Effect of Two-Step Sous Vide Cooking and Storage on Microbiological and Oxidative Stability of Chicken Breast
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Meat Sample Preparation
Preparation of Bacterial Strain and Inoculum
2.2. Experimental Design and Sous Vide Treatments
2.3. Microbiological Analysis
2.3.1. Determination of Enterococcus Faecalis
Bacterial Inoculation on Chicken Breast
Microbiological Enumeration
2.3.2. Determination of Total Mesophilic Aerobic Counts
2.4. Odor Acceptability
2.5. Lipid Oxidation
2.6. Statistical Analysis
3. Results
3.1. Effect of Sous Vide Treatments on Microbial Inactivation
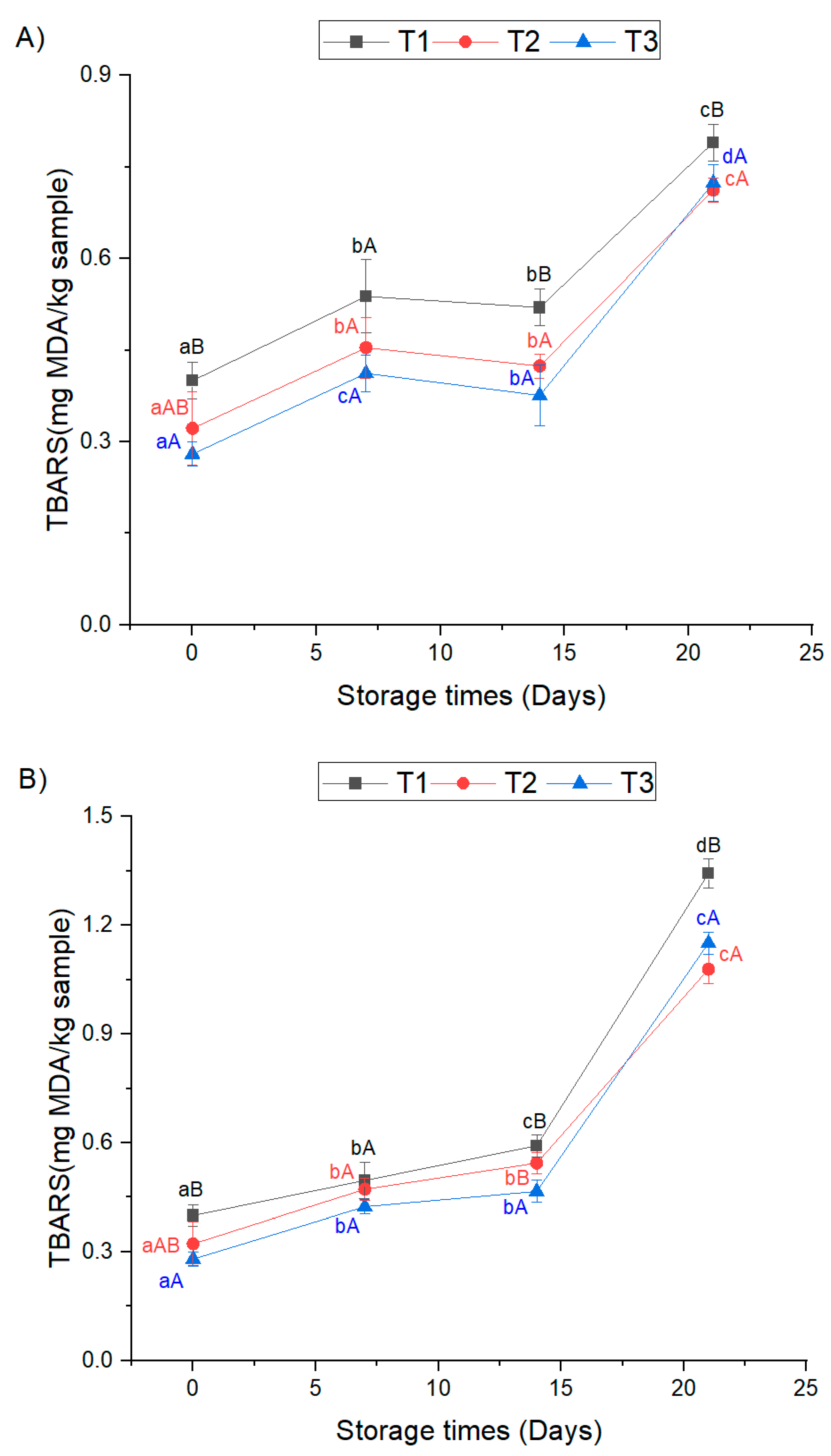
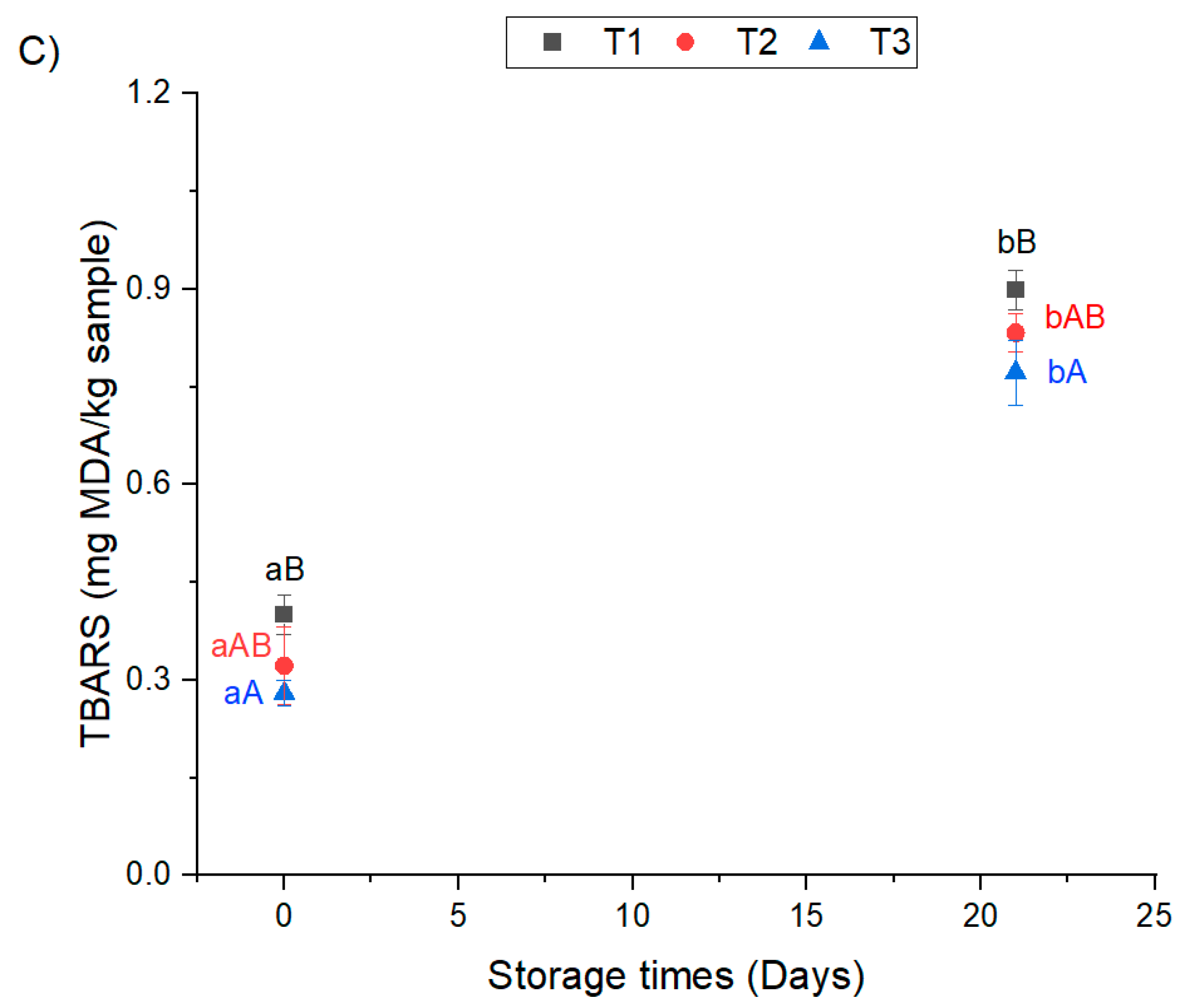
3.2. Oxidative Storage Stability
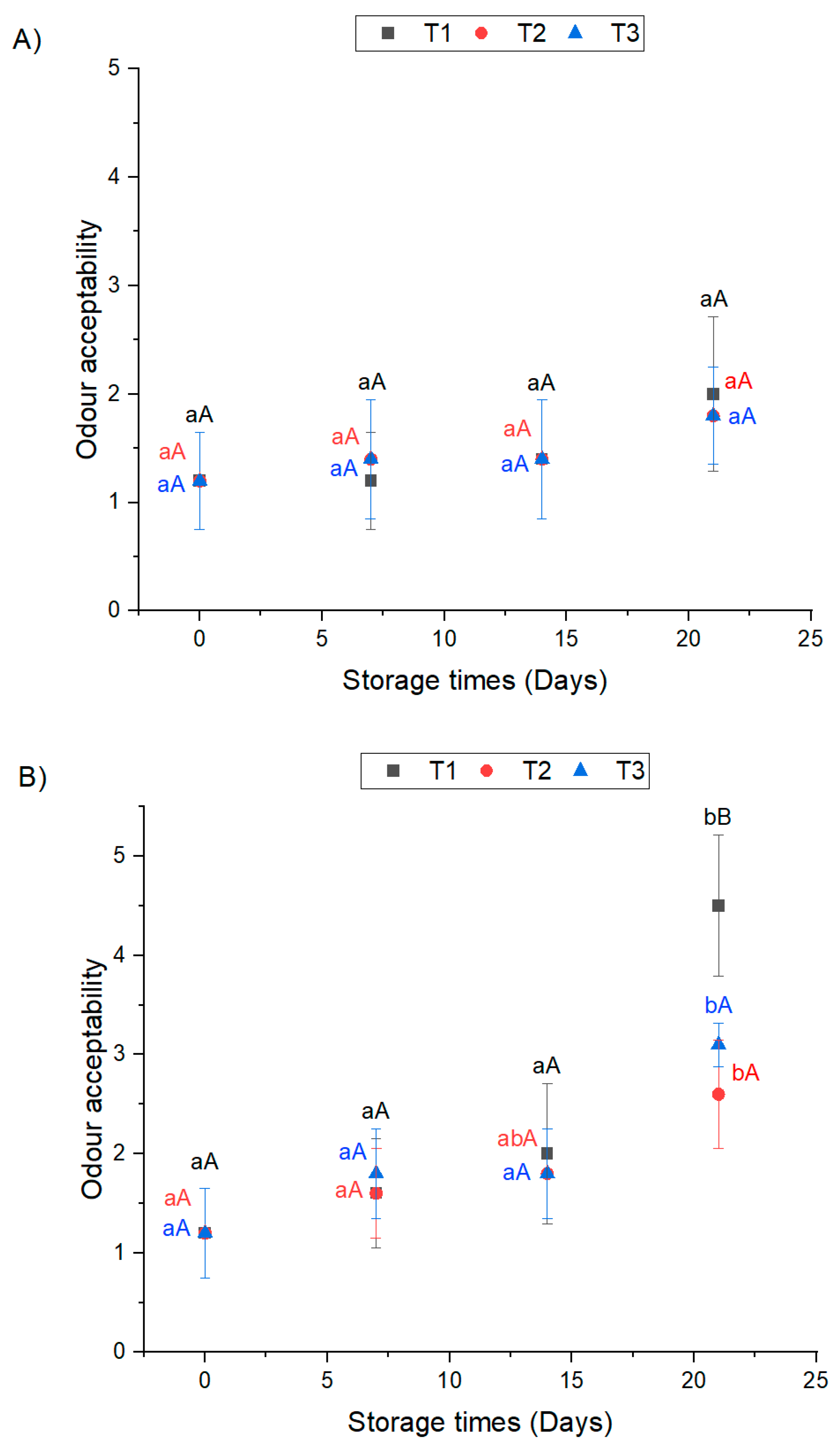
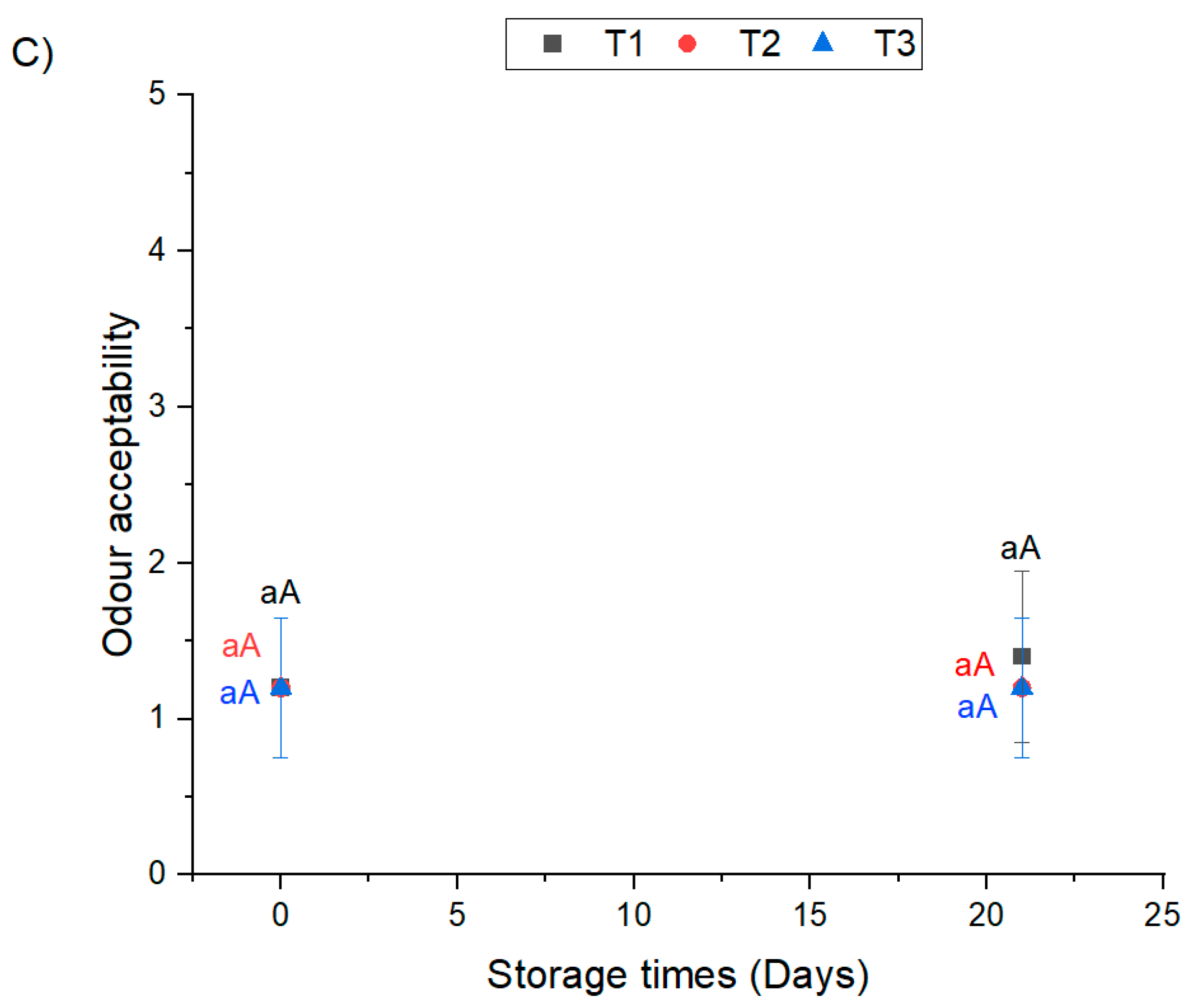
3.3. Odor Acceptability
3.4. Microbiological Storage Stability
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zavadlav, S.; Blažic, M.; Van de Velde, F.; Vignatti, C.; Fenoglio, C.; Piagentini, A.M.; Pirovani, M.E.; Perotti, C.M.; Bursac Kovacevic, D.; Putnik, P. Sous-Vide as a Technique for Preparing Healthy and High-Quality Vegetable and Seafood Products. Foods 2020, 9, 1537. [Google Scholar] [CrossRef]
- Díaz, P.; Nieto, G.; Garrido, M.D.; Bañón, S. Microbial, physical–chemical and sensory spoilage during the refrigerated storage of cooked pork loin processed by the sous vide method. Meat Sci. 2008, 80, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, D.E. Sous vide cooking: A review. Int. J. Gastron. Food Sci. 2012, 1, 15–30. [Google Scholar] [CrossRef]
- Rinaldi, M.; Dall’Asta, C.; Paciulli, M.; Cirlini, M.; Manzi, C.; Chiavaro, E. A Novel Time/Temperature Approach to Sous Vide Cooking of Beef Muscle. Food Bioprocess Technol. 2014, 7, 2969–2977. [Google Scholar] [CrossRef]
- Bıyıklı, M.; Akoğlu, A.; Kurhan, Ş.; Akoğlu, İ.T. Effect of Different Sous Vide Cooking Temperature-Time Combinations on the Physicochemical, Microbiological, and Sensory Properties of Turkey Cutlet. Int. J. Gastron. Food Sci. 2020, 20, 100204. [Google Scholar] [CrossRef]
- Christensen, L.; Ertbjerg, P.; Løje, H.; Risbo, J.; van den Berg, F.W.; Christensen, M. Relationship between meat toughness and properties of connective tissue from cows and young bulls heat treated at low temperatures for prolonged times. Meat Sci. 2013, 93, 787–795. [Google Scholar] [CrossRef]
- Ertbjerg, P.; Christiansen, L.S.; Pedersen, A.B.; Kristensen, L. The effect of temperature and time on activity of calpain and lysosomal enzymes and degradation of desmin in porcine longissimus muscle. In Proceedings of the 58th International Congress of Meat Science and Technology, Montreal, QC, Canada, 12–17 August 2012. [Google Scholar]
- Chang, Y.S.; Chou, R.G.R. Postmortem degradation of desmin and calpain in breast and leg and thigh muscles from Taiwan black-feathered country chickens. J. Sci. Food Agric. 2010, 90, 2664–2668. [Google Scholar] [CrossRef]
- Huang, M.; Huang, F.; Ma, H.; Xu, X.; Zhou, G. Preliminary study on the effect of caspase-6 and calpain inhibitors on postmortem proteolysis of myofibrillar proteins in chicken breast muscle. Meat Sci. 2012, 90, 536–542. [Google Scholar] [CrossRef]
- Lee, H.L.; Santé-Lhoutellier, V.; Vigouroux, S.; Briand, Y.; Briand, M. Role of calpains in postmortem proteolysis in chicken muscle. Poult. Sci. 2008, 87, 2126–2132. [Google Scholar] [CrossRef]
- Hasani, E.; Csehi, B.; Darnay, L.; Ladányi, M.; Dalmadi, I.; Kenesei, G. Effect of Combination of Time and Temperature on Quality Characteristics of Sous Vide Chicken Breast. Foods 2022, 11, 521. [Google Scholar] [CrossRef]
- Hasani, E.; Kenesei, G.; Dalmadi, I. Comparison of the single-step and double-step sous-vide treatment effect on the quality attributes of chicken breast: A novel approach to sous-vide. Prog. Agric. Eng. Sci. 2021, 17, 61–68. [Google Scholar] [CrossRef]
- Ismail, I.; Hwang, Y.H.; Joo, S.T. Effect of different temperature and time combinations on quality characteristics of sous-vide cooked goat gluteus medius and biceps femoris. Food Bioprocess Technol. 2019, 12, 1000–1009. [Google Scholar] [CrossRef]
- Stringer, S.C.; Fernandes, M.A.; Metris, A. Safety of Sous—Vide Foods: Feasibility of Extending Combase to Describe the Growth/Survival/Death Response of Bacterial Foodborne Pathogens between 40 °C and 60 °C; Final report on FSA project FS102028; Institute of Food Research: Norwich, UK, May 2012. [Google Scholar]
- Kharel, K.; Yemmireddy, V.K.; Graham, C.J.; Prinyawiwatkul, W.; Adhikari, A. Hot water treatment as a kill-step to inactivate Escherichia coli O157: H7, Salmonella enterica, Listeria monocytogenes and Enterococcus faecium on in-shell pecans. LWT 2018, 97, 555–560. [Google Scholar] [CrossRef]
- Oliveira, S.R.; Cruz, R.M.; Vieira, M.C.; Silva, C.L.; Gaspar, M.N. Enterococcus faecalis and Pseudomonas aeruginosa behaviour in frozen watercress (Nasturtium officinale) submitted to temperature abuses. Int. J. Refrig. 2009, 32, 472–477. [Google Scholar] [CrossRef]
- McAuley, C.M.; Gobius, K.S.; Britz, M.L.; Craven, H.M. Heat resistance of thermoduric enterococci isolated from milk. Int. J. Food Microbiol. 2012, 154, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Chajęcka-Wierzchowska, W.; Zadernowska, A.; Łaniewska-Trokenheim, Ł. Diversity of antibiotic resistance genes in Enterococcus strains isolated from ready-to-eat meat products. J. Food Sci. 2016, 81, 2799–2807. [Google Scholar] [CrossRef] [PubMed]
- Koluman, A.; Akan, L.S.; Çakiroğlu, F.P. Occurrence and antimicrobial resistance of enterococci in retail foods. Food Control 2009, 20, 281–283. [Google Scholar] [CrossRef]
- Yuksek, N.; Evrensel, S.S.; Temelli, S.; Sahsene, A.N. A microbiological evaluation on the ready-to-eat red meat and chicken donair kebabs from a local catering company in Bursa. J. Biol. Environ. Sci. 2009, 3, 7–10. [Google Scholar]
- Hong, G.-E.; Kim, J.-H.; Ahn, S.-J.; Lee, C.-H. Changes in Meat Quality Characteristics of the Sous-Vide Cooked Chicken Breast during Refrigerated Storage. Korean J. Food Sci. Anim. Resour. 2015, 35, 757–764. [Google Scholar] [CrossRef]
- Yang, X.; Wang, H.; Badoni, M.; Zawadski, S.; McLeod, B.; Holman, D.; Uttaro, B. Effects of a novel three-step sous-vide cooking and subsequent chilled storage on the microbiota of beef steaks. Meat Sci. 2020, 159, 107938. [Google Scholar] [CrossRef]
- Karyotis, D.; Skandamis, P.N.; Juneja, V.K. Thermal Inactivation of Listeria Monocytogenes and Salmonella Spp. In Sous-Vide Processed Marinated Chicken Breast. Food Res. Int. 2017, 100, 894–898. [Google Scholar] [CrossRef]
- Karpińska-Tymoszczyk, M.; Draszanowska, A.; Danowska-Oziewicz, M.; Kurp, L. The Effect of Low-Temperature Thermal Processing on the Quality of Chicken Breast Fillets. Food Sci. Technol. Int. 2020, 26, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Haugen, J.E.; Lundby, F.; Wold, J.P.; Veberg, A. Detection of rancidity in freeze stored turkey meat using a commercial gas-sensor array system. Sens. Actuators B Chem. 2006, 116, 78–84. [Google Scholar] [CrossRef]
- Archile-Contreras, A.C.; Purslow, P.P. Oxidative stress may affect meat quality by interfering with collagen turnover by muscle fibroblasts. Food Res. Int. 2011, 44, 582–588. [Google Scholar] [CrossRef]
- Akoğlu, I.; Bıyıklı, M.; Akoğlu, A.; Kurhan, Ş. Determination of the Quality and Shelf Life of Sous Vide Cooked Turkey Cutlet Stored at 4 and 12oC. Rev. Bras. Ciência Avícola 2018, 20, 1–8. [Google Scholar] [CrossRef]
- BC Centre for Disease Control 2016 Guidelines for Restaurant Sous Vide Cooking Safety in British Columbia. Available online: http://www.bccdc.ca/search?k=svguidelines_finalforweb.pdf (accessed on 9 February 2021).
- Belletti, N.; Garriga, M.; Aymerich, T.; Bover-Cid, S. High pressure inactivation of a virulent Enterococcus faecalis on dry-cured ham: Modeling the effect of processing parameters. Innov. Food Sci. Emerg. Technol. 2013, 18, 43–47. [Google Scholar] [CrossRef]
- Jridi, M.; Siala, R.; Fakhfakh, N.; Ayadi, M.A.; Elhatmi, M.; Taktak, M.A.; Nasri, M.; Zouari, N. Effect of rosemary leaves and essential oil on turkey sausage quality. Acta Aliment. 2015, 44, 534–541. [Google Scholar] [CrossRef]
- Gill, C.O.; Badoni, M. Microbiological and organoleptic qualities of vacuum-packaged ground beef prepared from pasteurized manufacturing beef. Int. J. Food Microbiol. 2002, 74, 111–118. [Google Scholar] [CrossRef]
- Youssef, M.K.; Gill, C.O.; Yang, X. Storage life at 2 °C or −1.5 °C of vacuum-packaged boneless and bone-in cuts from decontaminated beef carcasses. J. Sci. Food Agric. 2014, 94, 3118–3124. [Google Scholar] [CrossRef]
- Dias, M.V.; Nilda de Fátima, F.S.; Borges, S.V.; de Sousa, M.M.; Nunes, C.A.; de Oliveira, I.R.; Medeiros, E.A. Use of Allyl Isothiocyanate and Carbon Nanotubes in an Antimicrobial Film to Package Shredded, Cooked Chicken Meat. Food Chem. 2013, 141, 3160–3166. [Google Scholar] [CrossRef]
- Ganhão, R.; Estévez, M.; Morcuende, D. Suitability of the TBA Method for Assessing Lipid Oxidation in a Meat System with Added Phenolic-Rich Materials. Food Chem. 2011, 126, 772–778. [Google Scholar] [CrossRef]
- de Oliveira, T.L.; Junior, B.R.; Ramos, A.L.; Ramos, E.M.; Piccoli, R.H.; Cristianini, M. Phenolic carvacrol as a natural additive to improve the preservative effects of high pressure processing of low-sodium sliced vacuum-packed turkey breast ham. LWT-Food Sci. Technol. 2015, 64, 1297–1308. [Google Scholar] [CrossRef]
- NACMCF. Requisite scientific parameters for establishing the equivalence of alternative methods of pasteurization. J. Food Prot. 2006, 69, 1190. [Google Scholar] [CrossRef]
- Haghighi, H.; Belmonte, A.M.; Masino, F.; Minelli, G.; Lo Fiego, D.P.; Pulvirenti, A. Effect of time and temperature on physicochemical and microbiological properties of sous vide chicken breast fillets. Appl. Sci. 2021, 11, 3189. [Google Scholar] [CrossRef]
- Miranda, J.M.; Guarddon, M.; Mondragon, A.; Vázquez, B.I.; Fente, C.A.; Cepeda, A.; Franco, C.M. Antimicrobial resistance in Enterococcus spp. strains isolated from organic chicken, conventional chicken, and turkey meat: A comparative survey. J. Food Prot. 2007, 70, 1021–1024. [Google Scholar] [CrossRef] [PubMed]
- Leygonie, C.; Britz, T.J.; Hoffman, L.C. Impact of freezing and thawing on the quality of meat. Meat Sci. 2012, 91, 93–98. [Google Scholar] [CrossRef]
- Estévez, M.; Ventanas, S.; Heinonen, M. Formation of Strecker Aldehydes between Protein Carbonyls—α-Aminoadipic and γ-Glutamic Semialdehydes—and Leucine and Isoleucine. Food Chem. 2011, 128, 1051–1057. [Google Scholar] [CrossRef]
- Ingham, S.C.; Tautorus, C.L. Survival of Salmonella typhimurium, Listeria monocytogenes and indicator bacteria on cooked uncured turkey loaf stored under vacuum at 3 °C. J. Food Saf. 1991, 11, 285–292. [Google Scholar] [CrossRef]
- Moreno, M.F.; Sarantinopoulos, P.; Tsakalidou, E.; De Vuyst, L. The role and application of enterococci in food and health. Int. J. Food Microbiol. 2006, 106, 1–24. [Google Scholar] [CrossRef]
- Mohammed, H.H.; He, L.; Nawaz, A.; Jin, G.; Huang, X.; Ma, M.; Abdegadir, W.S.; Elgasim, E.A.; Khalifa, I. Effect of frozen and refrozen storage of beef and chicken meats on inoculated microorganisms and meat quality. Meat Sci. 2021, 175, 108453. [Google Scholar] [CrossRef]
| Group | Time at Temperature of 50 °C (Min) | Time at Temperature of 60 °C (Min) | Treatment Time Ratio 50 °C/60 °C | Total Treatment Time (Min) |
|---|---|---|---|---|
| T1 | 0 | 120 | 0:1 | 120 |
| T2 | 40 | 80 | 1:2 | 120 |
| T3 | 60 | 60 | 1:1 | 120 |
| Microorganism | Raw Sample | Treatments | p-Value | ||
|---|---|---|---|---|---|
| T1 | T2 | T3 | |||
| Total mesophilic aerobic counts (log CFU/g) | 5.72 ± 0.5 | ND | 3.77 ± 0.08 a | 4.03 ± 0.04 b | 0.06 |
| Enterococcus faecalis NCAIM B. 01312 (log CFU/g) | 2.69 ± 0.12 | ND | 3.01 ± 0.07 a | 3.37 ± 0.07 b | 0.02 |
| Microorganism | Storage Days | Treatments | ||
|---|---|---|---|---|
| T1 | T2 | T3 | ||
| Total mesophilic aerobic counts (log CFU/g) | 0 | ND | 3.77 ± 0.08 aA | 4.03 ± 0.04 aB |
| 7 | ND | 3.8 ± 0.03 aA | 4.12 ± 0.05 abB | |
| 14 | ND | 3.81 ±0.04 aA | 4.19 ± 0.08 bcB | |
| 21 | ND | 3.95 ±0.03 bA | 4.31 ± 0.03 cB | |
| p-value | - | 0.008 | 0.001 | |
| Enterococcus faecalis NCAIM B. 01312 (log CFU/g) | 0 | ND | 3.01 ± 0.07 aA | 3.37 ± 0.07 aB |
| 7 | ND | 3.07 ± 0.04 aA | 3.40 ± 0.02 aB | |
| 14 | ND | 3.11 ± 0.02 aA | 3.39 ± 0.03 aB | |
| 21 | ND | 3.09 ± 0.02 aA | 3.40 ± 0.02 aB | |
| p-value | - | 0.07 | 0.63 | |
| Microorganism | Storage Days | Treatments | ||
|---|---|---|---|---|
| T1 | T2 | T3 | ||
| Total mesophilic aerobic counts (log CFU/g) | 0 | ND | 3.77 ± 0.08 Aa | 4.03 ± 0.04 aB |
| 7 | ND | 3.89 ± 0.04 aA | 4.0 ± 0.1 aA | |
| 14 | ND | 5.02 ± 0.04 bA | 5.29 ± 0.03 bB | |
| 21 | ND | 6.64 ± 0.04 cA | 6.91 ± 0.04 cB | |
| p-value | - | <0.001 | <0.001 | |
| Enterococcus faecalis NCAIM B. 01312 (log CFU/g) | 0 | ND | 3.01 ± 0.07 aA | 3.37 ± 0.07 aB |
| 7 | ND | 3.24 ± 0.06 bA | 3.47 ± 0.03 aB | |
| 14 | ND | 4.22 ± 0.09 cA | 5.08 ± 0.05 bB | |
| 21 | ND | 6.37 ± 0.03 dA | 6.64 ± 0.04 cB | |
| p-value | - | <0.001 | <0.001 | |
| Microorganism | Storage Days | Treatments | ||
|---|---|---|---|---|
| T1 | T2 | T3 | ||
| Total mesophilic aerobic counts (log CFU/g) | 0 | ND | 3.77 ± 0.08 bA | 4.03 ± 0.04 bB |
| 21 | ND | 3.1 ± 0.05 aA | 3.35 ± 0.06 aB | |
| p-value | - | <0.001 | <0.001 | |
| Enterococcus faecalis NCAIM B. 01312 (log CFU/g) | 0 | ND | 3.01 ± 0.07 bA | 3.37 ± 0.07 bB |
| 21 | ND | 2.05 ± 0.05 aA | 2.91 ± 0.05 aB | |
| p-value | - | <0.001 | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasani, E.; Kiskó, G.; Dalmadi, I.; Hitka, G.; Friedrich, L.F.; Kenesei, G. Effect of Two-Step Sous Vide Cooking and Storage on Microbiological and Oxidative Stability of Chicken Breast. Foods 2023, 12, 1213. https://doi.org/10.3390/foods12061213
Hasani E, Kiskó G, Dalmadi I, Hitka G, Friedrich LF, Kenesei G. Effect of Two-Step Sous Vide Cooking and Storage on Microbiological and Oxidative Stability of Chicken Breast. Foods. 2023; 12(6):1213. https://doi.org/10.3390/foods12061213
Chicago/Turabian StyleHasani, Endrit, Gabriella Kiskó, István Dalmadi, Géza Hitka, László Ferenc Friedrich, and György Kenesei. 2023. "Effect of Two-Step Sous Vide Cooking and Storage on Microbiological and Oxidative Stability of Chicken Breast" Foods 12, no. 6: 1213. https://doi.org/10.3390/foods12061213
APA StyleHasani, E., Kiskó, G., Dalmadi, I., Hitka, G., Friedrich, L. F., & Kenesei, G. (2023). Effect of Two-Step Sous Vide Cooking and Storage on Microbiological and Oxidative Stability of Chicken Breast. Foods, 12(6), 1213. https://doi.org/10.3390/foods12061213





