Effects of Marinades Prepared from Food Industry By-Products on Quality and Biosafety Parameters of Lamb Meat
Abstract
1. Introduction
2. Materials and Methods
2.1. Ingredients Used for Experiments
2.2. Principal Scheme of the Experiments
2.3. Analysis of the Acidity Parameters of the Prepared Marinades
2.4. Analysis of the Microbiological Parameters of the Marinades and the Treated Lamb Meat
2.5. Analysis of the Antimicrobial Activity of the Prepared Marinades
2.6. Analysis of the Technological and Physicochemical Parameters of the LM
2.7. Analysis of the Biogenic Amine Contents in the Lamb Meat Samples
2.8. Analysis of the Fatty Acid Profiles of the Lamb Meat Samples
2.9. Analysis of the Overall Acceptability of the Treated Lamb Meat
2.10. Statistical Analysis
3. Results and Discussion
3.1. Microbial and Acidity Parameters of the Prepared Marinades
3.2. Antimicrobial Activity of the Developed Marinades
3.3. Influence of the Different Treatments on the Chemical Composition and Technological Parameters of the Lamb Meat Samples
3.3.1. Physicochemical Properties of the Lamb Meat
3.3.2. Technological Parameters of the Lamb Meat
3.3.3. Colour Coordinates, Tenderness, and Overall Acceptability of the Lamb Meat
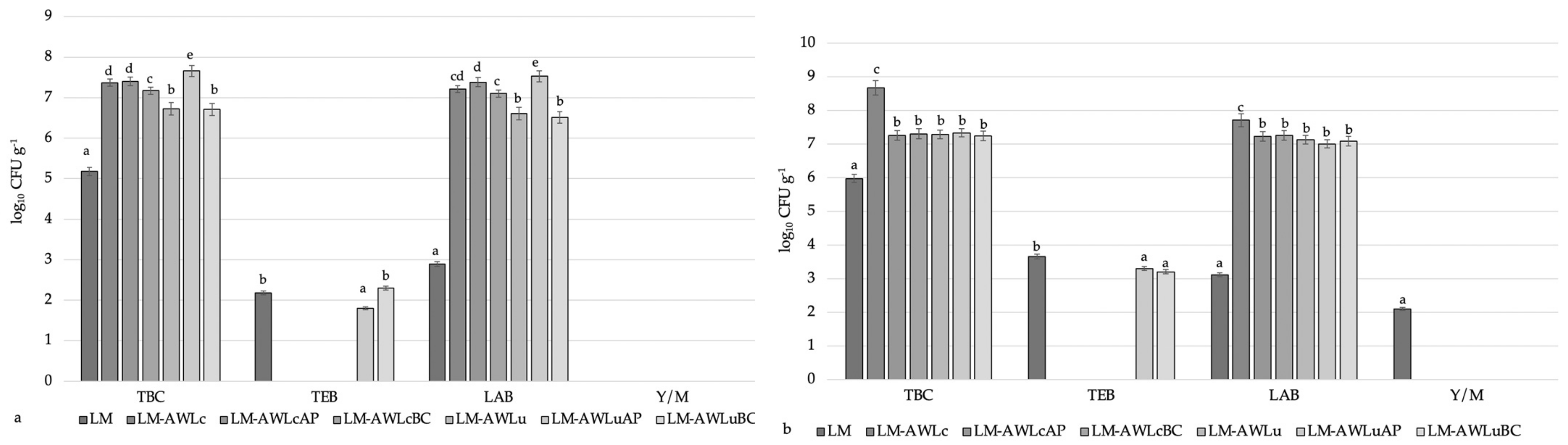
3.4. The Influence of Different Treatments on the Microbiological and Chemical Parameters of the Lamb Meat Samples
3.4.1. Biogenic Amine Content and Microbiological Parameters of the Lamb Meat
3.4.2. Fatty Acid (FA) Composition of the Lamb Meat
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mandolesi, S.; Naspetti, S.; Arsenos, G.; Caramelle-Holtz, E.; Latvala, T.; Martin-Collado, D.; Orsini, S.; Ozturk, E.; Zanoli, R. Motivations and Barriers for Sheep and Goat Meat Consumption in Europe: A Means–End Chain Study. Animals 2020, 10, 1105. [Google Scholar] [CrossRef]
- Lee, H.J.; Yong, H.I.; Kim, M.; Choi, Y.-S.; Jo, C. Status of Meat Alternatives and Their Potential Role in the Future Meat Market—A Review. Asian-Australas. J. Anim. Sci. 2020, 33, 1533–1543. [Google Scholar] [CrossRef]
- Lipinski, B. Why Does Animal-Based Food Loss and Waste Matter? Anim. Front. 2020, 10, 48–52. [Google Scholar] [CrossRef]
- Karwowska, M.; Łaba, S.; Szczepański, K. Food Loss and Waste in Meat Sector—Why the Consumption Stage Generates the Most Losses? Sustainability 2021, 13, 6227. [Google Scholar] [CrossRef]
- Hodges, R.J.; Buzby, J.C.; Bennett, B. Postharvest Losses and Waste in Developed and Less Developed Countries: Opportunities to Improve Resource Use. J. Agric. Sci. 2011, 149, 37–45. [Google Scholar] [CrossRef]
- Hicks, T.M.; Knowles, S.O.; Farouk, M.M. Global Provisioning of Red Meat for Flexitarian Diets. Front. Nutr. 2018, 5, 50. [Google Scholar] [CrossRef]
- Suleman, R.; Wang, Z.; Aadil, R.M.; Hui, T.; Hopkins, D.L.; Zhang, D. Effect of Cooking on the Nutritive Quality, Sensory Properties and Safety of Lamb Meat: Current Challenges and Future Prospects. Meat Sci. 2020, 167, 108172. [Google Scholar] [CrossRef]
- Battagin, H.V.; Panea, B.; Trindade, M.A. Study on the Lamb Meat Consumer Behavior in Brazil. Foods 2021, 10, 1713. [Google Scholar] [CrossRef]
- De Smet, S.; Vossen, E. Meat: The Balance between Nutrition and Health. A Review. Meat Sci. 2016, 120, 145–156. [Google Scholar] [CrossRef]
- Williams, P. Nutritional Composition of Red Meat. Nutr. Diet. 2007, 64, S113–S119. [Google Scholar] [CrossRef]
- Fowler, S.M.; Morris, S.; Hopkins, D.L. Nutritional Composition of Lamb Retail Cuts from the Carcases of Extensively Finished Lambs. Meat Sci. 2019, 154, 126–132. [Google Scholar] [CrossRef]
- Sulaiman, N.B.; Arief, I.I.; Budiman, C. Characteristic of Lamb Sausages Fermented by Indonesian Meat-Derived Probiotic, Lactobacillus plantarum IIA-2C12 and Lactobacillus acidophilus IIA-2B4. Media Peternak. 2016, 39, 104–111. [Google Scholar] [CrossRef]
- Klupsaite, D.; Buckiuniene, V.; Sidlauskiene, S.; Lele, V.; Sakiene, V.; Zavistanaviciute, P.; Klementaviciute, J.; Viskontaite, E.; Bartkiene, E. Comparison Studies of the Chemical, Physical, Technological, and Microbiological Characteristics of the European Roe Deer, Boar, Red Deer, and Beaver Hunted Wild Game Meat. Anim. Sci. J. 2020, 91, e13346. [Google Scholar] [CrossRef]
- Klupsaite, D.; Buckiuniene, V.; Bliznikas, S.; Sidlauskiene, S.; Dauksiene, A.; Klementaviciute, J.; Jurkevicius, A.; Zaborskiene, G.; Bartkiene, E. Impact of Romanov Breed Lamb Gender on Carcass Traits and Meat Quality Parameters Including Biogenic Amines and Malondialdehyde Changes during Storage. Food Sci. Nutr. 2022, 10, 1745–1755. [Google Scholar] [CrossRef]
- Alarcon-Rojo, A.D.; Carrillo-Lopez, L.M.; Reyes-Villagrana, R.; Huerta-Jiménez, M.; Garcia-Galicia, I.A. Ultrasound and Meat Quality: A Review. Ultrason. Sonochem. 2019, 55, 369–382. [Google Scholar] [CrossRef]
- Caraveo-Suarez, R.O.; Garcia-Galicia, I.A.; Santellano-Estrada, E.; Carrillo-Lopez, L.M.; Huerta-Jimenez, M.; Morales-Rodriguez, S.; Vargas-Bello-Pérez, E.; Alarcon-Rojo, A.D. Ultrasound as a Potential Technology to Improve the Quality of Meat Produced from a Mexican Autochthonous Bovine Breed. Sustainability 2022, 14, 3886. [Google Scholar] [CrossRef]
- Jeong, K.; Hyeonbin, O.; Shin, S.Y.; Kim, Y.-S. Effects of Different Marination Conditions on Quality, Microbiological Properties, and Sensory Characteristics of Pork Ham Cooked by the Sous-Vide Method. Korean J. Food Sci. Anim. Resour. 2018, 38, 506–514. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, M.; Wang, Y.; Bhandari, B. Novel Technologies for Flavor Formation in the Processing of Meat Products: A Review. Food Rev. Int. 2021, 10, 1–25. [Google Scholar] [CrossRef]
- Klupsaite, D.; Zavistanaviciute, P.; Sakiene, V.; Lele, V.; Mozuriene, E.; Klementaviciute, J.; Sidlauskiene, S.; Buckiuniene, V.; Tolpeznikaite, E.; Ruibys, R.; et al. Evaluation of the Use of Lactic Acid Bacteria and Thymus vulgaris Essential Oil on Suffolk and Ile de France Lamb Breed (MuscuIus gluteus) Quality Parameters. Int. J. Food Sci. Technol. 2020, 55, 3463–3474. [Google Scholar] [CrossRef]
- Vişan, V.-G.; Chiş, M.S.; Păucean, A.; Mureșan, V.; Pușcaș, A.; Stan, L.; Vodnar, D.C.; Dulf, F.V.; Țibulcă, D.; Vlaic, B.A.; et al. Influence of Marination with Aromatic Herbs and Cold Pressed Oils on Black Angus Beef Meat. Foods 2021, 10, 2012. [Google Scholar] [CrossRef]
- Shtonda, O.; Semeniuk, K. Aspects of the Influence of Vegetable-Oil-Based Marinade on Organoleptic and Physicochemical Indicators of the Quality of Semi-Finished Natural Marinated Meat Products. Potravin. Slovak J. Food Sci. 2021, 15, 513–520. [Google Scholar] [CrossRef]
- Yu, H.H.; Chin, Y.-W.; Paik, H.-D. Application of Natural Preservatives for Meat and Meat Products against Food-Borne Pathogens and Spoilage Bacteria: A Review. Foods 2021, 10, 2418. [Google Scholar] [CrossRef]
- Chaleshtori, F.S.; Arian, A.; Chaleshtori, R.S. Assessment of Sodium Benzoate and Potassium Sorbate Preservatives in Some Products in Kashan, Iran with Estimation of Human Health Risk. Food Chem. Toxicol. 2018, 120, 634–638. [Google Scholar] [CrossRef]
- Piper, J.D.; Piper, P.W. Benzoate and Sorbate Salts: A Systematic Review of the Potential Hazards of These Invaluable Preservatives and the Expanding Spectrum of Clinical Uses for Sodium Benzoate. Compr. Rev. Food Sci. Food Saf. 2017, 16, 868–880. [Google Scholar] [CrossRef]
- Crowe, W.; Elliott, C.T.; Green, B.D. A Review of the in Vivo Evidence Investigating the Role of Nitrite Exposure from Processed Meat Consumption in the Development of Colorectal Cancer. Nutrients 2019, 11, 2673. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Ingested Nitrate and Nitrite, and Cyanobacterial Peptide Toxins. IARC Monogr. Eval. Carcinog. Risks Hum. 2010, 94, 1–412. [Google Scholar]
- Zhong, Y.; Wu, L.; Chen, X.; Huang, Z.; Hu, W. Effects of Food-Additive-Information on Consumers’ Willingness to Accept Food with Additives. Int. J. Environ. Res. Public Health 2018, 15, 2394. [Google Scholar] [CrossRef]
- Cunha, L.C.M.; Monteiro, M.L.G.; Lorenzo, J.M.; Munekata, P.E.S.; Muchenje, V.; de Carvalho, F.A.L.; Conte-Junior, C.A. Natural Antioxidants in Processing and Storage Stability of Sheep and Goat Meat Products. Food Res. Int. 2018, 111, 379–390. [Google Scholar] [CrossRef]
- Alirezalu, K.; Pateiro, M.; Yaghoubi, M.; Alirezalu, A.; Peighambardoust, S.H.; Lorenzo, J.M. Phytochemical Constituents, Advanced Extraction Technologies and Techno-Functional Properties of Selected Mediterranean Plants for Use in Meat Products. A Comprehensive Review. Trends Food Sci. Technol. 2020, 100, 292–306. [Google Scholar] [CrossRef]
- Stéphane, F.F.Y.; Jules, B.K.J.; Batiha, G.E.-S.; Ali, I.; Bruno, L.N.; Stéphane, F.F.Y.; Jules, B.K.J.; Batiha, G.E.-S.; Ali, I.; Bruno, L.N. Extraction of Bioactive Compounds from Medicinal Plants and Herbs; IntechOpen: London, UK, 2021; ISBN 978-1-83969-276-5. [Google Scholar]
- Wang, Y.; Wu, J.; Lv, M.; Shao, Z.; Hungwe, M.; Wang, J.; Bai, X.; Xie, J.; Wang, Y.; Geng, W. Metabolism Characteristics of Lactic Acid Bacteria and the Expanding Applications in Food Industry. Front. Bioeng. Biotechnol. 2021, 9, 612285. [Google Scholar] [CrossRef]
- Bartkiene, E.; Lele, V.; Sakiene, V.; Zavistanaviciute, P.; Ruzauskas, M.; Bernatoniene, J.; Jakstas, V.; Viskelis, P.; Zadeike, D.; Juodeikiene, G. Improvement of the Antimicrobial Activity of Lactic Acid Bacteria in Combination with Berries/Fruits and Dairy Industry by-Products. J. Sci. Food Agric. 2019, 99, 3992–4002. [Google Scholar] [CrossRef]
- Bartkiene, E.; Lele, V.; Ruzauskas, M.; Domig, K.J.; Starkute, V.; Zavistanaviciute, P.; Bartkevics, V.; Pugajeva, I.; Klupsaite, D.; Juodeikiene, G.; et al. Lactic Acid Bacteria Isolation from Spontaneous Sourdough and Their Characterization Including Antimicrobial and Antifungal Properties Evaluation. Microorganisms 2020, 8, 64. [Google Scholar] [CrossRef]
- Mishra, B.; Mishra, A.K.; Kumar, S.; Mandal, S.K.; Nsv, L.; Kumar, V.; Baek, K.-H.; Mohanta, Y.K. Antifungal Metabolites as Food Bio-Preservative: Innovation, Outlook, and Challenges. Metabolites 2021, 12, 12. [Google Scholar] [CrossRef]
- Oliveira, M.; Ferreira, V.; Magalhães, R.; Teixeira, P. Biocontrol Strategies for Mediterranean-Style Fermented Sausages. Food Res. Int. 2018, 103, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Zavistanaviciute, P.; Zokaityte, E.; Starkute, V.; Ruzauskas, M.; Viskelis, P.; Bartkiene, E. Berry By-Products in Combination with Antimicrobial Lactic Acid Bacteria Strains for the Sustainable Formulation of Chewing Candies. Foods 2022, 11, 1177. [Google Scholar] [CrossRef]
- Zokaityte, E.; Lele, V.; Starkute, V.; Zavistanaviciute, P.; Cernauskas, D.; Klupsaite, D.; Ruzauskas, M.; Alisauskaite, J.; Baltrusaitytė, A.; Dapsas, M.; et al. Antimicrobial, Antioxidant, Sensory Properties, and Emotions Induced for the Consumers of Nutraceutical Beverages Developed from Technological Functionalised Food Industry By-Products. Foods 2020, 9, 1620. [Google Scholar] [CrossRef]
- Mozuriene, E.; Bartkiene, E.; Krungleviciute, V.; Zadeike, D.; Juodeikiene, G.; Damasius, J.; Baltusnikiene, A. Effect of Natural Marinade Based on Lactic Acid Bacteria on Pork Meat Quality Parameters and Biogenic Amine Contents. LWT Food Sci. Technol. 2016, 69, 319–326. [Google Scholar] [CrossRef]
- Barcenilla, C.; Ducic, M.; López, M.; Prieto, M.; Álvarez-Ordóñez, A. Application of Lactic Acid Bacteria for the Biopreservation of Meat Products: A Systematic Review. Meat Sci. 2022, 183, 108661. [Google Scholar] [CrossRef] [PubMed]
- Bartkiene, E.; Bartkevics, V.; Lele, V.; Pugajeva, I.; Zavistanaviciute, P.; Zadeike, D.; Juodeikiene, G. Application of Antifungal Lactobacilli in Combination with Coatings Based on Apple Processing By-Products as a Bio-Preservative in Wheat Bread Production. J. Food Sci. Technol. 2019, 56, 2989–3000. [Google Scholar] [CrossRef]
- Rocha-Mendoza, D.; Kosmerl, E.; Krentz, A.; Zhang, L.; Badiger, S.; Miyagusuku-Cruzado, G.; Mayta-Apaza, A.; Giusti, M.; Jiménez-Flores, R.; García-Cano, I. Invited Review: Acid Whey Trends and Health Benefits. J. Dairy Sci. 2021, 104, 1262–1275. [Google Scholar] [CrossRef]
- Augustyńska-Prejsnar, A.; Ormian, M.; Kluz, M.; Sokołowicz, Z. Effect of Using Acid Whey for Marinating Chicken Breast Muscles in Organic Production. Emir. J. Food Agric. 2019, 31, 281–287. [Google Scholar]
- Fritsch, C.; Staebler, A.; Happel, A.; Cubero Márquez, M.A.; Aguiló-Aguayo, I.; Abadias, M.; Gallur, M.; Cigognini, I.M.; Montanari, A.; López, M.J.; et al. Processing, Valorization and Application of Bio-Waste Derived Compounds from Potato, Tomato, Olive and Cereals: A Review. Sustainability 2017, 9, 1492. [Google Scholar] [CrossRef]
- Ferreira-Santos, P.; Zanuso, E.; Genisheva, Z.; Rocha, C.M.R.; Teixeira, J.A. Green and Sustainable Valorization of Bioactive Phenolic Compounds from Pinus By-Products. Molecules 2020, 25, 2931. [Google Scholar] [CrossRef]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive Compounds and Antioxidant Activity in Different Types of Berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef]
- Bartkiene, E.; Lele, V.; Starkute, V.; Zavistanaviciute, P.; Zokaityte, E.; Varinauskaite, I.; Pileckaite, G.; Paskeviciute, L.; Rutkauskaite, G.; Kanaporis, T.; et al. Plants and Lactic Acid Bacteria Combination for New Antimicrobial and Antioxidant Properties Product Development in a Sustainable Manner. Foods 2020, 9, 433. [Google Scholar] [CrossRef] [PubMed]
- Zavistanavičiūtė, P. Application of Food Industry By-Products for Microorganisms Encapsulation/Immobilization and Sustainable Antimicrobial Properties Feed Supplements Preparation: Doctoral Dissertation: Agricultural Sciences, Animal Sciences (A 003). Ph.D. Thesis, Lietuvos Sveikatos Mokslų Universiteto Leidybos Namai, Kaunas, Lithuania, 2021. [Google Scholar]
- Zokaityte, E.; Lele, V.; Starkute, V.; Zavistanaviciute, P.; Ruzauskas, M.; Mozuriene, E.; Cepiene, M.; Ceplinskas, V.; Kairaityte, G.; Lingyte, R.; et al. Antimicrobial Potential of Beverages Preparation Based on Fermented Milk Permeate and Berries/Vegetables. Beverages 2020, 6, 65. [Google Scholar] [CrossRef]
- Zokaityte, E.; Cernauskas, D.; Klupsaite, D.; Lele, V.; Starkute, V.; Zavistanaviciute, P.; Ruzauskas, M.; Gruzauskas, R.; Juodeikiene, G.; Rocha, J.M.; et al. Bioconversion of Milk Permeate with Selected Lactic Acid Bacteria Strains and Apple By-Products into Beverages with Antimicrobial Properties and Enriched with Galactooligosaccharides. Microorganisms 2020, 8, E1182. [Google Scholar] [CrossRef]
- Tarasevičienė, Ž.; Čechovičienė, I.; Paulauskienė, A.; Gumbytė, M.; Blinstrubienė, A.; Burbulis, N. The Effect of Berry Pomace on Quality Changes of Beef Patties during Refrigerated Storage. Foods 2022, 11, 2180. [Google Scholar] [CrossRef]
- Dey, D.; Richter, J.K.; Ek, P.; Gu, B.-J.; Ganjyal, G.M. Utilization of Food Processing By-Products in Extrusion Processing: A Review. Front. Sustain. Food Syst. 2021, 4, 603751. [Google Scholar] [CrossRef]
- Kim, Y.J.; Jin, S.K.; Park, W.Y.; Kim, B.W.; Joo, S.T.; Yang, H.S. The Effect of Garlic or Onion Marinade on the Lipid Oxidation and Meat Quality of Pork during Cold Storage. J. Food Qual. 2010, 33, 171–185. [Google Scholar] [CrossRef]
- Nour, V. Effect of Sour Cherry or Plum Juice Marinades on Quality Characteristics and Oxidative Stability of Pork Loin. Foods 2022, 11, 1088. [Google Scholar] [CrossRef]
- Rupasinghe, R.A.; Alahakoon, A.U.; Alakolanga, A.W.; Jayasena, D.D.; Jo, C. Oxidative Stability of Vacuum-Packed Chicken Wings Marinated with Fruit Juices during Frozen Storage. Food Sci. Anim. Resour. 2022, 42, 61. [Google Scholar] [CrossRef] [PubMed]
- Unal, K.; Alagöz, E.; Çelik, İ.; Sarıçoban, C. Marination with Citric Acid, Lemon, and Grapefruit Affects the Sensory, Textural, and Microstructure Characteristics of Poultry Meat. Br. Poult. Sci. 2022, 63, 31–38. [Google Scholar] [CrossRef]
- Augustyńska-Prejsnar, A.; Hanus, P.; Sokołowicz, Z.; Kačániová, M. Assessment of Technological Characteristics and Microbiological Quality of Marinated Turkey Meat with the Use of Dairy Products and Lemon Juice. Anim. Biosci. 2021, 34, 2003–2011. [Google Scholar] [CrossRef] [PubMed]
- Susanti, S.; Bintoro, V.P.; Hintono, A.; Nisa, K. Physical and Chemical Characteristics of Beef Marinated by Cashew Apple Extract. Int. J. Food Stud. 2022, 11, 98–105. [Google Scholar] [CrossRef]
- Sengun, I.Y.; Yildiz Turp, G.; Cicek, S.N.; Avci, T.; Ozturk, B.; Kilic, G. Assessment of the Effect of Marination with Organic Fruit Vinegars on Safety and Quality of Beef. Int. J. Food Microbiol. 2021, 336, 108904. [Google Scholar] [CrossRef]
- Demir, H.; Celik, S.; Sezer, Y.C. Effect of Ultrasonication and Vacuum Impregnation Pretreatments on the Quality of Beef Marinated in Onion Juice a Natural Meat Tenderizer. Food Sci. Technol. Int. 2022, 28, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Burke, R.M.; Monahan, F.J. The Tenderisation of Shin Beef Using a Citrus Juice Marinade. Meat Sci. 2003, 63, 161–168. [Google Scholar] [CrossRef]
- Bekhit, A.E.D.; Han, J.; Morton, J.; Sedcole, R. Effect of Kiwifruit Juice and Water Pre-Rigor Infusion on Lamb Quality. In Proceedings of the Proceedings 53rd International Congress of Meat Science and Technology, Beijing, China, 5–9 August 2007; pp. 377–378. [Google Scholar]
- Mahmud, A.B. Effect of Fruit Juice and Storage Period on Some Physio-Chemical, Microbial and Sensory Traits of Sheep Mutton Stored at Low Temperature. Mesop. J. Agric. 2019, 47, 70–89. [Google Scholar]
- Zavistanaviciute, P.; Lele, V.; Antanaitis, R.; Televičius, M.; Ruzauskas, M.; Zebeli, Q.; Bartkiene, E. Separate and Synergic Effects of Lactobacillus uvarum LUHSS245 and Arabinogalactan on the In Vitro Antimicrobial Properties as Well as on the Fecal and Metabolic Profile of Newborn Calves. Animals 2020, 10, 593. [Google Scholar] [CrossRef]
- Bartkiene, E.; Bartkevics, V.; Mozuriene, E.; Krungleviciute, V.; Novoslavskij, A.; Santini, A.; Rozentale, I.; Juodeikiene, G.; Cizeikiene, D. The Impact of Lactic Acid Bacteria with Antimicrobial Properties on Biodegradation of Polycyclic Aromatic Hydrocarbons and Biogenic Amines in Cold Smoked Pork Sausages. Food Control 2017, 71, 285–292. [Google Scholar] [CrossRef]
- ISO 15214:1998; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Mesophilic Lactic Acid Bacteria—Colony-Count Technique at 30 Degrees C. ISO: London, UK, 1998.
- ISO 4833-2:2013; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms—Part 2: Colony Count at 30 °C by the Surface Plating Technique. ISO: London, UK, 2013.
- ISO 21528-2:2017; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Enterobacteriaceae—Part 2: Colony-Count Technique. ISO: London, UK, 2017.
- ISO 21527-2:2008; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Yeasts and Moulds—Part 2: Colony Count Technique in Products with Water Activity Less than or Equal to 0,95. ISO: London, UK, 2008.
- ISO 1442:1997; Meat and Meat Products—Determination of Moisture Content (Reference Method). ISO: London, UK, 1997.
- Tkacz, K.; Modzelewska-Kapituła, M.; Więk, A.; Nogalski, Z. The Applicability of Total Color Difference ΔE for Determining the Blooming Time in Longissimus Lumborum and Semimembranosus Muscles from Holstein-Friesian Bulls at Different Ageing Times. Appl. Sci. 2020, 10, 8215. [Google Scholar] [CrossRef]
- Ben-Gigirey, B.; Vieites Baaptista de Sousa, J.M.; Villa, T.G.; Barros-Velazquez, J. Histamine and Cadaverine Production by Bacteria Isolated from Fresh and Frozen Albacore (Thunnus alalunga). J. Food Prot. 1999, 62, 933–939. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Guidelines for Single Laboratory Validation of Chemical Methods for Dietary Supplements and Botanicals. Obtenido de 2002, 74, 835–855. [Google Scholar] [CrossRef]
- ICH. Validation of Analytical Procedures: Text and Methodology. Q2 (R1) 2005, 1, 5. [Google Scholar]
- Pérez-Palacios, T.; Ruiz, J.; Ferreira, I.M.P.L.V.O.; Petisca, C.; Antequera, T. Effect of Solvent to Sample Ratio on Total Lipid Extracted and Fatty Acid Composition in Meat Products within Different Fat Content. Meat Sci. 2012, 91, 369–373. [Google Scholar] [CrossRef] [PubMed]
- ISO 6658:2017; Sensory Analysis—Methodology—General Guidance. ISO: London, UK, 2017.
- SO 8586:2012; Sensory Analysis—General Guidelines for the Selection, Training and Monitoring of Selected Assessors and Expert Sensory Assessors. ISO: London, UK, 2012.
- Du, H.; Yang, H.; Wang, X.; Zhu, F.; Tang, D.; Cheng, J.; Liu, X. Effects of Mulberry Pomace on Physicochemical and Textural Properties of Stirred-Type Flavored Yogurt. J. Dairy Sci. 2021, 104, 12403–12414. [Google Scholar] [CrossRef]
- Arai, A.; Igoshi, A.; Inoue, A.; Noda, K.; Tsutsuura, S.; Murata, M. Relationship between Lactose Utilization of Lactic Acid Bacteria and Browning of Cheese during Storage. Biosci. Biotechnol. Biochem. 2020, 84, 1886–1893. [Google Scholar] [CrossRef] [PubMed]
- Bylund, G. Dairy Processing Handbook; Tetra Pak Processing Systems AB: Lund, Switzerland, 2003; ISBN 91-631-3427-6. [Google Scholar]
- Ruiz Rodríguez, L.G.; Zamora Gasga, V.M.; Pescuma, M.; Van Nieuwenhove, C.; Mozzi, F.; Sánchez Burgos, J.A. Fruits and Fruit By-Products as Sources of Bioactive Compounds. Benefits and Trends of Lactic Acid Fermentation in the Development of Novel Fruit-Based Functional Beverages. Food Res. Int. 2021, 140, 109854. [Google Scholar] [CrossRef]
- Ziarno, M.; Cichońska, P. Lactic Acid Bacteria-Fermentable Cereal- and Pseudocereal-Based Beverages. Microorganisms 2021, 9, 2532. [Google Scholar] [CrossRef]
- George, F.; Daniel, C.; Thomas, M.; Singer, E.; Guilbaud, A.; Tessier, F.J.; Revol-Junelles, A.-M.; Borges, F.; Foligné, B. Occurrence and Dynamism of Lactic Acid Bacteria in Distinct Ecological Niches: A Multifaceted Functional Health Perspective. Front. Microbiol. 2018, 9, 2899. [Google Scholar] [CrossRef] [PubMed]
- Endo, A. Fructophilic Lactic Acid Bacteria Inhabit Fructose-Rich Niches in Nature. Microb. Ecol. Health Dis. 2012, 23, 18563. [Google Scholar] [CrossRef]
- Mokoena, M.P.; Omatola, C.A.; Olaniran, A.O. Applications of Lactic Acid Bacteria and Their Bacteriocins against Food Spoilage Microorganisms and Foodborne Pathogens. Molecules 2021, 26, 7055. [Google Scholar] [CrossRef]
- Bartkiene, E.; Ruzauskas, M.; Bartkevics, V.; Pugajeva, I.; Zavistanaviciute, P.; Starkute, V.; Zokaityte, E.; Lele, V.; Dauksiene, A.; Grashorn, M.; et al. Study of the Antibiotic Residues in Poultry Meat in Some of the EU Countries and Selection of the Best Compositions of Lactic Acid Bacteria and Essential Oils against Salmonella enterica. Poult. Sci. 2020, 99, 4065–4076. [Google Scholar] [CrossRef] [PubMed]
- Campagnollo, F.B.; Pedrosa, G.T.S.; Kamimura, B.A.; Furtado, M.M.; Baptista, R.C.; Nascimento, H.M.; Alvarenga, V.O.; Magnani, M.; Sant’Ana, A.S. Growth Potential of Three Strains of Listeria monocytogenes and Salmonella enterica in Frescal and Semi-Hard Artisanal Minas Microcheeses: Impact of the Addition of Lactic Acid Bacteria with Antimicrobial Activity. LWT 2022, 158, 113169. [Google Scholar] [CrossRef]
- Zhang, H.; HuangFu, H.; Wang, X.; Zhao, S.; Liu, Y.; Lv, H.; Qin, G.; Tan, Z. Antibacterial Activity of Lactic Acid Producing Leuconostoc mesenteroides QZ1178 against Pathogenic Gallibacterium anatis. Front. Vet. Sci. 2021, 8, 630294. [Google Scholar] [CrossRef]
- Atassi, F.; Pho Viet Ahn, D.L.; Lievin-Le Moal, V. Diverse Expression of Antimicrobial Activities against Bacterial Vaginosis and Urinary Tract Infection Pathogens by Cervicovaginal Microbiota Strains of Lactobacillus gasseri and Lactobacillus crispatus. Front. Microbiol. 2019, 10, 2900. [Google Scholar] [CrossRef]
- Pap, N.; Fidelis, M.; Azevedo, L.; do Carmo, M.A.V.; Wang, D.; Mocan, A.; Pereira, E.P.R.; Xavier-Santos, D.; Sant’Ana, A.S.; Yang, B.; et al. Berry Polyphenols and Human Health: Evidence of Antioxidant, Anti-Inflammatory, Microbiota Modulation, and Cell-Protecting Effects. Curr. Opin. Food Sci. 2021, 42, 167–186. [Google Scholar] [CrossRef]
- Staszowska-Karkut, M.; Materska, M. Phenolic Composition, Mineral Content, and Beneficial Bioactivities of Leaf Extracts from Black Currant (Ribes nigrum L.), Raspberry (Rubus idaeus), and Aronia (Aronia melanocarpa). Nutrients 2020, 12, 463. [Google Scholar] [CrossRef] [PubMed]
- Zare Mirzaei, E.; Lashani, E.; Davoodabadi, A. Antimicrobial Properties of Lactic Acid Bacteria Isolated from Traditional Yogurt and Milk against Shigella Strains. GMS Hyg. Infect. Control 2018, 13, Doc01. [Google Scholar] [CrossRef]
- Rodríguez-Sánchez, S.; Fernández-Pacheco, P.; Seseña, S.; Pintado, C.; Palop, M.L. Selection of Probiotic Lactobacillus Strains with Antimicrobial Activity to Be Used as Biocontrol Agents in Food Industry. LWT Food Sci. Technol. 2021, 143, 111142. [Google Scholar] [CrossRef]
- García-Lomillo, J.; González-SanJosé, M.L.; Del Pino-García, R.; Rivero-Pérez, M.D.; Muñiz-Rodríguez, P. Antioxidant and Antimicrobial Properties of Wine Byproducts and Their Potential Uses in the Food Industry. J. Agric. Food Chem. 2014, 62, 12595–12602. [Google Scholar] [CrossRef] [PubMed]
- Tolpeznikaite, E.; Ruzauskas, M.; Pilkaityte, R.; Bartkevics, V.; Zavistanaviciute, P.; Starkute, V.; Lele, V.; Zokaityte, E.; Mozuriene, E.; Ruibys, R.; et al. Influence of Fermentation on the Characteristics of Baltic Sea Macroalgae, Including Microbial Profile and Trace Element Content. Food Control 2021, 129, 108235. [Google Scholar] [CrossRef]
- Sharma, K.; Guleria, S.; Razdan, V.K.; Babu, V. Synergistic Antioxidant and Antimicrobial Activities of Essential Oils of Some Selected Medicinal Plants in Combination and with Synthetic Compounds. Ind. Crops Prod. 2020, 154, 112569. [Google Scholar] [CrossRef]
- Bartkiene, E.; Lele, V.; Sakiene, V.; Zavistanaviciute, P.; Ruzauskas, M.; Stankevicius, A.; Grigas, J.; Pautienius, A.; Bernatoniene, J.; Jakstas, V.; et al. Fermented, Ultrasonicated, and Dehydrated Bovine Colostrum: Changes in Antimicrobial Properties and Immunoglobulin Content. J. Dairy Sci. 2020, 103, 1315–1323. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Pop, A.; Georgescu, C.; Turcuş, V.; Olah, N.K.; Mathe, E. An Overview of Natural Antimicrobials Role in Food. Eur. J. Med. Chem. 2018, 143, 922–935. [Google Scholar] [CrossRef]
- Fernandes, J.C.; Tavaria, F.K.; Soares, J.C.; Ramos, Ó.S.; Monteiro, M.J.; Pintado, M.E.; Malcata, F.X. Antimicrobial Effects of Chitosans and Chitooligosaccharides, upon Staphylococcus aureus and Escherichia coli, in Food Model Systems. Food Microbiol. 2008, 25, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Hayek, S.A.; Gyawali, R.; Ibrahim, S.A. Antimicrobial Natural Products. Microbial Pathogens and Strategies for Combating Them. Science 2013, 2, 910–921. [Google Scholar]
- Sánchez-Gutiérrez, M.; Bascón-Villegas, I.; Rodríguez, A.; Pérez-Rodríguez, F.; Fernández-Prior, Á.; Rosal, A.; Carrasco, E. Valorisation of Olea europaea L. Olive Leaves through the Evaluation of Their Extracts: Antioxidant and Antimicrobial Activity. Foods 2021, 10, 966. [Google Scholar] [CrossRef]
- Tolpeznikaite, E.; Starkute, V.; Zokaityte, E.; Ruzauskas, M.; Pilkaityte, R.; Viskelis, P.; Urbonaviciene, D.; Ruibys, R.; Rocha, J.M.; Bartkiene, E. Effect of Solid-State Fermentation and Ultrasonication Processes on Antimicrobial and Antioxidant Properties of Algae Extracts. Front. Nutr. 2022, 9, 990274. [Google Scholar] [CrossRef]
- Roudbari, Z.; Coort, S.L.; Kutmon, M.; Eijssen, L.; Melius, J.; Sadkowski, T.; Evelo, C.T. Identification of Biological Pathways Contributing to Marbling in Skeletal Muscle to Improve Beef Cattle Breeding. Front. Genet. 2020, 10, 1370. [Google Scholar] [CrossRef] [PubMed]
- Shange, N.; Makasi, T.; Gouws, P.; Hoffman, L.C. Preservation of Previously Frozen Black Wildebeest Meat (Connochaetes gnou) Using Oregano (Oreganum vulgare) Essential Oil. Meat Sci. 2019, 148, 88–95. [Google Scholar] [CrossRef]
- Karabagias, I.; Badeka, A.; Kontominas, M.G. Shelf Life Extension of Lamb Meat Using Thyme or Oregano Essential Oils and Modified Atmosphere Packaging. Meat Sci. 2011, 88, 109–116. [Google Scholar] [CrossRef]
- Yusop, S.M.; O’Sullivan, M.G.; Kerry, J.P. 17-Marinating and Enhancement of the Nutritional Content of Processed Meat Products. In Processed Meats; Kerry, J.P., Kerry, J.F., Eds.; Woodhead Publishing Series in Food Science Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2011; pp. 421–449. ISBN 978-1-84569-466-1. [Google Scholar]
- Ruiz-Ramírez, J.; Arnau, J.; Serra, X.; Gou, P. Relationship between Water Content, NaCl Content, PH and Texture Parameters in Dry-Cured Muscles. Meat Sci. 2005, 70, 579–587. [Google Scholar] [CrossRef]
- Pang, B.; Yu, X.; Bowker, B.; Zhang, J.; Yang, Y.; Zhuang, H. Effect of Meat Temperature on Moisture Loss, Water Properties, and Protein Profiles of Broiler Pectoralis Major with the Woody Breast Condition. Poult. Sci. 2020, 100, 1283–1290. [Google Scholar] [CrossRef]
- Sokołowicz, Z.; Augustyńska-Prejsnar, A.; Krawczyk, J.; Kačániová, M.; Kluz, M.; Hanus, P.; Topczewska, J. Technological and Sensory Quality and Microbiological Safety of RIR Chicken Breast Meat Marinated with Fermented Milk Products. Animals 2021, 11, 3282. [Google Scholar] [CrossRef] [PubMed]
- Tänavots, A.; Põldvere, A.; Kerner, K.; Veri, K.; Kaart, T.; Torp, J. Effects of Mustard-Honey, Apple Vinegar, White Wine Vinegar and Kefir Acidic Marinades on the Properties of Pork. Vet. Zootech. 2018, 76, 9. [Google Scholar]
- Goli, T.; Bohuon, P.; Ricci, J.; Trystram, G.; Collignan, A. Mass Transfer Dynamics during the Acidic Marination of Turkey Meat. J. Food Eng. 2011, 104, 161–168. [Google Scholar] [CrossRef]
- Botinestean, C.; Hossain, M.; Mullen, A.M.; Auty, M.A.E.; Kerry, J.P.; Hamill, R.M. Optimization of Textural and Technological Parameters Using Response Surface Methodology for the Development of Beef Products for Older Consumers. J. Texture Stud. 2020, 51, 263–275. [Google Scholar] [CrossRef]
- Della Malva, A.; Albenzio, M.; Annicchiarico, G.; Caroprese, M.; Muscio, A.; Santillo, A.; Marino, R. Relationship between Slaughtering Age, Nutritional and Organoleptic Properties of Altamurana Lamb Meat. Small Rumin. Res. 2016, 135, 39–45. [Google Scholar] [CrossRef]
- Liu, D.; Ma, J.; Sun, D.-W.; Pu, H.; Gao, W.; Qu, J.; Zeng, X.-A. Prediction of Color and PH of Salted Porcine Meats Using Visible and Near-Infrared Hyperspectral Imaging. Food Bioprocess Technol. 2014, 7, 3100–3108. [Google Scholar] [CrossRef]
- Lee, H.-J.; Lee, J.-J.; Jung, M.-O.; Choi, J.-S.; Jung, J.-T.; Choi, Y.-I.; Lee, J.-K. Meat Quality and Storage Characteristics of Pork Loin Marinated in Grape Pomace. Korean J. Food Sci. Anim. Resour. 2017, 37, 726–734. [Google Scholar] [CrossRef]
- International Commission on Illumination (CIE). Recommendations on Uniform Colour Spaces, Colour Difference Equations, Psychometric Colour Terms; CIE Publ. No. 15; International Commission on Illumination (CIE): Peter Blattner; Switzerland, 1978; p. 3, Issue 2. [Google Scholar]
- Nieto, G.; Díaz, P.; Bañón, S.; Garrido, M.D. Effect on Lamb Meat Quality of Including Thyme (Thymus zygis ssp. gracilis) Leaves in Ewes’ Diet. Meat Sci. 2010, 85, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Corlett, M.T.; Pethick, D.W.; Kelman, K.R.; Jacob, R.H.; Gardner, G.E. Consumer Perceptions of Meat Redness Were Strongly Influenced by Storage and Display Times. Foods 2021, 10, 540. [Google Scholar] [CrossRef] [PubMed]
- Mancini, R.A.; Hunt, M. Current Research in Meat Color. Meat Sci. 2005, 71, 100–121. [Google Scholar] [CrossRef]
- Knight, M.I.; Linden, N.; Ponnampalam, E.N.; Kerr, M.G.; Brown, W.G.; Hopkins, D.L.; Baud, S.; Ball, A.J.; Borggaard, C.; Wesley, I. Development of VISNIR Predictive Regression Models for Ultimate PH, Meat Tenderness (Shear Force) and Intramuscular Fat Content of Australian Lamb. Meat Sci. 2019, 155, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Hah, K.H. Microbial and Physico-Chemical Properties of Seasoned Pork with Korean Traditional Sauces during Aging. Ph.D. Thesis, Gyeongsang National University, Jinju, Republic of Korea, 2005. [Google Scholar]
- Seleshe, S.; Kang, S.N. Effect of Different Pediococcus Pentosaceus and Lactobacillus plantarum Strains on Quality Characteristics of Dry Fermented Sausage after Completion of Ripening Period. Food Sci. Anim. Resour. 2021, 41, 636–649. [Google Scholar] [CrossRef] [PubMed]
- Onopiuk, A.; Kołodziejczak, K.; Marcinkowska-Lesiak, M.; Wojtasik-Kalinowska, I.; Szpicer, A.; Stelmasiak, A.; Poltorak, A. Influence of Plant Extract Addition to Marinades on Polycyclic Aromatic Hydrocarbon Formation in Grilled Pork Meat. Molecules 2021, 27, 175. [Google Scholar] [CrossRef] [PubMed]
- Stimbirys, A.; Bartkiene, E.; Siugzdaite, J.; Augeniene, D.; Vidmantiene, D.; Juodeikiene, G.; Maruska, A.; Stankevicius, M.; Cizeikiene, D. Safety and Quality Parameters of Ready-to-Cook Minced Pork Meat Products Supplemented with Helianthus tuberosus L. Tubers Fermented by BLIS Producing Lactic Acid Bacteria. J. Food Sci. Technol. 2015, 52, 4306–4314. [Google Scholar] [CrossRef] [PubMed]
- Bartkiene, E.; Mozuriene, E.; Juodeikiene, G.; Zadeike, D.; Maruska, A.; Stankevicius, M.; Ragazinskiene, O.; Cizeikiene, D. Pork Meat Products Functional Value and Safety Parameters Improving by Using Lactic Acid Fermentation of Savory Plants. J. Food Sci. Technol. 2015, 52, 7143–7152. [Google Scholar] [CrossRef]
- Ibrahim, S.A.; Ayivi, R.D.; Zimmerman, T.; Siddiqui, S.A.; Altemimi, A.B.; Fidan, H.; Esatbeyoglu, T.; Bakhshayesh, R.V. Lactic Acid Bacteria as Antimicrobial Agents: Food Safety and Microbial Food Spoilage Prevention. Foods 2021, 10, 3131. [Google Scholar] [CrossRef]
- Vázquez-Velázquez, R.; Salvador-Figueroa, M.; Adriano-Anaya, L.; DeGyves–Córdova, G.; Vázquez-Ovando, A. Use of Starter Culture of Native Lactic Acid Bacteria for Producing an Artisanal Mexican Cheese Safe and Sensory Acceptable. CyTA J. Food 2018, 16, 460–468. [Google Scholar] [CrossRef]
- Coelho, M.C.; Malcata, F.X.; Silva, C.C.G. Lactic Acid Bacteria in Raw-Milk Cheeses: From Starter Cultures to Probiotic Functions. Foods 2022, 11, 2276. [Google Scholar] [CrossRef]
- Bartkiene, E.; Zokaityte, E.; Zavistanaviciute, P.; Mockus, E.; Cernauskas, D.; Ruzauskas, M.; Tolpeznikaite, E.; Guiné, R.P.F. Nutraceutical Chewing Candy Formulations Based on Acetic, Alcoholic, and Lactofermented Apple Juice Products. Foods 2021, 10, 2329. [Google Scholar] [CrossRef] [PubMed]
- Bobinaitė, R.; Grootaert, C.; Van Camp, J.; Šarkinas, A.; Liaudanskas, M.; Žvikas, V.; Viškelis, P.; Rimantas Venskutonis, P. Chemical Composition, Antioxidant, Antimicrobial and Antiproliferative Activities of the Extracts Isolated from the Pomace of Rowanberry (Sorbus aucuparia L.). Food Res. Int. 2020, 136, 109310. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.C.; Ribeiro-Vidal, H.; Bartolomé, B.; Figuero, E.; Moreno-Arribas, M.V.; Sanz, M.; Herrera, D. New Evidences of Antibacterial Effects of Cranberry Against Periodontal Pathogens. Foods 2020, 9, 246. [Google Scholar] [CrossRef] [PubMed]
- Viskelis, P.; Rubinskienė, M.; Jasutienė, I.; Šarkinas, A.; Daubaras, R.; Česonienė, L. Anthocyanins, Antioxidative, and Antimicrobial Properties of American Cranberry (Vaccinium macrocarpon Ait.) and Their Press Cakes. J. Food Sci. 2009, 74, C157–C161. [Google Scholar] [CrossRef] [PubMed]
- Schirone, M.; Esposito, L.; D’Onofrio, F.; Visciano, P.; Martuscelli, M.; Mastrocola, D.; Paparella, A. Biogenic Amines in Meat and Meat Products: A Review of the Science and Future Perspectives. Foods 2022, 11, 788. [Google Scholar] [CrossRef] [PubMed]
- Triki, M.; Herrero, A.M.; Jiménez-Colmenero, F.; Ruiz-Capillas, C. Quality Assessment of Fresh Meat from Several Species Based on Free Amino Acid and Biogenic Amine Contents during Chilled Storage. Foods 2018, 7, E132. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Munekata, P.E.S.; Domínguez, R. Role of Autochthonous Starter Cultures in the Reduction of Biogenic Amines in Traditional Meat Products. Curr. Opin. Food Sci. 2017, 14, 61–65. [Google Scholar] [CrossRef]
- Stadnik, J.; Dolatowski, Z.J. Biogenic amines in meat and fermented meat products. ACTA Sci. Pol. Technol. Aliment. 2010, 9, 251–263. [Google Scholar]
- EFSA. Scientific Opinion on Risk Based Control of Biogenic Amine Formation in Fermented Foods-European Food Safety Authority Panel on Biological Hazards (BIOHAZ). EFSA J. 2011, 9, 2393–2486. [Google Scholar] [CrossRef]
- Zagorec, M.; Champomier-Vergès, M.-C. Meat Microbiology and Spoilage. In Lawrie’s Meat Science; Elsevier: Amsterdam, The Netherlands, 2023; pp. 195–218. [Google Scholar]
- Abdelhamid, A.S.; Martin, N.; Bridges, C.; Brainard, J.S.; Wang, X.; Brown, T.J.; Hanson, S.; Jimoh, O.F.; Ajabnoor, S.M.; Deane, K.H.; et al. Polyunsaturated Fatty Acids for the Primary and Secondary Prevention of Cardiovascular Disease. Cochrane Database Syst. Rev. 2018, 2018, CD012345. [Google Scholar] [CrossRef]
- Belhaj, K.; Mansouri, F.; Benmoumen, A.; Sindic, M.; Fauconnier, M.-L.; Boukharta, M.; Serghini, C.H.; Elamrani, A. Fatty Acids, Health Lipid Indices, and Cholesterol Content of Sheep Meat of Three Breeds from Moroccan Pastures. Arch. Anim. Breed 2020, 63, 471–482. [Google Scholar] [CrossRef]
- Borghi, T.H.; da Silva Sobrinho, A.G.; Zeola, N.M.B.L.; de Almeida, F.A.; Cirne, L.G.A.; Lima, A.R.C. Dietary Glycerin Does Not Affect Meat Quality of Ile de France Lambs. R. Bras. Zootec. 2016, 45, 554–562. [Google Scholar] [CrossRef]
- Romero-Bernal, J.; Almaraz, E.M.; Ortega, O.A.C.; Salas, N.P.; González-Ronquillo, M. Chemical Composition and Fatty Acid Profile in Meat from Grazing Lamb Diets Supplemented with Ryegrass Hay, Fishmeal and Soya Bean Meal as PUFA Sources. Cienc. Rural 2016, 47, e20160533. [Google Scholar] [CrossRef]
- Faria, P.B.; Bressan, M.C.; Vieira, J.O.; Vicente-Neto, J.; Ferrão, S.P.B.; Rosa, F.C.; Monteiro, M.; Cardoso, M.G.; Gama, L.T. Meat Quality and Lipid Profiles in Crossbred Lambs Finished on Clover-Rich Pastures. Meat Sci. 2012, 90, 733–738. [Google Scholar] [CrossRef]
- Polidori, P.; Pucciarelli, S.; Cammertoni, N.; Polzonetti, V.; Vincenzetti, S. The Effects of Slaughter Age on Carcass and Meat Quality of Fabrianese Lambs. Small Rumin. Res. 2017, 155, 12–15. [Google Scholar] [CrossRef]
- Sinanoglou, V.J.; Batrinou, A.; Mantis, F.; Bizelis, I.; Miniadis-Meimaroglou, S. Lipid Quality Indices: Differentiation of Suckling Lamb and Kid Breeds Reared by Traditional Sheep Farming. Small Rumin. Res. 2013, 113, 1–10. [Google Scholar] [CrossRef]
- Boschetti, E.; Bordoni, A.; Meluzzi, A.; Castellini, C.; Dal Bosco, A.; Sirri, F. Fatty Acid Composition of Chicken Breast Meat Is Dependent on Genotype-Related Variation of FADS1 and FADS2 Gene Expression and Desaturating Activity. Animal 2016, 10, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.Y.; Gou, Z.Y.; Abouelezz, K.F.M.; Li, L.; Lin, X.J.; Fan, Q.L.; Wang, Y.B.; Cheng, Z.G.; Ding, F.Y.; Jiang, S.Q. Alterations of the Fatty Acid Composition and Lipid Metabolome of Breast Muscle in Chickens Exposed to Dietary Mixed Edible Oils. Animal 2020, 14, 1322–1332. [Google Scholar] [CrossRef] [PubMed]
- Haak, L.; De Smet, S.; Fremaut, D.; Van Walleghem, K.; Raes, K. Fatty Acid Profile and Oxidative Stability of Pork as Influenced by Duration and Time of Dietary Linseed or Fish Oil Supplementation. J. Anim. Sci. 2008, 86, 1418–1425. [Google Scholar] [CrossRef] [PubMed]
- Soni-Guillermo, E.; Figueroa-Velasco, J.L.; Sánchez-Torres-Esqueda, M.T.; Cordero-Mora, J.L.; Hernández-Cázares, A.S.; Martínez-Aispuro, J.A.; Copado-Bueno, J.M.F.; Crosby-Galván, M.M. Efectividad del aceite de canola en dietas de cerdos para mejorar el perfil lipídico de la carne. Rev. Mex. Cienc. Pecu. 2021, 12, 1083–1097. [Google Scholar] [CrossRef]
- Dinçer, E.; Kıvanç, M. Lipolytic Activity of Lactic Acid Bacteria Isolated from Turkish pastirma. Anadolu Univ. J. Sci. Technol. C-Life Sci. Biotechnol. 2018, 7, 12–19. [Google Scholar] [CrossRef]
| Sample | LAB (%) | AW (mL) | Freeze-Dried AP Pomace (g) | Freeze-Dried BC Pomace (g) | |
|---|---|---|---|---|---|
| L. casei | L. uvarum | ||||
| M-AWLc | 3 | - | 120 | - | - |
| M-AWLcAP | - | 3.00 | |||
| M-AWLcBC | - | 3.00 | |||
| M-AWLu | - | 3 | - | - | |
| M-AWLuAP | - | 3.00 | - | ||
| M-AWLuBC | - | 3.00 | |||
| Samples | Lamb Meat (g) | AWLc (mL) | AWLu (mL) | Freeze-Dried AP Pomace (g) | Freeze-Dried BC Pomace (g) |
|---|---|---|---|---|---|
| LM-C | 400 | - | - | - | - |
| LM-AWLc | 120 | - | - | - | |
| LM-AWLcAP | - | 3.00 | - | ||
| LM-AWLcBC | - | - | 3.00 | ||
| LM-AWLu | - | 120 | - | - | |
| LM-AWLuAP | - | 3.00 | - | ||
| LM-AWLuBC | - | - | 3.00 |
| Parameter | Marinade | |||||
|---|---|---|---|---|---|---|
| M-AWLc | M-AWLcAP | M-AWLcBC | M-AWLu | M-AWLuAP | M-AWLuBC | |
| Log10 CFU mL−1 | ||||||
| After 24 h of fermentation | ||||||
| TBC | 7.34 ± 0.06 c | 6.79 ± 0.05 ab | 6.64 ± 0.14 ab | 6.80 ± 0.08 b | 6.67 ± 0.11 ab | 6.47 ± 0.12 a |
| LAB | 6.92 ± 0.04 e | 6.78 ± 0.06 d | 6.51 ± 0.05 c | 6.41 ± 0.03 b | 6.23 ± 0.04 a | 6.15 ± 0.05 a |
| TEB | nd | nd | nd | nd | nd | nd |
| M/Y | nd | nd | nd | nd | nd | nd |
| After 48 h of fermentation | ||||||
| TBC | 8.94 ± 0.09 d | 8.57 ± 0.07 b | 8.55 ± 0.04 b | 8.74 ± 0.06 c | 8.26 ± 0.10 a | 8.16 ± 0.06 a |
| LAB | 8.82 ± 0.13 d | 8.42 ± 0.15 c | 8.47 ± 0.06 c | 8.68 ± 0.05 d | 8.15 ± 0.07 b | 7.95 ± 0.09 a |
| TEB | nd | nd | nd | nd | nd | nd |
| M/Y | nd | nd | nd | nd | nd | nd |
| Acidity Parameters | ||||||
| After 24 h of fermentation | ||||||
| pH | 4.13 ± 0.06 a | 4.23 ± 0.03 b | 4.25 ± 0.02 b | 4.32 ± 0.02 c | 4.41 ± 0.03 d | 4.36 ± 0.04 cd |
| TA (N°) | 7.80 ± 0.10 d | 7.40 ± 0.10b c | 7.50 ± 0.10 c | 7.20 ± 0.10 b | 6.80 ± 0.20 a | 6.90 ± 0.10 a |
| After 48 h of fermentation | ||||||
| pH | 3.61 ± 0.03 b | 3.41 ± 0.02 a | 3.45 ± 0.03 a | 3.74 ± 0.01 c | 3.42 ± 0.04 a | 3.42 ± 0.02 a |
| TA (N°) | 8.60 ± 0.10 a | 9.70 ± 0.10 bc | 9.80 ± 0.20 bc | 9.50 ± 0.10 b | 10.5 ± 0.20 d | 10.6 ± 0.2 d |
| Inhibition Zone (mm) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Pathogenic Bacteria Strain | ||||||||
| Salmonellaenterica | Pseudomonas aeruginosa | Proteusmirabilis | Enterococcus faecalis | Enterococcus faecium | Bacilluscereus | Streptococcus mutans | Citrobacter freundii | |
| M-AWLc | 9.20 ± 0.3 a | nd | 10.3 ± 0.1 a | 10.1 ± 0.3 a | 9.40 ± 0.2 a | 14.3 ± 0.2 b | 15.2 ± 0.4 c | nd |
| M-AWLcAP | 10.3 ± 0.3 b | 10.1 ± 0.4 a | 12.1 ± 0.3 c | 9.90 ± 0.1 a | 9.80 ± 0.4 a | 15.2 ± 0.3 c | 14.3±0.2 b | 10.1 ± 0.2 a |
| M-AWLcBC | 8.90 ± 0.4 a | nd | 13.2 ± 0.5 d | nd | nd | 14.3 ± 0.1 b | 14.3 ± 0.3 b | 11.4 ± 0.3 b |
| M-AWLu | nd | nd | 11.4 ± 0.2 b | 12.4 ± 0.2 c | nd | 13.4 ± 0.1 a | 13.2 ± 0.4 a | nd |
| M-AWLuAP | 9.40 ± 0.3 a | 10.6 ± 0.2 a | 12.2 ± 0.1 c | 12.5 ± 0.4 c | 10.5 ± 0.3 b | 14.3 ± 0.3 b | 16.7 ± 0.4 d | 14.3 ± 0.2 c |
| M-AWLuBC | 10.30 ± 0.3 b | 10.6 ± 0.3 a | 13.5 ± 0.3 d | 11.8 ± 0.2 b | 11.7 ± 0.4 c | 13.7 ± 0.2 a | 15.5 ± 0.1 c | 14.2 ± 0.3 c |
| Sample | Physicochemical Properties | |||||||
|---|---|---|---|---|---|---|---|---|
| pH | Fat Content (%) | Ash Content (%) | Protein Content (%) | |||||
| Duration of Treatment (h) | ||||||||
| 24 | 48 | 24 | 48 | 24 | 48 | 24 | 48 | |
| C-LM | 5.40 ± 0.05 b | 5.51 ± 0.07 a | 10.5 ± 0.05 c | 9.62 ± 0.12 c | 1.32 ± 0.09 c | 1.24 ± 0.11 d | 17.1 ± 0.85 a | 16.3 ± 0.32 a |
| LM-AWLc | 5.89 ± 0.09 c | 5.77 ± 0.09 ab | 11.1 ± 0.25 d | 6.79 ± 0.14 a | 0.97 ± 0.05 b | 1.13 ± 0.06 c | 17.7 ± 0.88 a | 18.9 ± 0.94 c |
| LM-AWLcAP | 5.72 ± 0.08 c | 6.09 ± 0.10 c | 10.6 ± 0.25 c | 6.90 ± 0.25 a | 0.91 ± 0.05 ab | 1.10 ± 0.07 c | 20.6 ± 1.03 b | 21.0 ± 1.05 c |
| LM-AWLcBC | 5.87 ± 0.09 d | 6.01 ± 0.09 bc | 7.65 ± 0.28a | 6.99 ± 0.15 a | 0.98 ± 0.05 b | 1.07 ± 0.05 c | 19.4 ± 0.97b | 20.3 ± 1.02 c |
| LM-AWLu | 6.10 ± 0.06 e | 5.83 ± 0.09 b | 9.37 ± 0.17 b | 7.28 ± 0.06 b | 0.87 ± 0.04 a | 1.03 ± 0.05 c | 21.4 ± 1.07 b | 17.7 ± 0.89 b |
| LM-AWLuAP | 5.24 ± 0.06 a | 5.57 ± 0.08a | 11.1 ± 0.24 d | 10.5 ± 0.18 d | 0.83 ± 0.02 a | 0.70 ± 0.02 a | 20.8 ± 1.04 b | 17.5 ± 0.87 b |
| LM-AWLuBC | 5.62 ± 0.08 c | 5.69 ± 0.07 a | 13.4 ± 0.18 e | 12.7 ± 0.23 e | 0.81 ± 0.04 a | 0.81 ± 0.04 b | 16.7 ± 0.84 a | 17.9 ± 0.89 b |
| Technological Parameters (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| DM | MC | WHC | CL | |||||
| Duration of Treatment (h) | ||||||||
| 24 | 48 | 24 | 48 | 24 | 48 | 24 | 48 | |
| C-LM | 33.4 ± 1.13 b | 32.9 ± 0.95 b | 69.7 ± 1.15 a | 65.8 ± 1.22 a | 2.97 ± 0.15 a | 2.42 ± 0.18 a | 13.6 ± 0.56 a | 21.9 ± 0.56 a |
| LM-AWLc | 29.8 ± 1.49 b | 26.8 ± 1.34 a | 72.9 ± 1.10 a | 73.2 ± 0.68 b | 5.03 ± 0.0 8c | 4.81 ± 0.23 f | 25.3 ± 1.27 d | 23.0 ± 1.51 a |
| LM-AWLcAP | 26.6 ± 1.33 a | 27.0 ± 1.35 a | 74.2 ± 1.05 a | 72.5 ± 1.35 b | 3.15 ± 0.06 a | 2.91 ± 0.15 b | 19.7 ± 0.99 c | 33.8 ± 1.09 c |
| LM-AWLcBC | 26.0 ± 1.30 a | 26.3 ± 1.32 a | 72.3 ± 0.98 a | 71.8 ± 0.68 b | 5.50 ± 0.0 d | 4.74 ± 0.06 f | 23.1 ± 1.16 d | 29.3 ± 1.17 b |
| LM-AWLu | 31.6 ± 1.58 b | 26.1 ± 1.30 a | 71.4 ± 0.53 a | 71.5 ± 1.33 b | 5.81 ± 0.11 e | 4.43 ± 0.17 e | 25.5 ± 1.28 d | 29.6 ± 1.48 b |
| LM-AWLuAP | 42.7 ± 2.14 c | 35.3 ± 1.76 c | 71.6 ± 0.71 a | 72.0 ± 1.50 b | 4.81 ± 0.15 c | 3.59 ± 0.13 d | 22.0 ± 1.09 d | 32.7 ± 1.24 bc |
| LM-AWLuBC | 31.1 ± 1.56 b | 38.5 ± 1.93 c | 72.2 ± 0.61 a | 71.9 ± 0.95 b | 3.92 ± 0.21 b | 3.36 ± 0.07 c | 17.6 ± 0.88 b | 29.6 ± 1.48 b |
| Colour Coordinates | Shear Force (kg·cm−2) | Overall Acceptability Score | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | ΔE | |||||||||
| Duration of Treatment (h) | ||||||||||||
| 24 | 48 | 24 | 48 | 24 | 48 | 24 | 48 | 24 | 48 | 24 | 48 | |
| CLM | 51.9 ±1.29 d | 48.9 ±0.96 e | 19.0 ± 0.65 f | 15.7 ± 0.35 d | 12.1 ± 0.60 b | 10.5 ± 0.14 d | 0 | 0 | 1.75 ± 0.09 a | 1.83 ± 0.12 c | 3.42 ± 0.33 a | 2.13 ± 0.20 a |
| LM-AWLc | 40.5 ± 0.53 a | 45.3 ± 0.27 c | 6.60 ± 0.33 c | 9.93 ± 0.49 a | 10.1 ± 0.51 a | 7.27 ± 0.36 b | 16.95 | 7.53 | 1.70 ± 0.10 a | 1.64 ± 0.05 b | 5.68 ± 0.24 b | 6.02 ± 0.43 b |
| LM-AWLcAP | 43.3 ± 0.47 b | 49.3 ± 0.76 e | 7.60 ± 0.18 d | 10.2 ± 0.51 a | 13.0 ± 0.65 b | 9.37 ± 0.47 c | 14,30 | 5.63 | 1.61 ± 0.09 a | 1.54 ± 0.03 a | 6.07 ± 0.46 b | 9.04 ± 0.43 d |
| LM-AWLcBC | 39.6 ± 0.58 a | 41.9 ± 0.49 b | 2.94 ± 0.15 a | 11.6 ± 0.18 b | 10.3 ± 0.52 a | 4.86 ± 0.24 a | 20.31 | 9.88 | 1.59 ± 0.05 a | 1.52 ± 0.04 a | 7.23 ± 0.37 c | 8.12 ± 0.15 c |
| LM-AWLu | 44.6 ± 0.23 c | 46.8 ± 0.34 d | 6.48 ± 0.32 c | 10.8 ± 0.34 a | 11.6 ± 0.43 ab | 7.63 ± 0.38 b | 14.50 | 6.25 | 1.69 ± 0.07 a | 1.55 ± 0.03 a | 5.99 ± 0.61 b | 6.97 ± 0.31 b |
| LM-AWLuAP | 50.6 ± 0.53 d | 49.8 ± 0.49 e | 9.22 ± 0.46 e | 11.7 ± 0.39 b | 9.74 ± 0.49 a | 10.3 ± 0.22 d | 10.14 | 4.10 | 1.61 ± 0.10 a | 1.47 ± 0.06 a | 7.53 ± 0.22 c | 9.43 ± 0.61 d |
| LM-AWLuBC | 38.3 ± 0.91 a | 40.2 ± 0.71 a | 4.49 ± 0.22 b | 13.1 ± 0.66 c | 11.0 ± 0.55 a | 5.54 ± 0.28 a | 19.92 | 10.35 | 1.64 ± 0.11 a | 1.48 ± 0.04 a | 6.14 ± 0.19 b | 8.16 ± 0.28 c |
| Samples | Biogenic Amines (mg·kg−1) | |||||||
|---|---|---|---|---|---|---|---|---|
| Phenylethylamine | Tyramine | Spermidine | Spermine | |||||
| Duration of Treatment (h) | ||||||||
| 24 | 48 | 24 | 48 | 24 | 48 | 24 | 48 | |
| C-LM | nd | nd | nd | nd | nd | nd | 42.3 ± 0.55 d | 54.2 ± 0.15 e |
| LM-AWLc | nd | nd | nd | nd | nd | nd | 43.7 ± 0.29 e | 53.6 ± 0.17 d |
| LM-WLcAP | nd | nd | nd | 5.35 ± 0.05 a | nd | 23.4 ± 0.24 c | 41.2 ± 0.31 c | 51.8 ± 0.19 c |
| LM-AWLcBC | nd | nd | 5.58 ± 0.19 | 15.6 ± 0.0 b | 32.3 ± 0.21 | 22.7 ± 0.17 b | 55.2 ± 0.61 f | 64.8 ± 0.60 g |
| LM-AWLu | nd | nd | nd | 20.8 ± 0.20 c | nd | 37.2 ± 0.25 d | 60.6 ± 0.28 g | 57.8 ± 0.58 f |
| LM-AWLuAP | nd | 5.80 ± 0.0 b | nd | nd | nd | 9.48 ± 0.14 a | 26.4 ± 0.42 a | 42.7 ± 0.21 a |
| LM-AWLuBC | nd | 4.84 ± 0.08 a | nd | nd | nd | nd | 36.8 ± 0.16 b | 50.9 ± 0.20 b |
| Sample | Fatty Acid Composition (% of Total Fatty Acid Content) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total SFAs | Total MUFAs | Total PUFAs | Omega-3 (ω-3) | Omega-6 (ω-6) | Omega-9 (ω-9) | |||||||
| Duration of Treatment (h) | ||||||||||||
| 24 | 48 | 24 | 48 | 24 | 48 | 24 | 48 | 24 | 48 | 24 | 48 | |
| C-LM | 55.71 ±0.86 a | 54.61 ± 0.95 a | 37.63 ± 1.19 a | 37.15 ± 1.04 a | 6.56 ± 0.14 c | 5.51 ± 0.11 b | 0.25 ± 0.070 a | 0.23 ± 0.009 d | 6.31 ± 0.11 b | 5.28 ± 0.12 b | 34.67 ± 0.43 a | 34.23 ± 0.65 a |
| LM-AWLc | 57.87 ± 1.06 b | 58.78 ± 0.74 b | 36.22 ± 0.64 a | 36.36 ± 0.34 a | 5.83 ± 0.18 b | 4.63 ± 0.15 a | 0.20 ± 0.050 a | 0.17 ± 0.010 b | 5.63 ± 0.12 c | 4.46 ± 0.10 a | 34.25 ± 0.65 a | 34.04 ± 0.71 a |
| LM-AWLcAP | 57.33 ± 0.94 b | 57.99 ± 1.07 b | 37.09 ± 1.04 a | 36.91 ± 0.86 a | 5.98 ± 0.12 b | 4.82 ± 0.20 a | 0.30 ± 0.050 a | 0.39 ± 0.010 e | 5.68 ± 0.11 c | 4.43 ± 0.09 a | 34.34 ± 0.54 a | 34.6 ± 0.89 a |
| LM-AWLcBC | 59.00 ± 1.21 c | 58.19 ± 0.74 b | 37.00 ± 0.83 a | 36.61 ± 1.00 a | 4.42 ± 0.08 a | 4.78 ± 0.19 a | 0.27 ± 0.011 a | 0.22 ± 0.009 d | 4.15 ± 0.19 a | 4.56 ± 0.21 a | 34.96 ± 1.02 a | 34.45 ± 0.89 a |
| LM-AWLu | 57.32 ± 0.75 b | 56.48 ± 0.87 b | 36.50 ± 0.41 a | 36.66 ± 0.78 a | 6.03 ± 0.11 b | 6.02 ± 0.23 c | 0.34 ± 0.012 a | 0.20 ± 0.008 c | 5.69 ± 0.19 c | 5.82 ± 0.11 c | 34.17 ± 0.70 a | 34.75 ± 0.61 a |
| LM-AWLuAP | 56.71 ± 0.87 ab | 57.37 ± 0.91 b | 37.08 ± 0.97 a | 37.03 ± 0.75 a | 6.13 ± 0.17 b | 5.15 ± 0.17 a | 0.31 ± 0.070 a | 0.14 ± 0.004 a | 5.82 ± 0.11 c | 5.01 ± 0.22 b | 34.60 ± 0.54 a | 34.77 ± 0.85 a |
| LM-AWLuBC | 56.41 ± 0.93 b | 56.46 ± 0.74 b | 37.13 ± 1.10 a | 36.73 ± 0.84 a | 6.30 ± 0.10 b | 6.45 ± 0.25 c | 0.21 ± 0.021 a | 0.20 ± 0.011 d | 6.09 ± 0.12 d | 6.25 ± 0.19 d | 34.70 ± 0.66 a | 34.58 ± 0.39 a |
| Saturated Fatty Acids (SFAs) | Monounsaturated Fatty Acids (MUFAs) | Polyunsaturated Fatty Acids (PUFAs) | ||||||||||
| Palmitic C16:0 | Stearic C18:0 | Oleic C18:1 cis-9 | Palmitoleic C16:1 cis-9 | Linoleic C18:2 cis-9,12 | γ-Linolenic C18:3 cis-6,9,12 | |||||||
| 24 | 48 | 24 | 48 | 24 | 48 | 24 | 48 | 24 | 48 | 24 | 48 | |
| C-LM | 22.59 ± 0.75 a | 22.00 ± 0.66 a | 24.10 ± 0.43 a | 23.80 ± 0.32 a | 32.45 ± 0.84 a | 31.98 ± 0.72 a | 2.82 ± 0.17 b | 2.81 ± 0.11 b | 3.70 ± 0.23 b | 3.21 ± 0.11 a | 2.49 ± 0.13 c | 1.99 ± 0.12 b |
| LM-AWLc | 24.54 ± 0.81 a | 24.83 ± 0.53 b | 24.07 ± 0.20 a | 24.40 ± 0.25 a | 32.87 ± 1.25 a | 32.82 ± 0.94 a | 1.86 ± 0.16 a | 2.13 ± 0.09 a | 3.34 ± 0.19 a | 2.84 ± 0.14 a | 2.15 ± 0.150 b | 1.44 ± 0.16 a |
| LM-AWLcAP | 24.52 ± 0.85 a | 24.43 ± 0.45 b | 24.41 ± 0.64 ab | 25.75 ± 0.31 c | 33.16 ± 1.02 a | 33.43 ± 0.36 a | 2.62 ± 0.23 b | 2.19 ± 0.13 a | 3.24 ± 0.16 a | 2.88 ± 0.13 a | 2.12 ± 0.144 b | 1.20 ± 0.20 a |
| LM-AWLcBC | 24.81 ± 0.77 a | 24.97 ± 0.64 b | 24.73 ± 0.19 a | 24.83 ± 0.24 b | 33.78 ± 0.54 a | 33.29 ± 0.47 a | 1.97 ± 0.24 a | 2.09 ± 0.10 a | 2.83 ± 0.18 a | 2.96 ± 0.22 a | 1.14 ± 0.23 a | 1.43 ± 0.13 a |
| LM-AWLu | 24.67 ± 0.54 a | 23.94 ± 0.41 b | 24.67 ± 0.29 a | 24.29 ± 0.18 a | 32.45 ± 0.69 a | 32.45 ± 0.99 a | 2.24 ± 0.17 ab | 1.86 ± 0.14 a | 3.14 ± 0.23 a | 3.45 ± 0.36 b | 2.37 ± 0.12 bc | 2.22 ± 0.23 b |
| LM-AWLuAP | 23.28 ± 0.57 a | 24.45 ± 0.69 b | 24.64 ± 0.24 a | 24.71 ± 0.37 b | 32.77 ± 0.88 a | 33.19 ± 1.12 a | 2.33 ± 0.18 b | 2.17 ± 0.20 a | 3.20 ± 0.30 a | 2.99 ± 0.18 a | 2.46 ± 0.17 c | 1.85 ± 0.15 b |
| LM-AWLuBC | 23.38 ± 0.64 a | 23.32 ± 0.41 b | 24.04 ± 0.22 a | 24.18 ± 0.19 a | 32.74 ± 0.82 a | 32.59 ± 0.93 a | 2.33 ± 0.11 b | 2.03 ± 0.09 a | 3.64 ± 0.15 b | 3.87 ± 0.18 b | 2.29 ± 0.19 b | 2.26 ± 0.17 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zavistanaviciute, P.; Klementaviciute, J.; Klupsaite, D.; Zokaityte, E.; Ruzauskas, M.; Buckiuniene, V.; Viskelis, P.; Bartkiene, E. Effects of Marinades Prepared from Food Industry By-Products on Quality and Biosafety Parameters of Lamb Meat. Foods 2023, 12, 1391. https://doi.org/10.3390/foods12071391
Zavistanaviciute P, Klementaviciute J, Klupsaite D, Zokaityte E, Ruzauskas M, Buckiuniene V, Viskelis P, Bartkiene E. Effects of Marinades Prepared from Food Industry By-Products on Quality and Biosafety Parameters of Lamb Meat. Foods. 2023; 12(7):1391. https://doi.org/10.3390/foods12071391
Chicago/Turabian StyleZavistanaviciute, Paulina, Jolita Klementaviciute, Dovile Klupsaite, Egle Zokaityte, Modestas Ruzauskas, Vilija Buckiuniene, Pranas Viskelis, and Elena Bartkiene. 2023. "Effects of Marinades Prepared from Food Industry By-Products on Quality and Biosafety Parameters of Lamb Meat" Foods 12, no. 7: 1391. https://doi.org/10.3390/foods12071391
APA StyleZavistanaviciute, P., Klementaviciute, J., Klupsaite, D., Zokaityte, E., Ruzauskas, M., Buckiuniene, V., Viskelis, P., & Bartkiene, E. (2023). Effects of Marinades Prepared from Food Industry By-Products on Quality and Biosafety Parameters of Lamb Meat. Foods, 12(7), 1391. https://doi.org/10.3390/foods12071391









