Abstract
Bread wheat has traditionally been selected for whitish derived flours. As a consequence, the current varieties carry carotenogenic alleles associated with low grain carotenoid. In contrast, high grain yellow pigment content (YPC) has been a major target in durum wheat programs since yellow colour is an important aesthetic factor for pasta production. Phytoene synthase 1 (Psy1) genes have an important role in the determination of the carotenoid content in wheat. In this work, we have transferred the genes Psy1-A1 and Psy1-B1 from durum to bread wheat by inter-specific hybridization in order to evaluate the combined effect of these genes for the improvement of grain carotenoid content, as well as the development of carotenoid-enriched bread wheat lines. Inter-specific breeding coupled with a MAS approach based on Psy1-A1 and Psy1-B1 alleles has allowed the development of bread wheat pre-breeding lines with enhanced grain carotenoid content (16–23% mean). These biofortified lines have the potential to become new varieties or to be used as recurrent parents in bread wheat breeding programs.
1. Introduction
Wheat is one of the most important crops in the world. Both bread wheat (Triticum aestivum L.), and durum wheat (Triticum turgidum (L.) ssp. durum (Desf.) Husn.) are considered to be staple foods. The former is adapted to a wider range of environments, while the latter is more adapted to specific areas such as the Mediterranean basin. Both species differ in their chromosome constitution, bread wheat being a hexaploid (2n = 6x = 42) with three genomes (A, B and D) and durum wheat a tetraploid species (2n = 4x = 28) with the A and B genomes.
Improving yield and disease resistance are primary targets for both species. However, breeding strategies for quality are different and, for some traits, even opposite. For instance, bread wheat breeding has focused on obtaining grains with whitish endosperm to meet the industry requirements for the production of white flours. On the other hand, high grain yellow pigment content (YPC) is a main target in durum wheat programs since yellow colour is an important aesthetic factor influencing consumer choice for pasta acquisition [1,2]. Consumers usually prefer a bright yellow coloured pasta, so products that do not match these requirements are refused. Consequently, breeding programs worldwide have focused on the development of varieties with enhanced YPC [3]. The high heritability of YPC [4,5] has benefited wheat breeding for this trait using colorimetric determinations (CIE, colour-space system with positive b* index providing a measure of the yellowness) due to their simplicity and cost. Alternatively, spectrophotometric determinations using the AACC-approved method 14–50 are also used, although this method is more expensive, slower and has higher technical requirements than the previous one. In addition, many efforts have been dedicated to the identification of the genetic bases controlling YPC either by the identification of QTL using bi-parental populations, or by the detection of marker-trait associations using diversity panels reviewed by [6] with new target regions for specific carotenoids being identified in recent expanded analyses of wheat biodiversity [7,8]. However, it is widely known that the main QTLs for YPC content in both durum and bread wheat are located in the long arm of chromosomes 7A and 7B [4,5,9,10,11,12,13].
Carotenoids are isoprenoids synthesized by all photosynthetic organisms, and they are essential for photo-protection and participate in light harvesting [14]. These pigments are responsible for the colours in the range between yellow and red in non-photosynthetic tissues, and they confer the YPC in wheat and related species such as tritordeum. There are two types of carotenoids, considering their chemical composition: carotenes, which are exclusively formed by carbon and hydrogen, and xanthophylls, that also contain oxygen. These pigments are precursors of important hormones such as abscisic acid (ABA) and strigolactones, which are related to growth regulation and responses against biotic and abiotic stresses [14]. In addition, they also play important roles in relation to human nutrition. In general, all carotenoids have an antioxidant nature, but they also have specific functions such as provitamin A activity, which is exclusive to carotenoids with unsubstituted β-rings [15]. Other carotenoids, such as lutein and zeaxanthin, accumulate in the eye macula and their consumption has been associated with the alleviation of age macular degeneration [16]. The AACC 14–50 method expresses the total amount of carotenoids as β-carotene equivalent units, i.e., it indicates the total amount of carotenoids considering that all of them are β-carotene [17]. In the case of wheat, this has induced errors for farmers and millers on certain occasions since they assume that durum wheat has a high quantity of β-carotene, when this compound is a minor carotenoid in durum wheat grain since lutein accounts for 90% or higher of the carotenoid pool. This has even led to the incorrect labelling of commercial pasta as having high β-carotene content. Since this is untrue, it can be considered as misleading to potential consumers and it could have serious commercial consequences. Therefore, breeding for carotenoid biofortification should be coupled with analytical techniques (i.e., chromatographic), allowing the individual determination of carotenoids to provide accurate and specific information to millers and consumers.
Carotenoid biosynthesis is well known due to the importance of these compounds at the nutritional level. The first step of the carotenoid pathway is controlled by the Phytoene synthase. This enzyme condenses two molecules of geranylgeranyl pyrophosphate (GGPP) to produce a molecule of phytoene [18], and it is considered to be the limiting step for carotenoid biosynthesis [19,20]. Indeed, the development of Golden Rice 2 was founded on the hypothesis that PSY was the limiting step in carotenoid biosynthesis [21]. In grasses, there are three Psy genes (Psy1, Psy2, and Psy3) [22]. Studies in maize have shown that while Psy1 is critical for carotenoid accumulation in the endosperm, the roles of Psy2 and Psy3 were negligible. Indeed, candidate gene approaches allowed the demonstration that Psy1-A1 and Psy1-B1 were the genes underlying the genetic variation of QTL for YPC identified in chromosomes 7A and 7B of durum wheat [9,10]. The importance of this gene has promoted the development of diagnostic markers for alleles of both Psy1-A1 and Psy1-B1 [23,24,25], useful for marker-assisted selection for higher carotenoid content.
The divergent breeding strategies for grain colour deployed in durum and bread wheat offer an interesting opportunity to ascertain the real effects of Psy1 loci in carotenoid accumulation in wheat. Since bread wheat has been selected for a whitish endosperm, it usually carries alleles associated with low grain carotenoid content in Psy1 and other carotenogenic genes with minor effects. Accordingly, the transference of Psy1-A1 and Psy1-B1 from durum wheat to bread wheat by inter-specific hybridization would allow the evaluation of the combined effect of these genes for the improvement of grain carotenoid content, as well as the development of bread wheat lines enriched for grain carotenoid content.
The aims of this work were (1) to determine the effectiveness of marker-assisted selection (MAS) based exclusively on Psy1-A1 and Psy1-B1 to increase grain carotenoid content in bread wheat; (2) the determination of the combined effect of alleles for high carotenoid content from both genes; (3) to develop pre-breeding bread wheat lines with enriched carotenoid content.
2. Materials and Methods
2.1. Plant Material and Field Testing
A panel of durum wheat varieties and bread wheat (BW) advanced lines (kindly supplied by Dr. J.C. Sillero, IFAPA, Córdoba, Spain) were genotyped with markers for Psy1-A1 and Psy1-B1, as described below. Two BW lines (12THES4515 and ES07H044SWC29) and the durum wheat (DW) elite variety ‘Kiko Nick’ differed in the alleles at both genes, and they were selected as parental lines. Two inter-specific crosses were performed: ‘Kiko Nick’ × 12THES4515 (referred to as cross KN × THES), and ‘Kiko Nick’ × ES07H044SWC29 (referred to as cross KN × C29). Subsequent plant material generation and selection were developed as described in Figure 1 under greenhouse conditions at IAS-CSIC (Córdoba, Spain). Briefly, ‘Kiko Nick’ was crossed as the maternal parent with each of the BW lines. The F1 hybrids were backcrossed with their respective BW parents. After this, BC1 grains were sown and genotyped with markers for Psy1-A1 and Psy1-B1 genes, as described below. Plants carrying DW alleles simultaneously for both genes were selected and selfed. During the fourth year, heterozygous plants for both genes were identified by MAS and selfed. Progenies of these plants were genotyped at two leaf-stage at greenhouse for both Psy1-A1 and Psy1-B1 to identify contrasting siblings carrying either BW alleles or DW alleles for both genes. These plants were grown outdoors under an anti-bird netting structure and with an anti-weed net. Grain samples were independently harvested from each plant and used for carotenoid analysis.
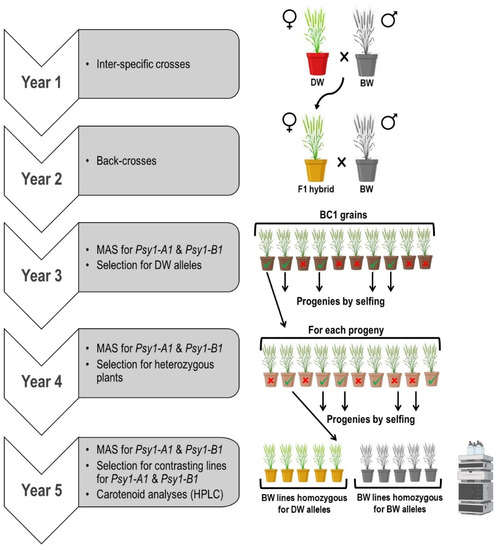
Figure 1.
Crossing scheme and selection for the transference of Psy1-A1 and Psy1-B1 from durum wheat (DW) to bread wheat (BW) (Image created with Biorender.com).
2.2. DNA Isolation and Marker-Assisted Selection
Genomic DNA was isolated using the CTAB method according to [26], with slight modifications. The detailed protocol used in our lab is shown in [27]. Primers YP7A-2, YP7B-1 and YP7B-4 were used for the amplification of the candidate genes since they allowed discrimination between the parental lines used in this work, as resulted from preliminary work [24,25]. PCR amplifications were performed with MyTaqTM DNA polymerase (Bioline, London, UK) following the manufacturer’s instructions. PCR conditions were as reported by [24,25]. Amplification products were resolved in agarose gels and visualized using SafeviewTM Nucleic Acid Stain (NBS biologicals, Ltd., Cambridgeshire, UK).
2.3. Extraction of Carotenoids and HPLC Analysis
Carotenoids pigments were extracted from wheat grains according to the method previously described in [27]. Briefly, 1 g of grain sample and 6 mL of HPLC grade acetone (containing 0.1% BHT) were milled in an oscillating ball mill Retsch Model MM400 (Retsch, Haan, Germany) with two stainless-steel balls (15 mm Ø) at 25 Hz for 1 min. The resulting slurry was placed in a centrifuge tube (15 mL) and centrifuged at 4500× g for 5 min at 4 °C. The acetone phase was transferred to another plastic centrifuge tube, and the solvent was evaporated under nitrogen stream. The concentrated residue containing the pigments was dissolved in 0.5 mL of HPLC grade acetone and stored at −30 °C until chromatographic analysis (HPLC). All samples were pre-cleaned by centrifugation at 13,000× g prior to the chromatographic analysis. All the steps of carotenoid extraction and analysis were performed under dimmed light to prevent carotenoid photo-degradation and isomerization.
The analysis of carotenoids was performed by HPLC as described in previous works [28,29]. The HPLC system consisted of a Waters e2695 Alliance chromatograph fitted with a Waters 2998 photodiode array detector, and controlled with Empower2 software (Waters Cromatografía, S.A., Barcelona, Spain). A reversed-phase column (Mediterranea SEA18, 3 μm, 20 × 0.46 cm; Teknokroma, Barcelona, Spain) was used. Pigment separation was achieved by a binary gradient elution, at a flow rate of 1 mL/min, using an initial composition of 75% acetone (HPLC-grade) and 25% deionized water (HPLC-grade), which was increased linearly to 95% acetone in 10 min, then raised to 100% in 2 min, and maintained at a constant. Detection was performed at 450 nm, and the online spectra were acquired in the 350–700 nm wavelength range.
Carotenoid quantification was performed using calibration curves (concentration range of 0.5–45 µg/mL) prepared from pure pigment standards. The concentration of (Z)-isomers of lutein was assessed by using the calibration curve for (all-E)-lutein. Similarly, lutein esters were determined as free lutein equivalents. All the analyses were carried out on the same day of the preparation of extracts and performed in duplicate. Data were expressed as µg/g fresh weight (µg/g fw).
2.4. Statistical Analyses
Analyses of variance for total carotenoid content and concentration of each compound were performed using Statistix version 10.0 (Analytical Software, Tallahassee, FL, USA). The use of p values followed the recommendations by [30], and thus they were reported as continuous quantities.
3. Results and Discussion
Before the initial crosses, the durum (DW) and bread wheat (BW) genotypes selected as parental lines were genotyped with the markers for Psy1-A1 and Psy1-B1 genes developed by [24]. The markers YP7A-2 and YP7B-4 allowed for distinguishing between the alleles of the DW and BW parental lines (Table 1).

Table 1.
Genotypic characterization for Psy1-A1 and Psy1-B1 and carotenoid composition of the parental lines.
The DW ‘Kiko Nick’ carried the Psy1-A1d allele as deduced from the 1001-bp fragment amplified with YP7A-2 primers [25]. Both BW lines amplified the 1684-bp fragment with the same primers. The Psy1-A1d allele found in ‘Kiko Nick’ is associated with high YPC content [24,25]. Regarding Psy1-B1, ‘Kiko Nick’ carries the Psy1-B1e allele as shown by the amplification of the dominant marker YP7B-4 [25]. This allele is also associated with high grain yellowness [25]. Therefore, we used the markers YP7A-2 and YP7B-4 for the development of the BW pre-breeding lines, as detailed in Figure 1.
The seeds obtained by selfing from the parental lines used for the initial crosses (year 1) were harvested and conserved at 4 °C. These seeds were later analysed for grain carotenoid content (Table 1, Figure 2). All three parental lines showed the typical carotenoid profile of wheat grains, as described in previous works [3,31,32,33,34].
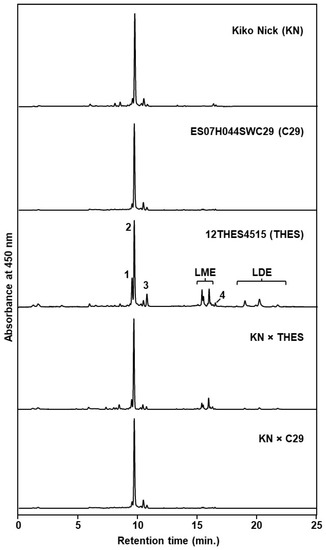
Figure 2.
HPLC chromatograms corresponding to the grain carotenoid profile of the DW ‘Kiko Nick’, the BW breeding lines ES07H044SWC29, 12THES4515 and representative individuals of KN × THES and KN × C29 crosses. Peak identities: 1, (all-E)-zeaxanthin; 2, (all-E)-lutein; 3, (Z)-lutein isomers; 4, (all-E)-β-carotene; LME, lutein monoesters; LDE, lutein diesters. Detection wavelength was 450 nm.
Lutein was the main carotenoid, accounting for 85–95% of the total carotenoids with minor amounts of other carotenoids such as zeaxanthin and β-carotene (see Supplementary Table S1 for the detailed grain carotenoid profile). This is in agreement with previous knowledge, showing that lutein represents between 86 and 94% of the total carotenoids in wheat and related cereal grains [3,17,32,35]. The BW parental 12THES4515 had both lutein monoesters and lutein diesters. DW ‘Kiko Nick’ and BW ES07H044SWC29 showed very small amounts of lutein monoesters (<1.5% of total carotenoids), while lutein diesters were completely absent in both genotypes. Considering the low contribution of lutein esters to the total carotenoid pool, these lines can be classified as zero/low esters genotypes, as suggested by [31]. Indeed, the presence of low amounts of lutein monoesters in these lines can be explained by the time these samples were conserved at 4 °C, since lutein esterification can occur during seed storage [31,36,37]. On the other hand, lutein esters accounted for 35% of the total carotenoids in 12THES4515, which indicates the existence of an important esterification ability in this genotype.
The existence of lutein esters in 12THES4515 is relevant since esterification is considered a new target for carotenoid biofortification [14,38]. Lutein esters are more stable than free lutein during grain and flour storage [31,39,40,41], although the protective effect of esterification seems not to be relevant at very high temperatures, at boiling [42], extrusion or other high temperature regimes [43]. Nevertheless, lutein diesters are much more resistant to degradation than free lutein and lutein monoesters during the processing of white salted noodles [44]. Therefore, the improvement of carotenoid retention during grain storage due to esterification constitutes an interesting opportunity for the industrial sector, and thus the potential of this genetic trait is currently being explored in international efforts. Lutein esterification is common in bread wheat due to the D genome inherited from Aegilops tauschii Coss. [31]. Indeed, lutein esters were found in 40 out of 45 accessions of Ae. tauschii and in four accessions of T. monococcum, but no lutein esters were detected in any of the 17 accessions of durum wheat analysed. This has promoted the development of breeding programs to transfer the esterification ability to durum wheat, either by transferring the XAT-7D from common wheat [45] or the XAT-7Hch gene identified in the wild barley H. chilense [46] using an inter-specific breeding approach using a translocation line T7HchS·7A/B [47] carrying XAT-7Hch as donor parent [27]. However, a recent survey of 156 Spanish landraces allowed the identification of four accessions with high esterification ability [48], which opens new opportunities for the improvement of carotenoid esterification in durum wheat since they provide an additional source of esterification genes to the currently known XAT-7D and XAT-7Hch, which require recombination.
In our case, the development of BW lines from the cross KN × THES, enriched for grain carotenoid content and with a high contribution of lutein esters, would accomplish a double biofortification strategy based on high carotenoid (lutein) content and stability.
After the crossing and selection steps detailed in Figure 1, BW lines carrying either DW or BW alleles simultaneously for both Psy1-A1 and Psy1-B1 alleles were obtained for each cross (year 5). Carotenoid determination was carried out in the harvested seeds of each plant (Supplementary Table S2). For each cross, the genotypes were grouped in two contrasting groups, either carrying DW or BW alleles for Psy1-A1 and Psy1-B1 genes (KN × C29-DW vs. KN × C29-BW and KN × THES-DW vs. KN × THES-BW). The mean values for carotenoid content were determined, as shown in Table 2.

Table 2.
Mean carotenoid composition of progenies contrasting for Psy1-A1 and Psy1-B1 alleles.
For the cross KN × C29, we obtained thirteen breeding lines simultaneously carrying DW alleles for Psy1-A1 and Psy1-B1, and seven lines carrying the alternative BW alleles (Supplementary Table S2). On average, the KN × C29-DW progeny had 2.48 µg/g grain carotenoid content, with a range between 1.05 and 3.10 µg/g (Figure 3, Supplementary Table S2). In contrast, the KN × C29-BW progeny showed a mean carotenoid content of 2.01 µg/g, with a range between 1.66 and 2.73 µg/g. Thus, the selection strategy for DW alleles increased the total grain carotenoid content by 23% (p = 0.080) (Figure 3).
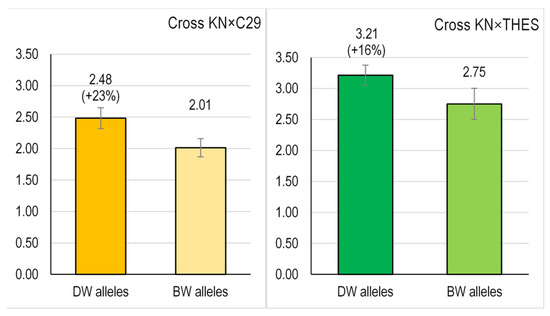
Figure 3.
Phenotypic effect of the simultaneous presence of DW alleles for Psy1-7A and Psy1-7B on the total grain carotenoid content (µg/g fresh weight) of bread wheat lines obtained in this work. For each cross, bars represent the mean total carotenoid content ± SE.
The same tendency was found in the KN × THES cross, where lineages carrying DW alleles (KN × THES-DW) outperformed those with BW alleles (KN × THES-BW) by 16% (mean values) (Figure 2). Indeed, the ten breeding lines with DW alleles yielded 3.21 µg/g of grain carotenoid mean content (range of 2.40–4.14 µg/g), whereas the seven lines with BW alleles showed a mean carotenoid content of 2.75 µg/g (p = 0.126) ranging from 1.66 to 3.52 (Figure 3). Considering both crosses, our results show an important effect of Psy1 alleles for total carotenoid content, since a breeding strategy focused exclusively in two genes allowed an enhancement of carotenoid content between 16 and 23%.
In addition to this, it is important to note that all the breeding lines from the cross KN × THES produced lutein esters. Carotenoid esterification is controlled by XAT-7D in bread wheat [49], and thus this result was expected, considering the carotenoid profile of the parental line 12THES4515 (Table 1) and that all the breeding lines obtained carried the chromosome 7D. Bread wheat lines without lutein esters, such as Haruhikari, have been previously described [31] and the absence of lutein esters in ES07H044SWC29 is not surprising.
Esterification is a mechanism for carotenoid accumulation and sequestration [50,51], and it has been related to the enhancement of carotenoid content [14]. Our results show that the progenies derived from the cross KN × THES had higher carotenoid content than those derived from the cross KN × C29 despite both ‘Kiko Nick’ and ES07H044SWC29 having almost double the carotenoid content of 12THES4515 (Table 1). These findings may be partly related to the esterification ability conferred by XAT-7D, that may improve carotenoid accumulation. However, the parental line 12THES4515 also carries a functional XAT-7D allele, but it has low carotenoid content. Thus, the higher carotenoid content of KN × THES progenies compared with KN × C29 suggests that other carotenoid-related genes are playing an important role in the cross KN × THES, in agreement with previous knowledge in wheat [6,8]. Indeed, a total of 124 significant marker-trait associations for lutein content were reported in common wheat, covering 18 out of 21 wheat chromosomes [7] and with six stable QTL on chromosomes 2AL, 2DS, 3BL, 3DL, 7AL and 7BS. Similar results have been obtained in durum wheat, with the identification of marker-trait associations for lutein and zeaxanthin grain content in several chromosomes [8]. These findings highlight the importance of other chromosome regions beyond Psy1 genes for lutein accumulation in wheat.
In addition to the presence of the D genome, durum and bread wheat differ in a small quality trait gene package which determines the distinct technological properties necessary for their different end-use products: pasta or couscous, and bread, respectively. These few quality traits, mainly controlled by major genes, include the puroindoline loci (Pin-a and Pin-b) related to grain hardness, gliadins (Gli) and glutenins (Glu) loci, which determine gluten properties, and the Psy1 gene, involved in carotenoid content/YPC reviewed by [52]. Through interspecific crosses, genes and/or alleles can be transferred in both directions to develop durum and bread wheat varieties with new or combined quality characteristics in order to obtain novel end-products, as demonstrated in this work.
Previous attempts have allowed the development of high lutein BW lines (>6 µg/g of lutein) [31], but to the best of our knowledge these lines have not reach commercial status. Similarly, highly pigmented yellow grains are obtained in the synthetic wheat program at CIMMYT and routinely discarded, since traditional consumer preferences are oriented towards white flour. In this context, the pre-breeding BW lines with durum wheat Psy1 alleles developed in this work constitute a good starting point towards the development of BW varieties with enhanced carotenoid content. In particular, pre-breeding lines with at least 3 µg/g of grain carotenoid content may cover an intermediate point between the current consumer preferences and the development of new products with creamy/yellow colour to fulfil new market demands. These pre-breeding lines outperform previous BW lines enriched in carotenoids [53] or other translocation lines developed throughout inter-specific breeding [34], where grain carotenoid content was 1.2 µg/g in the former case and below 2.1 µg/g in the latter, but they do not reach too much carotenoid content that may discourage potential consumers.
However, it is important to note the existence of an emerging market for yellow-pigmented bread grains. For instance, the renewed interest in the nutritional aspects of foods has promoted the development of new breeding programs for einkorn (Triticum monococcum L. subsp. monococcum) [54], whose flour has a higher carotenoid content than bread wheat. Similarly, the commercial success of the new crop × Tritordeum martinii A. Pujadas has been partly associated with its higher carotenoid content [55]. Indeed, the high lutein content provides the golden colour of tritordeum-derived products, which is one of the most highlighted characteristics of this new cereal (https://www.tritordeum.com/?lang=en#whatis, accessed on 3 February 2023) since this trait is highly appreciated by the consumers. In this case, the availability of wheat lines biofortified for lutein content is useful for the commercialization of wheat-derived products with enhanced nutritional quality. Indeed, some authors refer to lutein as ‘the eye vitamin’ [7] due to its role in the alleviation of eye disorders such as age-macular degradation. Accordingly, marketing strategies for wheat grain and flours should focus on lutein instead of β-carotene, as has been wrongly done in certain cases.
4. Conclusions
Psy1 genes have an important role in the determination of carotenoid content in wheat, although more attention should be paid to other genes of the carotenoid pathway. Inter-specific breeding between DW and BW, coupled with a MAS approach based exclusively on Psy1-A1 and Psy1-B1 alleles, has allowed the development of BW pre-breeding lines with enhanced grain carotenoid content (16–23% mean higher carotenoid content). This constitutes an interesting advance for wheat biofortification, considering that only two genes were subjected to selection. This approach offers new opportunities towards the development of new common wheat-derived products with a golden colour, as achieved in the new cereal crop tritordeum. Further evaluation of the agronomic performance should be performed in the future at several locations in order to determine their potential to become new varieties. In any case, they can be used as recurrent parents in BW breeding programs for carotenoid biofortification.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/foods12071365/s1, Table S1. Detailed carotenoid composition of the parental lines used in this work; Table S2. Detailed carotenoid composition and genotypic constitution for markers YP7A-2 and YP7B-4 of the BW lines obtained in this work through inter-specific hybridization.
Author Contributions
Conceptualization, S.G.A. and D.H.-M.; methodology, S.G.A., C.R.-S., D.H.-M. and M.D.R.-R.; investigation, M.D.R.-R., C.R.-S., C.M.Á., C.P., D.H.-M. and S.G.A.; writing—review and editing, M.D.R.-R., C.R.-S., C.M.Á., C.P., D.H.-M. and S.G.A.; funding acquisition, S.G.A. and D.H.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by projects AGL2017-85368-P and PID2021-122152NB-I00 funded by MCIN/AEI/10.13039/501100011033/ and by ERDF “ERDF A way of making Europe”. M.D.R.-R. was supported by PRE2018-084037 funded by MCIN/AEI/10.13039/501100011033 and ESF “ESF investing in your future”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and supplementary materials.
Acknowledgments
We thank J. C. Sillero, from the Spanish National Bread Wheat breeding program, for providing the seeds of the bread wheat parental lines used in this work.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Dexter, J.E.; Marchylo, B.A. Recent trends in durum wheat milling and pasta processing: Impact on durum wheat quality requirements. In Proceedings of the International Workshop on Durum Wheat, Semolina and Pasta Quality, Montpellier, France, 27 November 2000. [Google Scholar]
- Ficco, D.B.M.; Mastrangelo, A.M.; Trono, D.; Borrelli, G.M.; De Vita, P.; Fares, C.; Beleggia, R.; Platani, C.; Papa, R. The colours of durum wheat: A review. Crop Pasture Sci. 2014, 65, 1. [Google Scholar] [CrossRef]
- Digesù, A.M.; Platani, C.; Cattivelli, L.; Mangini, G.; Blanco, A. Genetic variability in yellow pigment components in cultivated and wild tetraploid wheats. J. Cereal Sci. 2009, 50, 210–218. [Google Scholar] [CrossRef]
- Parker, G.D.; Chalmers, K.J.; Rathjen, A.J.; Langridge, P. Mapping loci associated with flour colour in wheat (Triticum aestivum L.). Theor. Appl. Genet. 1998, 97, 238–245. [Google Scholar] [CrossRef]
- Elouafi, I.; Nachit, M.M.; Martin, L.M. Identification of a microsatellite on chromosome 7B showing a strong linkage with yellow pigment in durum wheat (Triticum turgidum L. var. durum). Hereditas 2004, 135, 255–261. [Google Scholar] [CrossRef]
- Colasuonno, P.; Marcotuli, I.; Blanco, A.; Maccaferri, M.; Condorelli, G.E.; Tuberosa, R.; Parada, R.; de Camargo, A.C.; Schwember, A.R.; Gadaleta, A. Carotenoid pigment content in durum wheat (Triticum turgidum L. var durum): An overview of quantitative trait loci and candidate genes. Front. Plant Sci. 2019, 10, 1347. [Google Scholar] [CrossRef]
- Guan, P.; Li, X.; Zhuang, L.; Wu, B.; Huang, J.; Zhao, J.; Qiao, L.; Zheng, J.; Hao, C.; Zheng, X. Genetic dissection of lutein content in common wheat via association and linkage mapping. Theor. Appl. Genet. 2022, 135, 3127–3141. [Google Scholar] [CrossRef]
- Requena-Ramírez, M.D.; Rodríguez-Suárez, C.; Flores, F.; Hornero-Méndez, D.; Atienza, S.G. Marker-trait associations for total carotenoid content and individual carotenoids in durum wheat identified by genome-wide association analysis. Plants 2022, 11, 2065. [Google Scholar] [CrossRef]
- Atienza, S.G.; Avila, C.M.; Martín, A. The development of a PCR-based marker for PSY1 from Hordeum chilense, a candidate gene for carotenoid content accumulation in tritordeum seeds. Aust. J. Agric. Res. 2007, 58, 767–773. [Google Scholar] [CrossRef]
- Pozniak, C.J.; Knox, R.E.; Clarke, F.R.; Clarke, J.M. Identification of QTL and association of a phytoene synthase gene with endosperm colour in durum wheat. Theor. Appl. Genet. 2007, 114, 525–537. [Google Scholar] [CrossRef]
- Patil, R.M.; Oak, M.D.; Tamhankar, S.A.; Sourdille, P.; Rao, V.S. Mapping and validation of a major QTL for yellow pigment content on 7AL in durum wheat (Triticum turgidum L. ssp durum). Mol. Breed. 2008, 21, 485–496. [Google Scholar] [CrossRef]
- Zhang, W.; Chao, S.; Manthey, F.; Chicaiza, O.; Brevis, J.C.; Echenique, V.; Dubcovsky, J. QTL analysis of pasta quality using a composite microsatellite and SNP map of durum wheat. Theor. Appl. Genet. 2008, 117, 1361–1377. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Wu, Y.P.; Xiao, Y.G.; He, Z.H.; Zhang, Y.; Yan, J.; Xia, X.C.; Ma, C.X. QTL mapping for flour and noodle colour components and yellow pigment content in common wheat. Euphytica 2009, 165, 435–444. [Google Scholar] [CrossRef]
- Rodriguez-Concepcion, M.; Avalos, J.; Bonet, M.L.; Boronat, A.; Gomez-Gomez, L.; Hornero-Mendez, D.; Limon, M.C.; Meléndez-Martínez, A.J.; Olmedilla-Alonso, B.; Palou, A.; et al. A global perspective on carotenoids: Metabolism, biotechnology, and benefits for nutrition and health. Prog. Lipid Res. 2018, 70, 62–93. [Google Scholar] [CrossRef]
- Giuliano, G.; Tavazza, R.; Diretto, G.; Beyer, P.; Taylor, M.A. Metabolic engineering of carotenoid biosynthesis in plants. Trends Biotech. 2008, 26, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.J. Role of lutein and zeaxanthin in visual and cognitive function throughout the lifespan. Nutr. Rev. 2014, 72, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Suárez, C.; Giménez, M.J.; Atienza, S.G. Progress and perspectives for carotenoid accumulation in selected Triticeae species. Crop Pasture Sci. 2010, 61, 743. [Google Scholar] [CrossRef]
- Hirschberg, J. Carotenoid biosynthesis in flowering plants. Curr. Opin. Plant Biol. 2001, 4, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Fraser, P.D.; Truesdale, M.R.; Bird, C.R.; Schuch, W.; Bramley, P.M. Carotenoid biosynthesis during tomato fruit development (evidence fort-specific gene expression). Plant Physiol. 1994, 105, 405–413. [Google Scholar] [CrossRef]
- Fraser, P.D.; Bramley, P.M. The biosynthesis and nutritional uses of carotenoids. Prog. Lipid Res. 2004, 43, 228–265. [Google Scholar] [CrossRef]
- Paine, J.A.; Shipton, C.A.; Chaggar, S.; Howells, R.M.; Kennedy, M.J.; Vernon, G.; Wright, S.Y.; Hinchlife, E.; Adams, J.L.; Silverstone, A.L.; et al. Improving the nutritional value of Golden Rice through increased pro-vitamin A content. Nat. Biotechnol. 2005, 23, 482–487. [Google Scholar] [CrossRef]
- Li, F.; Tsfadia, O.; Wurtzel, E.T. The phytoene synthase gene family in the Grasses: Subfunctionalization provides tissue-specific control of carotenogenesis. Plant Signal. Behav. 2009, 4, 208–211. [Google Scholar] [CrossRef] [PubMed]
- He, X.Y.; Zhang, Y.L.; He, Z.H.; Wu, Y.P.; Xiao, Y.G.; Ma, C.X.; Xia, X.C. Characterization of phytoene synthase 1 gene (Psy1) located on common wheat chromosome 7A and development of a functional marker. Theor. Appl. Genet. 2008, 116, 213–221. [Google Scholar] [CrossRef]
- He, X.Y.; He, Z.H.; Ma, W.; Appels, R.; Xia, X.C. Allelic variants of phytoene synthase 1 (Psy1) genes in Chinese and CIMMYT wheat cultivars and development of functional markers for flour colour. Mol. Breed. 2009, 23, 553–563. [Google Scholar] [CrossRef]
- He, X.Y.; Wang, J.W.; Ammar, K.; Pena, R.J.; Xia, X.C.; He, Z.H. Allelic variants at the Psy-A1 and Psy-B1 loci in durum wheat and their associations with grain yellowness. Crop Sci. 2009, 49, 2058–2064. [Google Scholar] [CrossRef]
- Murray, M.G.; Thompson, W.F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980, 8, 4321–4326. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Suárez, C.; Requena-Ramírez, M.D.; Hornero-Méndez, D.; Atienza, S.G. The breeder’s tool-box for enhancing the content of esterified carotenoids in wheat: From extraction and profiling of carotenoids to marker-assisted selection of candidate genes. In Carotenoids: Carotenoid and Apocarotenoid Biosynthesis, Metabolic Engineering and Synthetic Biology; Wurtzel, E.T., Ed.; Elsevier Academic Press: Amsterdam, The Netherlands, 2022; ISBN 9780323913539. [Google Scholar]
- Mellado-Ortega, E.; Hornero-Méndez, D. Carotenoid evolution during short-storage period of durum wheat (Triticum turgidum conv. durum) and tritordeum (×Tritordeum Ascherson et Graebner) whole-grain flours. Food Chem. 2016, 192, 714–723. [Google Scholar] [CrossRef]
- Mínguez-Mosquera, M.I.; Hornero-Méndez, D. Separation and quantification of the carotenoid pigments in red peppers (Capsicum annuum L.), paprika and oleoresin by reversed-phase HPLC. J. Agric. Food Chem. 1993, 41, 1616–1620. [Google Scholar] [CrossRef]
- Wasserstein, R.L.; Schirm, A.L.; Lazar, N.A. Moving to a World Beyond “p < 0.05”. Am. Stat. 2019, 73, 1–19. [Google Scholar] [CrossRef]
- Ahmad, F.T.; Mather, D.E.; Law, H.-Y.; Li, M.; Yousif, S.A.-J.; Chalmers, K.J.; Asenstorfer, R.E.; Mares, D.J. Genetic control of lutein esterification in wheat (Triticum aestivum L.) grain. J. Cereal Sci. 2015, 64, 109–115. [Google Scholar] [CrossRef]
- Atienza, S.G.; Ballesteros, J.; Martin, A.; Hornero-Mendez, D. Genetic variability of carotenoid concentration and degree of esterification among tritordeum (×Tritordeum Ascherson et Graebner) and durum wheat accessions. J. Agric. Food Chem. 2007, 55, 4244–4251. [Google Scholar] [CrossRef]
- Fratianni, A.; Irano, M.; Panfili, G.; Acquistucci, R. Estimation of color of durum wheat. Comparison of WSB, HPLC, and reflectance colorimeter measurements. J. Agric. Food Chem. 2005, 53, 2373–2378. [Google Scholar] [CrossRef]
- Mattera, M.G.; Cabrera, A.; Hornero-Méndez, D.; Atienza, S.G. Lutein esterification in wheat endosperm is controlled by the homoeologous group 7, and is increased by the simultaneous presence of chromosomes 7D and 7Hch from Hordeum chilense. Crop Pasture Sci. 2015, 66, 912–921. [Google Scholar] [CrossRef]
- Abdel-Aal, E.-S.M.; Young, J.C.; Rabalski, I.; Hucl, P.; Fregeau-Reid, J. Identification and quantification of seed carotenoids in selected wheat species. J. Agric. Food Chem. 2007, 55, 787–794. [Google Scholar] [CrossRef]
- Kaneko, S.; Nagamine, T.; Yamada, T. Esterification of endosperm lutein with fatty acids during the storage of wheat seeds. Biosci. Biotechnol. Biochem. 1995, 59, 1–4. [Google Scholar] [CrossRef]
- Mellado-Ortega, E.; Hornero-Méndez, D. Lutein esterification in wheat flour increases the carotenoid retention and is induced by storage temperatures. Foods 2017, 6, 111. [Google Scholar] [CrossRef] [PubMed]
- Watkins, J.L.; Pogson, B.J. Prospects for carotenoid biofortification targeting retention and catabolism. Trends Plant Sci. 2020, 25, 501–512. [Google Scholar] [CrossRef]
- Ahmad, F.T.; Asenstorfer, R.E.; Soriano, I.R.; Mares, D.J. Effect of temperature on lutein esterification and lutein stability in wheat grain. J. Cereal Sci. 2013, 58, 408–413. [Google Scholar] [CrossRef]
- Mellado-Ortega, E.; Atienza, S.G.; Hornero-Méndez, D. Carotenoid evolution during postharvest storage of durum wheat (Triticum turgidum conv. durum) and tritordeum (×Tritordeum Ascherson et Graebner) grains. J. Cereal Sci. 2015, 62, 134–142. [Google Scholar] [CrossRef]
- Mellado-Ortega, E.; Hornero-Méndez, D. Effect of long-term storage on the free and esterified carotenoids in durum wheat (Triticum turgidum conv. durum) and tritordeum (×Tritordeum Ascherson et Graebner) grains. Food Res. Int. 2017, 99, 877–890. [Google Scholar] [CrossRef]
- Burešová, B.; Paznocht, L.; Jarošová, V.; Doskočil, I.; Martinek, P. The effect of boiling and in vitro digestion on the carotenoid content of colored-grain wheat. J. Food Compos. Anal. 2023, 115, 105002. [Google Scholar] [CrossRef]
- Paznocht, L.; Burešová, B.; Kotíková, Z.; Martinek, P. Carotenoid content of extruded and puffed products made of colored-grain wheats. Food Chem. 2021, 340, 127951. [Google Scholar] [CrossRef] [PubMed]
- Mares, D.J.; Cheong, J.; Goonetilleke, S.N.; Mather, D.E. Lipoxygenase in wheat: Genetic control and impact on stability of lutein and lutein esters. Foods 2021, 10, 1149. [Google Scholar] [CrossRef] [PubMed]
- Watkins, J.; Pogson, B.; Mather, D. Available online: https://plantae.org/xat-catalyzes-carotenoid-esterification-in-wheat/ (accessed on 14 March 2023).
- Requena-Ramírez, M.D.; Atienza, S.G.; Hornero-Méndez, D.; Rodríguez-Suárez, C. Mediation of a GDSL esterase/lipase in carotenoid esterification in tritordeum suggests a common mechanism of carotenoid esterification in Triticeae species. Front. Plant Sci. 2020, 11, 2032. [Google Scholar] [CrossRef]
- Mattera, M.G.; Cabrera, A. Characterization of a set of common wheat–Hordeum chilense chromosome 7Hch introgression lines and its potential use in research on grain quality traits. Plant Breed. 2017, 136, 344–350. [Google Scholar] [CrossRef]
- Requena-Ramírez, M.D.; Hornero-Méndez, D.; Rodríguez-Suárez, C.; Atienza, S.G. Durum wheat (Triticum durum L.) landraces reveal potential for the improvement of grain carotenoid esterification in breeding programs. Foods 2021, 10, 757. [Google Scholar] [CrossRef] [PubMed]
- Watkins, J.L.; Li, M.; McQuinn, R.P.; Chan, K.X.; McFarlane, H.E.; Ermakova, M.; Furbank, R.T.; Mares, D.J.; Dong, C.; Chalmers, K.J.; et al. A GDSL esterase/lipase catalyzes the esterification of lutein in bread wheat. Plant Cell 2019, 31, 3092–3112. [Google Scholar] [CrossRef]
- Berry, H.M.; Rickett, D.V.; Baxter, C.J.; Enfissi, E.M.A.; Fraser, P.D. Carotenoid biosynthesis and sequestration in red chilli pepper fruit and its impact on colour intensity traits. J. Exp. Bot. 2019, 70, 2637–2650. [Google Scholar] [CrossRef]
- Hornero-Méndez, D. CHAPTER 7: Occurrence of Carotenoid Esters in Foods. In Carotenoid Esters in Foods: Physical, Chemical and Biological Properties; Mercadante, A.Z., Ed.; Royal Society of Chemistry: London, UK, 2019; pp. 182–284. ISBN 978178801242. [Google Scholar]
- Mastrangelo, A.M.; Cattivelli, L. What makes bread and durum wheat different? Trends Plant Sci. 2021, 26, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Rey, M.-D.; Calderón, M.-C.; Rodrigo, M.J.; Zacarías, L.; Alós, E.; Prieto, P. Novel bread wheat lines enriched in carotenoids carrying Hordeum chilense chromosome arms in the ph1b background. PLoS ONE 2015, 10, e0134598. [Google Scholar] [CrossRef]
- Brandolini, A.; Hidalgo, A. Einkorn (Triticum monococcum) flour and bread. In Flour and Breads Their Fortification in Health and Disease Prevention; Preedy, V.R., Ed.; Elsevier Academic Press: Amsterdam, The Netherlands, 2011; pp. 79–88. ISBN 978-0-12-380886-8. [Google Scholar] [CrossRef]
- Ávila, C.M.; Rodríguez-Suárez, C.; Atienza, S.G. Tritordeum: Creating a new crop species—The successful use of plant Genetic Resources. Plants 2021, 10, 1029. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).