Tamarind Xyloglucan Oligosaccharides Attenuate Metabolic Disorders via the Gut–Liver Axis in Mice with High-Fat-Diet-Induced Obesity
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Treatments
2.2. RNA Extraction and Quantitative PCR Analysis (qRT-PCR)
2.3. Biochemical Tests
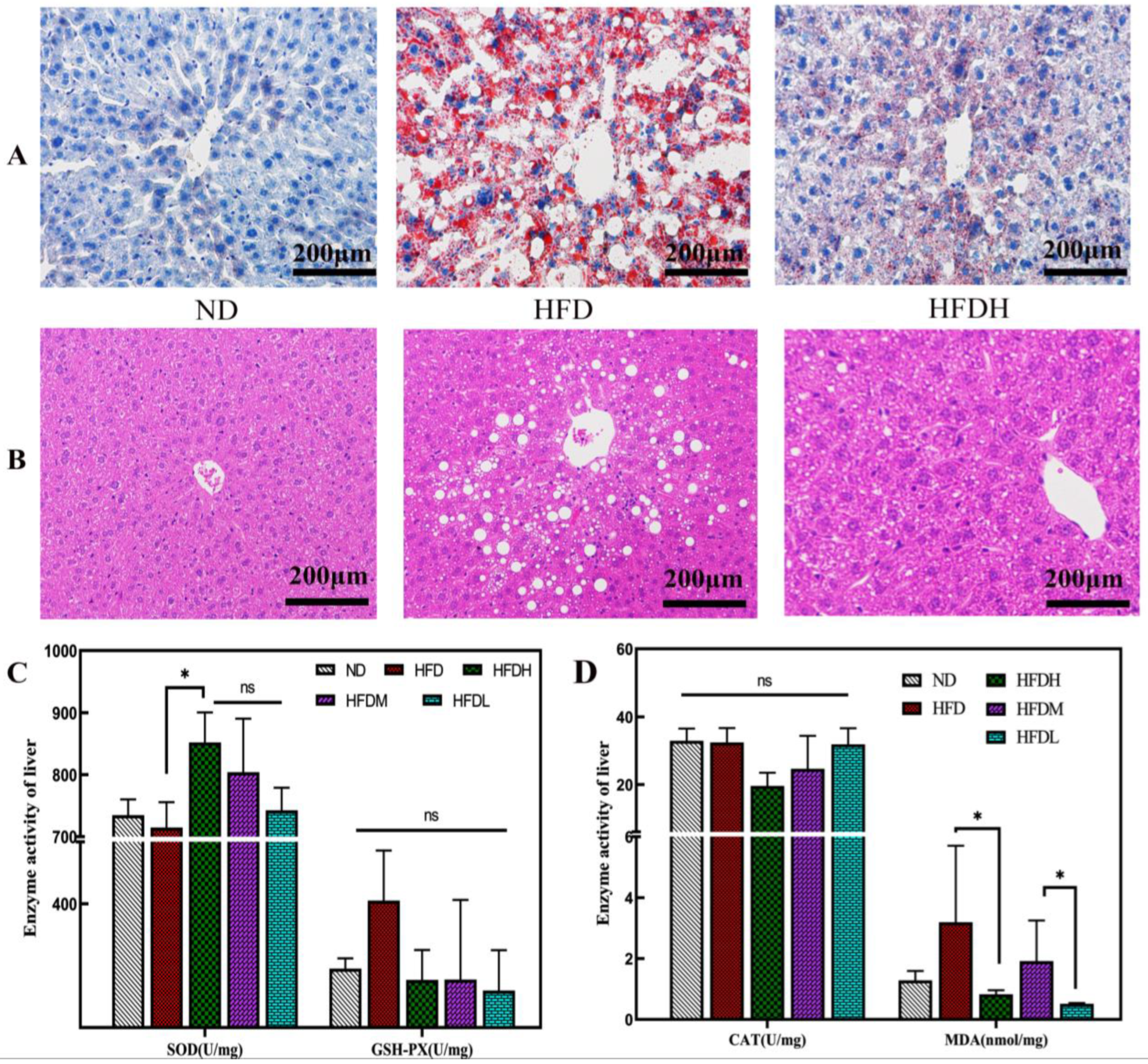
2.4. Histological Analysis
2.5. Fatty Acid Analysis
2.6. DNA Extraction and 16S rRNA Gene Sequencing of Mouse Faeces
2.7. Statistical Analysis
3. Results
3.1. Effects of TXOS on Bodyweight and the Serum of Obese Mice
3.2. Effect of TXOS on Adipose and Liver Tissues in Mice
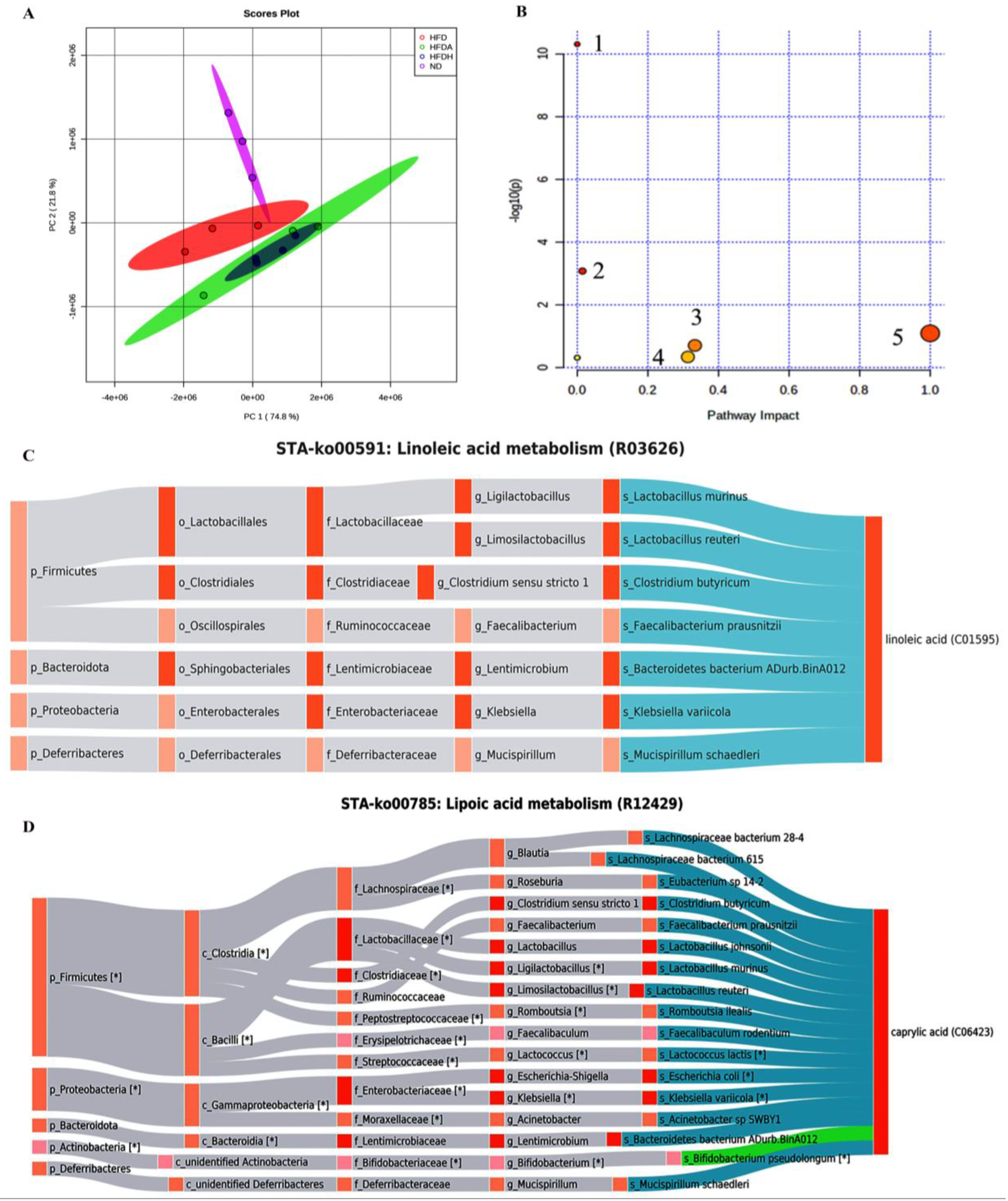
3.3. TXOS Supplementation Changed the Composition of Hepatic Fatty Acids
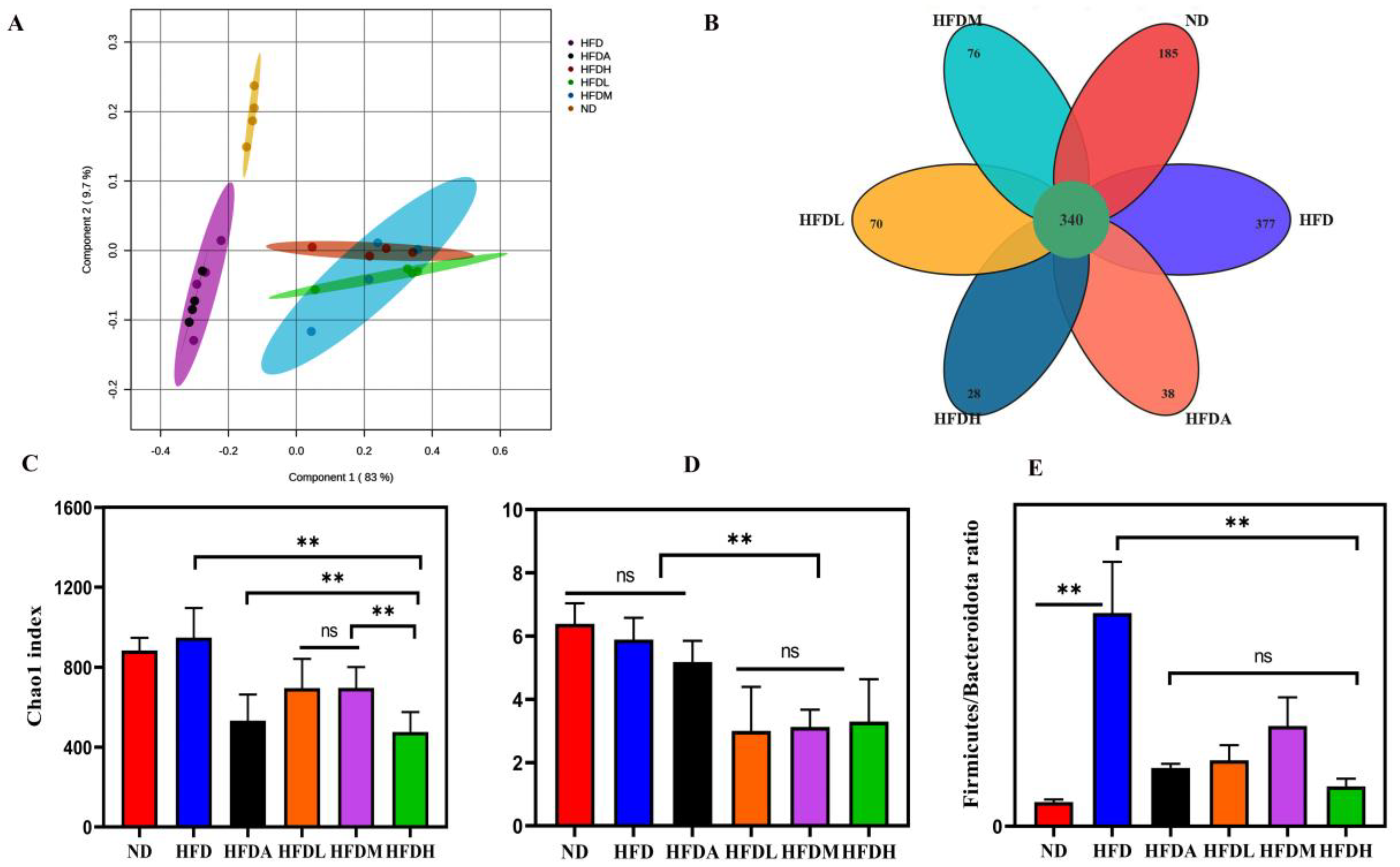
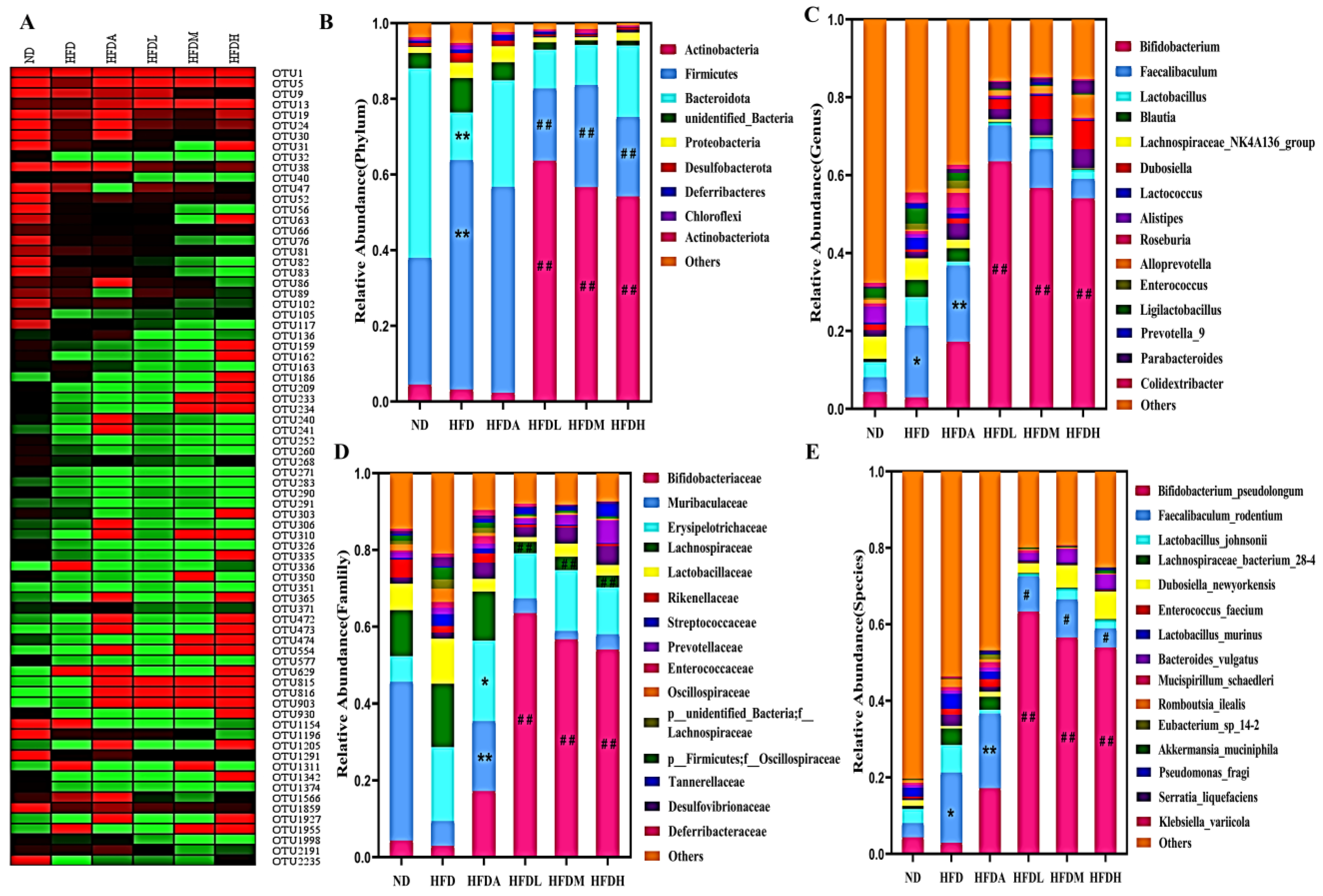
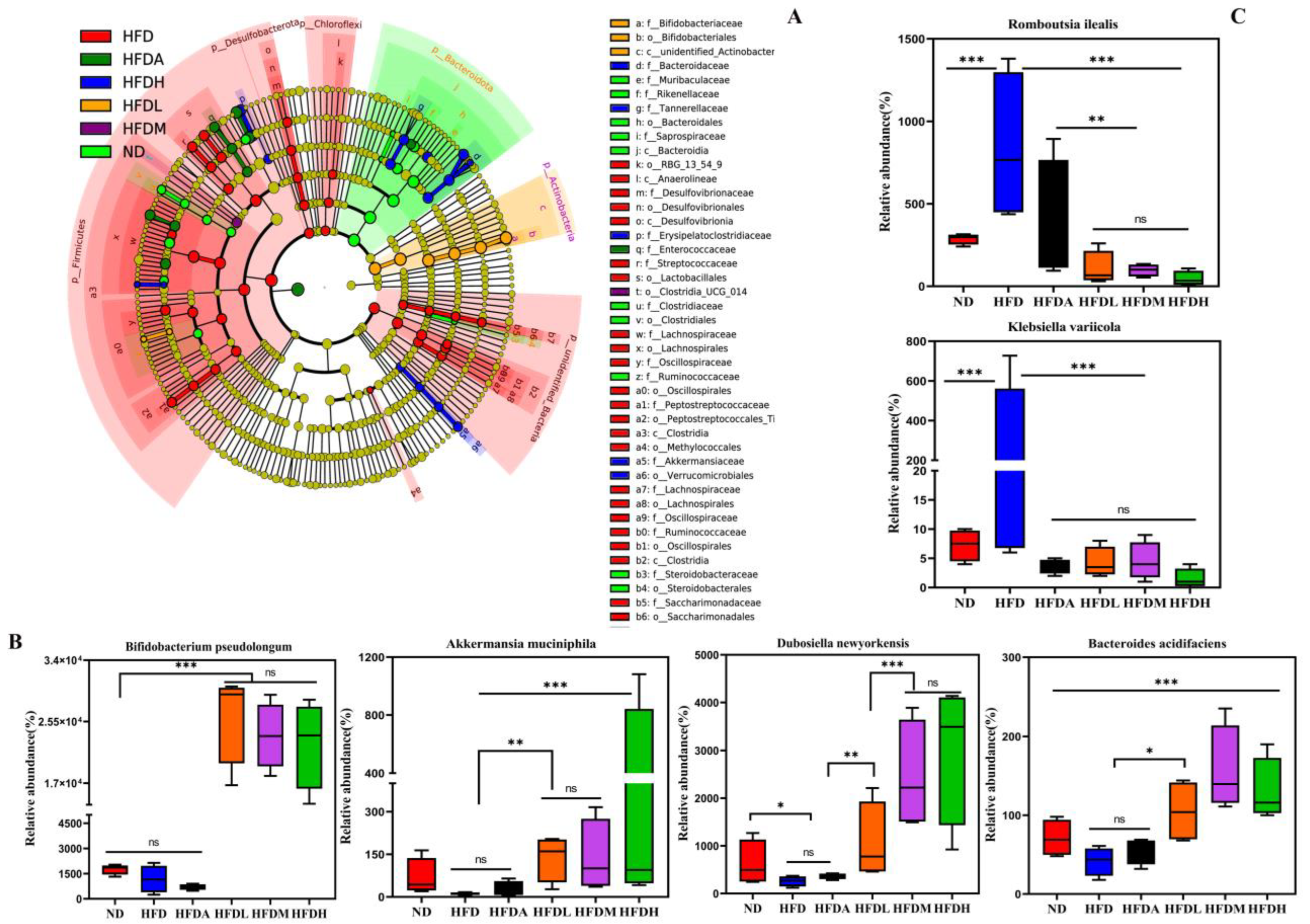
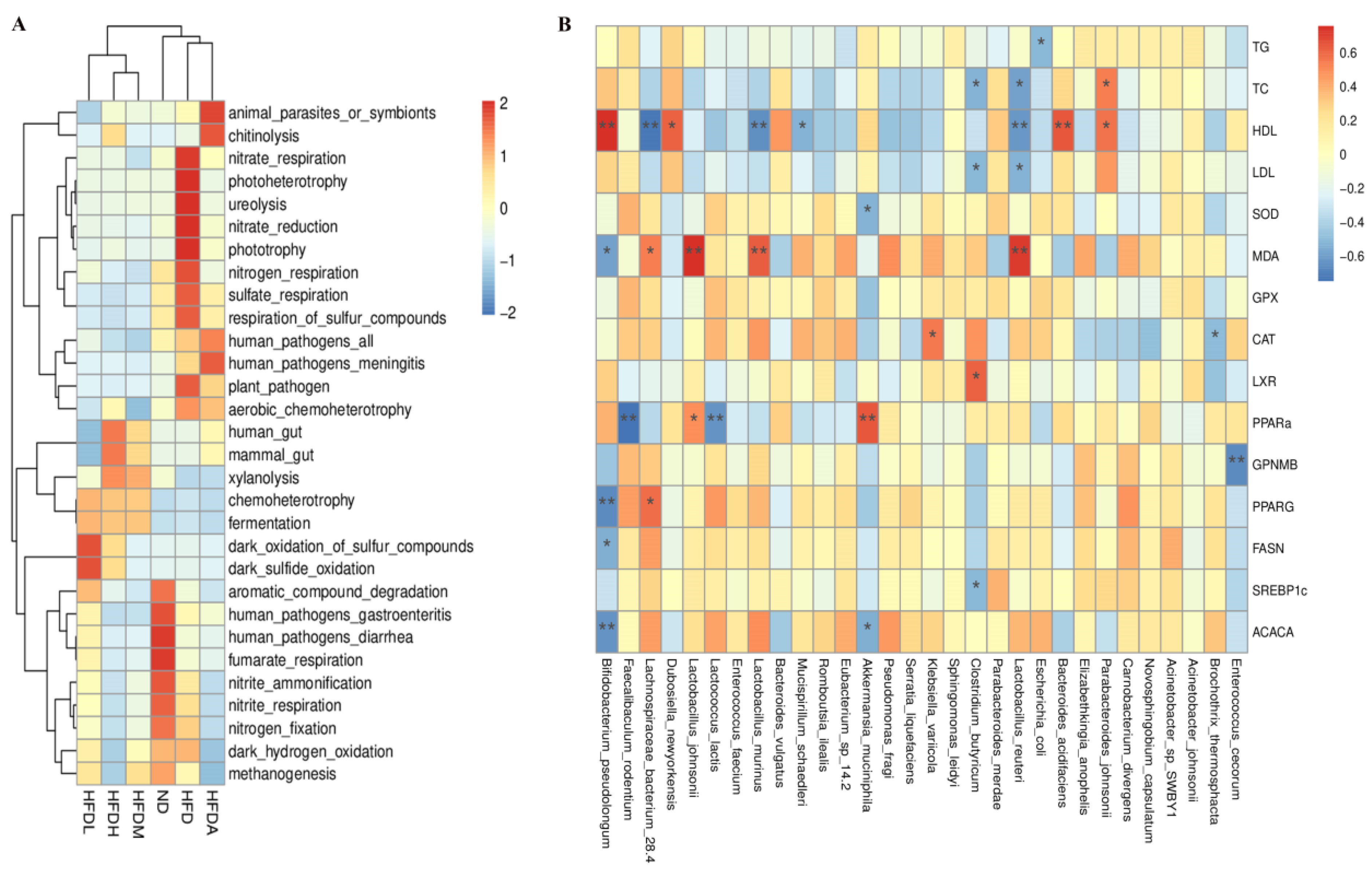
3.4. TXOS Alleviated Obesity Caused by HFD-Induced Gut Dysbiosis in Mice
3.5. Correlation between the Obesity Parameters and Gut Microbiota
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Arnoriaga-Rodriguez, M.; Mayneris-Perxachs, J.; Burokas, A.; Perez-Brocal, V.; Moya, A.; Portero-Otin, M.; Ricart, W.; Maldonado, R.; Fernandez-Real, J.M. Gut bacterial ClpB-like gene function is associated with decreased body weight and a characteristic microbiota profile. Microbiome 2020, 8, 59. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.R.; Lin, C.S.; Chang, C.J.; Lin, T.L.; Martel, J.; Ko, Y.F.; Ojcius, D.M.; Lu, C.C.; Young, J.D.; Lai, H.C. Gut commensal parabacteroides goldsteinii plays a predominant role in the anti-obesity effects of polysaccharides isolated from Hirsutella sinensis. Gut 2019, 68, 248–262. [Google Scholar] [CrossRef] [PubMed]
- Di Cesare, M.; Bentham, J.; Stevens, G.A.; Zhou, B.; Danaei, G.; Lu, Y.; Bixby, H.; Cowan, M.J.; Riley, L.M.; Hajifathalian, K.; et al. Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 2016, 387, 1377–1396. [Google Scholar] [CrossRef]
- Dahiya, D.K.; Renuka; Puniya, M.; Shandilya, U.K.; Dhewa, T.; Kumar, N.; Kumar, S.; Puniya, A.K.; Shukla, P. Gut microbiota modulation and its relationship with obesity using prebiotic fibers and probiotics: A review. Front. Microbiol. 2017, 8, 563. [Google Scholar] [CrossRef] [PubMed]
- Moorthy, M.; Sundralingam, U.; Palanisamy, U.D. Polyphenols as Prebiotics in the Management of High-Fat Diet-Induced Obesity: A Systematic Review of Animal Studies. Foods 2021, 10, 299. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Probert, H.M.; Loo, J.V.; Rastall, R.A.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Updating the concept of prebiotics. Nutr. Res. Rev. 2004, 17, 259–275. [Google Scholar] [CrossRef]
- Cockburn, D.W.; Koropatkin, N.M. Polysaccharide degradation by the intestinal microbiota and its influence on human health and disease. J. Mol. Biol. 2016, 428, 3230–3252. [Google Scholar] [CrossRef] [PubMed]
- Holmes, Z.C.; Silverman, J.D.; Dressman, H.K.; Wei, Z.; Dallow, E.P.; Armstrong, S.C.; Seed, P.C.; Rawls, J.F.; David, L.A. Short-chain fatty acid production by gut microbiota from children with obesity differs according to prebiotic choice and bacterial community composition. mBio 2020, 11, e00914–e00920. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J.; Tan, Q.; Deng, X.; Tsai, P.J.; Chen, P.H.; Ye, M.; Guo, J.; Su, Z. Nondigestible Oligosaccharides with Anti-Obesity Effects. J. Agric. Food Chem. 2020, 68, 4–16. [Google Scholar] [CrossRef]
- Vojinovic, D.; Radjabzadeh, D.; Kurilshikov, A.; Amin, N.; Wijmenga, C.; Franke, L.; Ikram, M.A.; Uitterlinden, A.G.; Zhernakova, A.; Fu, J.; et al. Relationship between gut microbiota and circulating metabolites in population-based cohorts. Nat. Commun. 2019, 10, 5813. [Google Scholar] [CrossRef]
- Bliesner, A.; Eccles-Smith, J.; Bates, C.; Hayes, O.; Ho, J.Y.; Martins, C.; Truby, H.; Nitert, M.D. Impact of Food-Based Weight Loss Interventions on Gut Microbiome in Individuals with Obesity: A Systematic Review. Nutrients 2022, 14, 1953. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.H.; Jiang, X.J.; Li, J.W.; Zhou, S.S.; Bai, T.M.; Qin, W.; Li, H.; Luo, Y.H.; Huang, Z.Q.; Liu, Y.T.; et al. Xyloglucan affects gut-liver circulating bile acid metabolism to improve liver damage in mice fed with high-fat diet. J. Funct. Foods 2020, 64, 103651. [Google Scholar] [CrossRef]
- Jiang, J.; Tang, M.; Han, M. Research progress of the structure, gel property and application of tamarind xyloglucan. J. For. Eng. 2020, 5, 11–19. [Google Scholar] [CrossRef]
- Yamatoya, K.; Yamazaki, K.; Shirakawa, M.; Baba, O. Effects of xyloglucan with the partial removal of galactose on plasma lipid concentration. J. Funct. Foods 2011, 3, 275–279. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, J.; Xiong, B.; Zhang, C.; Kang, B.; Gao, Y.; Li, Z.; Ge, W.; Cheng, S.; Hao, Y.; et al. Microbiota from alginate oligosaccharide-dosed mice successfully mitigated small intestinal mucositis. Microbiome 2020, 8, 112. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, Z.; Xu, X.; Jiang, H.; Cai, C.; Yu, G. Odd-numbered agaro-oligosaccharides alleviate type 2 diabetes mellitus and related colonic microbiota dysbiosis in mice. Carbohydr. Polym. 2020, 240, 116261. [Google Scholar] [CrossRef]
- Liu, Q.; Xi, Y.; Wang, Q.; Liu, J.; Li, P.; Meng, X.; Liu, K.; Chen, W.; Liu, X.; Liu, Z. Mannan oligosaccharide attenuates cognitive and behavioral disorders in the 5xFAD Alzheimer’s disease mouse model via regulating the gut microbiota-brain axis. Brain Behav. Immun. 2021, 95, 330–343. [Google Scholar] [CrossRef]
- Sookoian, S.; Salatino, A.; Castano, G.O.; Landa, M.S.; Fijalkowky, C.; Garaycoechea, M.; Pirola, C.J. Intrahepatic bacterial metataxonomic signature in non-alcoholic fatty liver disease. Gut 2020, 69, 1483–1491. [Google Scholar] [CrossRef]
- Silveira, M.A.D.; Bilodeau, S.; Greten, T.F.; Wang, X.W.; Trinchieri, G. The gut–liver axis: Host microbiota interactions shape hepatocarcinogenesis. Trends Cancer 2022, 8, 583–597. [Google Scholar] [CrossRef]
- Kindt, A.; Liebisch, G.; Clavel, T.; Haller, D.; Hormannsperger, G.; Yoon, H.; Kolmeder, D.; Sigruener, A.; Krautbauer, S.; Seeliger, C.; et al. The gut microbiota promotes hepatic fatty acid desaturation and elongation in mice. Nat. Commun. 2018, 9, 3760. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, J.; Xie, F.; He, H.; Johnston, L.J.; Dai, X.; Wu, C.; Ma, X. Dietary fiber-derived short-chain fatty acids: A potential therapeutic target to alleviate obesity-related nonalcoholic fatty liver disease. Obes. Rev. 2021, 22, e13316. [Google Scholar] [CrossRef] [PubMed]
- Adolph, T.E.; Grander, C.; Moschen, A.R.; Tilg, H. Liver-microbiome axis in health and disease. Trends Immunol. 2018, 39, 712–723. [Google Scholar] [CrossRef] [PubMed]
- Koziol, A.; Cybulska, J.; Pieczywek, P.M.; Zdunek, A. Evaluation of structure and assembly of xyloglucan from tamarind seed (Tamarindus indica L.) with atomic force microscopy. Food Biophys. 2015, 10, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.N.; Li, Y.X.; Miao, M.; Zhu, C.H.; Yan, Q.J.; Jiang, Z.Q. High level expression of a xyloglucanase from Rhizomucor miehei in Pichia pastoris for production of xyloglucan oligosaccharides and its application in yoghurt. Int. J. Biol. Macromol. 2021, 190, 845–852. [Google Scholar] [CrossRef]
- Li, X.; Guo, R.; Wu, X.; Liu, X.; Ai, L.; Sheng, Y.; Song, Z.; Wu, Y. Dynamic digestion of tamarind seed polysaccharide: Indigestibility in gastrointestinal simulations and gut microbiota changes in vitro. Carbohydr. Polym. 2020, 239, 116194. [Google Scholar] [CrossRef]
- Arnold, J.W.; Roach, J.; Fabela, S.; Moorfield, E.; Ding, S.; Blue, E.; Dagher, S.; Magness, S.; Tamayo, R.; Bruno-Barcena, J.M.; et al. The pleiotropic effects of prebiotic galacto-oligosaccharides on the aging gut. Microbiome 2021, 9, 31. [Google Scholar] [CrossRef]
- Li, S.; He, N.; Wang, L. Efficiently Anti-Obesity Effects of Unsaturated Alginate Oligosaccharides (UAOS) in High-Fat Diet (HFD)-Fed Mice. Mar. Drugs 2019, 17, 540. [Google Scholar] [CrossRef]
- Chi, Y.; Wu, Z.; Du, C.; Zhang, M.; Wang, X.; Xie, A.; Wang, P.; Li, R. Regulatory effects mediated by ulvan oligosaccharide and its zinc complex on lipid metabolism in high-fat diet-fed mice. Carbohydr. Polym. 2023, 300, 120249. [Google Scholar] [CrossRef]
- Sikalidis, A.K.; Fitch, M.D.; Fleming, S.E. Risk of colonic cancer is not higher in the obese Lep(ob) mouse model compared to lean littermates. Pathol. Oncol. Res. 2013, 19, 867–874. [Google Scholar] [CrossRef]
- Sikalidis, A.K.; Fitch, M.D.; Fleming, S.E. Diet induced obesity increases the risk of colonic tumorigenesis in mice. Pathol. Oncol. Res. 2013, 19, 657–666. [Google Scholar] [CrossRef]
- Cani, P.D. Microbiota and metabolites in metabolic diseases. Nat. Rev. Endocrinol. 2019, 15, 69–70. [Google Scholar] [CrossRef] [PubMed]
- Lundsgaard, A.M.; Fritzen, A.M.; Sjoberg, K.A.; Kleinert, M.; Richter, E.A.; Kiens, B. Small Amounts of dietary medium-chain fatty acids protect against insulin resistance during caloric excess in humans. Diabetes 2021, 70, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Papamandjaris, A.A.; MacDougall, D.E.; Jones, P.J. Medium chain fatty acid metabolism and energy expenditure: Obesity treatment implications. Life Sci. 1998, 62, 1203–1215. [Google Scholar] [CrossRef]
- Nagao, K.; Yanagita, T. Medium-chain fatty acids: Functional lipids for the prevention and treatment of the metabolic syndrome. Pharmacol. Res. 2010, 61, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Fedorenko, A.; Lishko, P.V.; Kirichok, Y. Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell 2012, 151, 400–413. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, G.; Ecker, J. The opposing effects of n-3 and n-6 fatty acids. Prog. Lipid Res. 2008, 47, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Nie, Q.; Chen, H.; Hu, J.; Tan, H.; Nie, S.; Xie, M. Effects of nondigestible oligosaccharides on obesity. Ann. Rev. Food Sci. Technol. 2020, 11, 205–233. [Google Scholar] [CrossRef] [PubMed]
- Schott, E.M.; Farnsworth, C.W.; Grier, A.; Lillis, J.A.; Soniwala, S.; Dadourian, G.H.; Bell, R.D.; Doolittle, M.L.; Villani, D.A.; Awad, H.; et al. Targeting the gut microbiome to treat the osteoarthritis of obesity. JCI Insight 2018, 3, e95997. [Google Scholar] [CrossRef]
- Backhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef]
- Li, Z.; Yang, S.; Lin, H.; Huang, J.; Watkins, P.A.; Moser, A.B.; Desimone, C.; Song, X.Y.; Diehl, A.M. Probiotics and antibodies to TNF inhibit inflammatory activity and improve nonalcoholic fatty liver disease. Hepatology 2003, 37, 343–350. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Meric, G.; Havulinna, A.S.; Teo, S.M.; Aberg, F.; Ruuskanen, M.; Sanders, J.; Zhu, Q.; Tripathi, A.; Verspoor, K.; et al. Early prediction of incident liver disease using conventional risk factors and gut-microbiome-augmented gradient boosting. Cell Metab. 2022, 34, 719–730. [Google Scholar] [CrossRef] [PubMed]
| Group | Initial Weight/g | Final Weight/g | Weight Gain/g | Perirenal Fat/g | Epididymal Fat/g | Subcutaneous Fat/g | Food Intake/kg |
|---|---|---|---|---|---|---|---|
| ND | 20.12 ± 0.60 | 26.98 ± 1.16 | 6.86 ± 0.82 | 0.21 ± 0.28 | 0.78 ± 0.68 | 0.36 ± 0.35 | 3.56 |
| HFD | 20.04 ± 0.68 | 42.58 ± 7.37 ## | 22.54 ± 6.96 ## | 1.04 ± 0.32 ## | 2.63 ± 0.88 ## | 1.83 ± 0.51 ## | 2.03 ## |
| HFDH | 19.86 ± 1.00 | 37.15 ± 7.43 * | 17.29 ± 7.82 * | 0.83 ± 0.35 | 2.08 ± 1.02 | 1.76 ± 1.01 | 2.03 |
| HFDM | 19.78 ± 1.61 | 40.46 ± 8.08 | 20.69 ± 7.14 | 0.78 ± 0.36 | 2.48 ± 0.66 | 1.96 ± 1.03 | 2.39 |
| HFDL | 20.05 ± 0.83 | 43.60 ± 3.90 | 23.55 ± 3.52 | 0.87 ± 0.41 | 2.28 ± 0.76 | 1.50 ± 0.74 | 2.30 |
| HFDA | 19.41 ± 0.38 | 33.40 ± 5.65 ** | 14.00 ± 5.75 ** | 0.60 ± 0.28 * | 1.66 ± 0.72 * | 0.84 ± 0.30 * | 2.24 |
| Num. | Compound | Formula | Abbreviation | RT (min) | HFD/ND | HFDA | HFDH | Pathway |
|---|---|---|---|---|---|---|---|---|
| 1 | Caprylic acid | C8H16O2 | C8:0 | 3.50 | ↓ ### | ↓ *** | Fatty acid biosynthesis | |
| 2 | Dodecanoic acid | C12H24O2 | C12:0 | 5.11 | ↓ ** | ↓ ** | Fatty acid biosynthesis | |
| 3 | Tridecanoic acid | C13H26O2 | C13:0 | 5.65 | ↓ * | Fatty acids and conjugates | ||
| 4 | Tetradecanoic acid | C14H28O2 | C14:0 | 6.23 | ↓ ** | Fatty acid biosynthesis | ||
| 5 | Myristelaidic acid | C14H26O2 | C14:1T | 5.47 | ↑ ## | ↓ ** | ↓ ** | |
| 6 | Palmitelaidic acid | C16H30O2 | C16:1T | 6.55 | ↑ # | ↓ * | ||
| 7 | Linoleic acid | C18H32O2 | C18:2(n-6) | 6.66 | ↓ * | Linolenic acid metabolism | ||
| 8 | Linoelaidic acid | C18H32O2 | C18:2(n-6) T | 6.95 | ↓ *** | ↓*** | ||
| 9 | a-Linolenic acid | C18H30O2 | C18:3(n-3) | 5.93 | ↓ ** | ↓ ** | α-Linolenic acid metabolism | |
| 10 | cis-5,8,11,14,17-Eicosapentaenoic acid | C20H30O2 | C20:5 | 5.77 | ↑ ### | ↓ *** | ↓ *** | Biosynthesis of unsaturated fatty acids |
| 11 | cis-7,10,13,16,19-Docosapentaenoic acid | C22H34O2 | C22:5(n-3) | 6.59 | ↓ * | Biosynthesis of unsaturated fatty acids | ||
| 12 | Tetracosanoic acid | C24H48O2 | C24:0 | 10.86 | ↓ ** | ↓ ** | Biosynthesis of unsaturated fatty acids | |
| 13 | Myristoleic acid | C14H26O2 | C14:1 | 5.32 | ↓ *** | Fatty acids and conjugates | ||
| 14 | Heneicosanoic acid | C21H42O2 | C21:0 | 9.76 | ↓ ## | |||
| 15 | Nervonic acid | C24H46O2 | C24:1 | 10.08 | ↓ # | Biosynthesis of unsaturated fatty acids | ||
| 16 | Petroselinic acid | C18H34O2 | C18:1(n-12) | 7.66 | ↑ ## | Fatty acids and conjugates |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, C.-H.; Li, Y.-X.; Xu, Y.-C.; Wang, N.-N.; Yan, Q.-J.; Jiang, Z.-Q. Tamarind Xyloglucan Oligosaccharides Attenuate Metabolic Disorders via the Gut–Liver Axis in Mice with High-Fat-Diet-Induced Obesity. Foods 2023, 12, 1382. https://doi.org/10.3390/foods12071382
Zhu C-H, Li Y-X, Xu Y-C, Wang N-N, Yan Q-J, Jiang Z-Q. Tamarind Xyloglucan Oligosaccharides Attenuate Metabolic Disorders via the Gut–Liver Axis in Mice with High-Fat-Diet-Induced Obesity. Foods. 2023; 12(7):1382. https://doi.org/10.3390/foods12071382
Chicago/Turabian StyleZhu, Chun-Hua, Yan-Xiao Li, Yun-Cong Xu, Nan-Nan Wang, Qiao-Juan Yan, and Zheng-Qiang Jiang. 2023. "Tamarind Xyloglucan Oligosaccharides Attenuate Metabolic Disorders via the Gut–Liver Axis in Mice with High-Fat-Diet-Induced Obesity" Foods 12, no. 7: 1382. https://doi.org/10.3390/foods12071382
APA StyleZhu, C.-H., Li, Y.-X., Xu, Y.-C., Wang, N.-N., Yan, Q.-J., & Jiang, Z.-Q. (2023). Tamarind Xyloglucan Oligosaccharides Attenuate Metabolic Disorders via the Gut–Liver Axis in Mice with High-Fat-Diet-Induced Obesity. Foods, 12(7), 1382. https://doi.org/10.3390/foods12071382





