Abstract
Morphine and codeine are the two principal opiates found in the opium poppy (Papaver somniferum L.) and are therapeutically used for pain management. Poppy seeds with low opiates are primarily used for culinary purposes due to their nutritional and sensory attributes. Intentional adulteration of poppy seeds is common, often combined with immature, less expensive, exhausted, or substituted with morphologically similar seeds, viz., amaranth, quinoa, and sesame. For a safer food supply chain, preventive measures must be implemented to mitigate contamination or adulteration. Moreover, the simultaneous analysis of P. somniferum and its adulterants is largely unknown. Pre- and post-processing further complicate the alkaloid content and may pose a significant health hazard. To address these issues, two independent methods were investigated with eight botanically verified and fifteen commercial samples. Microscopical features were established for the authenticity of raw poppy seeds. Morphine, codeine, and thebaine quantities ranged from 0.8–223, 0.2–386, and 0.1–176 mg/kg, respectively, using LC-QToF. In most cases, conventional opiates have a higher content than papaverine and noscapine. The analytical methodology provided a chemical profile of 47 compounds that can be effectively applied to distinguish poppy seeds from their adulterants and may serve as an effective tool to combat ongoing adulteration.
Keywords:
opiates; microscopy; LC-QToF; adulteration; quantification; validation; chemical profiling 1. Introduction
Poppy seeds are a product of the opium poppy (Papaver somniferum L.), which belongs to the Papaveraceae family. P. somniferum has been cultivated for its seeds and their recreational, analgesic, and narcotic properties since prehistoric times [1,2]. These are tiny, edible, kidney-shaped seeds harvested as a source of alkaloid compounds for the pharmaceutical industry and as a source of poppy seeds for the food industry [3]. The sap, or latex, of the poppy plant, when dried to yield opium, contains multiple alkaloids, including opiates (OAs) such as morphine, thebaine, codeine, papaverine, and narcotine [4,5]. About 218 species belong to the genus Papaver [6,7], and 170 alkaloids have been reported from this genus [8]. Among these species, only Papaver somniferum L. and Papaver setigerum DC. (a synonym of Papaver somniferum subsp. setigerum (DC.) Arcang.) [9,10,11,12] produce higher quantities of narcotic substances as their secondary metabolites. Hence, the former species has been commonly cultivated. Although the seeds may contain low levels of opiates, contamination of residual poppy latex during harvesting and processing is known to contribute to higher levels of opiates [3,4,5,13].
A wide spectrum of color variants of poppy seeds, from white to yellow–brown, red–brown, red–lilac, blue–grey, and dark blue to black, are found in commerce [14]. The blue or black seeds are primarily used in Europe and the US by sprinkling on baked goods, whereas white (closer to beige) or yellow seeds, which are milder than the black seeds, are commonly used in South Asia, specifically in Indian cuisine, for flavoring and thickening purposes. Finally, the lesser-known brown or maroon seeds come from Turkey. All these seed types come from multiple P. somniferum varieties, commonly called the poppy plant, and can easily substitute one for the other. The occurrence data on opium alkaloids show poppy seed samples with high concentrations of morphine, codeine, and thebaine. The opium alkaloid content and profile appear to be related to the country of origin of the poppy seed samples and ultimately, to the poppy variety [4]. Currently, 68 varieties are listed on the EU Plant Variety Catalogue [15]. European Regulation (EU) 2021/2142, published on 3 December 2021 and effective from 1 July 2022, sets maximum levels for OAs expressed in morphine equivalents (morphine + 0.2 codeine) as 1.5 mg/kg for bakery and (or) derived products and 20 mg/kg for whole, ground, or milled poppy seeds [16].
Poppy seeds are not currently controlled in the United States and Canada but are prohibited in many countries, including Korea, Taiwan, Singapore, China, and Saudi Arabia, as they primarily contain morphine, codeine, and other narcotics. On the contrary, the opium poppy is legally grown and widely used in many countries, especially in Central Europe, South Asia, and elsewhere. In 2014, the European Commission recommended good practices to prevent and reduce the presence of opium alkaloids in poppy seeds and their finished products [17]. Various factors, such as cultivar variety, geographical location, time of harvesting, and processing [18], are reported to affect the alkaloid content in opium poppy seeds. Mature poppy seeds obtained from capsules of different varieties of P. somniferum and seed oil are directly marketed to consumers for processed foods, such as baked goods, desserts, and other culinary products [4,18]. However, according to the reported studies [4,18,19,20], the content of OAs varied in poppy seed samples sourced from the European market.
The commonly used detection methods are high-performance liquid chromatography with ultraviolet detection (HPLC-UV) [21,22,23], gas chromatography–mass spectrometry (GC–MS) [12,18,24,25,26,27,28,29,30,31,32,33,34], liquid chromatography coupled with tandem mass spectrometry (LC–MS/MS) [19,20,35,36,37,38,39], and LC hyphenated with a time of flight mass spectrometry (LC–QToF-MS) [40,41,42]. GC–MS and LC–MS/MS are more selective and sensitive than HPLC–UV. GC–MS has the drawback of requiring an additional derivatization step to obtain the desired sensitivity. GC–MS, LC–MS/MS, or liquid chromatography combined with high-resolution mass spectrometry (LC–HRMS) techniques are widely applied for the determination of OAs and metabolites in human biological samples such as urine, plasma, serum, hair, saliva, and cerebrospinal fluids [41,42] where positive opiate drug test results have been found [27,28,29,30,31,35,43]. These techniques are also used for metabolite profiling of poppy cultivars or opium latex [12,36,40]. On the other hand, poppy seeds are used in food processing, including bakery products, toppings for dishes, cakes, desserts [23,32], and tea beverages [20,33,44]. Recent industrial uses of these seeds include preparing dairy products (yogurts) and snacks [45]. Schulz et al. used Fourier transform (FT) infrared spectroscopy and NIR-FT-Raman spectroscopy techniques to identify and quantify five important alkaloids (morphine, codeine, thebaine, papaverine, and noscapine) in forty-five samples of poppy capsules, milk, and extracts. Using this method, discrimination between “low-alkaloid” (0.10–0.26 mg/g) and “high-alkaloid” (3.5–10.8 mg/g) plants can be easily achieved [46].
The poppy variety, geographical origin, harvest time, and external contamination can significantly influence the OA content. [19,34,38]. Bjerver et al. determined morphine levels in five poppy seed samples (three blue and two white) and found considerable amounts of morphine, especially in the three blue poppy seeds (9.4–374 μmol/kg) [34]. Lopez et al. (2018) [19] reported results obtained for 41 retail samples from the Netherlands (32 samples), Germany (8 samples), and Italy (one sample). Of these, 32 blue samples, three white poppy seed samples, ground seed samples, one ready-to-use poppy seed filling for the bakery, and two ready-to-eat products were analyzed for six alkaloids. All analyzed samples contained morphine between 0.2–241 mg/kg. Codeine (<0.1–348 mg/kg) and thebaine (<0.1–106 mg/kg) were identified in 78% of the samples. In four samples of black poppy seeds, Hayes et al. (1987) [25] found morphine and codeine in the range of 17–294 mg/kg and 3–14 mg/kg, respectively. The results obtained by Starnska et al. (2013) [39] indicated that the alkaloids morphine, codeine, narcotine, papaverine, and thebaine ranged from 3327–17175, 172–1767, 24–1675, 2–689, and 0–1701 μg/g, respectively, in the selected 15 poppy cultivars.
Sometimes, to increase profits, there is a possibility that the poppy seeds have been adulterated with locally available, less expensive seeds such as amaranth, cumin, chia, sesame, or other seeds that resemble poppy seeds in color and size, thereby increasing the difficulty of determining the authenticity of the poppy seeds, including artificially colored seeds [47,48]. In addition, DNA barcoding methods were successfully implemented to detect adulterants of the Papaver genus and helped differentiate P. somniferum from other varieties [49,50]. Poppy seed pods are harvested to extract opiate-containing latex before maturity, while culinary poppy seeds are harvested after the seed pods have matured and dried. There have been cases of poppy seed adulteration when immature by-product seeds from pharmaceutical processing with a higher content of opium alkaloids were mixed with food-grade mature poppy seeds. Often, the poppy seeds are heterogenous, having differences in size, color, and taste, and may be contaminated with poppy latex or other impurities [51].
Culinary poppy seeds are frequently mixed with low-quality seeds of cultivars with higher alkaloid content or with exhausted seeds following the extraction of opiates. The mixed product’s decreased quality and increased OA content represent a potential health hazard for consumers [19,52,53]. Even if establishing the detailed external morphological features of botanically verified materials would serve for the proper identification of raw materials, the development of a sensitive analytical method would address the overall quality of the poppy seeds in commerce or in poppy-derived products. Moreover, assessing extraction efficiency with various solvents and removing opiates from post-processed poppy seeds would be beneficial to differentiate naturally latex-contaminated seeds from seeds artificially spiked with opiates. Nevertheless, an untargeted analysis and the development of comprehensive alkaloid profiles could help discriminate between species in seeds of the genus Papaver and for detecting any added adulterants or substituents.
2. Materials and Methods
2.1. Chemicals and Standards
Representative images of poppy seeds and the chemical structures of five OAs are shown in Figure 1 and Figure 2. Morphine, codeine, thebaine, noscapine, papaverine, morphine-d3, and codeine-d3 were purchased from Sigma (St. Louis, MO, USA). The reference compounds were more than 99% pure, with identity and purity confirmed by chromatographic and spectral data (HRMS). HPLC-grade solvents such as acetonitrile, methanol, isopropanol, and formic acid were procured from Fisher Scientific, Waltham, MA, USA. Ultrapure water (18.2 MΩ.cm) was purified using the Milli-Q system (Millipore, Bedford, MA, USA).

Figure 1.
Representative images with NCNPR # of (a) poppy seed samples and (b) adulterants used in this study.
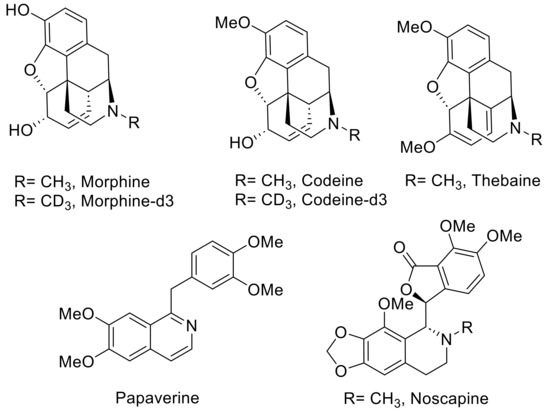
Figure 2.
Chemical structures of five alkaloids and two internal standards, morphine-d3, and codeine-d3.
2.2. Preparation of Standard Solutions
Stock solutions of standard compounds were prepared at a concentration of 1 μg/mL in methanol. Calibration curves ranged from 0.5–250 ng/mL at five different concentration levels. All standard solutions were stored in amber vials at 4 °C.
2.3. Plant Materials
Seed samples of P. somniferum (#2546, 7528, 12750, and 22469) were sourced from Hamdard University (Karachi, Pakistan), CRISM (Delhi, India), and the Missouri Botanical Garden (St. Louis, MO, USA). Samples of poppy seeds (#5552, 5558, 16725, 24790, 24791, 24792, 24793, 25064, 25107, and 25124) and other plant seed samples used as adulterants or substituents: Amaranthus cruentus L. (#22982 and 25225), A. caudatus L. (#23019 and 25226), A. paniculatus L. is a synonym of A. cruentus L. (#25223), Salvia hispanica L. (#22449 and 25145), and Nigella sativa L. (Black cumin) (#4978, 7516) seed samples were obtained from CRISM (Delhi, India), and two samples of Sesamum indicum L. (Sesame) (#2519 and 22448) seed samples were collected from Pakistan and Missouri Botanical Garden (St. Louis, MO, USA). Chenopodium quinoa Willd. (quinoa) seed samples (#6146 and 22444) were procured from the Missouri Botanical Garden (St. Louis, MO, USA). Nigella sativa (#22471) and Eschscholzia californica Cham. (golden poppy) seed samples (#24794) were obtained commercially.
Poppy seed samples (#2116–2021, 2123, and 2124) used in food were commercially procured from Amazon in November 2022. These samples were deposited at the NCNPR’s botanical repository, the University of Mississippi, MS, USA.
2.4. Plant Sample Preparation
Dry-ground seed samples were weighed separately for about 1000 mg, 100 mg, and 10 mg each. 100 μL of the internal standards (2.5 μg/mL) were added to these samples and sonicated in a 2.5 mL volume of methanol containing 1% formic acid for about 30 min, followed by their being centrifuged for 15 min at 959× g. The supernatant solution was transferred to a 10 mL volumetric flask. The procedure was repeated three more times with fresh solvent, and their supernatants were combined in a volumetric flask and adjusted to achieve a final volume of 10 mL. Before injection, approximately 2 mL of the solution was passed through a 0.45 µm PTFE membrane filter. The first 1.0 mL was discarded, and the remaining volume of about 1 mL was collected in an LC sample vial.
2.5. Micro Morphology Analysis
The external morphology of seeds was observed under low magnification using a NIKON SMZ-U (Japan) stereomicroscope with photo capture by the camera (NIKON DS-Fi1) attached to the microscope and processed with the software NIS-Element BR. Specimens were fixed in formaldehyde alcohol acetic acid (FAA) for high-level magnification using scanning electron microscopy (SEM) analysis. These samples were passed through a 100% ethanol solution and dehydrated using a 1 h and 15 min programmed critical point dryer (Leica CPD300, Wetzlar, Germany) under CO2. Dried samples were placed on double-sided adhesive carbon tape and pasted on the aluminum stubs. For platinum coating, these stubs were placed on a Desk V HP sputter coater (Denton Vacuum, NJ, USA) supplied with argon gas. The prepared samples were used for SEM analysis using a JSM-7200FLV field-emission SEM (JEOL Ltd., Tokyo, Japan).
2.6. Instrumentation and Analytical Conditions
Liquid Chromatography–Quadrupole Time of Flight Mass Spectrometry (LC–QToF)
The liquid chromatographic system was an Agilent Series 1290 system comprised of a binary pump, a vacuum solvent micro-degasser, a 100-well autosampler, and a thermostatically controlled column compartment. Separation was achieved on an Agilent Poroshell 120 EC-18 (2.1 × 150 mm, 2.7 µm) column at a 0.2 mL/min flow rate. The mobile phase consisted of water with 0.1% formic acid (A) and acetonitrile with 0.1% formic acid (B) with the following gradient elution: 99% A/1% B, isocratic for 3 min, 45% B in the next 27 min, and 100% B in the next 10 min. A 5 min wash followed each run with 100% acetonitrile and an equilibration period of 5 min with 99% A/1% B. One microliter of the sample was injected at a column temperature of 40 °C. The mass spectrometric analysis was performed with a QToF–MS/MS (Model #G6545A, Agilent Technologies, Palo Alto, CA, USA) equipped with an ESI source under the following conditions: drying gas (N2) flow, 13.0 L/min; drying gas temperature, 325 °C; nebulizer, 27 psig; sheath gas temperature, 325 °C; sheath gas flow, 11 L/min; capillary, 3500 V; skimmer, 65 V; Oct RF V, 750 V; and fragmentor voltage, 150 V. The sample collision energy was set at 45 eV. All operations, data acquisition, and data analysis were controlled by Agilent MassHunter Acquisition Software Ver. A.10.00, with further data processing by MassHunter Qualitative Analysis Software Ver. B.10.0. Each sample was analyzed in positive and negative modes in the range of m/z = 50–1700. Accurate mass measurements were obtained employing ion correction methodologies using mass references at m/z 121.0509 (protonated purine) and 922.0098 [protonated hexakis (1H, 1H, 3H-tetrafluoropropoxy) phosphazine or HP-921] in positive ion mode, and mass references at m/z 112.9856 (deprotonated trifluoroacetic acid-TFA) and 1033.9881 (TFA adducted HP-921) were used in negative ion mode. The compounds were confirmed in each spectrum.
2.7. Method Validation
The LC–QToF method was validated following International Conference on Harmonization (ICH) guidelines regarding precision, accuracy, carryover, stability, and linearity [54]. The limit of detection (LOD) and limit of quantification (LOQ) parameters were determined by injecting a series of diluted solutions with known concentrations. LOD and LOQ were defined as signal-to-noise ratios equal to three and ten, respectively. The accuracy of the assay method was verified in duplicate using two concentration levels of 10 and 100 ng/mL. Intra- and inter-day variation of the assay was determined on three consecutive days with three repetitions each [55].
2.8. Post-Processing of Poppy Seeds
2.8.1. Ground vs. Intact Poppy Seeds
To understand the possible role of grinding poppy seeds, 100 mg of intact seeds (#2120PR) and the corresponding ground mixture (#2120PR) were extracted separately with 1% formic acid in methanol (10 mL) in five replicates and analyzed for the content of the five alkaloids: morphine, codeine, thebaine, papaverine, and noscapine.
2.8.2. Washing Effect on Poppy Seeds
To understand the possible impact on OAs content of water washing intact poppy seeds, 500 milligrams of poppy seeds (#2546) were soaked in water (10 mL) and methanol with 1% formic acid (10 mL) separately at different temperatures (cold and hot, 70 °C) and at different time intervals (1, 10, 30, 60, and 120 min; 4, 8, 24, and 48 h; and 4, 6, and 8 days) with constant agitations. At each time increment, 100 µL of an aliquot was collected and analyzed for morphine, codeine, thebaine, papaverine, and noscapine. At the same time, 100 µL of water or methanol (1% formic acid) was added to maintain the constant final volume (10 mL). After 8 days of washing, all samples were dried, ground, and extracted with 10 mL water or methanol (1% formic acid) separately and analyzed. Effects of solvent, temperature, and extraction time factors on poppy seeds were also investigated.
In another experiment, ground poppy seeds (500 mg, #2546) were soaked in 10 mL each of water and methanol (1% formic acid) at different temperatures (cold and hot, 70 °C) and at different time intervals (1, 10, 30, 60, and 120 min; 4, 8, 24, 48 h; 4, 6, and 8 days) with constant agitations. At each time, 10 mL of solution was removed and analyzed for morphine, codeine, thebaine, papaverine, and noscapine. At the same time, 10 mL of water or acidified methanol was added. This process was continued for 8 days, and after 8 days of thorough washing, the samples were dried and ground. Samples were again extracted with water or methanol (1% formic acid) and analyzed separately. In this study, the OAs in each washing cycle were estimated.
3. Results and Discussion
3.1. Scanning Electron Microscopic Observation of Poppy and Its Adulteration/Substitution Seeds
P. somniferum seeds are of a tiny reniform shape and are noticeably blue/black and white-colored. Seeds are, on average, 1.65 mm long and 1.15 mm wide. The different color seeds are morphologically similar, with white seeds being 0.10–0.25 mm smaller than black seeds. Blue/black/white seeds show a rough surface with a pitted honeycomb-like structure, as shown in Figure 3. The surface is rough in blue–black seeds (Figure 3a–f), whereas in white seeds, the texture is less waxy coated (Figure 3g–h’). The hilum is at the center of the concave curve of the seed (Figure 3a–h). Known adulterant seeds can be differentiated via SEM microscopic characters (Figure 3i–p’) using the features highlighted in Table 1. Microscopically, these adulterant seeds have different seed coat (testa) textures from genuine poppy seeds. Eschscholzia californica seeds have a rough and microsculpted surface, but they can be differentiated by the absence of the characteristic honeycomb texture observed in poppy seeds (Figure 3m,m’) and also by the cylindrical shape of the seed. Amaranthus spp. seeds have smooth and polished dusty surfaces (Figure 3n,n’) and a reticulate hierarchical structure (Figure 3p,p’) using SEM observation. Other less common adulterants, such as Nigella sativa (Figure 3i,i’), Chenopodium quinoa (Figure 3j,j’), Sesamum indicum (Figure 3k,k’), and Salvia hispanica (Figure 3l,l’) have specific features starkly different from poppy seeds.
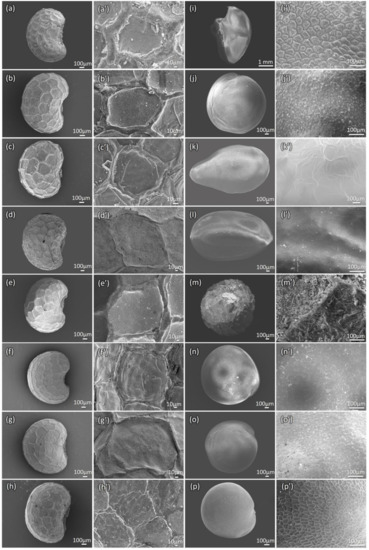
Figure 3.
Scanning electron microscopy (SEM) observation of different poppy seeds. #24792 (a,a’); #24790 (b,b’); #25124 (c,c’); #24793 (d,d’); #24791 (e,e’); #25107 (f,f’); #16725 (g,g’); and #5558 (h,h’) show the whole seed view and testa texture with a reticulate waxy plate in blue/black/brown seeds and mild in white seeds. The adulterant samples: N. sativa (i,i’) has a reticulate pattern of ridges with an ocellate spinulose testa texture; C. quinoa (j,j’) has a “dusty” surface, with no protuberances; S. indicum (k,k’) has a smooth texture with some nerve lines; S. hispanica (l,l’) has a smooth texture; E. californica (m,m’) has a rough and micro sculpted texture; A. cruentus (n,n’), A. caudatus (o,o’), are with smooth texture; A. paniculatus (p,p’), has a reticulate somewhat hierarchically structure. Scale bars are marked respectively for each image.

Table 1.
List of salient features to distinguish the poppy seeds from their adulterants.
3.2. Extraction from Poppy Seeds
Extraction efficiency for alkaloids was investigated using the following solvents and solvent mixtures covering a broad range of polarity index values: methanol, acidified methanol (0.1% and 1% formic acid), ethanol, water, and solvent mixtures such as acetonitrile–water (1:1, v/v), acetonitrile–water (1:1, v/v) with 0.1% formic acid, IPA–methanol (1:1, v/v), and IPA–ethanol (1:1, v/v) with 0.1% formic acid (Figure 4). Poppy seeds (# 2117PR) were homogenized using a grinder. Approximately 100 mg of the seeds were weighed in duplicate, and the solvent was added. A mixture of deuterated internal standards (morphine-d3 and codeine-d3) was added to each solution; tubes were capped, placed into an ultrasonic bath for 30 min, and centrifuged at 4000 rpm for 15 min. Ultrasound-assisted extraction was carried out and optimized for the above solvents, time of extraction, and concentration of constituents in each organic solvent. Among the different solvents, the optimum extraction conditions were realized when 100 mg of poppy seeds were extracted with 10 mL of methanol containing 1% formic acid (see under the Section 2).
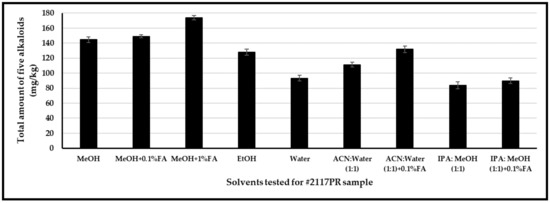
Figure 4.
Extraction efficiency of opiates in 2117PR with various solvents.
3.3. Optimization of Chromatographic Conditions
After several trials, optimized chromatographic conditions were achieved with acetonitrile and water with formic acid in different proportions for the mobile phase and column temperatures at ambient and 40 °C. Mass Hunter Workstation software, including Qualitative Analysis (version B.10.00), was used for processing both raw MS and MS/MS data. This includes molecular feature extraction, background subtraction, data filtering, and molecular formula estimation (using an exact mass of all isotopes, their relative abundances, and all detected adducts with their measured isotopes). A blank sample (methanol) was analyzed under identical instrument settings, and background molecular features (MFs) were removed. This method also involved using the [M + H]+ ions in the positive ion mode found in the extracted ion chromatogram (EIC).
3.4. Validation Procedure
According to ICH guidelines (Table 2), the newly developed LC–QToF method for the five opiates (OAs) was validated for selectivity, sensitivity, the limit of detection (LOD), the limit of quantification (LOQ), stability, precision, accuracy, specificity, and linearity [54].

Table 2.
LC-QToF-MS validation parameters for poppy seeds standards.
The specificity of the method was determined by comparing chromatograms of the blank (methanol) with poppy seed samples and spiked blanks (OAs added to methanol and code #2124PR, 25107). A comparison of methanol blanks with samples and spiked blanks demonstrated the specificity and selectivity of the chosen methodology.
The LOD and LOQ were determined by injecting diluted solutions with known concentrations of each standard. LOD and LOQ were defined as signal-to-noise ratios equal to three and ten, respectively. A five-point calibration curve for the five opioid alkaloids showed a linear correlation between concentration and peak area. Calibration data indicated the linearity (r2 > 0.99) of the detector response. The detection and quantification limits were 10–25 pg/mL and 25–100 pg/mL, respectively. All samples and standard solutions were analyzed in duplicate.
The accuracy of the assay method was evaluated by spiking two products (#2124PR and #25107) in duplicate using concentration levels of 10 and 100 ng/mL. The method’s accuracy was determined for the related compounds by spiking the sample (#2124PR and #25107) with a known amount of the five opiates standard. These samples were spiked with known amounts of the standard compound mixture and were extracted four times under optimized conditions. These sample’s percentage recovery (%RSD) ranged from 90–109% (1.7–4.4%).
The precision of a method is the degree of agreement among individual analytical results when the procedure is applied repeatedly to multiple samples of each product. The intra- and inter-day precisions were estimated by analyzing multiple replicates of two products (#2124PR and #25107). The intra-day precision of the assay was estimated by calculating the relative standard deviation (%RSD) for the analysis of samples in three replicates (n = 3) of each product, and the inter-day precision was determined by the analysis of three replicates of the same product on three consecutive days. The intra-day %RSD for the replicates was between 0.7 and 3.9%, and the %RSD for the day-to-day replicates was between 1.1 and 3.9%.
From the measured standard deviation (SD) and mean values, precision as relative standard deviation (%RSD) is calculated as %RSD = (SD/mean) × 100.
The sample solution (#2124PR and #25107) and standard solutions (10 and 50 ng/mL) were prepared by the proposed method and subjected to a stability study at room temperature for 72 h. The sample solution was analyzed at the initiation of the study period and at three additional time intervals before 72 h. No significant changes were observed.
3.5. Analysis of Poppy Seeds
LC–QToF–MS is a powerful technique offering high sensitivity and selectivity for qualitative and quantitative measurements of analytes of interest in a complex mixture. The high-resolution mass detection in the positive ion mode is useful for detecting alkaloids. In the positive ion mode, protonated species [M + H]+ at m/z 286.1440, 300.1596, 312.1597, 340.1541, and 414.1545 for morphine, codeine, thebaine, papaverine, and noscapine, respectively, were observed. The combination of retention time and exact mass results are helpful for the general identification of the compounds. The calibration curves were prepared for the compounds listed in Table 2. The validation parameters—retention time, molecular formula, and characteristic fragment ions observed for all compounds—are summarized in Table 3 and Table 4. Retention time, accurate mass, and MS/MS analysis are helpful in the identification of compounds.

Table 3.
Content of μg/g of different alkaloids in various ground poppy seeds using LC–QToF–MS.

Table 4.
RT, compound name, molecular formula, mass, m/z, and major fragment ions for reference compounds and other individual compounds detected in poppy seed samples using LC–QToF.
From each batch of poppy seeds, two different portions (each weighing ∼100 mg) were ground, extracted, and analyzed. Stranska et al. (2013) reported the alkaloids content from 15 different cultivar samples as 3327–17175 µg/g (morphine), 172–1767 µg/g (codeine), 0–1701 µg/g (thebaine), 2–689 µg/g (papaverine), and 24–1675 µg/g (noscapine) [39]. This is while Lopez et al. (2018) reported that the alkaloids’ content varies from 0.2–241 mg/kg (morphine), <0.1–3.48 mg/kg (codeine), and <0.1–106 mg/kg (thebaine) in 41 different commercially purchased samples from the Netherlands, Germany, and Italy [19]. In our study, 23 poppy seed samples (15 black/blue/brown mix and 8 white/brown mix) were analyzed for the content of five OAs. 17 of the 22 samples showed higher morphine content than any of the other four OAs analyzed. In these 17 samples, the morphine content ranged from 0.8 to 223 μg/g. Two samples (#24792 and #2120PR) showed high codeine content, while codeine content ranged from 0.2–386 μg/g across all 22 samples. One sample (#2117PR) showed a high thebaine content, whereas the thebaine content ranged from 0.1 to 176 μg/g across all 22 samples. Higher concentrations of morphine, codeine, and thebaine were detected in black and black–blue mix samples, while these OAs were lower in white and white–brown mix samples. The other two compounds, papaverine, and noscapine, ranged from DUL—28 μg/g to DUL—73 μg/g, respectively. Sample #6485, labeled “Papaver species,” contained all five OAs (Table 3) and showed a profile similar to that of P. somniferum. The mean levels of OAs are shown in Table 3, Figure 5 and Figure 6. Differences in the cultivar varieties, harvesting conditions, and post-processing procedures (viz., washing) affect the alkaloids content from the previously published reports. Furthermore, compared to previous reports, the developed method can perform quantitative analysis of opiates as well as chemical profiling to differentiate authentic poppy seeds from adulterants. Nevertheless, high OAs variation in these samples suggests that these poppy seeds may not meet the limits set by European authorities.
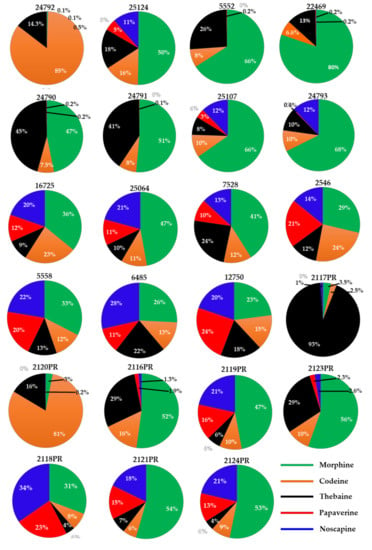
Figure 5.
Content of alkaloids (μg/g) observed in different poppy seed samples. The area of a slice in each pie chart is proportional to the individual alkaloid’s content (μg/g).
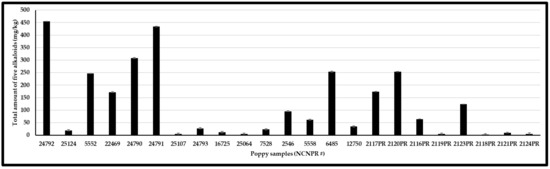
Figure 6.
Total five OAs content (mg/kg) of different poppy seed samples.
3.6. Post-Processing Studies of Alkaloids
3.6.1. Alkaloid Content in Ground vs. Intact Poppy Seeds
This experiment was aimed at evaluating alkaloid degradation during the processing of food. Unground and ground seeds were extracted separately with methanol (1% formic acid) in five replicates and analyzed for alkaloid content. The influence of grinding on the OA content was studied. Insignificantly lower concentrations of OAs were found in the ground seeds compared to the unground, intact seeds. Around 10–15% depletion of three alkaloids was observed, accounting for the formation of pseudomorphine (C34H36N2O6, m/z 569.2646 [M + H]+) [56,57], morphine-N-oxide (C17H19NO4, m/z 302.1387 [M + H]+) [56,57], codeine-N-oxide (C18H21NO4, m/z 316.1543 [M + H]+) and thebaine N-oxide (C19H21NO4, m/z 328.1543 [M + H]+). The data also suggested that temperature and light have minor effects on the content of the five studied opiates.
Besides the poppy variety, the harvest method has the strongest influence on the morphine content and leads to the greatest variability in the alkaloid concentration. High OAs concentrations can be attributed to insufficient harvesting practices leading to seed contamination with latex, inadequate cleaning and handling, or intentional spiking with pure standard morphine. The non-uniformity of this contamination leads to inhomogeneities observed within the sample replicates. Similar observations were seen by Sproll et al. (2006 and 2007) [4,19,38,56,58]. When the levels of OAs in unground poppy seeds from five replicates within each batch (#2120) were compared, it was found that there was much variation with the levels of morphine, codeine, thebaine, and noscapine ranging from 0.6–7.6 μg/g, 218–424 μg/g, 12.4–68.2 μg/g, and 0.5–1.4 μg/g, respectively, suggesting possible anomalies in post-harvesting. While in the ground seed samples, there was little variation between the five replicates (#2120), indicating the homogeneity of ground samples. These wide variations observed within the same seed sample suggest the presence of OAs may be due to inadequate cleaning of the seeds, with most of the opium alkaloids coming from debris or residual latex on the seed surface.
3.6.2. Removal of Free Alkaloids by Soaking
Only 40–50% of the five alkaloids are removed with cold water and acidified methanol washes. Seed washing at 70 °C significantly reduced OAs content by 70–80% (Figure 7), and extended washing for 48 h resulted in the complete removal of OAs. Similar observations were made by Sproll et al. (2007) [56].
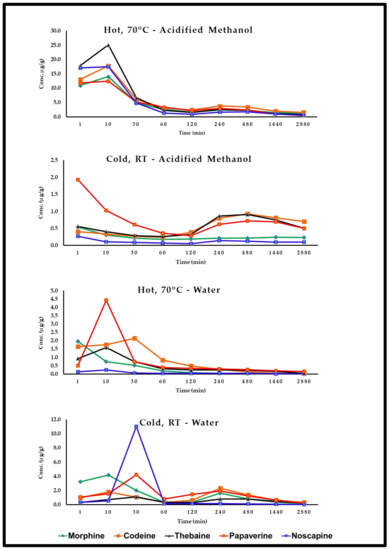
Figure 7.
Change in alkaloid content (μg/g) during washing at different conditions and time intervals.
Very low levels of OAs were detected in studied poppy seed samples using the current analytical methodology. This study confirms that poppy seed morphine or other OAs originate predominantly from external contamination. This was further confirmed by several seed-washing experiments that significantly reduced the OA content. Bjerver et al. (1982) [34] showed that 40% of the total morphine could be removed by a single washing with slightly acidified water. Soaking poppy seeds in water for five minutes removed about 46% of their free morphine and 48% of their codeine [59]. Moreover, the results presented in Figure 6 shows that about 40% of the total opioid content in the seed samples could be isolated from the seeds by a single, simple washing procedure. Alkaloid content in poppy seeds found in this study agrees with prior reports [34,56,59].
3.7. Identification and Characterization of Chemical Constituents
In this study, positive ESI tandem mass spectra of the [M + H]+ ions of alkaloids were obtained using LC–QToF. High-resolution mass resolving power and accurate mass measurement for MS and MS–MS experiments enable rapid dereplication of various alkaloids within the genus Papaver. It generates characteristic fragment ions, which help confirm the known compound in a complex mixture. Possible fragment ions are shown in Table 4. Accurate mass measurements were performed to obtain each analyte’s elemental composition. The diverse class of alkaloids, the major components of poppy seeds, have been categorized into seven groups based on their structural motifs and MS/MS fragmentation patterns, including morphinane, benzylisoquinoline, rhoeadine, tetrahydroisoquinoline, promorphinane, protopine, and benzophenanthridine types. The mass spectral fragmentation patterns of five reference compounds were studied, and based on these patterns, other alkaloids were investigated. Figure 8 shows the extracted ion chromatograms (EIC) of reference standards and poppy seeds (#24792) that have five opioids (OAs).
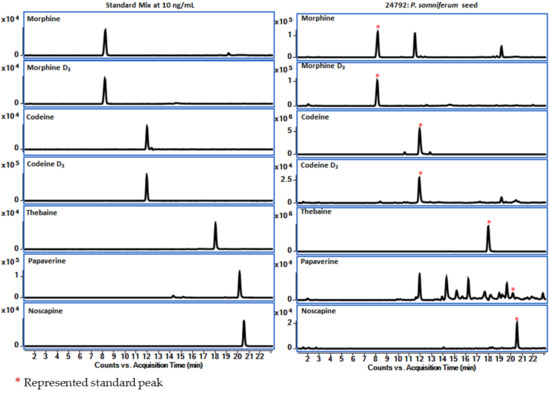
Figure 8.
Extracted ion chromatograms of five OAs and internal standards (*) from the standard mixture and poppy seed sample (#24792).
3.7.1. Fragmentation Patterns of Compounds 1–6 (Table 4)
Full-scan mass spectra in the positive ion mode of the LC peak at 3.3 min provided a quasimolecular ion at 𝑚/z 154.0863, suggesting a molecular formula with [C8H11NO2]+ (calculated to be 154.0863). The MS2 spectrum of the ion at 𝑚/z 154.0863 generated a series of ions at 𝑚/z 137.0598, 119.0488, 109.0647, and 91.0543, which agree with the fragmentation pattern of dopamine. The formation of product ions m/z 137 and 119 is attributable to the neutral loss of the amine group [M + H-NH3]+ and water molecule from the terminus of the dopamine molecule (Table 4) [60].
The major fragmentation pathway of protonated phenylalanine (m/z 166.0861) starts with the loss of water and CO to form a fragment ion at m/z 120.0801. Further loss of ammonia resulted in a fragment ion with m/z 103.0545. Leucine and isoleucine (m/z 132.1015) produced two fragments by sequential losses of H2O and CO (m/z 86.0966) and NH3 (m/z 69.0696) [60].
Protonated adenosine and guanosine dissociated through decompositions of base-protonated [B + H]+ ions by the cleavage of the glycosidic bonds to give the protonated bases with a sugar moiety as the neutral fragment. Fragment ions m/z 268.1042 and 284.0890 correspond to the protonated molecular ions of adenosine and guanosine. Ions at m/z 136.0619 [M + H-132]+ and 152.0567 [M + H-132]+ are protonated ions of adenine and guanine, respectively. Typical neutral losses correspond to the molecules -CO, -NH3, -CH2CH2, -NHCH2, -NHCO, and -NH2CN. The glycosidic C–N bond cleavage is characteristic of two nucleosides. The major fragmentation pathways are ring contraction (RC) and retro-Diels-Alder (RDA) [61].
3.7.2. Fragmentation Patterns of Alkaloids (Compounds 7–47) (Table 4)
Morphinane: The fragments are formed by cleavage of the piperidine ring and loss of an amine (CH2CHNHCH3, [M-57]+). Such a fragment is found for all morphine-like compounds and is crucial in further fragmentation pathways [62].
Morphine and codeine exhibit similar fragmentation patterns, and the major product ions obtained were m/z 201.0910 and m/z 215.1067, respectively, which were derived from the precursor ion [M + H]+ by cleavage of the piperidine ring and consecutive losses of ethylene methylamine [CH2CHNHCH3]+ and CO [62]. Consecutive losses of two water molecules in the case of morphine or methanol and water for codeine result in a cyclopentadiene naphthalene product ion with m/z 165.0704 and the elemental composition [C13H9]+. Loss of formaldehyde (H2CO) in place of H2O produces a dicyclopentadiene benzene product ion with m/z 153.0704 and the elemental composition [C12H9]+. The major product ions for thebaine are [M + H-31]+, [M + H-46]+, and [M + H-61]+, which correspond to the loss of methylamine and one and two methyl groups, respectively [63].
Five cleavages are still found, however, corresponding to those in codeine or morphine, giving ions at M-15 (loss of methyl), M-29, M-43, M-57, M-58, M-59, and M-17 (loss of hydroxyl) in neopine [64].
Promorphinane alkaloid: Salutaridine and salutaridinol differ from the morphine type since the 4,5-epoxy bridge is opened and additional oxygen is present at position 7. The difference between the keto and hydroxy groups at the C-7 position revealed through fragments reveals the characterization of salutaridine and salutaridinol. Initially, the fragmentation of salutaridine is characterized by the loss of methylamine [M + H-31]+ from the precursor ion m/z 328.1540 [M + H]+ followed by the subsequential loss of two methanol groups to form m/z 265.0857 and 233.0597. In addition, the key fragment loss of -HCHO (m/z 298.1430) confirms the presence of the keto group. Whereas, the formation of the m/z 298.1455 fragment reveals the loss of a methanol group followed by methylamine loss (m/z 267.1020). The absence of the epoxy bridge facilitates the formation of an isoquinoline fragment at m/z 192 [62].
Phthalideisoquinoline: The product ion of noscapine at m/z 220.0972 indicates the loss of meconine or 6, 7-dimethoxy-3H-1-isobenzofuranone (C10H10O4, 194.0579 Da) from the precursor ion [M + H]+, and subsequent loss of azirine (m/z 41.0265, C2H3N) produces a fragment at m/z 179.0716. A minor product ion of noscapine at m/z 353.1001 [M + H-61]+ is also detected, which can be explained by the loss of aziridine (m/z 43.0422, (CH2)2NH) and water [64].
Benzyltetrahydroisoquinoline and aporphine alkaloids were distinguished by characteristic losses of the NHR1R2 (R1 and R2 represent the substituent groups of the nitrogen atom) radical and the fragment ions below m/z 200. The MS/MS investigation of benzyltetrahydroisoquinoline and aporphine alkaloids results in fragment ions [M-45]+, [M + H-31]+, and [M + H-17]+ always being observed initially depending on the number of N-methyl groups. These fragments are attributed to a loss of the NH(CH3)2 (R1 = R2 = CH3), NH2CH3 (R1 = H, R2 = CH3), and NH3 (R1 = R2 = H) radicals, respectively. These characteristic ions play a diagnostic role in the discrimination of other alkaloid types. However, the mass spectrum fragmentation behaviors of magnoflorine and corydaline were different from those of the benzyltetrahydroisoquinoline alkaloid because of the conjugate structure. No characteristic ions exist below m/z 200, and the product ions are formed mainly by the loss of some substituent groups [65].
Protopine alkaloids (41–44): Product ions were generated by dehydration (m/z 336), retro-Diels-Alder (RDA) fragmentation (m/z 149 and 206), α-cleavage forming small fragment molecules, and subsequent losses of H2O (m/z 188) and OH (m/z 189), according to Shim et al. 2013 [66], Sai et al., 2020 [67], and Schmidt et al. 2007 [68]. Fragment ions at m/z 206.0823 and 149.0603 in the MS/MS spectrum are generated by RDA C ring opening, but given the presence of hydroxyl groups, the product ions at m/z 336.1209 and m/z 188.0721 are probably formed by loss of H2O from the molecular ion and the m/z 206.0823 ion.
Sanguinarine (benzophenanthridine-type) was also characterized by the fragmentation of their substituents, with the most abundant ions being formed by the loss of CH3 (m/z 317.0685), CH2O (m/z 302.0811), and CO (m/z 276). Benzophenanthridine alkaloids contain a large π conjugate system, making the parent nucleus difficult to fragment. The benzophenanthridine alkaloid sanguinarine, containing a methylenedioxy group, would lose carbon monoxide to form a stable ternary oxygen ring [67].
The alkaloids of the rhoeadine type represent a new group of natural bases isolated from plants of the Papaveraceae. These cyclic acetal compounds represent the molecular ion at [M-15]+ and the base fragment ion at m/z 177. This shows the stable fragment cleavage at C-1 and C-2 positions (C- and D-rings) [69].
Stilbenes: The product ions of narceine from the precursor ions at m/z 428.1707 [M + H-18]+, m/z 383 [M + H-63]+, and m/z 365 [M + H-81]+ can be rationalized by the losses of dimethylamine and one or two water molecules, respectively [19].
Benzylisoquinolines: These correspond to ion types a, [M + H-NH3]+, and ion types b, c, the protonated parental molecule [M + H]+, and ion type d, respectively. The resulting ion at m/z 123.04390 constitutes the signature fragment obtained for 1-benzylisoquinolines containing hydroxyl groups at C3′ and C4′. Increases in 14 or 28Da (m/z 137 or m/z 151) for the equivalent ions indicate single- or double-methylation at C3′ and C4′ [70].
Papaverine is a non-narcotic alkaloid found to be endemic and selectively localized in the latex of the opium poppy. Papaverine readily produced an m/z 340.1543 [M + H]+ protonated pseudomolecular ion. The fragmentation of the precursor ion can be explained by the loss of a methyl group, resulting in a product ion at m/z 324.1236, and the loss of the dimethoxy benzyl moiety to yield the prominent isoquinoline moiety at m/z 202.0868 via the C–C bond between the dimethoxy benzyl and isoquinoline ring systems. The subsequent loss of a methoxy group results in one or more fragments with m/z 171.0682 [64]. The product ion at m/z 187.0628 was an isoquinoline ring, and the product ion at m/z 156.0443 was produced by demethylation of the fragment ion at m/z 171. The product ions and the corresponding neutral fragment loss noticed in our study agree with the work demonstrated by Wickens et al. (2006) [64] and Peng et al., 2007 [71] for the characteristic structural information of papaverine.
Tetrahydroisoquinoline alkaloid: The reticuline was identified at RT 15.8 min by m/z 192.1022, resulting from fragmentation of [M + H]+ 330.1701 corresponding to reticuline (C19H23NO4 (calculated [M + H]+ 330.1700). The fragmentations indicated the initial loss of methylamine to form an ion of m/z 299.1281, followed by fragmentation of the benzyl moiety (m/z 137.0600). The stable naphthalene ion (m/z 175.0750) was formed from the rearrangement of the ion m/z 299. The benzylisoquinoline ion (m/z 192.1022) was formed directly from the quasimolecular ion [M + H]+ and was the diagnostic ion for tetrahydroisoquinoline type of alkaloids [65,72].
Benzo[c]phenanthridines: Sanguinarine ([M]+; m/z 332.0917) is benzo[c]phenanthridines, most of whose fragmentation ions form by cleavage of the substituted groups rather than ring fusion. As a derivative of protopine, sanguinarine contains two methylenedioxy rings. The base peak at m/z 304.0969 results from the rearrangement of one methylenedioxy ring and the consequent loss of CO. Consecutive losses of CH2O and CO generate other fragments at m/z 274.0865 and 246.0915, respectively [68].
3.8. Poppy Seed Adulteration or Substitution
Poppy seeds are relatively expensive and are sometimes mixed with seeds that closely resemble poppy seeds, such as seeds of Amaranthus species [48,73]. The adulterants are often similar in look, color, and texture to authentic poppy seeds. Even though some adulterant seeds may not present a health hazard, they are less expensive, and their use results in economic losses for food poppy seed producers and places fraudulent products in the marketplace.
Adulterated seeds were observed under 10× to 50× magnifications, and the salient features were noted to differentiate the adulterants from the genuine poppy seeds (Figure 9 and Table 1). Poppy seeds from different cultivars vary in size, color, and appearance, with the black/blue/brown seeds having a rough waxy coat while the white seeds have a smooth non-waxy coat. Many adulterants, such as Amaranthus seeds, are used commercially to increase the weight of poppy seeds, leading to lower-grade materials in commerce.

Figure 9.
External morphology of poppy seeds and their adulterants. (a–h) P. somniferum (black, brown, white, and blue); (i) A. paniculatus; (j) A. cruentus; (k,k’) A. caudatus (pink and white, respectively); (l) E. californica; (m) C. quinoa; (n). N. sativa; (o) S. hispanica; and (p) S. indicum. Scale bar: 0.5 mm: (i–k’); 1 mm: (a–h,l,m); 2 mm: (n–p).
In this study, an LC–ESI–MS/MS technique provided applicable information to characterize 47 compounds from P. somniferum and major components from adulterants using authenticated plant materials. With this methodology, ground plant material could be analyzed to confirm or deny the presence of P. somniferum, which should aid in detecting adulteration or preventing the use of potentially mislabeled or misidentified “P. somniferum” material. Poppy seeds containing varying amounts of adulterants were analyzed. Adulteration as low as 10% could be easily detected based on the constituents shown in Table 5. Other possible adulterants of poppy seeds (Table 5) are:

Table 5.
Adulterants derived from plants in poppy seed adulteration: plant name, compound name, retention time in minutes, molecular formula, mass, m/z, and major ion fragments for adulterants detected using LC–QToF.
Salvia hispanica, commonly known as chia, is a species of flowering plant of the mint family, Lamiaceae, native to Central and Southern Mexico and Guatemala [74]. Ground or whole chia seeds are consumed in Paraguay, Bolivia, Argentina, Mexico, and Guatemala for nutritious drinks and as a food source. S. hispanica is a source of omega-3 fatty acids—linolenic acid, linoleic acid, oleic acid, pantothenic acid, myricetin, the main flavanol, quercetin, and kaempferol are found in chia seeds.
Nigella sativa of the Ranunculaceae family is an indigenous herbaceous plant native to Asia and the Middle East. The plant is also known as black cumin (in English) and black caraway (in the USA). It grows throughout Mediterranean countries and is cultivated extensively in India and Pakistan. In Egypt and the Middle East, the black seed oil is commonly used to relieve cough and bronchial asthma [75,76,77]. The seeds contain fixed and volatile oils, proteins, flavonoid glycosides (kaempferol 3-O-β-D-glucopyranosyl-(1→2)-O-β-D-galactopyranosyl-(1→2)-O-β-D-glucopyranoside), alkaloids (mainly magnoflorine), and saponins (sieboldianoside A, tauroside H2, tauroside G3, decaisoside D, sapindoside B, tauroside E). The most important active compounds were thymoquinone, thymohydroquinone, and dithymoquinone [77,78,79]. Salvia and Nigella seeds contain terpenoids and quinones that are reported to interact with opiate receptors [80].
Amaranthus caudatus, A. paniculatus, and A. cruentus: Amaranthus of the family Amaranthaceae, which includes quinoa and amaranth species, is a valuable food source of nutrients with high-quality proteins, vitamins, minerals, and bioactive compounds such as phenolics [81,82]. Approximately 35 to 55 Amaranthus species are native to the Americas, and at least 15 species are native to Africa, Europe, and Asia. Amaranthus is an important nutritional crop. It is a rare plant, with leaves consumed as a vegetable while seeds are eaten as a cereal [83,84]. A. caudatus, A. hypochondriacus, and A. cruentus, used for grain purposes, have tremendous potential to increase food production. Amaranth is commonly used in the bakery for cookies, biscuits, candies, pancakes, pasta, noodles, etc. [85]. In addition to its nutritional properties, amaranth offers an attractive source of lysine and other bioactive compounds such as phenolics, squalene, folate, phytates, and tocopherols [86]. Studies have also revealed the presence of olean triterpene saponins in the seeds.
Sesame (Sesamum indicum) has long been used extensively as a traditional food in the Middle East and South Asia. Sesame seed and sesame oil are widely used in cooking and as ingredients in sweet and confectionery foods. The potent antioxidant properties of seed extracts from S. indicum and sesame oil are attributed mainly to the presence of the lignans, including sesamin, sesamolin, sesamolinol, sesaminol, and lignan glycosides [87].
Chenopodium quinoa: Due to their high protein content, quinoa seeds have high nutritional value and are known to possess several flavonoids, including kaempferol glycosides, quercetin glycosides, and saponins (Table 5) [88].
Eschscholzia californica, the California golden poppy, California sunlight, or cup of gold, is a flowering plant in the family Papaveraceae, native to the United States and Mexico. The Californian poppy has a sap that contains a different class of alkaloids from that of the addictive “opium poppy” and does not have a narcotic effect on the human body. Similar to all poppy alkaloids, these have a sedative and relaxant effect on the body and mind, but they are perceived to be gentle and mild. Analysis showed the presence of morphinan alkaloids in opium poppy (P. somniferum) and benzophenanthridine alkaloids in E. californica. Several chemical compounds have been identified in E. californica, including californidine, cryptopine, eschscholtzine (californidine), sanguinarine, chelirubine, and other similar (Papaveraceae) alkaloids [89].
4. Conclusions
Two independent methodologies (microscopy and LC-QToF) were investigated with twenty-three poppy seed samples and eight adulterants. Detailed micromorphology observations with salient features highlighted may be used to differentiate poppy seeds from adulterant seeds. The developed LC–QToF method was identified as a sensitive and selective analytical method to determine five opiates in various colored and noncolored poppy seeds. The method was validated in terms of linearity, accuracy, selectivity, LOD, LOQ, and precision. A variation in total alkaloid content was noted among different color poppy seeds, specifically morphine, codeine, and thebaine, ranging from 0.8–223, 0.2–386, and 0.1–176 mg/kg, respectively. In most cases, the phenanthrene alkaloids morphine (dominant), codeine, and thebaine are in higher concentrations than the benzylisoquinoline alkaloids papaverine (<LOD-28 mg/kg) and noscapine (<LOD-73 mg/kg). The optimal treatment for reducing morphine and other OAs in poppy seed consists of washing, heating, and grinding. The application of LC–QToF provided useful information to characterize forty-seven compounds in poppy seed samples. Cleavage of the piperidine ring with consecutive losses of an amine and CO were some of the characteristic fragment ions for morphine, thebaine, and codeine, although this fragmentation process is not applicable for noscapine and papaverine. The developed simple and accurate method will be useful for determining and confirming unknown molecules, contaminants, or adulterants via a targeted or non-targeted approach. It is essential for identifying P. somniferum. There were no adulterants found in any of the poppy seed samples studied, which included seeds of amaranthus, golden poppy, black cumin, chia, sesame, and quinoa. However, the developed methods should serve as proactive tools to combat potential adulteration with seeds that closely mimic poppy seeds.
Author Contributions
B.A.: methodology, investigation, data curation, writing—original draft preparation. K.K.: methodology, data curation, visualization, investigation. S.J.A.: data curation, writing–reviewing, and editing. A.G.C. and Y.-H.W.: visualization, writing–reviewing and editing, I.A.K.: conceptualization, resources, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research is supported in part by “Science-Based Authentication of Botanical Ingredients,” funded by the Center for Food Safety and Applied Nutrition, US Food and Drug Administration grant number 5U01FD004246, and “Discovery and Development of Natural Products for Pharmaceutical and Agricultural Applications,” funded by the United States Department of Agriculture, Agricultural Research Service, Specific Cooperative Agreement No. 58-6060-6-015.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The scanning electron microscopy analysis was performed at the University of Mississippi Microscopy and Imaging Center, which is partly supported by the National Science Foundation through a Major Research Instrumentation grant (#1726880). The authors thank Jeff Solomon for his support and native English proofreading.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Merlin, M.D. Archaeological evidence for the tradition of psychoactive plant use in the old world. Econ. Bot. 2003, 57, 295–323. [Google Scholar] [CrossRef]
- Mirsafavi, R.Y.; Lai, K.; Kline, N.D.; Fountain, A.W., III; Meinhart, C.D.; Moskovits, M. Detection of papaverine for the possible of illicit opium cultivation. Anal. Chem. 2017, 89, 1684–1688. [Google Scholar] [CrossRef] [PubMed]
- ElSohly, M.A.; Jones, A.B. Morphine and Codeine in biological fluids: Approaches to source differentiation. Forensic Sci. Rev. 1989, 1, 13–22. [Google Scholar]
- Chain, E.; Panel, O.C.I.T.F.; Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; et al. Update of the scientific opinion on opium alkaloids in poppy seeds. EFSA J. 2018, 16, e05243. [Google Scholar] [CrossRef]
- Reisfield, G.M.; Teitelbaum, S.A.; Jones, J.T. Poppy seed consumption may be associated with codeine-only urine drug test results. J. Anal. Toxicol. 2022, 47, bkac079. [Google Scholar] [CrossRef]
- Hosokawa, K.; Shibata, T.; Nakamura, I.; Hishida, A. Discrimination among species of Papaver based on the plastid rpl16 gene and the rpl16-rpl14 spacer sequence. Forensic Sci. Int. 2004, 139, 195–199. [Google Scholar] [CrossRef]
- WFO. The World flora online—Papaver L. Available online: http://www.worldfloraonline.org/taxon/wfo-4000027914 (accessed on 31 March 2023).
- Langlois, A.; Mulholland, D.A.; Crouch, N.R.; Grace, O.M. Aporphine alkaloid from Papaver aculeatum (sect. Horrida; Papaveraceae) of Southern Africa. Biochem. Syst. Ecol. 2004, 32, 1087–1090. [Google Scholar] [CrossRef]
- Garnock-Jones, P.J.; Scholes, P. Alkaloid content of Papaver somniferum subsp. setigerum from New Zealand. N. Z. J. Bot. 1990, 28, 367–369. [Google Scholar] [CrossRef]
- Yoshimatsu, K.; Kiuchi, F.; Shimomura, K.; Makino, Y. A rapid and reliable solid-phase extraction method for high-performance liquid chromatographic analysis of opium alkaloids from Papaver plants. Chem. Pharm. Bull. 2005, 53, 1446–1450. [Google Scholar] [CrossRef]
- Mohsin, H.; Abdul Wahab, I.; Nasir, N.; Hidayah, N.; Nasir, N. The chemical investigation of Papaver seeds. Int. J. Adv. Sci. Eng. Inform. Technol. 2012, 2, 309–312. [Google Scholar] [CrossRef]
- Choe, S.; Kim, S.; Lee, C.; Yang, W.; Park, Y.; Choi, H.; Chung, H.; Lee, D.; Hwang, B.Y. Species identification of Papaver by metabolite profiling. Forensic Sci. Int. 2011, 211, 51–60. [Google Scholar] [CrossRef]
- Tenore, P.L. Advanced urine toxicology testing. J. Addict. Dis. 2010, 29, 436–448. [Google Scholar] [CrossRef]
- Swarbrick, J.T.; Raymond, J.C. The identification of the seeds of the British Papaveraceae. Ann. Bot. 1970, 34, 1115–1122. [Google Scholar] [CrossRef]
- EU Database of Registered Plant Varieties. Available online: https://ec.europa.eu/food/plant-variety-portal/ (accessed on 31 March 2023).
- L 433/8; Maximum Levels of Opium Alkaloids in Certain Foodstuffs. The Publications Office of the Europena Union: Brussel, Belgium, 2021. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32021R2142 (accessed on 31 March 2023).
- L 271/96; Good Practices to Prevent and to Reduce the Presence of Opium Alkaloids in Poppy Seeds and Poppy Seed Products. The Publications Office of the Europena Union: Brussel, Belgium, 2014. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32014H0662&from=SK (accessed on 31 March 2023).
- Meadway, C.; George, S.; Braithwaite, R. Opiate concentrations following the ingestion of poppy seed products—Evidence for ‘the poppy seed defence’. Forensic Sci. Int. 1998, 96, 29–38. [Google Scholar] [CrossRef]
- López, P.; Pereboom-de Fauw, D.P.K.H.; Mulder, P.P.J.; Spanjer, M.; de Stoppelaar, J.; Mol, H.G.J.; de Nijs, M. Straightforward analytical method to determine opium alkaloids in poppy seeds and bakery products. Food Chem. 2018, 242, 443–450. [Google Scholar] [CrossRef]
- Powers, D.; Erickson, S.; Swortwood, M.J. Quantification of morphine, codeine, and thebaine in home-brewed poppy seed tea by LC-MS/MS. J. Forensic Sci. 2018, 63, 1229–1235. [Google Scholar] [CrossRef]
- Croitoru, M.D.; Fülöp, I.; Irimia-Constantin, M.-R.; Varga, E.; Kelemen, H.; Fogarasi, E.; Faliboga, L.-M. The risk of using poppy seed tea made from several varieties available on the Romanian market. Acta Marisiensis-Ser. Med. 2017, 63, 62–65. [Google Scholar] [CrossRef]
- Soares, M.E.; Seabra, V.; Bastos, M.D.L.A. Comparative study of different extractive procedures to quantify morphine in urine by HPLC-UV. J. Liq. Chromatogr. 1992, 15, 1533–1541. [Google Scholar] [CrossRef]
- Ghafoor, K.; Özcan, M.M.; Al-Juhaimi, F.; Babiker, E.E.; Fadimu, G.J. Changes in quality, bioactive compounds, fatty acids, tocopherols, and phenolic composition in oven- and microwave-roasted poppy seeds and oil. LWT 2019, 99, 490–496. [Google Scholar] [CrossRef]
- Azcan, N.; Ozturk Kalender, B.; Kara, M. Investigation of Turkish poppy seeds and seed oils. Chem. Nat. Comp. 2004, 40, 370–372. [Google Scholar] [CrossRef]
- Hayes, L.W.; Krasselt, W.G.; Mueggler, P.A. Concentrations of morphine and codeine in serum and urine after ingestion of poppy seeds. Clin. Chem. 1987, 33, 806–808. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.H.; Taylor, E.H.; Pappas, A.A. Comparison of derivatives for determination of codeine and morphine by gas chromatography/mass spectrometry. J. Anal. Toxicol. 1990, 14, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Moeller, M.R.; Hammer, K.; Engel, O. Poppy seed consumption and toxicological analysis of blood and urine samples. Forensic Sci. Int. 2004, 143, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Özbunar, E.; Aydoğdu, M.; Döğer, R.; Bostancı, H.İ.; Koruyucu, M.; Akgür, S.A. Morphine concentrations in human urine following poppy seed paste consumption. Forensic Sci. Int. 2019, 295, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Rohrig, T.P.; Moore, C. The determination of morphine in urine and oral fluid following ingestion of poppy seeds*. J. Anal. Toxicol. 2003, 27, 449–452. [Google Scholar] [CrossRef]
- Wong, R.C.; Tran, M.; Tung, J.K. Oral fluid drug tests: Effects of adulterants and foodstuffs. Forensic Sci. Int. 2005, 150, 175–180. [Google Scholar] [CrossRef]
- Jankovičová, K.; Ulbrich, P.; Fuknová, M. Effect of poppy seed consummation on the positive results of opiates screening in biological samples. Leg. Med. 2009, 11, S416–S418. [Google Scholar] [CrossRef]
- Musa Özcan, M.; Atalay, Ç. Determination of seed and oil properties of some poppy (Papaver somniferum L.) varieties. Grasas Y Aceites 2006, 57, 169–174. [Google Scholar] [CrossRef]
- Van Thuyne, W.; Van Eenoo, P.; Delbeke, F.T. Urinary concentrations of morphine after the administration of herbal teas containing papaveris fructus in relation to doping analysis. J. Chromatogr. B 2003, 785, 245–251. [Google Scholar] [CrossRef]
- Bjerver, K.; Jonsson, J.; Nilsson, A.; Schuberth, J.; Schuberth, J. Morphine intake from poppy seed food. J. Pharm. Pharmacol. 1982, 34, 798–801. [Google Scholar] [CrossRef]
- Newmeyer, M.N.; Concheiro, M.; da Costa, J.L.; LoDico, C.; Gorelick, D.A.; Huestis, M.A. Simultaneous plasma and oral fluid morphine and codeine concentrations after controlled administration of poppy seeds with known opiate content. Forensic Toxicol. 2015, 33, 235–243. [Google Scholar] [CrossRef]
- Desgagné-Penix, I.; Farrow, S.C.; Cram, D.; Nowak, J.; Facchini, P.J. Integration of deep transcript and targeted metabolite profiles for eight cultivars of opium poppy. Plant Mol. Biol. 2012, 79, 295–313. [Google Scholar] [CrossRef]
- Zentai, A.; Sali, J.; Szeitzné-Szabó, M.; Szabó, I.J.; Ambrus, Á. Exposure of consumers to morphine from poppy seeds in Hungary. Food Addit. Contam Part A 2012, 29, 403–414. [Google Scholar] [CrossRef]
- Sproll, C.; Perz, R.C.; Lachenmeier, D.W. Optimized LC/MS/MS analysis of morphine and codeine in poppy seed and evaluation of their fate during food processing as a basis for risk analysis. J. Agric. Food Chem. 2006, 54, 5292–5298. [Google Scholar] [CrossRef]
- Stranska, I.; Skalicky, M.; Novak, J.; Matyasova, E.; Hejnak, V. Analysis of selected poppy (Papaver somniferum L.) cultivars: Pharmaceutically important alkaloids. Ind. Crop. Prod. 2013, 41, 120–126. [Google Scholar] [CrossRef]
- Liu, C.; Hua, Z.; Bai, Y. Classification of opium by UPLC-Q-TOF analysis of principal and minor alkaloids. J. Forensic Sci. 2016, 61, 1615–1621. [Google Scholar] [CrossRef]
- Bosch, M.E.; Sánchez, A.R.; Rojas, F.S.; Ojeda, C.B. Morphine and its metabolites: Analytical methodologies for its determination. J. Pharm. Biomed. Anal. 2007, 43, 799–815. [Google Scholar] [CrossRef]
- Barroso, M.; Gallardo, E.; Vieira, D.N.; Queiroz, J.A.; López-Rivadulla, M. Bioanalytical procedures and recent developments in the determination of opiates/opioids in human biological samples. Anal. Bioanal. Chem. 2011, 400, 1665–1690. [Google Scholar] [CrossRef]
- Lachenmeier, D.W.; Sproll, C.; Musshoff, F. Poppy seed foods and opiate drug testing-where are we today? Ther. Drug Monit. 2010, 32, 11–18. [Google Scholar] [CrossRef]
- Haber, I.; Pergolizzi, J., Jr.; LeQuang, J.A. Poppy seed tea: A short review and case study. Pain. Ther. 2019, 8, 151–155. [Google Scholar] [CrossRef]
- Lebrero, E.A.; Baviera, J.M.B.; Moreno, M.P.C.; Riba, R.E.; Pérez, M.A.F.; Pérez, G.F.; Arnau, S.G.; de la Torre, A.H.; Gallego, A.J.; Sánchez, A.M.; et al. Report of the scientific committee of the Spanish agency for consumer affairs, food safety and nutrition (AECOSAN) in relation to the assessment of the exposure of the Spanish population to morphine resulting from the consumption of poppy seeds. Rev. Del Com. Científico AECOSAN 2016, 23, 11–20. [Google Scholar] [CrossRef]
- Schulz, H.; Baranska, M.; Quilitzsch, R.; Schütze, W. Determination of alkaloids in capsules, milk and ethanolic extracts of poppy (Papaver somniferum L.) by ATR-FT-IR and FT-Raman spectroscopy. Analyst 2004, 129, 917–920. [Google Scholar] [CrossRef] [PubMed]
- Jillian, L. Poppy Seeds: Healthy, Beneficial Food or Dangerous Opiate? 2018. Available online: https://draxe.com/nutrition/poppy-seeds/ (accessed on 31 March 2023).
- Singhal, R.S.; Kulkarni, P.R. Detection of adultration of the spice poppy seeds (Papaver somniferum) with Amaranthus paniculatus (Rajgeera) seeds. J. Food Qual. 1990, 13, 375–381. [Google Scholar] [CrossRef]
- Čermáková, E.; Zdeňková, K.; Demnerová, K.; Ovesna, J.; Svoboda, P.; Vašek, J. Authentication of opium poppy (Papaver somniferum L.) using DNA analysis. Arh. Za Hig. Rada I Toksikol. 2022, 73, 38. [Google Scholar]
- Cheng, L.; Lin, Z.; Xin, C.; Sun, H.; Li, X. Molecular identification and phylogenetic analysis of Papaver based on ITS2 barcoding. J. Forensic Sci. 2022, 67, 712–719. [Google Scholar] [CrossRef]
- Krikorian, A.D.; Ledbetter, M.C. Some observations on the cultivation of opium poppy (Papaver somniferum L.) for its latex. Bot. Rev. 1975, 41, 30–103. [Google Scholar] [CrossRef]
- Eisenreich, A.; Sachse, B.; Gürtler, R.; Dusemund, B.; Lindtner, O.; Schäfer, B. What do we know about health risks related to thebaine in food? Food Chem. 2020, 309, 125564. [Google Scholar] [CrossRef]
- Svoboda, P.; Vasek, J.; Vejl, P.; Ovesna, J. Genetic features of Czech blue poppy (Papaver somniferum L.) revealed by DNA polymorphism. Czech J. Food Sci. 2020, 38, 198–202. [Google Scholar] [CrossRef]
- European Medicines Agency. ICH. Q2 (R1),Validation of analytical procedures: Text and methodology. In Proceedings of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, Chicago, IL, USA, 2–4 March 2005. [Google Scholar]
- Avula, B.; Cohen, P.A.; Wang, Y.-H.; Sagi, S.; Feng, W.; Wang, M.; Zweigenbaum, J.; Shuangcheng, M.; Khan, I.A. Chemical profiling and quantification of monacolins and citrinin in red yeast rice commercial raw materials and dietary supplements using liquid chromatography-accurate QToF mass spectrometry: Chemometrics application. J. Pharm. Biomed. Anal. 2014, 100, 243–253. [Google Scholar] [CrossRef]
- Sproll, C.; Perz, R.C.; Buschmann, R.; Lachenmeier, D.W. Guidelines for reduction of morphine in poppy seed intended for food purposes. Eur. Food Res. Technol. 2007, 226, 307–310. [Google Scholar] [CrossRef]
- Vermeire, A.; Remon, J.P. Stability and compatibility of morphine. Int. J. Pharm. 1999, 187, 17–51. [Google Scholar] [CrossRef]
- Carlin, M.G.; Dean, J.R.; Ames, J.M. Opium alkaloids in harvested and thermally processed poppy seeds. Front. Chem. 2020, 8, 737. [Google Scholar] [CrossRef]
- Lo, D.S.T.; Chua, T.H. Poppy Seeds: Implications of consumption. Med. Sci. Law 1992, 32, 296–302. [Google Scholar] [CrossRef]
- Zhang, P.; Chan, W.; Ang, I.L.; Wei, R.; Lam, M.M.T.; Lei, K.M.K.; Poon, T.C.W. Revisiting fragmentation reactions of protonated α-amino acids by high-resolution electrospray ionization tandem mass spectrometry with collision-induced dissociation. Sci. Rep. 2019, 9, 6453. [Google Scholar] [CrossRef]
- Liu, R.; Ye, Y.; Qiang, L.; Liao, X.; Zhao, Y. The fragmentation pathway of the nucleosides under the electrospray ionization multi-stage mass spectrometry. Life Sci. J. 2008, 5, 37–40. Available online: http://www.lifesciencesite.com/lsj/life0502/07_life0502_37_40_fragmentation.pdf (accessed on 31 March 2023).
- Raith, K.; Neubert, R.; Poeaknapo, C.; Boettcher, C.; Zenk, M.H.; Schmidt, J. Electrospray tandem mass spectrometric investigations of morphinans. J. Am. Soc. Mass Spectrom. 2003, 14, 1262–1269. [Google Scholar] [CrossRef]
- Zhang, Z.; Yan, B.; Liu, K.; Bo, T.; Liao, Y.; Liu, H. Fragmentation pathways of heroin-related alkaloids revealed by ion trap and quadrupole time-of-flight tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2008, 22, 2851–2862. [Google Scholar] [CrossRef]
- Wickens, J.R.; Sleeman, R.; Keely, B.J. Atmospheric pressure ionisation mass spectrometric fragmentation pathways of noscapine and papaverine revealed by multistage mass spectrometry and in-source deuterium labelling. Rapid Commun. Mass Spectrom. 2006, 20, 473–480. [Google Scholar] [CrossRef]
- Schmidt, J.; Raith, K.; Boettcher, C.; Zenk, M.H. Analysis of benzylisoquinoline-type alkaloids by electrospray tandem mass spectrometry and atmospheric pressure photoionization. Eur. J. Mass Spectrom. 2005, 11, 325–333. [Google Scholar] [CrossRef]
- Shim, H.; Lee, J.Y.; Kim, B.; Hong, J. General fragmentations of alkaloids in electrospray ionization tandem mass spectrometry. Mass Spectrom. Lett. 2013, 4, 79–82. [Google Scholar] [CrossRef]
- Sai, C.; Wang, J.a.; Li, B.; Ding, L.; Wang, H.; Wang, Q.; Hua, H.; Zhang, F.; Ren, Q. Isolation and identification of alkaloids from Macleaya microcarpa by UHPLC–Q-TOF-MS and their cytotoxic activity in vitro, antiangiogenic activity in vivo. BMC Chem. 2020, 14, 5. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.; Boettcher, C.; Kuhnt, C.; Kutchan, T.M.; Zenk, M.H. Poppy alkaloid profiling by electrospray tandem mass spectrometry and electrospray FT-ICR mass spectrometry after [ring-13C6]-tyramine feeding. Phytochemistry 2007, 68, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Dolejš, L.; Hanuš, V. Mass spectrometry of rhoeadine type alkaloids. Tetrahedron 1967, 23, 2997–3005. [Google Scholar] [CrossRef]
- Menéndez-Perdomo, I.M.; Hagel, J.M.; Facchini, P.J. Benzylisoquinoline alkaloid analysis using high-resolution orbitrap LC-MSn. J. Mass Spectrom. 2021, 56, e4683. [Google Scholar] [CrossRef]
- Peng, Z.; Song, W.; Han, F.; Chen, H.; Zhu, M.; Chen, Y. Chromatographic tandam mass spectrometric detection of papaverine and its major metabolites in rat urine. Int. J. Mass Spectrom. 2007, 266, 114–121. [Google Scholar] [CrossRef]
- Amazonas, D.R.; Oliveira, C.; Barata, L.E.S.; Tepe, E.J.; Kato, M.J.; Mourão, R.H.V.; Yamaguchi, L.F. Chemical and genotypic variations in Aniba rosiodora from the Brazilian amazon forest. Molecules 2021, 26, 69. [Google Scholar] [CrossRef]
- Singhal, R.S.; Kulkarni, P.R.; Rege, D.V. Chapter 1—The development of the concept of food quality, safety, and authenticity. In Handbook of Indices of Food Quality and Authenticity; Woodhead Publishing: Cambridge, UK, 1997; pp. 9–34. [Google Scholar] [CrossRef]
- Kintzios, S.E. The Genus Salvia, 1st ed.; CRC Press: London, UK, 2000; p. 318. [Google Scholar]
- Khan, M.A. Chemical composition and medicinal properties of Nigella sativa Linn. Inflammopharmacology 1999, 7, 15–35. [Google Scholar] [CrossRef]
- Ali, B.H.; Blunden, G. Pharmacological and toxicological properties of Nigella sativa. Phytother. Res. 2003, 17, 299–305. [Google Scholar] [CrossRef]
- Avula, B.; Wang, Y.-H.; Ali, Z.; Khan, I.A. Quantitative determination of chemical constituents from seeds of Nigella sativa L. Using HPLC-UV and Identification by LC-ESI-TOF. J. AOAC Int. 2010, 93, 1778–1787. [Google Scholar] [CrossRef]
- Ramadan, M.F. Nutritional value, functional properties and nutraceutical applications of black cumin (Nigella sativa L.): An overview. Int. J. Food Sci. Technol. 2007, 42, 1208–1218. [Google Scholar] [CrossRef]
- Elbandy, M.; Kang, O.-H.; Kwon, D.-Y.; Rho, J.-R. Two new antiinflammatory triterpene saponins from the Egyptian edicinal food black cumin (Seeds of Nigella sativa). Bull. Korean Chem. Soc. 2009, 30, 1811–1816. [Google Scholar] [CrossRef]
- Paula, C.-P.; Pablo, M.-R.; Salvador, H.-N.; Iosody, S.-C.; Manuela, R.-S.; Jesús, M.-G. Vibrational analysis and thermal behavior of Salvia hispanica, Nigella sativa and Papaver somniferum seeds. Pharmacog. J. 2017, 9, 157–162. [Google Scholar] [CrossRef]
- Silva-Sánchez, C.; de la Rosa, A.P.B.; León-Galván, M.F.; de Lumen, B.O.; de León-Rodríguez, A.; de Mejía, E.G. Bioactive peptides in amaranth (Amaranthus hypochondriacus) seed. J. Agric. Food Chem. 2008, 56, 1233–1240. [Google Scholar] [CrossRef]
- Klimczak, I.; Małecka, M.; Pachołek, B. Antioxidant activity of ethanolic extracts of Amaranth seeds. Food Nahr. 2002, 46, 184–186. [Google Scholar] [CrossRef]
- Kauffman, C.S.; Hass, P.W. Grain Amaranth: A crop with low water requirements and high nutritional value. In Environmentally Sound Agriculture; Keretz, W.L., Ed.; Praeger Press: New York, NY, USA, 1983; p. 299. [Google Scholar]
- Saunders, R.M.; Becker, R. Amaranthus: A potential food and feed resource. Adv. Cereal Sci. Technol. 1984, 6, 357–396. [Google Scholar]
- Rastogi, A.; Shukla, S. Amaranth: A new millennium crop of nutraceutical values. Crit. Rev. Food Sci. Nutr. 2013, 53, 109–125. [Google Scholar] [CrossRef]
- Burgos, V.; Armada, M. Characterization and nutritional value of precooked products of kiwicha grains (Amaranthus caudatus). Food Sci. Technol. 2015, 35, 531–538. [Google Scholar] [CrossRef]
- Rangkadilok, N.; Pholphana, N.; Mahidol, C.; Wongyai, W.; Saengsooksree, K.; Nookabkaew, S.; Satayavivad, J. Variation of sesamin, sesamolin and tocopherols in sesame (Sesamum indicum L.) seeds and oil products in Thailand. Food Chem. 2010, 122, 724–730. [Google Scholar] [CrossRef]
- Gómez-Caravaca, A.M.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Caboni, M.F. Simultaneous determination of phenolic compounds and saponins in quinoa (Chenopodium quinoa Willd) by a liquid chromatography–diode array detection–electrospray ionization–time-of-flight mass spectrometry methodology. J. Agric. Food Chem. 2011, 59, 10815–10825. [Google Scholar] [CrossRef]
- Fabre, N.; Claparols, C.; Richelme, S.; Angelin, M.-L.; Fourasté, I.; Moulis, C. Direct characterization of isoquinoline alkaloids in a crude plant extract by ion-pair liquid chromatography–electrospray ionization tandem mass spectrometry: Example of Eschscholtzia californica. J. Chromatogr. A 2000, 904, 35–46. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).