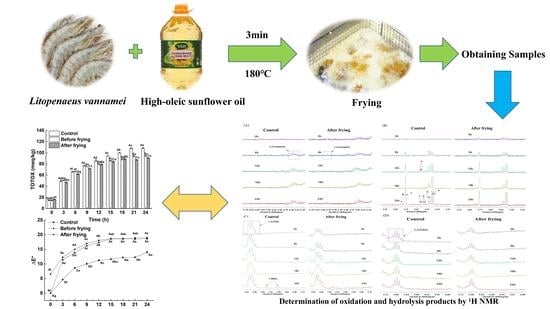
Changes in the Quality of High-Oleic Sunflower Oil during the Frying of Shrimp (Litopenaeus vannamei)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Sample Preparation
2.3. Determination of Moisture and Fat Content
2.4. Determination of Oil Color
2.5. Determination of Acid Value (AV)
2.6. Determination of Carbonyl Value (CV)
2.7. Determination of Total Oxidation (TOTOX) Value
2.8. Proton Nuclear Magnetic Resonance (1H NMR) Spectroscopy
2.9. Statistical Analysis
3. Results and Discussion
3.1. Components of Litopenaeus vannamei
3.2. Effect of Frying Time on the Color of Frying Oil
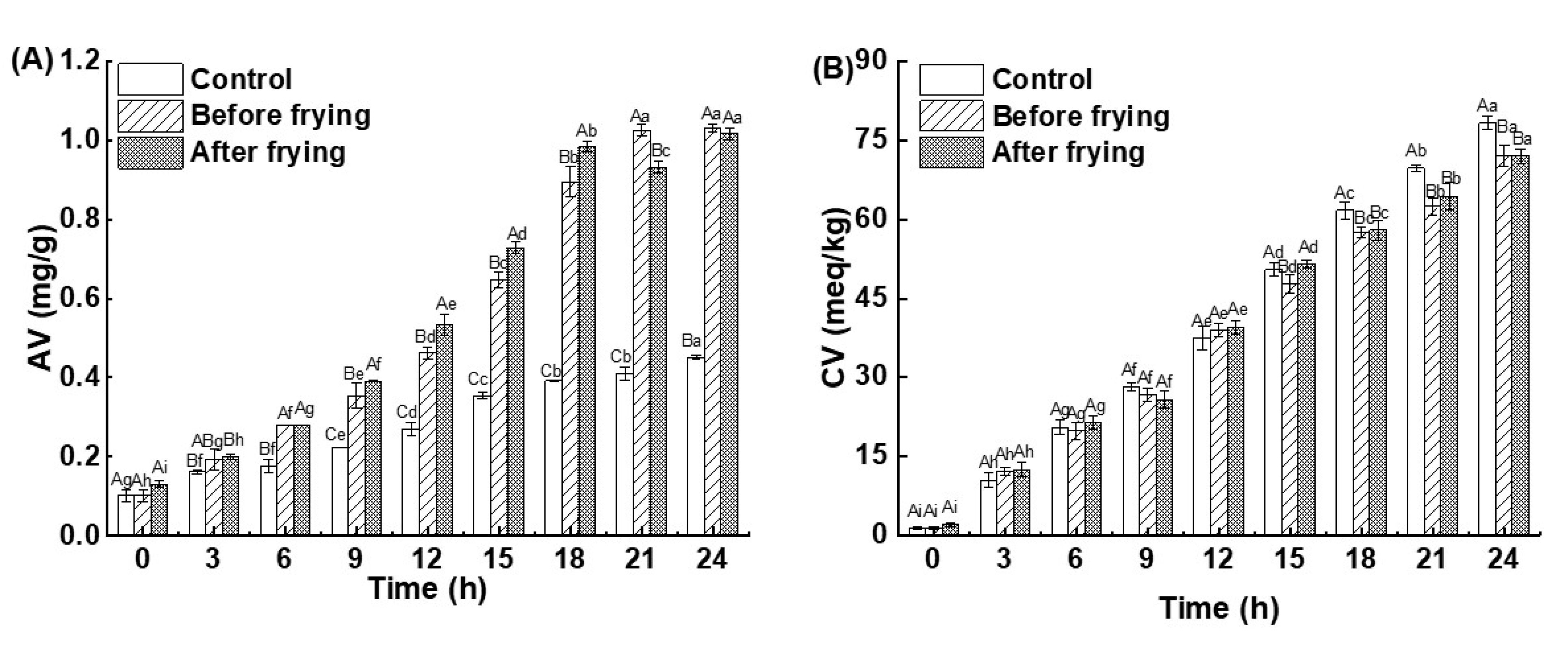
3.3. Effect of Frying Time on the AV and CV of Oil
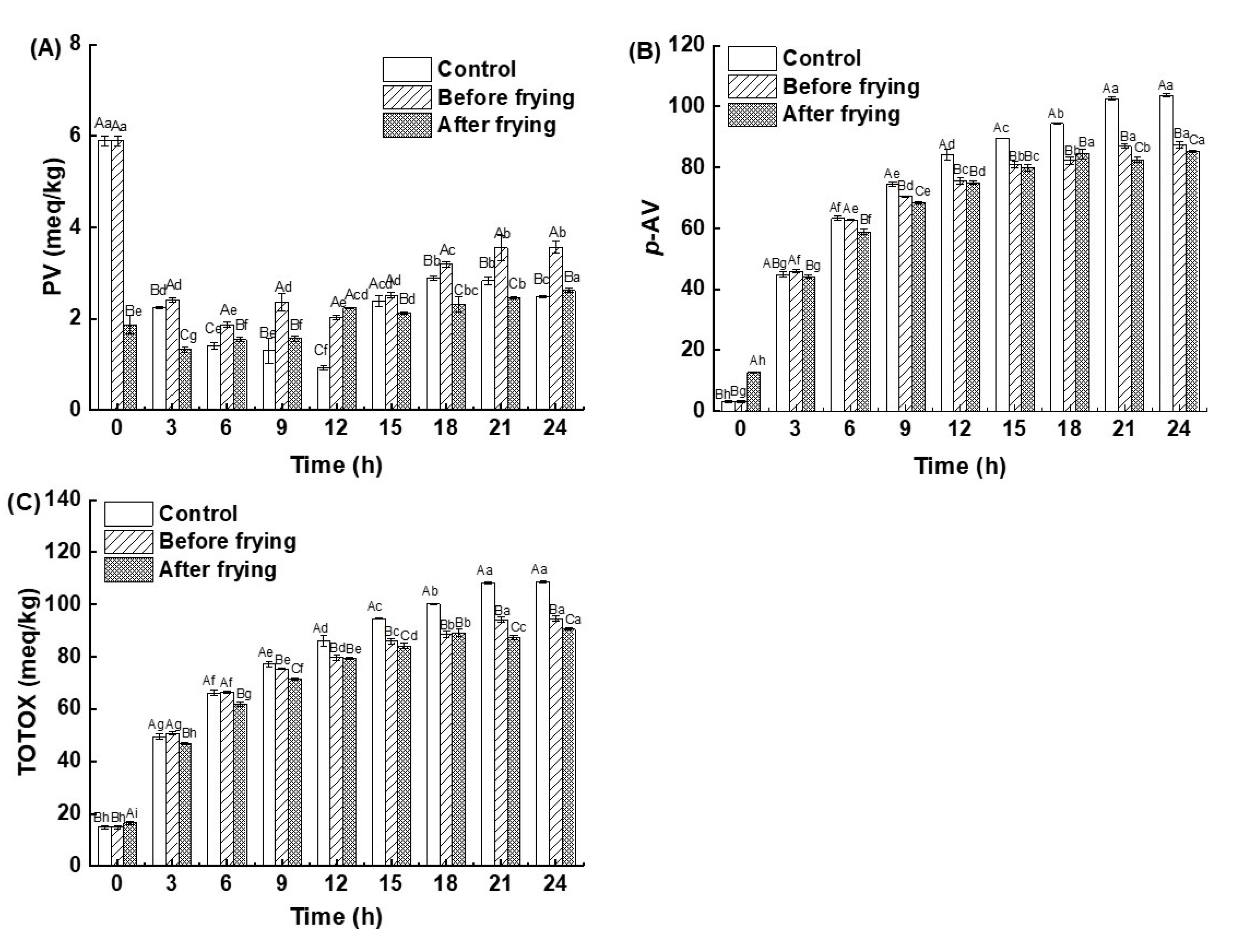
3.4. Determination of Total Oxidation (TOTOX) Value
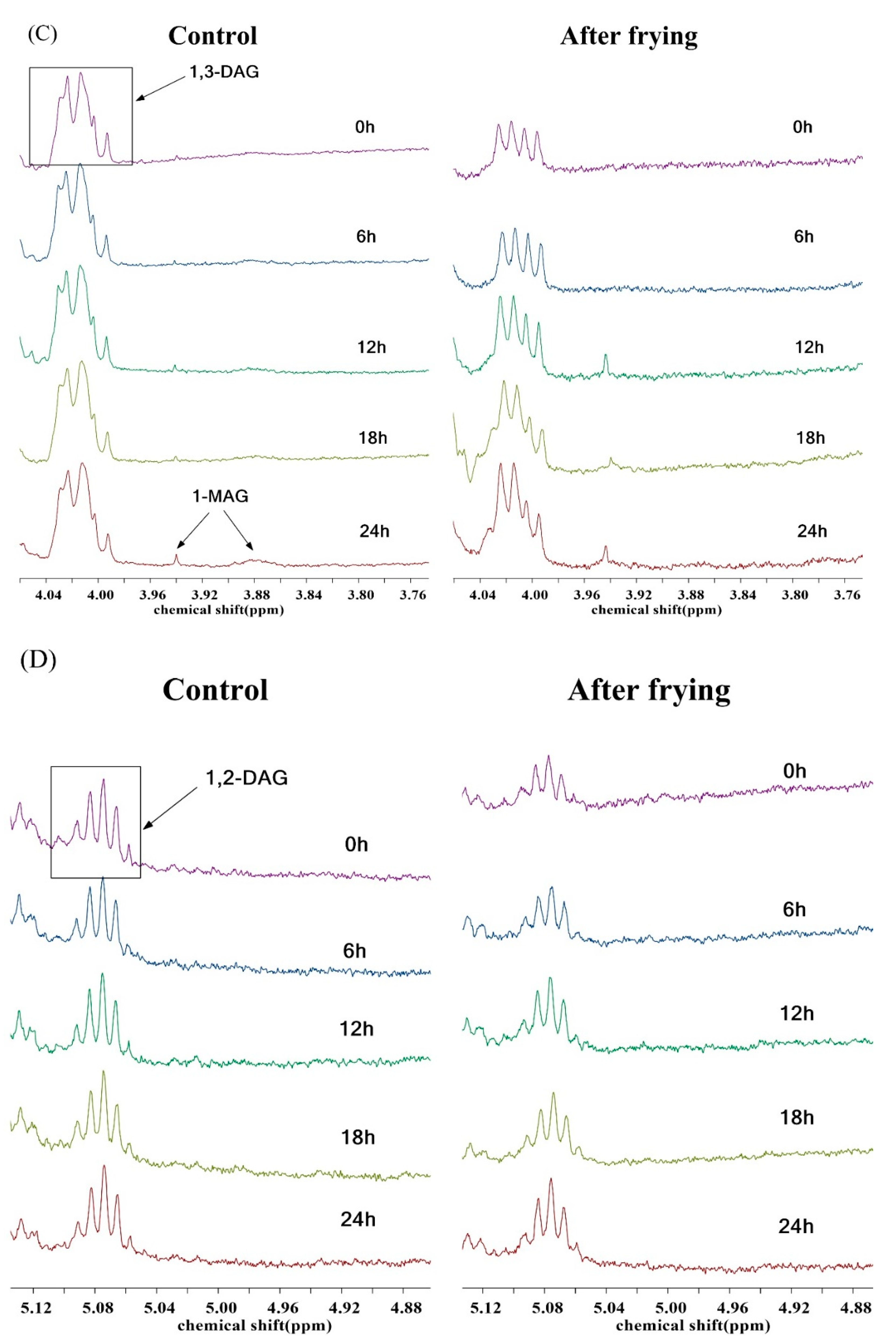
3.5. 1H NMR Study during Deep-Frying
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ahromrit, A.; Nema, P.K. Heat and mass transfer in deep-frying of pumpkin, sweet potato and taro. J. Food Sci. Technol. 2010, 47, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Bou, R.; Navas, J.A.; Tres, A.; Codony, R.; Guardiola, F. Quality assessment of frying fats and fried snacks during continuous deep-fat frying at different large-scale producers. Food Control 2012, 27, 254–267. [Google Scholar] [CrossRef]
- Houhoula, D.P.; Oreopoulou, V.; Tzia, C. The effect of process time and temperature on the accumulation of polar compounds in cottonseed oil during deep-fat frying. J. Sci. Food Agric. 2003, 83, 314–319. [Google Scholar] [CrossRef]
- Onacik-Gür, S.; Żbikowska, A. Effect of high-oleic rapeseed oil oleogels on the quality of short-dough biscuits and fat migration. J. Food Sci. Technol. 2019, 57, 1609–1618. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Shi, X.; Wang, Z.; Gao, W.; Jiang, L.; Xiao, Q.; Liu, X.; Deng, F. Effects of metal ions on formation of acrylamide and 5-hydroxymethylfurfural in asparagine-glucose model system. Int. J. Food Sci. Technol. 2015, 51, 279–285. [Google Scholar] [CrossRef]
- Farhoosh, R.; Tavassoli-Kafrani, M.H. Simultaneous monitoring of the conventional qualitative indicators during frying of sunflower oil. Food Chem. 2011, 125, 209–213. [Google Scholar] [CrossRef]
- Uchida, K.; Shiraishi, M.; Naito, Y.; Torii, Y.; Nakamura, Y.; Osawa, T. Activation of stress signaling pathways by the end product of lipid peroxidation: 4-hydroxy-2-nonenal is a potential inducer of intracellular peroxide production. J. Biol. Chem. 1999, 274, 2234–2242. [Google Scholar] [CrossRef]
- Zarkovic, N. 4-Hydroxynonenal as a bioactive marker of pathophysiological processes. Mol. Asp. Med. 2003, 24, 281–291. [Google Scholar] [CrossRef]
- Niemela, J.R.K.; Wester, I.; Lahtinen, R.M. Industrial frying trials with high oleic sunflower oil. Grasas Y Aceites 1996, 47, 1–4. [Google Scholar] [CrossRef]
- Roman, O.; Heyd, B.; Broyart, B.; Castillo, R.; Maillard, M.-N. Oxidative reactivity of unsaturated fatty acids from sunflower, high oleic sunflower and rapeseed oils subjected to heat treatment, under controlled conditions. LWT-Food Sci. Technol. 2013, 52, 49–59. [Google Scholar] [CrossRef]
- Guillén, M.D.; Ruiz, A. Monitoring of heat-induced degradation of edible oils by proton NMR. Eur. J. Lipid Sci. Technol. 2008, 110, 52–60. [Google Scholar] [CrossRef]
- Abdulkarim, S.M.; Long, K.; Lai, O.M.; Muhammad, S.K.S.; Ghazali, H.M. Frying quality and stability of high-oleic Moringa oleifera seed oil in comparison with other vegetable oils. Food Chem. 2007, 105, 1382–1389. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific opinion on dietary reference values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA J. 2010, 8, 1461. [Google Scholar]
- Sayyad, R. Effects of deep-fat frying process on the oil quality during French fries preparation. J. Food Sci. Technol. 2017, 54, 2224–2229. [Google Scholar] [CrossRef]
- Horwitz, W.; Latimer, G. Official Methods of Analysis; Association of Official Analytical Chemists Washington: Washington, DC, USA, 1975; Volume 222. [Google Scholar]
- Bansal, G.; Zhou, W.; Barlow, P.J.; Lo, H.-L.; Neo, F.-L. Performance of palm olein in repeated deep frying and controlled heating processes. Food Chem. 2010, 121, 338–347. [Google Scholar] [CrossRef]
- AOCS. Official Method Cd 3d-63. In Acid value of fats and oils, official methods and recommended practices of the AOCS; American Oil Chemists Society Press: Champaign, IL, USA, 2017. [Google Scholar]
- Farhoosh, R.; Moosavi, S.M.R. Determination of carbonyl value in rancid oils: A critical reconsideration. J. Food Lipids 2006, 13, 298–305. [Google Scholar] [CrossRef]
- Shantha, N.C.; Decker, E.A. Rapid, sensitive, iron-based spectrophotometric methods for determination of peroxide values of food lipids. J. AOAC Int. 1994, 77, 421–424. [Google Scholar] [CrossRef]
- AOCS. AOCS Official Method Cd 18-90: P-Anisidine Value. In Official Methods and Recommended Practices of the American Oil Chemists’ Society; AOCS Press: Champaign, IL, USA, 1996. [Google Scholar]
- Chapman, K.W.; Sagi, I.; Regenstein, J.M.; Bimbo, T.; Crowther, J.B.; Stauffer, C.E. Oxidative stability of hydrogenated menhaden oil shortening blends in cookies, crackers, and snacks. J. Am. Oil Chem. Soc. 1996, 73, 167–172. [Google Scholar] [CrossRef]
- Jia, C.; Zhang, M.; Ma, W.; Li, J.; Zhao, S.; Xiong, S.; Hu, X.; Li, X. Evaluation of antioxidant properties of the different tissues of vine tea (Ampelopsis grossedentata) in stripped canola oil and sunflower oil. J. Food Sci. 2020, 85, 1082–1089. [Google Scholar] [CrossRef]
- Guillén, M.D.; Ruiz, A. Rapid simultaneous determination by proton NMR of unsaturation and composition of acyl groups in vegetable oils. Eur. J. Lipid Sci. Technol. 2003, 105, 688–696. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, J.; Choe, E. Effects of flour storage conditions on the lipid oxidation of fried products during storage in the dark. Food Sci. Biotechnol. 2006, 15, 399–403. [Google Scholar]
- Quintana-López, A.; Hurtado-Oliva, M.A.; Manzano-Sarabia, M.; López-Peraza, D.J.; Hernández, C.; García, A.; Palacios, E. Effect of rearing conditions on astaxanthin accumulation in the white shrimp Penaeus vannamei (Boone, 1931). Lat. Am. J. Aquat. Res. 2019, 47, 303–309. [Google Scholar] [CrossRef]
- Pu, J.; Sathivel, S. Kinetics of Lipid Oxidation and Degradation of Flaxseed Oil Containing Crawfish (Procambarus clarkii) Astaxanthin. J. Am. Oil Chem. Soc. 2010, 88, 595–601. [Google Scholar] [CrossRef]
- Warner, K.; Dunlap, C. Effects of expeller-pressed/physically refined soybean oil on frying oil stability and flavor of french-fried potatoes. J. Am. Oil Chem. Soc. 2006, 83, 435–441. [Google Scholar] [CrossRef]
- Aladedunye, F.; Przybylski, R.; Matthaus, B. Performance of antioxidative compounds under frying conditions: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 1539–1561. [Google Scholar] [CrossRef]
- Berrios, M.; Siles, J.; Martin, M.; Martin, A. A kinetic study of the esterification of free fatty acids (FFA) in sunflower oil. Fuel 2007, 86, 2383–2388. [Google Scholar] [CrossRef]
- Ma, Y.; Hou, L.; Liu, Y.; Wang, Y. Quality changes of corn oil during frying dough sticks. Food Mach. 2016, 32, 16–19. [Google Scholar]
- Dongrui, L.I.; Yanlan, B.I.; Xinsheng, X.; Guolong, Y.; Dandan, C.A.O. Research on the change of edible oil quality during frying process. China Oils Fats 2006, 31, 34–36. [Google Scholar]
- Farhoosh, R.; Khodaparast, M.H.H.; Sharif, A.; Hoseini-Yazdi, S.Z.; Zamani-Ghalehshahi, A. Antioxidative and synergistic effects of bene kernel and hull oils during oxidation of virgin olive oil. Eur. J. Lipid Sci. Technol. 2012, 114, 1284–1291. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, Y.; Liang, J. Home Cooking Oxidative Stability of n-3 LC-PUFA Enriched Blended Oil. J. Chin. Cereals Oils Assoc. 2012, 27, 57. [Google Scholar]
- Shahidi, F.; Wanasundara, U.N. Methods for measuring oxidative rancidity in fats and oils. Food Lipids Chem. Nutr. Biotechnol. 2002, 3, 387–403. [Google Scholar]
- Swern, D. Bailey’s Industrial Oil and Fat Products, 4th ed.; John Wiley & Sons Inc.: New York, NY, USA, 1979; Volume 1. [Google Scholar]
- Pokorný, J.; Kovářová, H.; Voženílková, B.; Marcïn, A.; Davídek, J. Effect of frying oil on the quality of fried chicken muscle. Food/Nahrung 1982, 26, 681–687. [Google Scholar] [CrossRef]
- Xiaoli, Z.; Liu, J. Review on Antioxidant Effects of Astaxanthin and Its Application in Nutriology and Pharmacology. Food Sci. 2006, 27, 258–262. [Google Scholar]
- Dong, Z.; Wang, Q.; Sun, L.; Li, X.; Chen, H.; Mu, W.; Li, Y.; Li, J. Effect of heat treatment methods on the astaxanthin content, the amino acid composition and the fatty acid composition of Penaeus vannamei. Sci. Technol. Food Ind. 2017, 38, 14. [Google Scholar]
- Alimentarius, C. Codex standard for named vegetable oils. Codex Stan 1999, 210, 1–13. [Google Scholar]
- Alireza, S.; Tan, C.P.; Hamed, M.; Che Man, Y. Effect of frying process on fatty acid composition and iodine value of selected vegetable oils and their blends. Int. Food Res. J. 2010, 17, 295–302. [Google Scholar]
- Goicoechea, E.; Guillen, M.D. Analysis of hydroperoxides, aldehydes and epoxides by 1H nuclear magnetic resonance in sunflower oil oxidized at 70 and 100 C. J. Agric. Food Chem. 2010, 58, 6234–6245. [Google Scholar] [CrossRef]
- Nieva-Echevarría, B.; Goicoechea, E.; Manzanos, M.J.; Guillén, M.D. 1H NMR and SPME-GC/MS study of hydrolysis, oxidation and other reactions occurring during in vitro digestion of non-oxidized and oxidized sunflower oil. Formation of hydroxy-octadecadienoates. Food Res. Int. 2017, 91, 171–182. [Google Scholar] [CrossRef]
- Frankel, E.N. Recent advances in lipid oxidation. J. Sci. Food Agric. 1991, 54, 495–511. [Google Scholar] [CrossRef]
- Guillén, M.D.; Ruiz, A. Study by means of 1H nuclear magnetic resonance of the oxidation process undergone by edible oils of different natures submitted to microwave action. Food Chem. 2006, 96, 665–674. [Google Scholar] [CrossRef]
- Li, D.; Xie, H.; Liu, Z.; Li, A.; Li, J.; Liu, B.; Liu, X.; Zhou, D. Shelf life prediction and changes in lipid profiles of dried shrimp (Penaeus vannamei) during accelerated storage. Food Chem. 2019, 297, 124951. [Google Scholar] [CrossRef] [PubMed]
- Marmesat, S.; Velasco, L.; Ruiz-Méndez, M.V.; Fernández-Martínez, J.M.; Dobarganes, C. Thermostability of genetically modified sunflower oils differing in fatty acid and tocopherol compositions. Eur. J. Lipid Sci. Technol. 2008, 110, 776–782. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, X.; Huang, J.; Zhao, C.; Qi, J.; Jin, Q.; Wang, X. Correlations between polycyclic aromatic hydrocarbons and polar components in edible oils during deep frying of peanuts. Food Control. 2018, 87, 109–116. [Google Scholar] [CrossRef]
- Zhang, Q.; Saleh, A.S.M.; Chen, J.; Shen, Q. Chemical alterations taken place during deep-fat frying based on certain reaction products: A review. Chem. Phys. Lipids 2012, 165, 662–681. [Google Scholar] [CrossRef] [PubMed]
- Dobarganes, C.; Márquez-Ruiz, G. Analysis of used frying oils. Lipid Technol. 2013, 25, 159–162. [Google Scholar] [CrossRef]
- Bordin, K.; Tomihe Kunitake, M.; Kazue Aracava, K.; Silvia Favaro Trindade, C. Changes in food caused by deep fat frying-A review. Arch. Latinoam. Nutr. 2013, 63, 5–13. [Google Scholar]
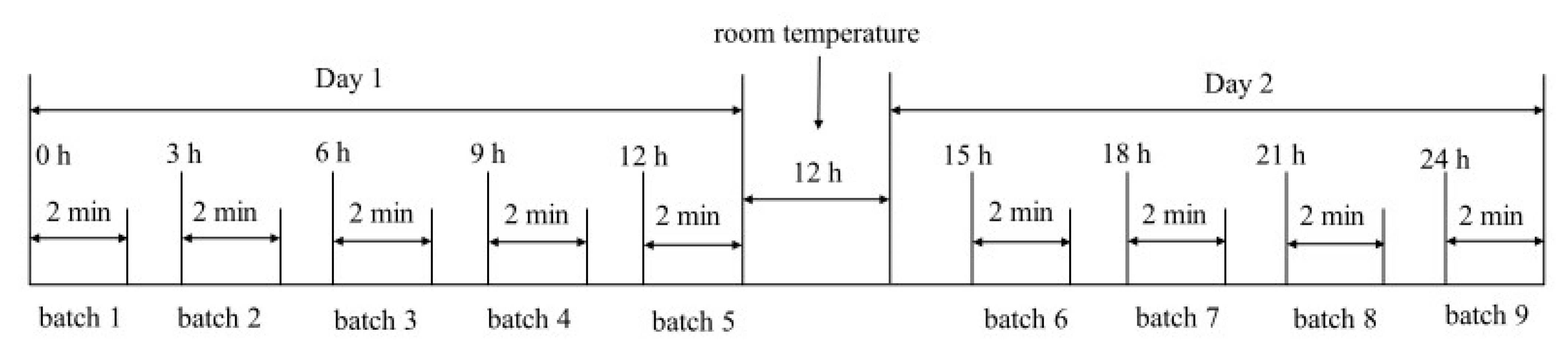



| Batch | Oil Volume (L) | Weight of Shrimp (kg) |
|---|---|---|
| 1 | 6.0 | 0.40 |
| 2 | 5.7 | 0.38 |
| 3 | 5.4 | 0.36 |
| 4 | 5.1 | 0.34 |
| 5 | 4.8 | 0.32 |
| 6 | 4.5 | 0.30 |
| 7 | 4.2 | 0.28 |
| 8 | 3.9 | 0.26 |
| 9 | 3.6 | 0.24 |
| Time/h | C18:1 | C18:2 | C18:3 | Saturated Fatty Acid | |
|---|---|---|---|---|---|
| Control | 0 | 77.42 ± 0.23 b | 11.8 ± 0.01 a | 1.04 ± 0.05 a | 9.75 ± 0.18 a |
| 6 | 77.43 ± 0.02 b | 11.4 ± 0.02 a | 0.82 ± 0.01 b | 10.34 ± 0.02 a | |
| 12 | 77.80 ± 1.79 ab | 10.23 ± 0.20 b | 1.04 ± 0.08 a | 10.94 ± 2.07 a | |
| 18 | 78.97 ± 0.43 a | 9.45 ± 0.12 c | 1.09 ± 0.15 a | 10.49 ± 0.45 a | |
| 24 | 78.67 ± 0.36 ab | 8.95 ± 0.56 c | 0.98 ± 0.16 a | 11.39 ± 0.04 a | |
| Frying | 0 | 75.78 ± 0.08 d | 12.22 ± 0.41 a | 0.95 ± 0.02 ab | 11.06 ± 0.50 a |
| 6 | 76.97 ± 0.36 c | 11.17 ± 0.07 a | 0.88 ± 0.03 c | 10.98 ± 0.31 a | |
| 12 | 78.57 ± 0.49 b | 12.26 ± 2.25 a | 0.97 ± 0.05 a | 8.20 ± 1.70 b | |
| 18 | 78.84 ± 0.16 b | 10.5 ± 0.94 ab | 0.9 ± 0.00 bc | 9.76 ± 1.10 ab | |
| 24 | 80.05 ± 0.19 a | 8.79 ± 0.25 b | 0.87 ± 0.03 c | 10.29 ± 0.03 a |
| Primary Oxidation Products | Secondary Oxidation Products | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Time/h | E,E-Conjugated Hydroperoxides | Z,E-Conjugated Hydroperoxides | (E)-2-Alkenals | (E,E)-2,4-Alkadienals | n-Alkanals | 4-Hydroxy-(E)-2-alkenals + 4-Hydroperoxy-(E)-2-alkenals | 4,5-Epoxy-(E)-2-alkenals | 4-Oxoalkanals | |
| Control | 0 | ND | ND | ND | ND | ND | ND | ND | ND |
| 6 | 1.90 ± 0.04 b | ND | 1.49 ± 0.02 d | 1.52 ± 0.12 b | 1.28 ± 0.02 d | 0.54 ± 0.02 a | 0.25 ± 0.01 c | ND | |
| 12 | 2.62 ± 0.36 ab | ND | 3.28 ± 0.08 c | 1.98 ± 0.06 a | 3.06 ± 0.10 c | 0.56 ± 0.01 a | 0.25 ± 0.01 c | 0.12 ± 0.02 c | |
| 18 | 2.62 ± 0.24 ab | ND | 5.14 ± 0.06 b | 1.96 ± 0.20 a | 5.64 ± 0.14 b | 0.76 ± 0.03 a | 0.60 ± 0.10 b | 0.41 ± 0.11 b | |
| 24 | 3.33 ± 0.52 a | ND | 6.05 ± 0.09 a | 1.82 ± 0.12 a | 6.77 ± 0.17 a | 1.15 ± 0.69 a | 0.77 ± 0.05 a | 0.74 ± 0.26 a | |
| Frying | 0 | 0.74 ± 0.12 e | ND | 0.75 ± 0.33 d | 0.72 ± 0.19 b | 0.47 ± 0.01 e | 0.50 ± 0.06 c | 0.04 ± 0.03 c | ND |
| 6 | 2.08 ± 0.08 cd | ND | 2.77 ± 0.04 c | 1.46 ± 0.45 a | 1.04 ± 0.05 d | 1.25 ± 0.12 b | 0.23 ± 0.04 a | 0.13 ± 0.04 b | |
| 12 | 3.59 ± 0.07 a | 0.55 ± 0.11 a | 2.93 ± 0.32 c | 1.62 ± 0.02 a | 3.44 ± 0.37 c | 1.32 ± 0.06 b | 0.16 ± 0.03 ab | 0.14 ± 0.02 b | |
| 18 | 2.46 ± 0.88 bc | 0.40 ± 0.10 a | 3.51 ± 0.18 b | 1.38 ± 0.33 a | 4.39 ± 0.03 b | 1.47 ± 0.05 a | 0.12 ± 0.01 bc | 0.19 ± 0.12 b | |
| 24 | 3.41 ± 0.12 ab | 0.49 ± 0.01 a | 4.97 ± 0.28 a | 1.66 ± 0.03 a | 5.44 ± 0.31 a | 1.24 ± 0.01 b | 0.23 ± 0.11 a | 0.44 ± 0.13 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Zhao, Y.; Wu, R.; Yin, T.; You, J.; Hu, B.; Jia, C.; Rong, J.; Liu, R.; Zhang, B.; et al. Changes in the Quality of High-Oleic Sunflower Oil during the Frying of Shrimp (Litopenaeus vannamei). Foods 2023, 12, 1332. https://doi.org/10.3390/foods12061332
Chen J, Zhao Y, Wu R, Yin T, You J, Hu B, Jia C, Rong J, Liu R, Zhang B, et al. Changes in the Quality of High-Oleic Sunflower Oil during the Frying of Shrimp (Litopenaeus vannamei). Foods. 2023; 12(6):1332. https://doi.org/10.3390/foods12061332
Chicago/Turabian StyleChen, Jiechang, Yuanyuan Zhao, Runlin Wu, Tao Yin, Juan You, Benlun Hu, Caihua Jia, Jianhua Rong, Ru Liu, Binjia Zhang, and et al. 2023. "Changes in the Quality of High-Oleic Sunflower Oil during the Frying of Shrimp (Litopenaeus vannamei)" Foods 12, no. 6: 1332. https://doi.org/10.3390/foods12061332
APA StyleChen, J., Zhao, Y., Wu, R., Yin, T., You, J., Hu, B., Jia, C., Rong, J., Liu, R., Zhang, B., & Zhao, S. (2023). Changes in the Quality of High-Oleic Sunflower Oil during the Frying of Shrimp (Litopenaeus vannamei). Foods, 12(6), 1332. https://doi.org/10.3390/foods12061332