Postharvest Biology and Technology of Loquat (Eriobotrya japonica Lindl.)
Abstract
1. Background
2. Determination of Harvest Maturity
3. Fruit Physiology Modifications during Ripening
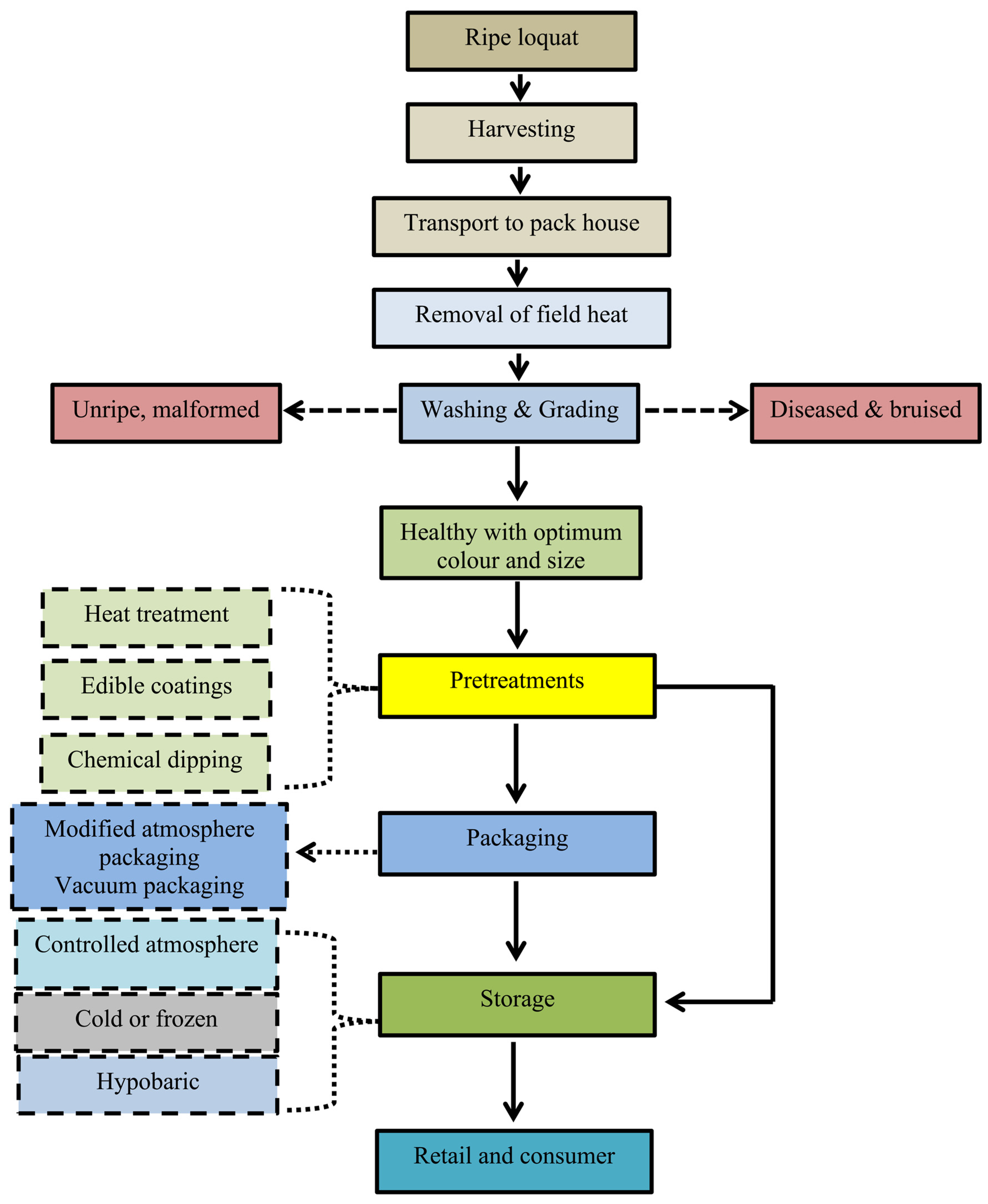
4. Harvest and Handling
5. Postharvest Handling and Storage Techniques
5.1. Heat Treatment
5.2. Postharvest Chemical Treatments
5.3. Edible Coatings
5.4. Cold Storage
5.5. Modified Atmosphere Packaging (MAP) and Controlled Atmosphere (CA) Storage
5.6. Hypobaric Storage
6. Postharvest Physiological Disorders
6.1. Chilling Injury (CI)
Control of CI
6.2. Enzymatic Browning
7. Diseases
7.1. Purple Spot
7.2. Anthracnose
7.3. Loquat Canker
8. Conclusions and Future Prospects
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martínez-Calvo, J.; Badenes, M.; Llácer, G.; Bleiholder, H.; Hack, H.; Meier, U. Phenological growth stages of loquat tree (Eriobotrya japonica (Thunb.) Lindl.). Ann. Appl. Biol. 1999, 134, 353–357. [Google Scholar] [CrossRef]
- Cai, J.; Chen, T.; Zhang, Z.; Li, B.; Qin, G.; Tian, S. Metabolic dynamics during loquat fruit ripening and postharvest technologies. Front. Plant Sci. 2019, 10, 619. [Google Scholar] [CrossRef] [PubMed]
- Koba, K.; Matsuoka, A.; Osada, K.; Huang, Y.S. Effect of loquat (Eriobotrya japonica) extracts on LDL oxidation. Food Chem. 2007, 104, 308–316. [Google Scholar] [CrossRef]
- Ferreres, F.; Gomes, D.; Valentão, P.; Gonçalves, R.; Pio, R.; Chagas, E.A.; Seabra, R.M.; Andrade, P.B. Improved loquat (Eriobotrya japonica Lindl.) cultivars: Variation of phenolics and antioxidative potential. Food Chem. 2009, 114, 1019–1027. [Google Scholar] [CrossRef]
- Alós, E.; Martinez-Fuentes, A.; Reig, C.; Mesejo, C.; Zacarías, L.; Agustí, M.; Rodrigo, M. Involvement of ethylene in color changes and carotenoid biosynthesis in loquat fruit (Eriobotrya japonica Lindl. cv. Algerie). Postharvest Biol. Technol. 2019, 149, 129–138. [Google Scholar] [CrossRef]
- Hadjipieri, M.; Georgiadou, E.C.; Marin, A.; Diaz-Mula, H.M.; Goulas, V.; Fotopoulos, V.; Tomás-Barberán, F.A.; Manganaris, G.A. Metabolic and transcriptional elucidation of the carotenoid biosynthesis pathway in peel and flesh tissue of loquat fruit during on-tree development. BMC Plant Biol. 2017, 17, 1–12. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J.; Niu, X.-Q.; Zheng, X.-L.; Chen, X.; Zheng, G.-H.; Wu, J.-C. Comparative transcriptome analysis reveals key genes potentially related to organic acid and sugar accumulation in loquat. PLoS ONE 2021, 16, e0238873. [Google Scholar] [CrossRef]
- Deng, H.; Wang, X.; Wang, Y.; Xiang, Y.; Chen, M.; Zhang, H.; Luo, X.; Xia, H.; Liang, D.; Lv, X. Dynamic changes in cell wall polysaccharides during fruit development and ripening of two contrasting loquat cultivars and associated molecular mechanisms. Foods 2023, 12, 309. [Google Scholar] [CrossRef]
- Barreto, G.P.; Benassi, M.T.; Mercadante, A.Z. Bioactive compounds from several tropical fruits and correlation by multivariate analysis to free radical scavenger activity. J. Braz. Chem. Soc. 2009, 20, 1856–1861. [Google Scholar] [CrossRef]
- Badenes, M.L.; Janick, J.; Lin, S.; Zhang, Z.; Liang, G.L.; Wang, W. Breeding loquat. Plant Breed. Rev. 2013, 37, 259–296. [Google Scholar]
- Janick, J. Important world cultivars of loquat. Acta Hortic. 2014, 1092, 25–32. [Google Scholar] [CrossRef]
- Cai, C.; Li, X.; Chen, K. Acetylsalicylic acid alleviates chilling injury of postharvest loquat (Eriobotrya japonica Lindl.) fruit. Eur. Food Res. Technol. 2006, 223, 533–539. [Google Scholar] [CrossRef]
- Cai, C.; Xu, C.; Shan, L.; Li, X.; Zhou, C.; Zhang, W.; Ferguson, I.; Chen, K. Low temperature conditioning reduces postharvest chilling injury in loquat fruit. Postharvest Biol. Technol. 2006, 41, 252–259. [Google Scholar] [CrossRef]
- Shao, X.; Zhu, Y.; Cao, S.; Wang, H.; Song, Y. Soluble sugar content and metabolism as related to the heat-induced chilling tolerance of loquat fruit during cold storage. Food Bioproc. Technol. 2013, 6, 3490–3498. [Google Scholar] [CrossRef]
- Yonghua, Z.; Xinguo, S.; Sanyu, L.; Yufang, X. Quality, active oxygen and polyamines metabolic changes in cold-stored loquat fruits as affected by postharvest SO_ (2) treatment. Acta Phytophysiologica Sinica 2000, 26, 397–401. [Google Scholar]
- Zheng, Y.H.; Li, S.Y.; Xi, Y.F.; Su, X.G.; Yi, Y.B. Polyamine changes and chilling injury in cold-stored loquat fruits. J. Integ. Plant Biol. 2000, 42, 824. [Google Scholar]
- Zhang, M.; Shi, Y.; Liu, Z.; Zhang, Y.; Yin, X.; Liang, Z.; Huang, Y.; Grierson, D.; Chen, K. An EjbHLH14-EjHB1-EjPRX12 module is involved in methyl jasmonate alleviation of chilling-induced lignin deposition in loquat fruit. J. Exp. Bot. 2022, 73, 1668–1682. [Google Scholar] [CrossRef]
- Hadjipieri, M.; Gavriel, K.; Sismanidis, G.; Manganaris, G.A. The effect of modified atmosphere packaging on postharvest performance of two loquat cultivars. Acta Hortic. 2016, 1242, 729–734. [Google Scholar] [CrossRef]
- Jin, P.; Zhang, Y.; Shan, T.; Huang, Y.; Xu, J.; Zheng, Y. Low-temperature conditioning alleviates chilling injury in loquat fruit and regulates glycine betaine content and energy status. J. Agric. Food Chem. 2015, 63, 3654–3659. [Google Scholar] [CrossRef]
- Gariglio, N.; Martinez-Fuentes, A.; Mesejo, C.; Agustí, M. Control of purple spot of loquat fruit (Eriobotrya japonica) by means of mineral compounds. Ann. Appl. Biol. 2005, 146, 415–420. [Google Scholar] [CrossRef]
- Cai, C.; Chen, K.; Xu, W.; Zhang, W.; Li, X.; Ferguson, I. Effect of 1-MCP on postharvest quality of loquat fruit. Postharvest Biol. Technol. 2006, 40, 155–162. [Google Scholar] [CrossRef]
- Öz, A.T.; Kafkas, N.E.; Bozdoğan, A. Combined effects of oxalic acid treatment and modified atmosphere packaging on postharvest quality of loquats during storage. Turk. J. Agric. Fores. 2016, 40, 433–440. [Google Scholar] [CrossRef]
- Akhtar, A.; Abbasi, N.A.; Hussain, A. Effect of calcium chloride treatments on quality characteristics of loquat fruit during storage. Pak. J. Bot. 2010, 42, 181–188. [Google Scholar]
- Cao, S.; Zheng, Y.; Yang, Z.; Tang, S.; Jin, P.; Wang, K.; Wang, X. Effect of methyl jasmonate on the inhibition of Colletotrichum acutatum infection in loquat fruit and the possible mechanisms. Postharvest Biol. Technol. 2008, 49, 301–307. [Google Scholar] [CrossRef]
- Xu, M.; Dong, J.; Zhang, M.; Xu, X.; Sun, L. Cold-induced endogenous nitric oxide generation plays a role in chilling tolerance of loquat fruit during postharvest storage. Postharvest Biol. Technol. 2012, 65, 5–12. [Google Scholar] [CrossRef]
- Ding, C.-K.; Chachin, K.; Ueda, Y.; Wang, C.Y. Inhibition of loquat enzymatic browning by sulfhydryl compounds. Food Chem. 2002, 76, 213–218. [Google Scholar] [CrossRef]
- Ding, Z.; Tian, S.; Wang, Y.; Li, B.; Chan, Z.; Han, J.; Xu, Y. Physiological response of loquat fruit to different storage conditions and its storability. Postharvest Biol. Technol. 2006, 41, 143–150. [Google Scholar] [CrossRef]
- Ding, C.-K.; Chachin, K.; Ueda, Y.; Imahori, Y.; Wang, C.Y. Modified atmosphere packaging maintains postharvest quality of loquat fruit. Postharvest Biol. Technol. 2002, 24, 341–348. [Google Scholar] [CrossRef]
- Amoros, A.; Pretel, M.; Zapata, P.; Botella, M.; Romojaro, F.; Serrano, M. Use of modified atmosphere packaging with microperforated polypropylene films to maintain postharvest loquat fruit quality. Int. J. Food Sci. Technol. 2008, 14, 95–103. [Google Scholar] [CrossRef]
- Ding, C.K.; Chachin, K.; Hamauzu, Y.; Ueda, Y.; Imahori, Y. Effects of storage temperatures on physiology and quality of loquat fruit. Postharvest Biol. Technol. 1998, 14, 309–315. [Google Scholar] [CrossRef]
- Ghasemnezhad, M.; Nezhad, M.A.; Gerailoo, S. Changes in postharvest quality of loquat (Eriobotrya japonica) fruits influenced by chitosan. Hortic. Environ. Biotechnol. 2011, 52, 40–45. [Google Scholar] [CrossRef]
- Zargar, B.; Mir, M.M.; Ganai, S.A.; Mir, S.A.; Shah, M.A.; Banday, S.A. Postharvest Biology and Technology of Loquat. Postharvest Biol. Technol. Temp. Fruits 2018, 285–298. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, H.; Wang, J.; Shen, J.; He, F.; Chen, D.; Wang, Y. The regulatory mechanisms and control technologies of chilling injury and fungal diseases of postharvest loquat fruit. Plants 2022, 11, 3472. [Google Scholar] [CrossRef]
- Cañete, M.; Hueso, J.; Pinillos, V.; Cuevas, J. Ripening degree at harvest affects bruising susceptibility and fruit sensorial traits of loquat (Eriobotrya japonica Lindl.). Sci. Hortic. 2015, 187, 102–107. [Google Scholar] [CrossRef]
- Pinillos, V.; Hueso, J.J.; Marcon Filho, J.L.; Cuevas, J. Changes in fruit maturity indices along the harvest season in ‘Algerie’ loquat. Sci. Hortic. 2011, 129, 769–776. [Google Scholar] [CrossRef]
- Hadjipieri, M.; Christofi, M.; Goulas, V.; Manganaris, G.A. The impact of genotype and harvesting day on qualitative attributes, postharvest performance and bioactive content of loquat fruit. Sci. Hortic. 2020, 263, 108891. [Google Scholar] [CrossRef]
- Chen, F.X.; Liu, X.H.; Chen, L.S. Developmental changes in pulp organic acid concentration and activities of acid-metabolising enzymes during the fruit development of two loquat (Eriobotrya japonica Lindl.) cultivars differing in fruit acidity. Food Chem. 2009, 114, 657–664. [Google Scholar] [CrossRef]
- Kader, A.A. Recommendations for Maintaining Postharvest Quality; Postharvest Technology Research and Information Center: UC Davis, CA, USA, 2009. [Google Scholar]
- Cuevas, J.; Salvador-Sola, F.; Gavilán, J.; Lorente, N.; Hueso, J.; González-Padierna, C. Loquat fruit sink strength and growth pattern. Sci. Hortic. 2003, 98, 131–137. [Google Scholar] [CrossRef]
- González, L.; Lafuente, M.; Zacarías, L. Maturation of loquat fruit (Eriobotrya japonica Lindl.) under Spanish growing conditions and its postharvest performance. Opt. Mediterr. 2003, 58, 171–179. [Google Scholar]
- Hirai, M. Sugar accumulation and development of loquat fruit. J. Jpn. Soc. Hortic. Sci. 1980, 49, 347–353. [Google Scholar] [CrossRef]
- Hamauzu, Y.; Chachin, K.; Ding, C.; Kurooka, H. Differences in surface color, flesh firmness, physiological activity, and some components of loquat [Eriobotrya japonica] fruits picked at various stages of maturity. J. Jpn. Soc. Hortic. Sci. 1997, 65, 859–865. [Google Scholar] [CrossRef]
- Hirai, M. Accelerated sugar accumulation and ripening of loquat fruit by exogenously applied ethylene. J. Jpn. Soc. Hortic. Sci. 1982, 51, 159–164. [Google Scholar] [CrossRef]
- Gariglio, N.; Reig, C.; Martinez-Fuentes, A.; Mesejo, C.; Agusti, M. Purple spot in loquat (Eriobotrya japonica Lindl.) is associated to changes in flesh-rind water relations during fruit development. Sci. Hortic. 2008, 119, 55–58. [Google Scholar] [CrossRef]
- Undurraga, P.; Olaeta, J. Effect of ethephon (2-chloro ethylphosphonic acid) applied to the trees on ripening of Golden Nugget loquat (Eriobotrya japonica Lindl.). Options Mediterr 2004, 58, 123–128. [Google Scholar]
- Reig, C.; Martinez-Fuentes, A.; Juan, M.; Gariglio, N.; Marti, G.; Mesejo, C.; Agustí, M. Tecnicas para anticipar la recoleccion del fruto del nispero japones (Eriobotrya japonica Lindl.). In Proceedings of the XI Congress National SECH Abstract 4D01, 2007.
- Jiang, T.M.; Wang, P.; Yin, X.R.; Zhang, B.; Xu, C.J.; Li, X.; Chen, K.S. Ethylene biosynthesis and expression of related genes in loquat fruit at different developmental and ripening stages. Sci. Hortic. 2011, 130, 452–458. [Google Scholar] [CrossRef]
- Hasegawa, P.N.; de Faria, A.F.; Mercadante, A.Z.; Chagas, E.A.; Pio, R.; Lajolo, F.M.; Cordenunsi, B.R.; Purgatto, E. Chemical composition of five loquat cultivars planted in Brazil. Food Sci. Technol. 2010, 30, 552–559. [Google Scholar] [CrossRef]
- Zhou, C.H.; Xu, C.J.; Sun, C.D.; Li, X.; Chen, K.S. Carotenoids in white-and red-fleshed loquat fruits. J. Agric. Food Chem. 2007, 55, 7822–7830. [Google Scholar] [CrossRef]
- Serrano, M.; Guillén, F.; Martínez-Romero, D.; Castillo, S.; Valero, D. Chemical constituents and antioxidant activity of sweet cherry at different ripening stages. J. Agric. Food Chem. 2005, 53, 2741–2745. [Google Scholar] [CrossRef]
- Pinillos, V.; Cañete, M.; Sanchez, R.; Cuevas, J.; Hueso, J. Fruit development and maturation phenological stages of ‘Algerie’ loquat. Acta Hortic. 2006, 750, 331–336. [Google Scholar] [CrossRef]
- Goulas, V.; Minas, I.; Kourdoulas, P.; Vicente, A.R.; Manganaris, G.A. Phytochemical content, antioxidants and cell wall metabolism of two loquat (Eriobotrya japonica) cultivars under different storage regimes. Food Chem. 2014, 155, 227–234. [Google Scholar] [CrossRef]
- Iida, M.; Bantog, N.A.; Yamada, K.; Shiratake, K.; Yamaki, S. Sorbitol-and other sugar-induced expressions of the NAD+-dependent sorbitol dehydrogenase gene in Japanese pear fruit. J. Am. Soc. Hortic. Sci. 2004, 129, 870–875. [Google Scholar] [CrossRef]
- Sotiras, M.; Papadakis, I.; Landi, M.; Tsaniklidis, G.; Tsiantas, P.; Psychoyou, M. Allocation pattern, photosynthetic performance and sugar metabolism in hydroponically grown seedlings of loquat (Eriobotrya japonica Lindl.) subjected to salinity. Photosynthetica 2019, 57, 258–267. [Google Scholar] [CrossRef]
- Bruneau, J.M.; Worrell, A.C.; Cambou, B.; Lando, D.; Voelker, T.A. Sucrose phosphate synthase, a key enzyme for sucrose biosynthesis in plants: Protein purification from corn leaves and immunological detection. Plant Physiology 1991, 96, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Sumitani, H.; Inada, Y.; Mori, D.; Nakano, Y. Potent aroma volatiles in fresh loquat and its canned product. J. Jpn. Soc. Food Sci. Technol. 2000, 47, 302–310. [Google Scholar] [CrossRef]
- Blumenfeld, A. Fruit growth of loquat. J. Am. Soc. Hortic. Sci. 1980, 105, 747–750. [Google Scholar] [CrossRef]
- Sultan, M.; Khalefa, S.; Elhamamsy, S.; Mostafa, Y. Effect of postharvest anti-oxidant treatments on loquat fruit deterioration during storage at room temperature. Acta Hortic. 2014, 1092, 173–179. [Google Scholar] [CrossRef]
- Barchi, G.; Berardinelli, A.; Guarnieri, A.; Ragni, L.; Fila, C.T. pH—Postharvest technology: Damage to loquats by vibration-simulating intra-state transport. Biosyst. Eng. 2002, 82, 305–312. [Google Scholar] [CrossRef]
- Chen, F.; Wu, G.; Li, C. Effects of modified atmosphere packaging on respiration and quality attributes of loquat fruit during cold storage. Trans. Chi. Soc. Agri. Engrr 2003, 19, 147–151. [Google Scholar]
- Amorós, A.; Zapata, P.; Pretel, M.; Botella, M.; Serrano, M. Physico-chemical and physiological changes during fruit development and ripening of five loquat (Eriobotrya japonica Lindl.) cultivars. Int. J. Food Sci. Technol. 2003, 9, 43–51. [Google Scholar] [CrossRef]
- Rui, H.; Cao, S.; Shang, H.; Jin, P.; Wang, K.; Zheng, Y. Effects of heat treatment on internal browning and membrane fatty acid in loquat fruit in response to chilling stress. J. Sci. Food Agric. 2010, 90, 1557–1561. [Google Scholar] [CrossRef]
- Rui, H.; Wang, K.; Shang, H.; Tang, S.; Jin, P.; Cao, S.; Zheng, Y. Effects of heat treatment on flesh leatheriness and related enzyme activities of loquat fruits during cold storage. Trans. Chi. Soc. Agric. Engrr. 2009, 25, 294–298. [Google Scholar] [CrossRef]
- Rui, H.; Shang, H.; Wang, K.; Jin, P.; Tang, S.; Cao, S.; Zheng, Y. Effects of heat treatment on active oxygen metabolism and flesh lignification in cold-stored loquat fruits. Food Science 2009, 25, 276–281. [Google Scholar] [CrossRef]
- Liu, F.; Shao, X.; Tu, K.; Zhao, Y. Effect of postharvest heat treatment on the quality of loquat fruit in cold storage. J. Fruit Sci. 2009, 26, 649–653. [Google Scholar]
- Edagi, F.K.; Sestari, I.; Sasaki, F.F.; Cabral, S.M.; Meneghini, J.; Kluge, R.A. Potential increasing in the cold-storage of ‘Fukuhara’ loquat using heat treatments. Pesq. Agro. Bras. 2009, 44, 1270–1276. [Google Scholar] [CrossRef]
- Wu, G.B.; Chen, F.H.; Zhang, Q.B.; Yang, J. Effects of heat shock treatment on chilling injury and physiological responses of Eriobotrya japonica fruit during cold storage. J. Plant Res. Environ. 2004, 13, 1–5. [Google Scholar]
- Liu, F.; Tu, K.; Shao, X.; Zhao, Y.; Tu, S.; Su, J.; Hou, Y.; Zou, X. Effect of hot air treatment in combination with Pichia guilliermondii on postharvest anthracnose rot of loquat fruit. Postharvest Biol. Technol. 2010, 58, 65–71. [Google Scholar] [CrossRef]
- Shao, X.; Tu, K. Hot air treatment improved the chilling resistance of loquat fruit under cold storage. J. Food Proc. Pres. 2014, 38, 694–703. [Google Scholar] [CrossRef]
- Song, Y.; Liao, X.; Shao, X. Effects of postharvest heat treatment on the fatty acid composition and chilling injury of loquat fruits during the cold storage. In Proceedings of the 2011 International Conference on New Technology of Agricultural, Zibo, China, 27–29 May 2011; pp. 945–948. [Google Scholar]
- Jin, P.; Duan, Y.; Wang, L.; Wang, J.; Zheng, Y. Reducing chilling injury of loquat fruit by combined treatment with hot air and methyl jasmonate. Food Bioproc. Technol. 2014, 7, 2259–2266. [Google Scholar] [CrossRef]
- Zhang, Y.; Jin, P.; Huang, Y.; Shan, T.; Wang, L.; Li, Y.; Zheng, Y. Effect of hot water combined with glycine betaine alleviates chilling injury in cold-stored loquat fruit. Postharvest Biol. Technol. 2016, 118, 141–147. [Google Scholar] [CrossRef]
- Khan, A.S.; Singh, Z. 1-methylcyclopropene application and modified atmosphere packaging affect ethylene biosynthesis, fruit softening, and quality of ‘Tegan Blue’ Japanese plum during cold storage. J. Am. Soc. Hortic. Sci. 2008, 133, 290–299. [Google Scholar] [CrossRef]
- Liu, H.; Cao, J.; Jiang, W. Changes in phenolics and antioxidant property of peach fruit during ripening and responses to 1-methylcyclopropene. Postharvest Biol. Technol. 2015, 108, 111–118. [Google Scholar] [CrossRef]
- Lee, J.; Jeong, M.C.; Ku, K.H. Chemical, physical, and sensory properties of 1-MCP-treated Fuji apple (Malus domestica Borkh.) fruits after long-term cold storage. Appl. Biol. Chem. 2017, 60, 363–374. [Google Scholar] [CrossRef]
- Shah, H.M.S.; Khan, A.S.; Ali, S. Pre-storage kojic acid application delays pericarp browning and maintains antioxidant activities of litchi fruit. Postharvest Biol. Technol. 2017, 132, 154–161. [Google Scholar] [CrossRef]
- Tomala, K.; Grzęda, M.; Guzek, D.; Głąbska, D.; Gutkowska, K. The effects of preharvest 1-methylcyclopropene (1-MCP) treatment on the fruit quality parameters of cold-stored ‘Szampion’ cultivar apples. Agric. 2020, 10, 80. [Google Scholar] [CrossRef]
- Cao, S.; Zheng, Y.; Yang, Z. Effect of 1-MCP treatment on nutritive and functional properties of loquat fruit during cold storage. NZ J. Crop Hortic Sci. 2011, 39, 61–70. [Google Scholar] [CrossRef]
- Cao, S.; Zheng, Y.; Wang, K.; Rui, H.; Tang, S. Effects of 1-methylcyclopropene on oxidative damage, phospholipases and chilling injury in loquat fruit. J. Sci. Food Agric. 2009, 89, 2214–2220. [Google Scholar] [CrossRef]
- Cao, S.; Zheng, Y.; Wang, K.; Rui, H.; Tang, S. Effect of 1-methylcyclopropene treatment on chilling injury, fatty acid and cell wall polysaccharide composition in loquat fruit. J. Agric. Food Chem. 2009, 57, 8439–8443. [Google Scholar] [CrossRef]
- Cao, S.; Zheng, Y. Effect of 1-methylcyclopropene on anthracnose rot caused by Colletotrichum acutatum and disease resistance in loquat fruit. J. Sci. Food Agric. 2010, 90, 2289–2294. [Google Scholar] [CrossRef]
- Yang, S.; Sun, C.; Wang, P.; Shan, L.; Cai, C.; Zhang, B.; Zhang, W.; Li, X.; Ferguson, I.; Chen, K. Expression of expansin genes during postharvest lignification and softening of ‘Luoyangqing’and ‘Baisha’ loquat fruit under different storage conditions. Postharvest Biol. Technol. 2008, 49, 46–53. [Google Scholar] [CrossRef]
- Liguori, G.; Farina, V.; Corona, O.; Mazzaglia, A.; Barone, E.; Inglese, P. Effects of 1-MCP on postharvest quality and internal browning of white-flesh loquat fruit during cold storage. Fruits 2017, 72, 67–73. [Google Scholar] [CrossRef]
- Liguori, G.; Farina, V.; Sortino, G.; Mazzaglia, A.; Inglese, P. Effects of 1-methylcyclopropene on postharvest quality of white-and yellow-flesh loquat (Eriobotrya japonica Lindl.) fruit. Fruits 2014, 69, 363–370. [Google Scholar] [CrossRef]
- Cao, S.; Zheng, Y.; Wang, K.; Rui, H.; Shang, H.; Tang, S. The effects of 1-methylcyclopropene on chilling and cell wall metabolism in loquat fruit. J. Hortic. Sci. Biotechnol. 2010, 85, 147–153. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, S. Effects of benzothiadiazole on diseases and quality of postharvest loquat fruit. Food Sci. 2009, 30, 264–267. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, Z.; Xu, J.; Ma, L.; Tang, W.; Liu, D. Effect of BTH treatment on storability and activity of related enzymes of harvested loquat fruit. Acta Hortic. 2006, 750, 445–450. [Google Scholar] [CrossRef]
- Hussain, S.; Zahoor, H.; Khadijac, F.; Salikc, M.R.; Alia, M.; Hayatc, A.; Mustafad, G. Postharvest calcium chloride application maintains shelf life and quality of loquat (Eriobotrya japonica L.) fruit. J. Hortic. Sci. Technol. 2021, 4, 1–6. [Google Scholar] [CrossRef]
- Mostafa, Y.; Sultan, M. Calcium chloride combined with antioxidants increases keeping quality and limits postharvest decay of loquat fruit. Acta Hortic. 2016, 1194, 157–164. [Google Scholar] [CrossRef]
- Hou, Y.; Li, Z.; Zheng, Y.; Jin, P. Effects of CaCl2 treatment alleviates chilling injury of loquat fruit (Eribotrya japonica) by modulating ROS homeostasis. Foods 2021, 10, 1662. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Xie, B.; Hu, S.; Zheng, Y.; Jin, P. Effects of exogenous calcium and calcium chelant on cold tolerance of postharvest loquat fruit. Sci. Hortic. 2020, 269, 109391. [Google Scholar] [CrossRef]
- Tzortzakis, N.; Sergentani, C. Determination of heat stress and calcium chloride application in loquat storage. Acta Hortic. 2014, 1079, 581–587. [Google Scholar] [CrossRef]
- Song, H.; Zheng, Y.; Yuan, W.; Zhang, R.; Yang, L. Effects of peracetic acid (PAA) combined with calcium treatments on storage quality of loquat fruit. Agric. Sci. Technol. 2013, 14, 1476. [Google Scholar]
- Wang, K.; Cao, S.; Di, Y.; Liao, Y.; Zheng, Y. Effect of ethanol treatment on disease resistance against anthracnose rot in postharvest loquat fruit. Sci. Hortic. 2015, 188, 115–121. [Google Scholar] [CrossRef]
- Shan, T.; Sun, Y.; Jin, P.; Xu, J.; Zheng, Y. Effects of glycine betaine on loquat fruit quality during cold storage. Acta Hortic. 2014, 1092, 131–137. [Google Scholar] [CrossRef]
- Cao, S.; Zheng, Y.; Yang, Z.; Wang, K.; Rui, H. Effect of methyl jasmonate on quality and antioxidant activity of postharvest loquat fruit. J. Sci. Food Agric. 2009, 89, 2064–2070. [Google Scholar] [CrossRef]
- Cao, S.; Zheng, Y.; Wang, K.; Jin, P.; Rui, H. Methyl jasmonate reduces chilling injury and enhances antioxidant enzyme activity in postharvest loquat fruit. Food Chem. 2009, 115, 1458–1463. [Google Scholar] [CrossRef]
- Cao, S.; Zheng, Y.; Wang, K.; Rui, H.; Tang, S. Effect of methyl jasmonate on cell wall modification of loquat fruit in relation to chilling injury after harvest. Food Chem. 2010, 118, 641–647. [Google Scholar] [CrossRef]
- Cao, S.; Zheng, Y.; Yang, Z.; Tang, S.; Jin, P. Control of anthracnose rot and quality deterioration in loquat fruit with methyl jasmonate. J. Sci. Food Agric. 2008, 88, 1598–1602. [Google Scholar] [CrossRef]
- Cao, S.; Zheng, Y.; Wang, K.; Tang, S.; Rui, H. Effect of yeast antagonist in combination with methyl jasmonate treatment on postharvest anthracnose rot of loquat fruit. Biol. Cont. 2009, 50, 73–77. [Google Scholar] [CrossRef]
- Cai, Y.; Cao, S.; Yang, Z.; Zheng, Y. MeJA regulates enzymes involved in ascorbic acid and glutathione metabolism and improves chilling tolerance in loquat fruit. Postharvest Biol. Technol. 2011, 59, 324–326. [Google Scholar] [CrossRef]
- Cao, S.; Cai, Y.; Yang, Z.; Zheng, Y. MeJA induces chilling tolerance in loquat fruit by regulating proline and γ-aminobutyric acid contents. Food Chem. 2012, 133, 1466–1470. [Google Scholar] [CrossRef]
- Cao, S.; Cai, Y.; Yang, Z.; Joyce, D.C.; Zheng, Y. Effect of MeJA treatment on polyamine, energy status and anthracnose rot of loquat fruit. Food Chem. 2014, 145, 86–89. [Google Scholar] [CrossRef]
- Wang, D.; Chen, Q.; Chen, W.; Guo, Q.; Xia, Y.; Wu, D.; Jing, D.; Liang, G. Melatonin treatment maintains quality and delays lignification in loquat fruit during cold storage. Sci. Hortic. 2021, 284, 110126. [Google Scholar] [CrossRef]
- Hussain, Z.; Hussain, S.; Sarwar, G.; Latif, M.; Anwar, R.; Khadija, F.; Bilal, M.; Sherani, J.; Yaseen, M. Postharvest application of salicylic acid to improve the shelf life and quality of loquat (Eriobotrya japonica L.) fruit. J. Pure Appl. Agric. 2021, 6, 31–38. [Google Scholar]
- Wu, J.; Chen, Q.; Tang, C.; Xia, H. Effects of exogenous salicylic acid on lignification and related enzymes activities of loquat during cold storage. Trans. Chi. Soc. Agric. Engrr. 2006, 22, 175–179. [Google Scholar]
- Huang, Z.; Wu, J.; Chen, W.; Cai, L.; Xie, C.; Lin, L.; Huang, S.; Ye, M. Effects of SA on enzymes of ascorbate-glutathione cycle in young loquat fruits after low temperature stress. Sci. Sil. Sin. 2011, 47, 36–42. [Google Scholar]
- Chen, G.; Hou, Y.; Zheng, Y.; Jin, P. 2, 4-epibrassinolide enhance chilling tolerance of loquat fruit by regulating cell wall and membrane fatty acid metabolism. Sci. Hortic. 2022, 295, 110813. [Google Scholar] [CrossRef]
- Gao, H.; Tao, F.; Song, L.; Chen, H.; Chen, W.; Zhou, Y.; Mao, J.; Zheng, Y. Effects of short-term N2 treatment on quality and antioxidant ability of loquat fruit during cold storage. J. Sci. Food Agric. 2009, 89, 1159–1163. [Google Scholar] [CrossRef]
- Creelman, R.A.; Mullet, J.E. Biosynthesis and action of jasmonates in plants. Annu. Rev. Plant Biol. 1997, 48, 355–381. [Google Scholar] [CrossRef]
- Besson-Bard, A.; Pugin, A.; Wendehenne, D. New insights into nitric oxide signaling in plants. Annu. Rev. Plant Biol. 2008, 59, 21–39. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, S.; Huang, Z.; Cai, B.; Wu, G.; Pan, Z.; Wu, J. Response of endogenous nitric oxide and Jasmonate acid to low temperature stress in young loquat fruits. Plant Sci. J. 2012, 30, 611–617. [Google Scholar] [CrossRef]
- Zheng, Y.; Su, X.; Yi, Y.; Li, S.; Xi, Y. Effects of SO2 on loquat fruits stored at 1° C. J. Nanj. Agric. Uni. 2000, 23, 89–92. [Google Scholar]
- Tang, J.; Ren, H.; Chen, X.; Ma, F.; Jiang, F.; Sun, B. Effects of short-term N2 anaerobic treatment on respiratory metabolism and oxidation status of Agaricus bisporus. Postharvest Biol. Technol. 2021, 181, 111692. [Google Scholar] [CrossRef]
- Larbat, R.; Olsen, K.M.; Slimestad, R.; Løvdal, T.; Bénard, C.; Verheul, M.; Bourgaud, F.; Robin, C.; Lillo, C. Influence of repeated short-term nitrogen limitations on leaf phenolics metabolism in tomato. Phytochemistry 2012, 77, 119–128. [Google Scholar] [CrossRef]
- Zhang, Z.; Fu, T.; Li, Y.; Peng, C.; Qin, W. Effects of ozone treatment on the preservation of loquat cultivar big five-pointed star during storage. Food Sci. 2011, 32, 282–285. [Google Scholar]
- Piechowiak, T.; Grzelak-Błaszczyk, K.; Sójka, M.; Balawejder, M. One-time ozone treatment improves the postharvest quality and antioxidant activity of Actinidia arguta fruit. Phytochemistry 2022, 203, 113393. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Perez, M.; Aldon, D.; Galaud, J.P. Respective contribution of CML8 and CML9, two arabidopsis calmodulin-like proteins, to plant stress responses. Plant Signal. Behav. 2017, 12, e1322246. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, L.; Zhao, L.; Xie, B.; Hu, S.; Chen, G.; Zheng, Y.; Jin, P. CaCl2 mitigates chilling injury in loquat fruit via the CAMTA5-mediated transcriptional repression of membrane lipid degradation genes. Food Res. Int. 2022, 162, 111966. [Google Scholar] [CrossRef] [PubMed]
- Bo, H. A preliminary report on the effect of calcium on some physiological indexes of postharvest loquat fruit. Subt. Plant Sci. 2000, 29, 31. [Google Scholar]
- Riseh, R.S.; Vatankhah, M.; Hassanisaadi, M.; Kennedy, J.F. Chitosan-based nanocomposites as coatings and packaging materials for the postharvest improvement of agricultural product: A review. Carbohydrate Polymers 2023, 309, 120666. [Google Scholar] [CrossRef]
- Márquez, C.J.; Cartagena, J.R.; Pérez-Gago, M.B. Effect of edible coatings on japanese loquat (Eriobotrya japonica T.) postharvest quality. Vitae 2009, 16, 304–310. [Google Scholar] [CrossRef]
- Petriccione, M.; Pasquariello, M.S.; Mastrobuoni, F.; Zampella, L.; Di Patre, D.; Scortichini, M. Influence of a chitosan coating on the quality and nutraceutical traits of loquat fruit during postharvest life. Sci. Hortic. 2015, 197, 287–296. [Google Scholar] [CrossRef]
- Bahadırlı, N.P.; Kahramanoğlu, İ.; Wan, C. Exposure to volatile essential oils of myrtle (Myrtus communis L.) leaves for improving the postharvest storability of fresh loquat fruits. J. Food Qual. 2020, 2020, 1–10. [Google Scholar] [CrossRef]
- Liguori, G.; Greco, G.; Gaglio, R.; Settanni, L.; Inglese, P.; Allegra, A. Influence of cactus pear mucilage-based edible coating on marketability and edibility parameters of minimally processed loquat fruits. Agronomy 2022, 12, 2120. [Google Scholar] [CrossRef]
- Kahramanoğlu, İ. Preserving postharvest storage quality of fresh loquat fruits by using different bio-materials. J. Food Sci. Technol. 2020, 57, 3004–3012. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Yuan, W.; Jin, P.; Wang, W.; Wang, X.; Yang, L.; Zhang, Y. Effects of chitosan/nano-silica on postharvest quality and antioxidant capacity of loquat fruit during cold storage. Postharvest Biol. Technol. 2016, 119, 41–48. [Google Scholar] [CrossRef]
- Ergin, S.Ö.; Yaman, H.; Dilek, M. The usage of edible films extracted from cherry and apricot tree gums for coating of strawberry (Fragaria ananassa) and loquat (Eriobotrya japonica) fruits. Turk. J. Agric. Food Sci. Technol. 2018, 6, 561–569. [Google Scholar] [CrossRef]
- Hanif, J.; Khalid, N.; Khan, R.S.; Bhatti, M.F.; Hayat, M.Q.; Ismail, M.; Andleeb, S.; Mansoor, Q.; Khan, F.; Amin, F. Formulation of active packaging system using Artemisia scoparia for enhancing shelf life of fresh fruits. Mat. Sci. Engnr. 2019, 100, 82–93. [Google Scholar] [CrossRef]
- Tian, S.; Li, B.; Ding, Z. Physiological properties and storage technologies of loquat fruit. In Fresh Produce; da Teixeira, S., Ed.; Global Science Books: Isleworth, UK, 2007; Volume 1, pp. 76–81. [Google Scholar]
- Cao, S.; Yang, Z.; Zheng, Y. Sugar metabolism in relation to chilling tolerance of loquat fruit. Food Chem. 2013, 136, 139–143. [Google Scholar] [CrossRef]
- Öz, T.; Kafkas, E.; Eker, T. Effects of active modified atmosphere packaging with argon and nitrogen on postharvest quality of loquat. Acta Hortic. 2016, 1242, 335–342. [Google Scholar] [CrossRef]
- Oz, A.; Ulukanil, Z. Effects of 1-methylcylopropene (1-MCP) and modifed atmopshere packing (MAP) on postharvest browning and microbial growth of loquat fruit. J. Appl. Bot. Food Qual. 2012, 84, 125. [Google Scholar]
- Akhtar, A.; Abbasi, N.A.; Hussain, A.; Bakhsh, A. Preserving quality of loquat fruit during storage by modified atmosphere packaging. Pak. J. Agric. Sci. 2012, 49, 419–423. [Google Scholar]
- Palma, A.; Mura, D.; D’Aquino, S.; Continella, G.; Continella, A. Storability of ‘Algerie’ and ‘Golden Nugget’ loquats in modified atmosphere packaging. Acta Hortic. 2012, 934, 245–252. [Google Scholar] [CrossRef]
- Çandir, E.; Polat, A.; Özdemir, A.; Caliskan, O.; Temizyürek, F. The effects of modified atmosphere packaging on quality of loquat fruits. Acta Hortic. 2010, 887, 363–367. [Google Scholar] [CrossRef]
- Hashemabadi, D.; Najmabadi, N.; Kaviani, B.; Ghasemnejad, M.; Zaredoost, F.; Jadid Soleymandarabi, M. The effect of antioxidant compounds and packaging materials on quality and storage life of loquat (Eriobotrya japonica) fruits. Ira. J. Hortic. Sci. Technol. 2018, 19, 143–156. [Google Scholar]
- Wang, L.; Shao, S.; Madebo, M.P.; Hou, Y.; Zheng, Y.; Jin, P. Effect of nano-SiO2 packing on postharvest quality and antioxidant capacity of loquat fruit under ambient temperature storage. Food Chem. 2020, 315, 126295. [Google Scholar] [CrossRef] [PubMed]
- Pica, A.; Continella, G.; Agabbio, M.; D’Aquino, S. Effect of packaging and coating on fruit quality changes of loquat during three cold storage regimes. Adv. Hortic. Sci. 1996, 10, 1000–1006. [Google Scholar]
- Zheng, Y.H.; Su, X.G.; Li, Q.J.; Li, S.Y.; Xi, Y.F. Effect of high oxygen on respiration rate, polyphenol oxidase activity and quality in postharvest loquat fruits. Plant Physiol. Commun. 2000, 36, 318–320. [Google Scholar]
- Haiyan, G.; Lili, S.; Yongjun, Z.; Ying, Y.; Wenxuan, C.; Hangjun, C.; Yonghua, Z. Effects of hypobaric storage on quality and flesh leatheriness of cold-stored loquat fruit. Trans. Chi. Soc. Agric. Engrr. 2008, 24, 245–249. [Google Scholar]
- Chen, W.; Yang, Y.; Song, L.; Jiang, Y.; Zheng, Y.; Gao, H.; Chen, H. Effect of hypobaric storage on physiological and quality attributes of loquat fruit at low temperature. Acta Hortic. 2006, 712, 269–274. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, H.; Cao, J.; Jiang, W. Advances in biochemical mechanisms and control technologies to treat chilling injury in postharvest fruits and vegetables. Trands Food Sci. Technol. 2021, 113, 355–365. [Google Scholar] [CrossRef]
- Lyons, J.M. Chilling injury in plants. Annu. Rev. Plant Physiol. 1973, 24, 445–466. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, B.; Li, X.; Xu, C.; Yin, X.; Shan, L.; Ferguson, I.; Chen, K. Ethylene signal transduction elements involved in chilling injury in non-climacteric loquat fruit. J. Exp. Bot. 2010, 61, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Xu, F.; Shao, X. Changes in soluble sugar metabolism in loquat fruit during different cold storage. J. Food Sci. Technol. 2017, 54, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.L.; Li, X.; Wang, P.; Cai, C.; Zhang, B.; Sun, C.D.; Zhang, W.S.; Xu, C.J.; Ferguson, I.; Chen, K.S. Characterization of cDNAs associated with lignification and their expression profiles in loquat fruit with different lignin accumulation. Planta 2008, 227, 1243–1254. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, W.; Zeng, J.; Zhang, J.; Grierson, D.; Li, X.; Yin, X.; Chen, K. A NAC transcription factor, EjNAC1, affects lignification of loquat fruit by regulating lignin. Postharvest Biol. Technol. 2015, 102, 25–31. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.; Zheng, T.; Wei, W.; Zhu, Y.; Gao, Y.; Yang, X.; Lin, S. Characterization of carotenoid accumulation and carotenogenic gene expression during fruit development in yellow and white loquat fruit. Hortic. Plant. J. 2016, 2, 9–15. [Google Scholar] [CrossRef]
- Hofman, P.J.; Stubbings, B.A.; Adkins, M.F.; Corcoran, R.J.; White, A.; Woolf, A.B. Low temperature conditioning before cold disinfestation improves ‘Hass’ avocado fruit quality. Postharvest Biol. Technol. 2003, 28, 123–133. [Google Scholar] [CrossRef]
- Zeng, J.K.; Li, X.; Zhang, J.; Ge, H.; Yin, X.R.; Chen, K.S. Regulation of loquat fruit low temperature response and lignification involves interaction of heat shock factors and genes associated with lignin biosynthesis. Plant Cell Environ. 2016, 39, 1780–1789. [Google Scholar] [CrossRef]
- Li, X.; Zang, C.; Ge, H.; Zhang, J.; Grierson, D.; Yin, X.R.; Chen, K.S. Involvement of PAL, C4H, and 4CL in chilling injury-induced flesh lignification of loquat fruit. HortScience 2017, 52, 127–131. [Google Scholar] [CrossRef]
- Jin, P.; Sun, C.; Zheng, Y.; Sun, M. Effects of methyl jasmonate in combination with low temperature conditioning on chilling injury and active oxygen metabolism in loquat fruits. Acta Hortic. Sin. 2012, 39, 461–468. [Google Scholar]
- Xu, M.; Zhang, M.; Shi, Y.; Liu, X.; Li, X.; Grierson, D.; Chen, K. EjHAT1 participates in heat alleviation of loquat fruit lignification by suppressing the promoter activity of key lignin monomer synthesis gene EjCAD5. J. Agric. Food Chem. 2019, 67, 5204–5211. [Google Scholar] [CrossRef]
- Jiang, Y.; Duan, X.; Joyce, D.; Zhang, Z.; Li, J. Advances in understanding of enzymatic browning in harvested litchi fruit. Food Chem. 2004, 88, 443–446. [Google Scholar] [CrossRef]
- Sapers, G.M.; Miller, R.L. Browning inhibition in fresh-cut pears. J. Food Sci. 1998, 63, 342–346. [Google Scholar] [CrossRef]
- Walker, J.R. Enzymatic browning in fruits: Its biochemistry and control. In Enzymatic Browning and Its Prevention; Lee, C., Whitaker, J., Eds.; ACS Publications: Washington, DC, USA, 1995; pp. 8–22. [Google Scholar]
- Zauberman, G.; Ronen, R.; Akerman, M.; Weksler, A.; Rot, I.; Fuchs, Y. Post-harvest retention of the red colour of litchi fruit pericarp. Sci. Hortic. 1991, 47, 89–97. [Google Scholar] [CrossRef]
- Gariglio, N.; Fonfría, M.A. Effect of fruit thinning on the mineral composition of loquat (“Eriobotrya japonica” Lindl.) fruit and its connection with purple spot. Span. J. Agric. Res. 2005, 4, 439–446. [Google Scholar] [CrossRef]
- Gariglio, N.; Almela, V.; Agusti, M. Physiological factors related to purple spot of loquat fruit. Plant Physiol. Biochem. 2000, 38, 58. [Google Scholar]
- Gariglio, N.; Juan, M.; Castillo, A.; Almela, V.; Agustı, M. Histological and physiological study of purple spot of loquat fruit. Sci. Hortic. 2002, 92, 255–263. [Google Scholar] [CrossRef]
- Villar, P.C. El nispero y su expansion, posibilidades y limitaciones. Fruit. Prof. 1993, 54, 35–40. [Google Scholar]
- Ojima, M.; Rigitano, O.; Simao, S.; Ique, T. The effect of the type of fruit protection on the incidence of purple spot and fruit development in loquats. Bragantia 1976, 35, 1–44. [Google Scholar]
- Hadjipieri, M.; Georgiadou, E.C.; Costa, F.; Fotopoulos, V.; Manganaris, G.A. Dissection of the incidence and severity of purple spot physiological disorder in loquat fruit through a physiological and molecular approach. Plant Physiol. Biochem. 2020, 155, 980–986. [Google Scholar] [CrossRef]
- Juárez-Vázquez, S.B.; Silva-Rojas, H.V.; Rebollar-Alviter, A.; Maidana-Ojeda, M.; Osnaya-González, M.; Fuentes-Aragón, D. Phylogenetic and morphological identification of Colletotrichum godetiae, a novel pathogen causing anthracnose on loquat fruits (Eriobotrya japonica). H. Plant Dis. Prot. 2019, 126, 593–598. [Google Scholar] [CrossRef]
- Damm, U.; Sun, Y.C.; Huang, C.J. Colletotrichum eriobotryae sp. nov. and C. nymphaeae, the anthracnose pathogens of loquat fruit in central Taiwan, and their sensitivity to azoxystrobin. Mycol. Prog. 2020, 19, 367–380. [Google Scholar] [CrossRef]
- Yan, F.; Li, C.; Ye, X.; Lian, Y.; Wu, Y.; Wang, X. Antifungal activity of lipopeptides from Bacillus amyloliquefaciens MG3 against Colletotrichum gloeosporioides in loquat fruits. Biol. Cont. 2020, 146, 104281. [Google Scholar] [CrossRef]
- Wang, Q.H.; Ji, Y.P.; Qu, Y.Y.; Qi, Y.K.; Li, D.W.; Liu, Z.Y.; Wu, X.Q. The response strategies of Colletotrichum gloeosporioides s.s. due to the stress caused by biological control agent Bacillus amyloliquefaciens deciphered by transcriptome analyses. Biol. Cont. 2020, 150, 104372. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Wang, J.; Jin, P.; Liu, H.; Zheng, Y. Bacillus cereus AR156-induced resistance to Colletotrichum acutatum is associated with priming of defense responses in loquat fruit. PLoS ONE 2014, 9, e112494. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Zheng, Y.; Tang, S.; Wang, K. Improved control of anthracnose rot in loquat fruit by a combination treatment of Pichia membranifaciens with CaCl2. Int. J. Food Microbiol. 2008, 126, 216–220. [Google Scholar] [CrossRef]
- Lin, S.; Sharpe, R.H.; Janick, J. Loquat: Botany and horticulture. Hortic. Rev. 1999, 23, 233–276. [Google Scholar]
- Fukuda, S.; Ishimoto, K.; Sato, S.; Terakami, S.; Yamamoto, T.; Hiehata, N. Genetic mapping of the loquat canker resistance locus in bronze loquat (Eriobotrya deflexa). Tree. Gen. Geno. 2014, 10, 875–883. [Google Scholar] [CrossRef]
- Hiehata, N.; Fukuda, S.; Sato, Y.; Tominaga, Y.; Terai, O.; Yamada, M. Quantitative inheritance of resistance to loquat canker (Pseudomonas syringae pv. eriobotryae, Group C) in loquat progenies from crosses between a resistant cultivar, ‘Champagne’, and susceptible cultivars. HortScience 2014, 49, 1486–1491. [Google Scholar] [CrossRef]
- Fukuda, S.; Ishimoto, K.; Terakami, S.; Yamamoto, T.; Hiehata, N. Genetic mapping of the loquat canker resistance gene pse-c in loquat (Eriobotrya japonica). Sci. Hortic. 2016, 200, 19–24. [Google Scholar] [CrossRef]
| Cultivar | Harvest Season | Flesh Colour | Fruit Shape | Peel Colour | Fruit Flavour |
|---|---|---|---|---|---|
| Advance | Mid | White | Pear | Yellow | Acidic sweet |
| Ahdar | Late | White | Oval | Greenish yellow | Sweet Tart |
| Ahmar | Early | Yellow | Pear | Radish orange | Sweet Tart |
| Baiyu | Early | White | Oval | Radish blushed | Sweet to subacid |
| Blush | Mid | White | Pear | Yellow | Subacid |
| Compagne | Early | Yellowish white | Elongated pear | Golden yellow | Subacid to sweet |
| Dahongpao | Mid | Yellow | Oval | Orange yellow | Subacid |
| Fire Ball | Mid | White or straw | Ovate | Orange | Acidic |
| Jiefangzhong | Mid | Yellow | Oval large | Yellow | Sweet to subacid |
| Lyuyangqing | Early to mid | Deep yellow | Ovate | Yellowish orange | Acidic |
| Mammoth | Mid | Orange | Rounded oval | Orange | subacid |
| Matchless | Mid | Pale orange | Pear | Golden yellow | Subacid |
| Mogi | Early | Light yellow | Elliptical | Yellow | Sweet |
| Premier | Mid | White | Oblong | Salmon orange with dots | Acidic |
| Safeda | Early to mid | White creamy | Pear | Yellow | Acidic |
| Tanaka | Late | Brownish orange | Ovoid or round | Orange yellow | Sweet |
| Thales | Late | Orange yellow | Oblong | Yellow | Sweet |
| Thames Pride | Early | Pale orange | Ovate | Yellow | Subacid |
| Victor | Very late | White | Oblong | Deep yellow | Sweet |
| Zhaozong | Mid | White | Pear | Yellow | Acidic |
| Zhaozong No. 6 | Late | Yellow | Oval | Light yellow | Sweet |
| Parameter | Ripe Fruit Concentration |
|---|---|
| Respiration rate at 20 °C (µL CO2 g−1 h−1) | 36.59–48.13 |
| Ethylene production at 20 °C (nL g−1 h−1) | 0.62–1.00 |
| Fruit firmness (N) | 3.33–3.83 |
| Lightness | 61.56–63.11 |
| a * | 8.82–10.57 |
| b * | 49.64–52.05 |
| Total soluble solids (°Brix) | 7.63–12.97 |
| Titratable acidity (%) | 7.04–9.78 |
| TSS/TA ratio | 0.86–1.11 |
| Glucose (g 100 g−1 FW) | 1.4–1.7 |
| Fructose (g 100 g−1 FW) | 3.08–3.60 |
| Sucrose (g 100 g−1 FW) | 5.2–5.4 |
| Malic acid (mg 100 g−1 FW) | 101.97–150.14 |
| Citric acid (mg 100 g−1 FW) | 895.58–988.05 |
| Ascorbic acid (µg g−1 FW) | 15.7–18.3 |
| Total phenolic content (µg GAE/g FW) | 427.9–450.7 |
| Total antioxidant content (% inhibition DPPH) | 60–65 |
| Total flavonoid content (µg rutin/g FW) | 43.5–51.0 |
| Total carotenoids (β-carotene/g FW) | 35.7–48.3 |
| Cultivar | Treatment | Inference | Reference |
|---|---|---|---|
| ‘Fukuhara’ | Hot air treatment 37 °C for 3- and 6 h | Higher AsA, TA, and TSS content with less activity of PAL, POD, and PPO enzymes were observed. CI was suppressed. | [66] |
| ‘Jiefangzhong’ | Hot air (38 °C) for 36- and 48 h | Less fruit rot, FWL, FD, and internal browning with higher TA and TSS content. Burning symptoms were observed when stored for 48 h. | [65] |
| ‘Jiefangzhong’ | Hot air exposure (38 °C) for 5 h | Reduced MDA and H2O2 contents, higher juice content and less fruit firmness, enhanced APX, SOD, and CAT enzyme activities. Lowered LOX and superoxide radical production. | [63] |
| ‘Jiefangzhong’ | Hot air treatment (38 °C) for 5 h | Delayed the activities of PG, PPO, POD, and PAL enzymes, reduced lignin deposition and FD, maintained higher sensory quality. | [64] |
| ‘Jiefangzhong’ | Exposure to hot air at 38 °C for 5 h | Inhibition of PLD and LOX enzymes, decline in membrane leakage, higher linolenic and linoleic acid content with less stearic and oleic acid. Maintained higher unsaturated/saturated fatty acid content with less MDA content. | [62] |
| ‘Jiefangzhong’ | Hot air (38 °C) for 36 h + Pichia guilliermondii | C. acutatum was lowered with higher SOD and CAT enzymes and less ROS. Higher lignin deposition due to higher PAL enzyme. | [68] |
| ‘Jiefangzhong’ | Hot air treatment at 38 °C for 36 h | Reduced FWL, FD, internal browning, and POD, PPO, and PAL enzymes. Higher APX, CAT, and SOD enzymes with less membrane leakage. Higher TPC with less CI and ROS. | [69] |
| ‘Jiefangzhong’ | Hot air exposure (35 °C) for 3 h | Lowered MDA and H2O2 contents, maintained membrane integrity, higher NI, AI, SS, and SPS enzyme assays with less sucrose and higher fructose and glucose level. Alleviated CI symptoms. | [70] |
| ‘Jiefangzhong’ | Hot air (38 °C, 5 h) + MeJA 16 μmol L−1 | Less protopectin, pectin, and lignin content, less PPO, POD, and PAL with higher PG, APX, CAT and SOD enzyme activities. | [71] |
| ‘Jiefangzhong’ | Hot water treatment (45 °C) for 10 min + GB 10 mmol L−1 | Reduced MDA content and electrolyte leakage, higher CAT, SOD, and APX enzymes, higher proline, and GABA due to higher OAT, P5CS, and GAD enzymes. | [72] |
| Chemical | Cultivar | Treatment | Inference | Reference |
|---|---|---|---|---|
| 1-MCP | ‘Baisha’, ‘Luoyangqing’ | 1 μL L−1 + LTC at 5 °C for 6d | Less G-POD, CAD, and PAL enzyme activities with less superoxide radicals, lignin deposition, FD at 5 °C LTC, and 1-MCP in both cultivars. ‘Luoyangqing’ was a better respondent than ‘Baisha’ | [82] |
| ‘Claudia’ | 1, 2, 3, 4 and 5 μL L−1 | CI, browning, FD, and fruit softening were delayed with better fruit sensory attributes. Best treatment was 1 μL L−1 1-MCP. | [83] | |
| ‘Claudia’ ‘Nespolone di Trabia’ | 0.50 and 1 μL L−1 | Fruit firmness was maintained with better TA and lower browning in both cultivars. However, better results were presented by ‘Nespolone di Trabia’ with 1 μL L−1 1-MCP treatment. | [84] | |
| ‘Fuyang’ | 10, 50 and 100 nL L−1 | CI was inhibited with a decrease in MDA, H2O2, and superoxide radicals, lower LOX, and PLC enzymes. However, CAT enzyme activity was maintained. | [79] | |
| ‘Fuyang’ | 2.32 nmol L−1 | CI was reduced. Higher linolenic and linoleic acid content, leading to higher unsaturated/saturated fatty acid ratio. Less hemicellulose and cellulose content with higher water- and CDTA-soluble pectin content. | [80] | |
| ‘Fuyang’ | 50 nL L−1 | Suppressed PAL, CAD, C4H, 4CL coenzyme A, POD, and PPO enzymes, and high PG enzyme activity. CI was also lowered. Inhibition of FD, browning, and lignin deposition were observed. | [85] | |
| ‘Jiefangzhong’ | 50 nL L−1 | Suppressed browning, FD, H2O2 content, and superoxide radicals. Improved APX, SOD, and CAT enzymes with higher chitinase and β-1,3-glucanase enzymes. Higher juice content, TSS, and TA were also exhibited. C. acutatum infection was inhibited. | [81] | |
| ‘Luoyangqing’ | 0.5, 5 and 50 μL L−1 | Inhibited browning and ethylene production. Lowered PPO and LOX enzymes. Maintained higher TPC and polyphenol content with reduced superoxide anion radicals. | [21] | |
| ‘Qingzhong’ | 50 nL L−1 | Lowered FD, enhanced TSS, TA, sucrose, glucose and TPC. Reduced PPO activity with higher DPPH-radical scavenging activity. Inhibited ROS production. | [78] | |
| BTH | ‘Jiefangzhong’ | 10, 30 and 60 mg L−1 | Higher TSS and TA were maintained. Suppressed C. acutatum infestation and PAL enzyme activity. Lignin deposition was minimized, and there was aggravation of the disease tolerance mechanism. | [86] |
| ‘Jiefangzhong’, ‘Zhaozhong 6′ | 10, 30 and 60 mg L−1 | Higher chitinase and β-1,3-glucanase, SOD, CAT, POD, and PPO enzyme activities but suppressed PAL enzyme. Reduced LOX and ROS production. | [87] | |
| 2, 3 and 4% CaCl2 | Maintained a lower browning index, weight loss, and TA while increasing juice content, pH, and TSS. | [88] | ||
| ‘Advance’ | 4% CaCl2 + 11 mM AsA + 5 mM CA + 5 mmol SA | Higher hue angle was exhibited. Less FWL, DI, FD, and firmness were seen. There was higher TA, TSS, and AsA content. | [89] | |
| ‘Changhong’ | 1% CaCl2 | Reduced CI, superoxide anion, H2O2, and MDA content while exhibiting higher activities of DHAR, GR, MDHAR, APX, CAT, and SOD enzymes, as well as a higher DPPH radical assay. | [90] | |
| ‘Changhong’ | 1% CaCl2, 10 mmol L−1 EGTA | Prevented CI, ion leakage, and MDA content with higher ATP, ADP, and EC. Increased Ca2+-ATPase and H+-ATPase, CCO, SDH, PAO, DAO, GAD, OAT, and P5CS enzymes. There were higher proline, polyamine, and GABA contents. | [91] | |
| ‘Surkh’ | 1, 2 and 3% CaCl2 | Reduction in EC, FWL, browning, and firmness increase. Higher TA, TSS, and AsA contents. | [23] | |
| ‘Trouloti’ | 2% CaCl2 | Sweetness was increased, and acidity declined with a lower TPC level. There was no impact on FWL and dry matter. There was a reduction in respiration rate. | [92] | |
| ‘Qingzhong’ | 0.8% CaCl2 + 0.2, 0.4 and 0.8% PAA | DI, FD, FWL, respiration rate, and membrane leakage was decreased. Higher TSS, TA, and AsA content with better sensory attributes were observed. | [93] | |
| Ethanol | ‘Jiefangzhong’ | 300 μL L−1 | Boosted activities of PAL, SOD, PPO, POD, chitinase, and β-1,3-glucanase enzymes. H2O2 was increased which initiated a defense mechanism against spores and mycelium of C. acutatum. | [94] |
| GB | ‘Jiefangzhong’ | 10 mmol L−1 GB + HT 45 °C for 10 min | Higher CAT, SOD, APX, P5CS, OAT, and GAD enzymes with higher GABA and proline content. Reduction in MDA content and CI symptoms. | [72] |
| ‘Jiefangzhong’ | 1, 5, 10, 20 mmol L−1 GB | Higher TFC and TPC content, less FWL, and browning. Higher CAT and SOD enzymes with less MDA content. A 10 mmolL−1 concentration was best. | [95] | |
| MeJA | ‘Fuyang’ | 10 mmol L−1 MeJA | Reduction in respiration, ethylene production, PPO, and PAL enzymes activities. Higher total sugar and organic acid content with higher TPC and TFC was maintained. Browning and FD were lessened. | [96] |
| ‘Fuyang’ | 10 mmol L−1 MeJA | Reduced CI, saturated fatty acids, H2O2 content, and superoxide radicals. Fruit firmness and colour was maintained. | [97] | |
| ‘Fuyang’ | 10 mmol L−1 MeJA | CI was lowered with less pectin, hemicellulose, pectin, alcohol content, and lignin deposition by suppressing PAL and PPO enzymes. CDTA and water soluble pectin were increased. ROS production was lowered with a remarkable decline in the POD enzyme. | [98] | |
| ‘Jiefangzhong’ | 10 μmol L−1 MeJA | Exhibited higher level of chitinase and β-1,3-glucanase enzymes, higher TSS and TA content, less browning, FD, and spore germination of C. acutatum. | [24] | |
| ‘Jiefangzhong’ | 10 μmol L−1 MeJA | Reduced lignin deposition, inhibited PPO and PAL enzyme activities. MeJA induced H2O2 content that suppressed the fungal growth of C. acutatum with higher CAT and APX enzymes. | [99] | |
| ‘Jiefangzhong’ | 10 μmol L−1 MeJA + Pichia membranefaciens 1 × 108 colony-forming units mL−1 | Inhibited spore germination and germ tube elongation of C. acutatum. There was less disease incidence and higher chitinase and β-1,3-glucanase enzymes activities were maintained. | [100] | |
| ‘Jiefangzhong’ | 10 μmol L−1 MeJA | GSH-POD, APX, and GST enzymes were enhanced with a higher AsA content. GSH content was decreased due to more MDHAR, DHAR, and GR enzyme activities, and inhibition of AO enzyme activity. Browning was also delayed. | [101] | |
| ‘Jiefangzhong’ | 10 μmol L−1 MeJA | Higher GABA and proline content were shown with higher P5CS, OAT, and GAD enzymes and reduced PDH enzyme. CI was suppressed with less browning. | [102] | |
| ‘Jiefangzhong’ | 10 μmol L−1 MeJA | Higher ATP, Put, Spd, and Spm content was maintained. Suppressed AMP and ADP production by increasing energy status with less anthracnose symptoms. | [103] | |
| ‘Jiefangzhong’ | MeJA 16 μmol L−1 + HT at 38 °C for 5 h | Less protopectin, pectin and lignin content, less PPO, POD, and PAL with higher PG, APX, CAT, and SOD enzyme activities. | [71] | |
| ‘Luoyangqing’ | 10 µmol L−1 | Induced EjbHLH14, EjHB1, and EjPRX12 gene expression, thereby decreasing CI and lignin content. | [17] | |
| NO | ‘Luoyangqing’ | 0.5 mmol L−1 cPTIO, 0.1 mmol L−1 TUN, 10 m mmol L−1 Gln, 0.5 m mmol L−1, L-NAME and 0.5 m mmol L−1 PBITU | All these NO inhibitors reduced APX, CAT, POD, and SOD enzyme activities with higher MDA and H2O2 content. Membrane leakage and browning were increased. The role of NO in fruit quality has been confirmed. | [25] |
| Melatonin | ‘Dawuxing’ | 50 µL melatonin | Reduced weight loss and MDA content while maintaining higher firmness, ABTS, FRAP, and DPPH radical assays | [104] |
| SA | 1500, 2000 and 3000 ppm | Reduced browning and increased TSS, TA, and pH. | [105] | |
| ‘Jiefangzhong’ | 1 gL−1 SA | FD, lignin deposition, and browning were decreased. Reduction in CAD, PAL, and PPO enzymes were also observed. | [106] | |
| ‘Luoyangqing’ | 1 mmol L−1 ASA | Reduced the CI symptoms with reduced G-POD, CAD, and PAL enzyme activities and prohibited superoxide radical accumulation. | [12] | |
| ‘Zaozhong’ | 40 and 70 mg L−1 SA | GSH was increased with higher GST, G-POD, MDHAR, DHAR, and APX enzymes, which alleviated the POD enzyme and decreased MDA and H2O2 contents with less DHA activities. | [107] | |
| 2, 4-epibrassinolide | ‘Changhong’ | 10 µmol L−1 EBR | The symptoms of lignification were alleviated because of the delay in the rise in lignin concentration in loquat fruit. | [108] |
| Short term N2 | ‘Dahongpao’ | 100% N2 | Changes in membrane permeability, MDA levels, and ROS production rates were all significantly slowed down. Furthermore, SOD and CAT activities were considerably higher, whereas LOX activity was significantly lower, in N2-treated fruits compared to control fruits. | [109] |
| Cultivar | Treatment | Inference | Reference |
|---|---|---|---|
| ‘Algerie, ‘Golden Nugget’, | PLA tray at 5 or 10 °C | Reduced browning, weight loss, and sugar content with improved organoleptic attributes. | [135] |
| ‘Algerie’ | Microperforated PE at 2 °C storage and a 4 day shelf life at 20 °C | Lowered fruit weight loss, reduced firmness increase, and maintained higher sugar and acid levels. | [29] |
| ‘Champagne de Grasse’ | (I) 312.5 ppb of 1-MCP+MAP (II) 625.5 ppb of 1-MCP+MAP | Reduced internal browning, microbial activity, better fruit colour, and higher TSS content. | [133] |
| ‘Golden Nugget’ ‘Syeda’ | 12.5 µm, 14 µm, or 16 µm thick PVC films at 0 °C | Browning increased with increased PE film thickness, lowered weight loss, and firmness increase maintained higher TSS and TA content. | [136] |
| ‘Hafif Çukurgöbek’ | MAP (20 µm thick; LifePack) with 3- and 6-mM OA | Higher TPC, TFC, organic acids, and delayed browning and firmness increase was maintained, especially in 6 mM and MAP storage. | [132] |
| ‘Japanese Azalea’ | MAP with CA (0.5, 1.0%), AsA (1, 2%), NaHMP (0.5, 1.0%) | Higher DPPH radical scavenging activity with a better TPC, TFC, TA, TSS, and TSS/TA ratio was exhibited with less browning and weight loss. | [137] |
| ‘Jiefangzhong’ | PE packaging with 6 at 10 °C | Less respiration and fruit weight loss at 10 °C than at 6 °C with better sensory attributes. | [27] |
| ‘Jiefangzhong’ | Perforated low density PE with (4.8 ± 0.67)% CO2 and (11.5 ± 0.85)% O2. | Reduced weight loss, respiration rate, firmness increased, and maintained higher TSS, TA, and vitamin C content. | [60] |
| ‘Karantoki’ ‘Morphitiki’ | Xtend® packaging + 4 °C storage + 20 °C for 2d. | ‘Morphitiki’ was better in colour, TSS, TA, and higher browning was observed in ‘Karantoki’ | [18] |
| ‘Mogi’ | 0.15% perforated PE + 1, 5, 10, 20 and 30 °C | Suppressed respiration rate and ethylene production, reduction in malic acid and sucrose content, fruit weight loss and fruit decay were lowered. | [28] |
| ‘Ottawianni’ | MAP with (12% O2 + 3% CO2 + 85% Ar), (12% O2 + 3% CO2 + 85% N), (15% O2 + 5% CO2 + 80% Ar), (15% O2 + 5% CO2 + 80% N) | Ar-treated MAP stored fruits exhibited higher TPC and TFC content, while there was less browning in N treated MAP stored fruits. | [132] |
| ‘Surkh’ | HDPE (0.09 mm), LDPE (0.03 mm), 0.25% LDPEP at 4 °C | LDPE exhibited the least browning, HDPE maintained the lowest TSS and firmness, and LDPEP maintained the lowest TA and highest firmness. | [134] |
| ‘Qingzhong’ ‘Dawuxing’ | Nano-SiO2 packing (0.10% of nano-SiO2 with film thickness of 40 mm | Substantial reduction in the rate of internal browning, as well as slowed declines in TSS, TA, and vitamin C contents and extractable juice in both cultivars. | [138] |
| ‘Palermitana’ | PVC plastic film, transmission rates of water vapor, O2 and CO2 through CX film are 350 g m−2·24 h at 38 °C, 18,000 cm3 m−2·24 h,and 47,000 cm3 m−2·24 h at 23 °C. | Loquat fruits kept in cold storage and SL with film packaging significantly prevented shriveling and maintained interior quality and flavour. | [139] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, H.M.S.; Khan, A.S.; Singh, Z.; Ayyub, S. Postharvest Biology and Technology of Loquat (Eriobotrya japonica Lindl.). Foods 2023, 12, 1329. https://doi.org/10.3390/foods12061329
Shah HMS, Khan AS, Singh Z, Ayyub S. Postharvest Biology and Technology of Loquat (Eriobotrya japonica Lindl.). Foods. 2023; 12(6):1329. https://doi.org/10.3390/foods12061329
Chicago/Turabian StyleShah, Hafiz Muhammad Shoaib, Ahmad Sattar Khan, Zora Singh, and Saqib Ayyub. 2023. "Postharvest Biology and Technology of Loquat (Eriobotrya japonica Lindl.)" Foods 12, no. 6: 1329. https://doi.org/10.3390/foods12061329
APA StyleShah, H. M. S., Khan, A. S., Singh, Z., & Ayyub, S. (2023). Postharvest Biology and Technology of Loquat (Eriobotrya japonica Lindl.). Foods, 12(6), 1329. https://doi.org/10.3390/foods12061329





