Intended and Unintended Benefits of Folic Acid Fortification—A Narrative Review
Abstract
1. Introduction
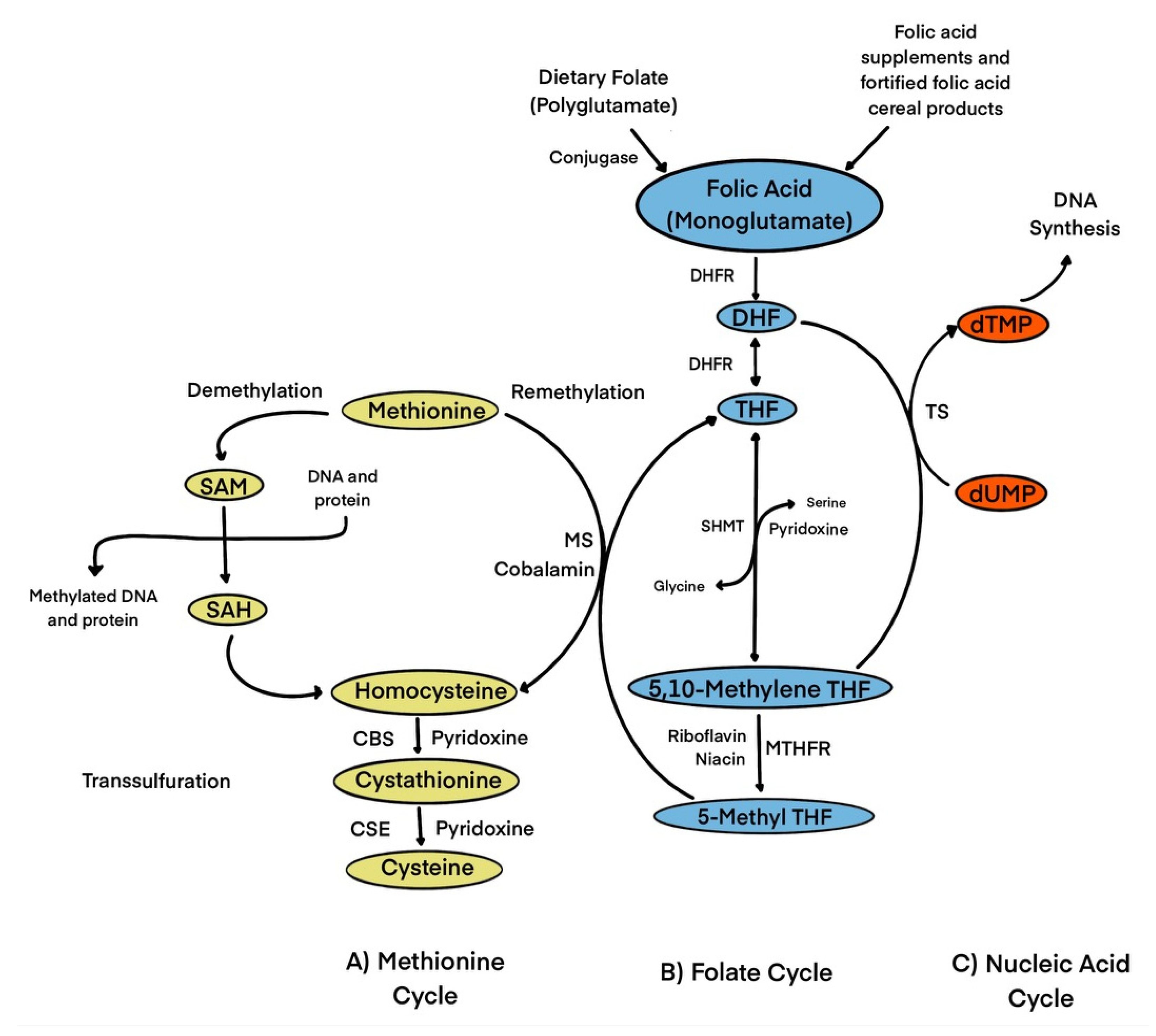
1.1. Biochemical Role of Folic Acid/Folate
1.2. Dietary Reference Intakes (DRI), Adequate Intakes (AI), and Tolerable Upper Intake Levels (UL) of Folate/Folic Acid
1.3. Biomarkers of Folic Acid Status
1.4. Folic Acid Fortification
2. Methods
3. Results and Discussion
3.1. Intended Benefits of Folic Acid Fortification
3.2. Unintended Benefits of Folic Acid Fortification
3.2.1. Reduction in tHcy
3.2.2. Impact on Stroke and CVD
3.2.3. Impact on Cognitive Health and Depression
3.2.4. Depression
3.2.5. Reduction in Anemia
3.2.6. Diabetes
4. Conclusions
- Increased intake of folic acid: excessive consumption of folic acid can mask the symptoms of vitamin B-12 deficiency, which can lead to vitamin B-12-induced anemia and neurological damage due to possible delayed diagnosis.
- Increased cancer risk: some studies have suggested that high folic acid intakes may increase the risk of certain types of cancer. However, the evidence for this is not very conclusive.
- Unmetabolized folic acid (UMFA) concentrations: there are some concerns that UMFA may have adverse effects on health, although the evidence for this is not very conclusive yet.
- Fortification of unhealthy foods: mandatory folic acid fortification may lead to the fortification of unhealthy foods or the displacement of other essential nutrients.
- Individual choice: some individuals may choose to avoid foods that have been fortified with folic acid due to personal beliefs or dietary restrictions.
- Socioeconomic disparities: Fortification may not be accessible to all segments of the population leading to socioeconomic disparities in folic acid intakes.
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Lyon, P.; Strippoli, V.; Fang, B.; Cimmino, L. B vitamins and one-carbon metabolism: Implications in human health and disease. Nutrients 2020, 12, 2867. [Google Scholar] [CrossRef]
- Allen, L.H. Causes of Vitamin B12 and Folate Deficiency. Food Nutr. Bull. 2008, 29, S20–S34. [Google Scholar] [CrossRef] [PubMed]
- Menezo, Y.; Elder, K.; Clement, A.; Clement, P. Folic Acid, Folinic Acid, 5 Methyl TetraHydroFolate Supplementation for Mutations That Affect Epigenesis through the Folate and One-Carbon Cycles. Biomolecules 2022, 12, 197. [Google Scholar] [CrossRef] [PubMed]
- Obeid, R.; Holzgreve, W.; Pietrzik, K. Is 5-methyltetrahydrofolate an alternative to folic acid for the prevention of neural tube defects? J. Perinat. Med. 2013, 41, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Czeizel, A.E.; Dudás, I.; Vereczkey, A.; Bánhidy, F. Folate deficiency and folic acid supplementation: The prevention of neural-tube defects and congenital heart defects. Nutrients 2013, 5, 4760–4775. [Google Scholar] [CrossRef] [PubMed]
- Martinez, H.; Pachón, H.; Kancherla, V.; Oakley, G.P. Food Fortification with Folic Acid for Prevention of Spina Bifida and Anencephaly: The Need for a Paradigm Shift in Evidence Evaluation for Policy-Making. Am. J. Epidemiol. 2021, 190, 1972–1976. [Google Scholar] [CrossRef]
- Crider, K.S.; Bailey, L.B.; Berry, R.J. Folic acid food fortification-its history, effect, concerns, and future directions. Nutrients 2011, 3, 370–384. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services. Folate Fact Sheet for Health Professionals. Available online: https://ods.od.nih.gov/factsheets/Folate-HealthProfessional/ (accessed on 1 February 2023).
- Novaković, R.; Geelen, A.; Ristić-Medić, D.; Nikolić, M.; Souverein, O.W.; McNulty, H.; Duffy, M.; Hoey, L.; Dullemeijer, C.; Renkema, J.M.S.; et al. Systematic Review of Observational Studies with Dose-Response Meta-Analysis between Folate Intake and Status Biomarkers in Adults and the Elderly. Ann. Nutr. Metab. 2018, 73, 30–43. [Google Scholar] [CrossRef]
- Mentch, S.J.; Locasale, J.W. One-carbon metabolism and epigenetics: Understanding the specificity. Ann. N. Y. Acad. Sci. 2016, 1363, 91–98. [Google Scholar] [CrossRef]
- Ebara, S. Nutritional role of folate. Congenit. Anom. 2017, 57, 138–141. [Google Scholar] [CrossRef]
- Crider, K.S.; Qi, Y.P.; Devine, O.; Tinker, S.C.; Berry, R.J. Modeling the impact of folic acid fortification and supplementation on red blood cell folate concentrations and predicted neural tube defect risk in the United States: Have we reached optimal prevention? Am. J. Clin. Nutr. 2018, 107, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Jin, J. Folic Acid Supplementation for Prevention of Neural Tube Defects. JAMA 2017, 317, 222. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.E.; Westmark, C.J. Folic acid fortification and neural tube defect risk: Analysis of the food fortification initiative dataset. Nutrients 2020, 12, 247. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Folate and Folic Acid on the Nutrition and Supplement Facts Labels. Available online: https://www.fda.gov/food/new-nutrition-facts-label/folate-and-folic-acid-nutrition-and-supplement-facts-labels#:~:text=Foods%20that%20are%20fortified%20with,found%20in%20certain%20dietary%20supplements (accessed on 6 April 2023).
- Imbard, A.; Benoist, J.F.; Blom, H.J. Neural tube defects, folic acid, and methylation. Int. J. Environ. Res. Public Health 2013, 10, 4352–4389. [Google Scholar] [CrossRef] [PubMed]
- Atta, C.A.M.; Fiest, K.M.; Frolkis, A.D.; Jette, N.; Pringsheim, T.; St Germaine-Smith, C.; Rajapakse, T.; Kaplan, G.G.; Metcalfe, A. Global Birth Prevalence of Spina Bifida by Folic Acid Fortification Status: A Systematic Review and Meta-Analysis. Am. J. Public Health 2016, 106, e24–e34. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Lancellotti, C.; Tur, J.A.; Uauy, R. Impact of folic acid fortification of flour on neural tube defects: A systematic review. Public Health Nutr. 2013, 16, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Van Gool, J.D.; Hirche, H.; Lax, H.; De Schaepdrijver, L. Folic acid and primary prevention of neural tube defects: A review. Reprod. Toxicol. 2018, 80, 73–84. [Google Scholar] [CrossRef]
- Kancherla, V.; Wagh, K.; Pachón, H.; Oakley, G.P. A 2019 global update on folic acid-preventable spina bifida and anencephaly. Birth Defects Res. 2021, 113, 77–89. [Google Scholar] [CrossRef]
- Grosse, S.D.; Berry, R.J.; Mick Tilford, J.; Kucik, J.E.; Waitzman, N.J. Retrospective Assessment of Cost Savings from Prevention. Am. J. Prev. Med. 2016, 50, S74–S80. [Google Scholar] [CrossRef]
- Bol, K.A.; Collins, J.S.; Kirby, R.S. Survival of Infants with Neural Tube Defects in the Presence of Folic Acid Fortification. Pediatrics 2006, 117, 803–813. [Google Scholar] [CrossRef]
- Ho, P.; Quigley, M.A.; Tatwavedi, D.; Britto, C.; Kurinczuk, J.J. Neonatal and infant mortality associated with spina bifida: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0250098. [Google Scholar] [CrossRef]
- Shin, M.; Kucik, J.E.; Siffel, C.; Lu, C.; Shaw, G.M.; Canfield, M.A.; Correa, A. Improved Survival among Children with Spina Bifida in the United States. J. Pediatr. 2012, 161, 1132–1137.e3. [Google Scholar] [CrossRef]
- Dolin, C.D.; Deierlein, A.L.; Evans, M.I. Folic Acid Supplementation to Prevent Recurrent Neural Tube Defects: 4 Milligrams Is Too Much. Fetal Diagn. Ther. 2018, 44, 161–165. [Google Scholar] [CrossRef]
- Ganji, V.; Kafai, M.R. Trends in Serum Folate, RBC Folate, and Circulating Total Homocysteine Concentrations in the United States: Analysis of Data from National Health and Nutrition Examination Surveys, 1988–1994, 1999–2000, and 2001–2002. J. Nutr. 2006, 136, 153–158. [Google Scholar] [CrossRef]
- Holmes, M.V.; Newcombe, P.; Hubacek, J.A.; Sofat, R.; Ricketts, S.L.; Cooper, J.; Breteler, M.M.; Bautista, L.E.; Sharma, P.; Whittaker, J.C.; et al. Effect modification by population dietary folate on the association between MTHFR genotype, homocysteine, and stroke risk: A meta-analysis of genetic studies and randomised trials. Lancet 2011, 378, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Botto, L.D.; Erickson, J.D.; Berry, R.J.; Sambell, C.; Johansen, H.; Friedman, J.M. Improvement in Stroke Mortality in Canada and the United States, 1990 to 2002. Circulation 2006, 113, 1335–1343. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.S.; Jacques, P.F.; Rosenberg, I.H.; Selhub, J. Folate and vitamin B-12 status in relation to anemia, macrocytosis, and cognitive impairment in older Americans in the age of folic acid fortification. Am. J. Clin. Nutr. 2007, 85, 193–200. [Google Scholar] [CrossRef]
- Biemi, F.D.; Ganji, V. Temporal Relation between Double Fortification of Wheat Flour with Iron and Folic Acid, and Markers and Prevalence of Anemia in Children. Nutrients 2021, 13, 2013. [Google Scholar] [CrossRef]
- Ganji, V.; Kafai, M.R. Hemoglobin and hematocrit values are higher and prevalence of anemia is lower in the post–folic acid fortification period than in the pre–folic acid fortification period in US adults. Am. J. Clin. Nutr. 2009, 89, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Del Carrasco Quintero, M.R.; Ortiz Hernández, L.; Roldán Amaro, J.A.; Chávez Villasana, A.; Aguirre Arenas, J.; Aguilar Carrasco, F.R. Efecto del consumo de una harina de maíz enriquecida con soja en el estado de nutrición de mujeres indígenas de México. Rev. Española Salud Pública 2013, 87, 293–302. [Google Scholar] [CrossRef]
- Li, Z.; Gueant-Rodriguez, R.-M.; Quilliot, D.; Sirveaux, M.-A.; Meyre, D.; Gueant, J.-L.; Brunaud, L. Folate and vitamin B12 status is associated with insulin resistance and metabolic syndrome in morbid obesity. Clin. Nutr. 2018, 37, 1700–1706. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.P.; Shang, X.X.; Zhao, Z.T. Low maternal vitamin B 12 is a risk factor for neural tube defects: A meta-analysis. J. Matern. -Fetal Neonatal Med. 2012, 25, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Meng, X.; Song, Z. Homocysteine and psoriasis. Biosci. Rep. 2019, 39, BSR20190867. [Google Scholar] [CrossRef] [PubMed]
- Kaye, A.D.; Jeha, G.M.; Pham, A.D.; Fuller, M.C.; Lerner, Z.I.; Sibley, G.T.; Cornett, E.M.; Urits, I.; Viswanath, O.; Kevil, C.G. Folic Acid Supplementation in Patients with Elevated Homocysteine Levels. Adv. Ther. 2020, 37, 4149–4164. [Google Scholar] [CrossRef]
- Carlsson, C.M. Homocysteine Lowering with Folic Acid and Vitamin B Supplements. Drugs Aging 2006, 23, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Almassinokiani, F.; Kashanian, M.; Akbari, P.; Mossayebi, E.; Sadeghian, E. Folic acid supplementation reduces plasma homocysteine in postmenopausal women. J. Obs. Gynaecol. 2016, 36, 492–495. [Google Scholar] [CrossRef]
- Zeng, R.; Xu, C.-H.; Xu, Y.-N.; Wang, Y.-L.; Wang, M. The effect of folate fortification on folic acid-based homocysteine-lowering intervention and stroke risk: A meta-analysis. Public Health Nutr. 2015, 18, 1514–1521. [Google Scholar] [CrossRef]
- Asbaghi, O.; Ashtary-Larky, D.; Bagheri, R.; Moosavian, S.P.; Olyaei, H.P.; Nazarian, B.; Rezaei Kelishadi, M.; Wong, A.; Candow, D.G.; Dutheil, F.; et al. Folic Acid Supplementation Improves Glycemic Control for Diabetes Prevention and Management: A Systematic Review and Dose-Response Meta-Analysis of Randomized Controlled Trials. Nutrients 2021, 13, 2355. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, Y.; Wang, Y.; Li, L.; Liao, Y.; Zhang, Y.; Yu, D. The effect of folic acid in patients with cardiovascular disease. Medicine 2019, 98, e17095. [Google Scholar] [CrossRef]
- Tian, T.; Yang, K.-Q.; Cui, J.-G.; Zhou, L.-L.; Zhou, X.-L. Folic Acid Supplementation for Stroke Prevention in Patients with Cardiovascular Disease. Am. J. Med. Sci. 2017, 354, 379–387. [Google Scholar] [CrossRef]
- Klerk, M.; Verhoef, P.; Clarke, R.; Blom, H.J.; Kok, F.J.; Schouten, E.G.; The MTHFR Studies Collaboration Group. MTHFR 677C→T Polymorphism and Risk of Coronary Heart Disease. JAMA 2002, 288, 2023. [Google Scholar] [CrossRef] [PubMed]
- De Santos, I.S.; Suemoto, C.K.; Valladão-Junior, J.B.R.; Liu, S.; Barreto, S.M.; Fedeli, L.M.G.; Lotufo, P.A.; Bensenor, I.M. Serum folate levels and cognitive performance in the ELSA-Brasil baseline assessment. Arq. Neuro-Psiquiatr. 2020, 78, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Sadat-Hossieny, Z.; Robalino, C.P.; Pennell, P.B.; Cohen, M.J.; Loring, D.W.; May, R.C.; Block, T.; Swiatlo, T.; Meador, K.J. Folate fortification of food: Insufficient for women with epilepsy. Epilepsy Behav. 2021, 117, 107688. [Google Scholar] [CrossRef] [PubMed]
- Eryilmaz, H.; Dowling, K.F.; Huntington, F.C.; Rodriguez-Thompson, A.; Soare, T.W.; Beard, L.M.; Lee, H.; Blossom, J.C.; Gollub, R.L.; Susser, E.; et al. Association of Prenatal Exposure to Population-Wide Folic Acid Fortification with Altered Cerebral Cortex Maturation in Youths. JAMA Psychiatry 2018, 75, 918. [Google Scholar] [CrossRef] [PubMed]
- Ramos, M.I.; Allen, L.H.; Mungas, D.M.; Jagust, W.J.; Haan, M.N.; Green, R.; Miller, J.W. Low folate status is associated with impaired cognitive function and dementia in the Sacramento Area Latino Study on Aging. Am. J. Clin. Nutr. 2005, 82, 1346–1352. [Google Scholar] [CrossRef]
- Wang, B.; Sahyoun, N.R.; Shao, K.; Dutta, E.; Clarke, J. Assessment of the Dose–Response Relationship Between Folate Exposure and Cognitive Impairment: Synthesizing Data from Documented Studies. Risk Anal. 2020, 40, 276–293. [Google Scholar] [CrossRef]
- Brody, D.J.; Pratt, L.A.; Hughes, J.P. Prevalence of Depression Among Adults Aged 20 and Over: United States, 2013–2016. NCHS Data Brief 2018, 303, 1–8. [Google Scholar]
- Bottiglieri, T.; Laundy, M.; Crellin, R.; Toone, B.K.; Carney, M.W.; Reynolds, E.H. Homocysteine, folate, methylation, and monoamine metabolism in depression. J. Neurol. Neurosurg. Psychiatry 2000, 69, 228–232. [Google Scholar] [CrossRef]
- Khalili, P.; Asbaghi, O.; Aghakhani, L.; Clark, C.C.T.; Haghighat, N. The effects of folic acid supplementation on depression in adults: A systematic review and meta-analysis of randomized controlled trials. Nutr. Food Sci. 2023, 53, 521–534. [Google Scholar] [CrossRef]
- Gilbody, S.; Lightfoot, T.; Sheldon, T. Is low folate a risk factor for depression? A meta-analysis and exploration of heterogeneity. J. Epidemiol. Community Health 2007, 61, 631–637. [Google Scholar] [CrossRef]
- Peña-Rosas, J.P.; Mithra, P.; Unnikrishnan, B.; Kumar, N.; De-Regil, L.M.; Nair, N.S.; Garcia-Casal, M.N.; Solon, J.A. Fortification of rice with vitamins and minerals for addressing micronutrient malnutrition. Cochrane Database Syst. Rev. 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Centeno Tablante, E.; Pachón, H.; Guetterman, H.M.; Finkelstein, J.L. Fortification of wheat and maize flour with folic acid for population health outcomes. Cochrane Database Syst. Rev. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, S.; de la Maza, P.; Barrera, G.; Gattás, V.; Petermann, M.; Bunout, D. The Chilean Flour Folic Acid Fortification Program Reduces Serum Homocysteine Levels and Masks Vitamin B-12 Deficiency in Elderly People. J. Nutr. 2002, 132, 289–291. [Google Scholar] [CrossRef]
- Huo, J.; Sun, J.; Huang, J.; Li, W.; Wang, L.; Selenje, L.; Gleason, G.R.; Yu, X. Effectiveness of Fortified Flour for Enhancement of Vitamin and Mineral Intakes and Nutrition Status in Northwest Chinese Villages. Food Nutr. Bull. 2012, 33, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Muzurović, E.; Kraljević, I.; Solak, M.; Dragnić, S.; Mikhailidis, D.P. Homocysteine and diabetes: Role in macrovascular and microvascular complications. J. Diabetes Complicat. 2021, 35, 107834. [Google Scholar] [CrossRef] [PubMed]
- Valdes-Ramos, R.; Laura, G.-L.; Elina, M.-C.; Donaji, B.-A. Vitamins and Type 2 Diabetes Mellitus. Endocr. Metab. Immune Disord. Drug Targets 2015, 15, 54–63. [Google Scholar] [CrossRef]
- Sudchada, P.; Saokaew, S.; Sridetch, S.; Incampa, S.; Jaiyen, S.; Khaithong, W. Effect of folic acid supplementation on plasma total homocysteine levels and glycemic control in patients with type 2 diabetes: A systematic review and meta-analysis. Diabetes Res. Clin. Pract. 2012, 98, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Lind, M.V.; Lauritzen, L.; Kristensen, M.; Ross, A.B.; Eriksen, J.N. Effect of folate supplementation on insulin sensitivity and type 2 diabetes: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2019, 109, 29–42. [Google Scholar] [CrossRef]
- Sikalidis, A.K.; Maykish, A. The Gut Microbiome and Type 2 Diabetes Mellitus: Discussing A Complex Relationship. Biomedicines 2020, 8, 8. [Google Scholar] [CrossRef]
- Gérard, P. Gut microbiota and obesity. Cell. Mol. Life Sci. 2016, 73, 147–162. [Google Scholar] [CrossRef]
- Aron-Wisnewsky, J.; Warmbrunn, M.V.; Nieuwdorp, M.; Clément, K. Metabolism and Metabolic Disorders and the Microbiome: The Intestinal Microbiota Associated with Obesity, Lipid Metabolism, and Metabolic Health-Pathophysiology and Therapeutic Strategies. Gastroenterology 2021, 160, 573–599. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Chen, Q.; Sun, Y.; Zeng, L.; Wu, H.; Gu, Q.; Li, P. Probiotic Potential of a Folate-Producing Strain Latilactobacillus sakei LZ217 and Its Modulation Effects on Human Gut Microbiota. Foods 2022, 11, 234. [Google Scholar] [CrossRef] [PubMed]
- Uebanso, T.; Shimohata, T.; Mawatari, K.; Takahashi, A. Functional roles of B-vitamins in the gut and gut microbiome. Mol. Nutr. Food Res. 2020, 64, 2000426. [Google Scholar] [CrossRef] [PubMed]
- Berding, K.; Vlckova, K.; Marx, W.; Schellekens, H.; Stanton, C.; Clarke, G.; Jacka, F.; Dinan, T.G.; Cryan, J.F. Diet and the Microbiota-Gut-Brain Axis: Sowing the Seeds of Good Mental Health. Adv. Nutr. 2021, 12, 1239–1285. [Google Scholar] [CrossRef] [PubMed]
| Reference (Author/s and Year) | Study Design | Intervention/Data Collection | Outcome Measurements | Findings | Conclusions |
|---|---|---|---|---|---|
| Intended benefits of folic acid fortification | |||||
| Bol et al., 2006 [22] | Retrospective cohort | Between 1995 to 2001 | Spina bifida and encephalocele | ↑Dietary Folic acid, ↑First-year survival rate; no difference in encephalocele | In addition to preventing the occurrence of NTDs, folic acid may have a role in a role in reducing the severity of NTDs |
| Ho et al., 2021 [23] | Systematic review | Evidence searched from 1 January 1990 to 31 August 2020 | Spina bifida and infant mortality rate | ↓spina bifida and associated infant and neonatal mortality rates | Significant declines in spina bifida associated infant/neonatal mortality and case fatality. Likely due to folic acid fortification |
| Unintended benefits of folic acid fortification | |||||
| Ganji et al., 2006 [26] | Retrospective cohort | From 1988 to 2002 pre- and post-fortification | Serum folic acid, RBC, RBC folate, and tHcy concertation | ↑Dietary folic acid, ↓tHcy | Folic acid plays a role in the reduction in tHcy. |
| Holmes et al., 2011 [27] | Meta-analysis | Mean follow-duration of 4.7 y | Risk of stroke; effect modification by population | ↑OR stroke was higher in Asia than in America, Australia, and New Zealand | Stroke risk in Asia was higher in comparison to areas with folate fortification such as America, Australia, and New Zealand. |
| Yang et al., 2006 [28] | Cohort study | From 1990–2002 | Stroke mortality | ↓Stroke mortality with folate fortification | Stroke mortality decreased after mandatory folic acid fortification. |
| Morris et al., 2007 [29] | Cohort study | From 1999–2002; folic acid fortification in elderly ˃60 y of age | Cognitive impairment | ↑High serum folate; ↓B12 deficiency; ↑anemia and cognition; Normal B12 ↑serum folate, ↓cognitive impairment | Folic acid has two-sided effects on cognitive health depending on the serum B12 serum concentration. |
| Biemi et al., 2021 [30] | Retrospective, observational | 2004–2010 pre- and post-fortification in children 5–14 y of age | Hemoglobin, hematocrit, RBC, MCV, and anemia | No difference in mean hemoglobin; ↑MCV concentrations post-fortification | No change in anemia in post-fortification; however, MCV significantly increased suggesting an increase in B12 deficiency. |
| Ganji et al., 2009 [31] | Cohort study | 1988–2004, pre and post-folic acid fortification | Anemia and macrocytosis | ↑Dietary folic acid; ↓anemia in women | Improvement in hemoglobin and decreased prevalence of anemia after folic acid fortification. |
| Carrasco Quintero et al., 2013 [32] | Randomized control trial | In 2010, maize flour fortified with folic acid | Hemoglobin in women | ↑Hemoglobin | Fortified flour is a good option for regionalized women in rural areas who are underweight, undernourished, and have anemia. |
| Li et al., 2018 [33] | Cross-sectional study | 2011–2012, serum folic acid | Serum folate and insulin resistance (fasting plasma glucose, OGTT, serum insulin, and HOMA-IR) | ↑Serum folate; ↓HOMA-IR | Serum folate was inversely associated with insulin resistance |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismail, S.; Eljazzar, S.; Ganji, V. Intended and Unintended Benefits of Folic Acid Fortification—A Narrative Review. Foods 2023, 12, 1612. https://doi.org/10.3390/foods12081612
Ismail S, Eljazzar S, Ganji V. Intended and Unintended Benefits of Folic Acid Fortification—A Narrative Review. Foods. 2023; 12(8):1612. https://doi.org/10.3390/foods12081612
Chicago/Turabian StyleIsmail, Shrooq, Sereen Eljazzar, and Vijay Ganji. 2023. "Intended and Unintended Benefits of Folic Acid Fortification—A Narrative Review" Foods 12, no. 8: 1612. https://doi.org/10.3390/foods12081612
APA StyleIsmail, S., Eljazzar, S., & Ganji, V. (2023). Intended and Unintended Benefits of Folic Acid Fortification—A Narrative Review. Foods, 12(8), 1612. https://doi.org/10.3390/foods12081612





