [PubMed]
| Storage (Days) | Group | Raw Sequences | Effective Tags | N90 | OTUs | Effective Ratio (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bacterial | Fungal | Bacterial | Fungal | Bacterial | Fungal | Bacterial | Fungal | Bacterial | Fungal | ||
| 0 | CK | 126,481 ± 2018 a | 109,728 ± 3229 a | 122,473 ± 1798 a | 106,061 ± 3246 a | 441 | 319 ± 1 | 342 ± 11 b | 365 ± 16 a | 96.84 ± 0.14 a | 96.65 ± 0.17 a |
| 21 | T25 | 130,028 ± 3704 a | 89,113 ± 483 b | 125,765 ± 3842 a | 82,275 ± 396 c | 441 | 326 ± 4 | 364 ± 8 b | 236 ± 13 b | 96.71 ± 0.29 a | 92.34 ± 0.91 b |
| T4 | 106,744 ± 3293 b | 95,803 ± 2514 b | 102,646 ± 3010 b | 90,820 ± 2629 b | 441 | 317 ± 0 | 472 ± 24 a | 386 ± 17 a | 96.17 ± 0.22 a | 94.79 ± 0.31 a | |
| Group | Shannon | Simpson | Chao1 | Sobs | Good’s Coverage | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Bacterial | Fungal | Bacterial | Fungal | Bacterial | Fungal | Bacterial | Fungal | Bacterial | Fungal | |
| CK | 3.85 ± 0.49 b | 4.57 ± 0.16 b | 0.76 ± 0.064 b | 0.9 ± 0.013 a b | 425.77 ± 20.93 b | 414.24 ± 14.9 b | 341.67 ± 11.35 b | 365 ± 16.44 b | 0.999 | 0.999 |
| T25 | 2.64 ± 0.26 c | 3.63 ± 0.097 a | 0.69 ± 0.034 b | 0.86 ± 0.003 b | 453.06 ± 10.68 b | 291.8 ± 21.09 a | 363.67 ± 8.455 b | 236 ± 12.77 a | 0.999 | 0.999 |
| T4 | 5.22 ± 0.2 a | 4.47 ± 0.046 b | 0.92 ± 0.013 a | 0.91 ± 0.004 a | 528.33 ± 4.84 b | 434.78 ± 16.86 b | 472.33 ± 23.95 a | 386 ± 17.01 b | 0.999 | 0.999 |
| CK vs. T25 | 0.12 | 0.011 * | 0.37 | 0.08 | 0.33 | 0.012 * | 0.2 | 0.0042 ** | 0.21 | 0.036 * |
| CK vs. T4 | 0.092 | 0.6 | 0.12 | 0.43 | 0.034 * | 0.41 | 0.018 * | 0.42 | 0.31 | 0.17 |
| T4 vs. T25 | 0.0017 ** | 0.0051 ** | 0.011 * | 0.0007 ** | 0.0095 ** | 0.007 ** | 0.034 * | 0.0028 ** | 0.15 | 0.89 |
| CK vs. T4 vs. T25 | 0.0042 ** | 0.0021 ** | 0.017 * | 0.0082 ** | 0.0047 ** | 0.0031 ** | 0.0029 ** | 0.0012 ** | 0.13 | 0.14 |
| Storage (Days) | Group Name | Firmness (N) | Decay Rate (%) | L* | a* | b* |
|---|---|---|---|---|---|---|
| 0 | CK | 137.27 ± 39.91 | 13.64 ± 0.53 | 26.42 ± 1.50 | −3.12 ± 1.40 | −1.56 ± 0.90 |
| 21 | T25 | 76.20 ± 2.73 b | 9.31 ± 0.67 b | 27.62 ± 3.317 a | 7.70 ± 3.10 a | 3.82 ± 3.79 a |
| T4 | 155.73 ± 29.06 a | 12.33 ± 1.01 b | 26.76 ± 2.675 a | 4.20 ± 3.42 b | 0.76 ± 3.31 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Shi, C.; Fang, D.; Che, J.; Wu, W.; Lyu, L.; Li, W. The Impact of Storage Temperature on the Development of Microbial Communities on the Surface of Blueberry Fruit. Foods 2023, 12, 1611. https://doi.org/10.3390/foods12081611
Wang J, Shi C, Fang D, Che J, Wu W, Lyu L, Li W. The Impact of Storage Temperature on the Development of Microbial Communities on the Surface of Blueberry Fruit. Foods. 2023; 12(8):1611. https://doi.org/10.3390/foods12081611
Chicago/Turabian StyleWang, Junying, Chong Shi, Donglu Fang, Jilu Che, Wenlong Wu, Lianfei Lyu, and Weilin Li. 2023. "The Impact of Storage Temperature on the Development of Microbial Communities on the Surface of Blueberry Fruit" Foods 12, no. 8: 1611. https://doi.org/10.3390/foods12081611
APA StyleWang, J., Shi, C., Fang, D., Che, J., Wu, W., Lyu, L., & Li, W. (2023). The Impact of Storage Temperature on the Development of Microbial Communities on the Surface of Blueberry Fruit. Foods, 12(8), 1611. https://doi.org/10.3390/foods12081611





