Selenium Speciation in Se-Enriched Soybean Grains from Biofortified Plants Grown under Different Methods of Selenium Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Area Characterization
2.2. Selenium Application Methods
2.3. Selenium Extraction: Soluble and Protease
2.4. Total Selenium, Selenium Recovery, Selenium Speciation, and Total Free Amino Acids
2.5. Statistical Analyses
3. Results
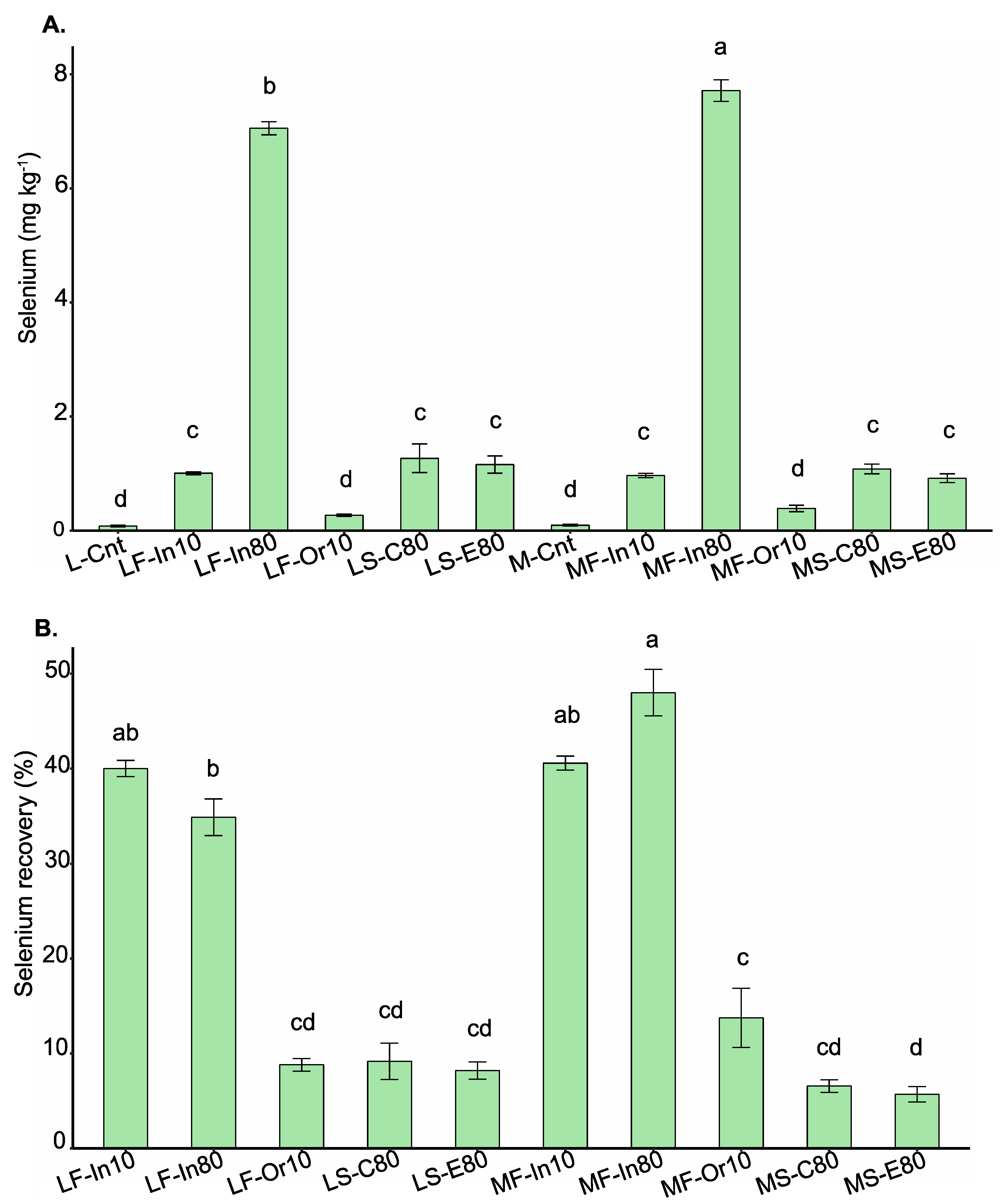
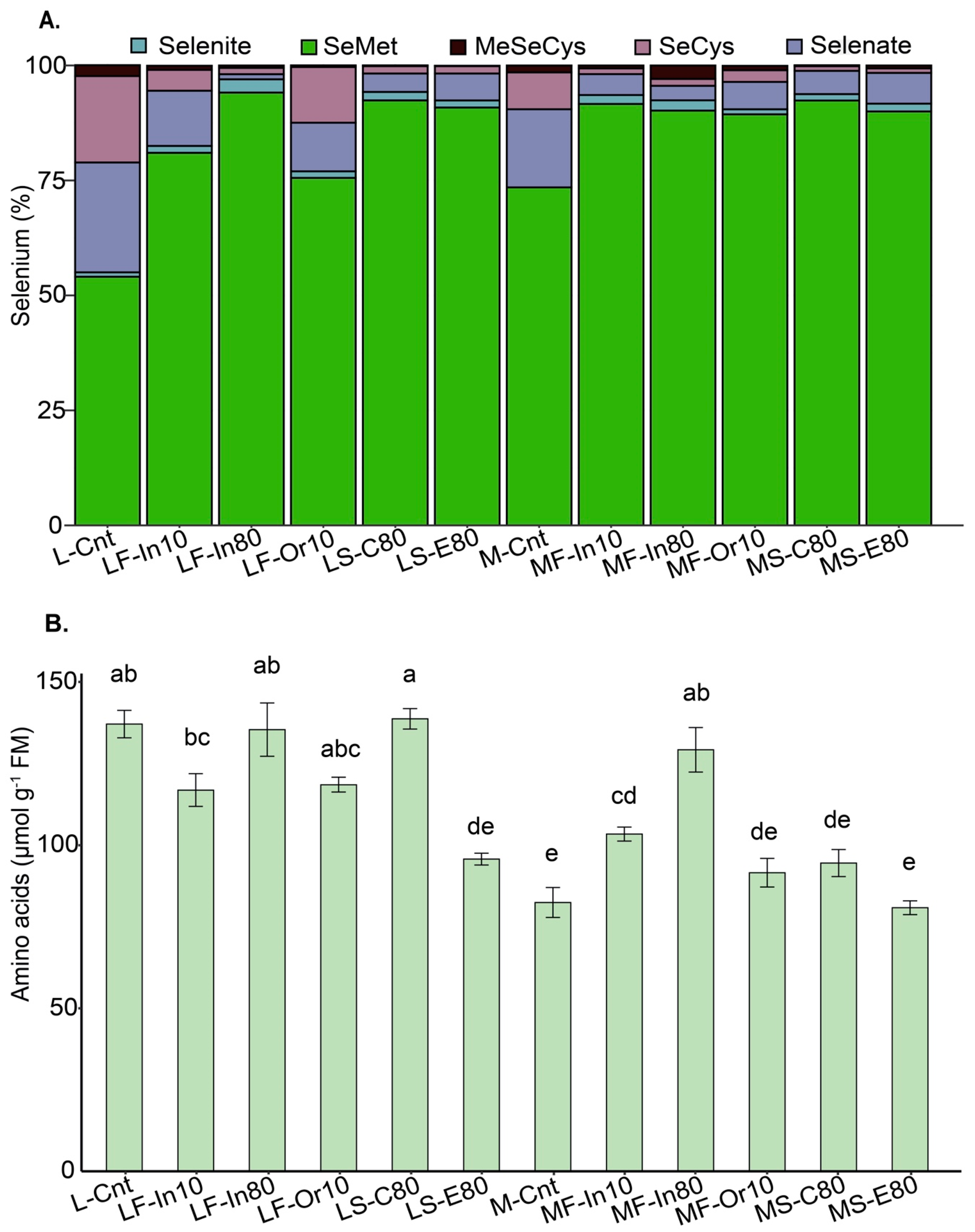
3.1. Selenium Accumulation, Recovery, and Speciation in Soybean Grains
3.2. Total Free Amino Acids
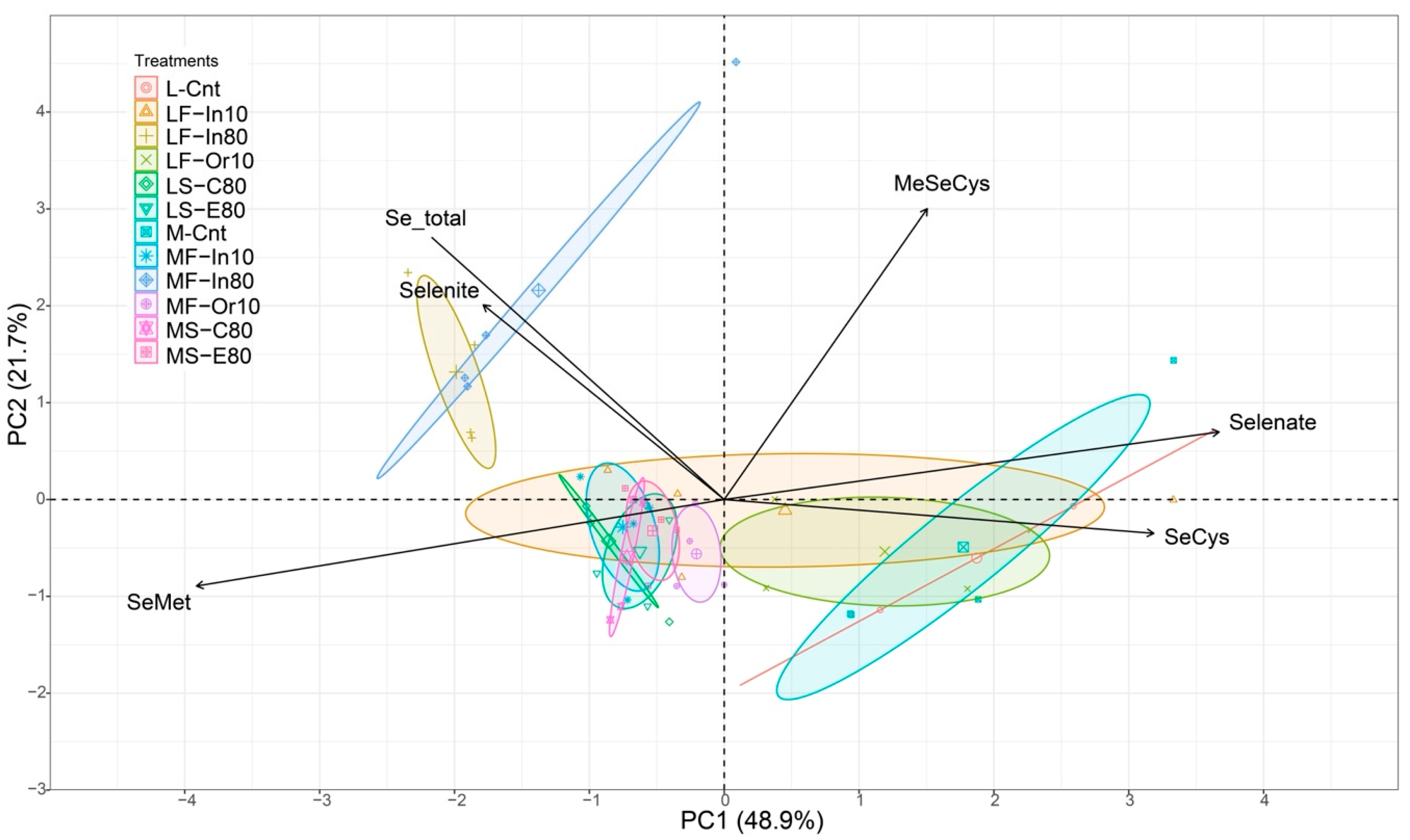
3.3. Principal Component Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rayman, M.P. Food-Chain Selenium and Human Health: Emphasis on Intake. Br. J. Nutr. 2008, 100, 254–268. [Google Scholar] [CrossRef]
- Combs, G.F. Selenium in Global Food Systems. Br. J. Nutr. 2001, 85, 517–547. [Google Scholar] [CrossRef]
- Jones, G.D.; Droz, B.; Greve, P.; Gottschalk, P.; Poffet, D.; McGrath, S.P.; Seneviratne, S.I.; Smith, P.; Winkel, L.H.E. Selenium Deficiency Risk Predicted to Increase under Future Climate Change. Proc. Natl. Acad. Sci. USA 2017, 114, 2848–2853. [Google Scholar] [CrossRef] [PubMed]
- Garg, M.; Sharma, N.; Sharma, S.; Kapoor, P.; Kumar, A.; Chunduri, V.; Arora, P. Biofortified Crops Generated by Breeding, Agronomy, and Transgenic Approaches Are Improving Lives of Millions of People around the World. Front. Nutr. 2018, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Natasha; Shahid, M.; Niazi, N.K.; Khalid, S.; Murtaza, B.; Bibi, I.; Rashid, M.I. A Critical Review of Selenium Biogeochemical Behavior in Soil-Plant System with an Inference to Human Health. Environ. Pollut. 2018, 234, 915–934. [Google Scholar] [CrossRef] [PubMed]
- Lopes, G.; Ávila, F.W.; Guilherme, L.R.G. Selenium Behavior in the Soil Environment and Its Implication for Human Health. Ciênc. Agrotec. 2017, 41, 605–615. [Google Scholar] [CrossRef]
- Garousi, F. The Essentiality of Selenium for Humans, Animals, and Plants, and the Role of Selenium in Plant Metabolism and Physiology. Acta Univ. Sapientiae Aliment. 2017, 10, 75–90. [Google Scholar] [CrossRef]
- Schomburg, L. Selenium Deficiency Due to Diet, Pregnancy, Severe Illness, or COVID-19—A Preventable Trigger for Autoimmune Disease. Int. J. Mol. Sci. 2021, 22, 8532. [Google Scholar] [CrossRef]
- Cakmak, I. Enrichment of Cereal Grains with Zinc: Agronomic or Genetic Biofortification? Plant Soil 2008, 302, 1–17. [Google Scholar] [CrossRef]
- D’Amato, R.; Regni, L.; Falcinelli, B.; Mattioli, S.; Benincasa, P.; Dal Bosco, A.; Pacheco, P.; Proietti, P.; Troni, E.; Santi, C.; et al. Current Knowledge on Selenium Biofortification to Improve the Nutraceutical Profile of Food: A Comprehensive Review. J. Agric. Food Chem. 2020, 68, 4075–4097. [Google Scholar] [CrossRef]
- Broadley, M.R.; White, P.J.; Bryson, R.J.; Meacham, M.C.; Bowen, H.C.; Johnson, S.E.; Hawkesford, M.J.; McGrath, S.P.; Zhao, F.-J.; Breward, N.; et al. Biofortification of UK Food Crops with Selenium. Proc. Nutr. Soc. 2006, 65, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Lara, T.S.; de Lessa, J.H.L.; de Souza, K.R.D.; Corguinha, A.P.B.; Martins, F.A.D.; Lopes, G.; Guilherme, L.R.G. Selenium Biofortification of Wheat Grain via Foliar Application and Its Effect on Plant Metabolism. J. Food Compos. Anal. 2019, 81, 10–18. [Google Scholar] [CrossRef]
- Wang, M.; Ali, F.; Wang, M.; Dinh, Q.T.; Zhou, F.; Bañuelos, G.S.; Liang, D. Understanding Boosting Selenium Accumulation in Wheat (Triticum aestivum L.) Following Foliar Selenium Application at Different Stages, Forms, and Doses. Environ. Sci. Pollut. Res. 2020, 27, 717–728. [Google Scholar] [CrossRef]
- Lessa, J.; Raymundo, J.F.; Branco Corguinha, A.P.; Dias Martins, F.A.; Araujo, A.M.; Melo Santiago, F.E.; Pereira de Carvalho, H.W.; Guimarães Guilherme, L.R.; Lopes, G. Strategies for Applying Selenium for Biofortification of Rice in Tropical Soils and Their Effect on Element Accumulation and Distribution in Grains. J. Cereal Sci. 2020, 96, 103125. [Google Scholar] [CrossRef]
- De Araújo, S.N.; Raymundo, J.F.; Costa, F.F.R.; de Lima Lessa, J.H.; Guilherme, L.R.G.; Lopes, G. Selenium Application Methods and Rates for Biofortification of Common Bean and Their Residual Effects on Mombaça Grass. Crop Pasture Sci. 2022, 73, 792–803. [Google Scholar] [CrossRef]
- Ravello, R.A.V.; de Oliveira, C.; Lessa, J.; Boas, L.V.V.; de Castro, E.M.; Guilherme, L.R.G.; Lopes, G. Selenium Application Influenced Selenium Biofortification and Physiological Traits in Water-Deficit Common Bean Plants. Crop Pasture Sci. 2021, 73, 44–55. [Google Scholar] [CrossRef]
- Cipriano, P.E.; da Silva, R.F.; de Lima, F.R.D.; de Oliveira, C.; de Lima, A.B.; Celante, G.; Dos Santos, A.A.; Archilha, M.V.L.R.; Pinatto-Botelho, M.F.; Faquin, V.; et al. Selenium Biofortification via Soil and Its Effect on Plant Metabolism and Mineral Content of Sorghum Plants. J. Food Compos. Anal. 2022, 109, 104505. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, L.; Xin, Z.; Zhao, L.; An, X.; Hu, Q. Effect of Foliar Application of Zinc, Selenium, and Iron Fertilizers on Nutrients Concentration and Yield of Rice Grain in China. J. Agric. Food Chem. 2008, 56, 2079–2084. [Google Scholar] [CrossRef]
- Li, J.; Yang, W.; Guo, A.; Qi, Z.; Chen, J.; Huang, T.; Yang, Z.; Gao, Z.; Sun, M.; Wang, J. Combined Foliar and Soil Selenium Fertilizer Increased the Grain Yield, Quality, Total Se, and Organic Se Content in Naked Oats. J. Cereal Sci. 2021, 100, 103265. [Google Scholar] [CrossRef]
- Araujo, A.M.; de Lima Lessa, J.H.; de Lima, F.R.D.; Raymundo, J.F.; Curi, N.; Guilherme, L.R.G.; Lopes, G. Adsorption of Selenite in Tropical Soils as Affected by Soil Management, Ionic Strength, and Soil Properties. J. Soil Sci. Plant Nutr. 2020, 20, 139–148. [Google Scholar] [CrossRef]
- Curtin, D.; Hanson, R.; Lindley, T.N.; Butler, R.C. Selenium Concentration in Wheat (Triticum aestivum) Grain as Influenced by Method, Rate, and Timing of Sodium Selenate Application. N. Z. J. Crop Hortic. Sci. 2006, 34, 329–339. [Google Scholar] [CrossRef]
- Bañuelos, G.S.; Freeman, J.L.; Arroyo, I.S. Selenium Content and Speciation Differences in Selenium Enriched Soups Made from Selenium Biofortified Plants. J. Food Compos. Anal. 2022, 105, 104255. [Google Scholar] [CrossRef]
- Khanam, A.; Platel, K. Bioaccessibility of Selenium, Selenomethionine and Selenocysteine from Foods and Influence of Heat Processing on the Same. Food Chem. 2016, 194, 1293–1299. [Google Scholar] [CrossRef] [PubMed]
- Šindelářová, K.; Száková, J.; Tremlová, J.; Mestek, O.; Praus, L.; Kaňa, A.; Najmanová, J.; Tlustoš, P. The Response of Broccoli (Brassica oleracea Convar. Italica) Varieties on Foliar Application of Selenium: Uptake, Translocation, and Speciation. Food Addit. Contam. Part A 2015, 32, 2027–2038. [Google Scholar] [CrossRef]
- Zhang, M.; Xing, G.; Tang, S.; Pang, Y.; Yi, Q.; Huang, Q.; Huang, X.; Huang, J.; Li, P.; Fu, H. Improving Soil Selenium Availability as a Strategy to Promote Selenium Uptake by High-Se Rice Cultivar. Environ. Exp. Bot. 2019, 163, 45–54. [Google Scholar] [CrossRef]
- Hu, J.; Zhao, Q.; Cheng, X.; Selomulya, C.; Bai, C.; Zhu, X.; Li, X.; Xiong, H. Antioxidant Activities of Se-SPI Produced from Soybean as Accumulation and Biotransformation Reactor of Natural Selenium. Food Chem. 2014, 146, 531–537. [Google Scholar] [CrossRef]
- Silva, M.A.; de Sousa, G.F.; Corguinha, A.P.B.; de Lima Lessa, J.H.; Dinali, G.S.; Oliveira, C.; Lopes, G.; Amaral, D.; Brown, P.; Guilherme, L.R.G. Selenium Biofortification of Soybean Genotypes in a Tropical Soil via Se-Enriched Phosphate Fertilizers. Front. Plant Sci. 2022, 13, 988140. [Google Scholar] [CrossRef]
- Soil Survey Staff. Keys to Soil Taxonomy, 13th ed.; USDA-Natural Resources Conservation Service: Washington, DC, USA, 2022.
- Brazilian Agricultural Research Company (EMBRAPA). Manual de Métodos de Análise de Solo, 3rd ed.; Embrapa: Brasília, Brazil, 2017; ISBN 978-85-7035-771-7. [Google Scholar]
- Bañuelos, G.S.; Walse, S.S.; Yang, S.I.; Pickering, I.J.; Fakra, S.C.; Marcus, M.A.; Freeman, J.L. Quantification, Localization, and Speciation of Selenium in Seeds of Canola and Two Mustard Species Compared to Seed-Meals Produced by Hydraulic Press. Anal. Chem. 2012, 84, 6024–6030. [Google Scholar] [CrossRef]
- Montes-Bayón, M.; Yanes, E.G.; Ponce de León, C.; Jayasimhulu, K.; Stalcup, A.; Shann, J.; Caruso, J.A. Initial Studies of Selenium Speciation in Brassica juncea by LC with ICPMS and ES-MS Detection: An Approach for Phytoremediation Studies. Anal. Chem. 2002, 74, 107–113. [Google Scholar] [CrossRef]
- Yemm, E.W.; Cocking, E.C.; Ricketts, R.E. Ricketts Estimation of Amino Acids with Ninhydrin. Analyst 1955, 80, 209–214. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2022. [Google Scholar]
- Brown, P.H.; Zhao, F.-J.; Dobermann, A. What Is a Plant Nutrient? Changing Definitions to Advance Science and Innovation in Plant Nutrition. Plant Soil 2022, 476, 11–23. [Google Scholar] [CrossRef]
- Boldrin, P.F.; Faquin, V.; Ramos, S.J.; Boldrin, K.V.F.; Ávila, F.W.; Guilherme, L.R.G. Soil and Foliar Application of Selenium in Rice Biofortification. J. Food Compos. Anal. 2013, 31, 238–244. [Google Scholar] [CrossRef]
- Zhu, Y.-G.; Pilon-Smits, E.A.H.; Zhao, F.-J.; Williams, P.N.; Meharg, A.A. Selenium in Higher Plants: Understanding Mechanisms for Biofortification and Phytoremediation. Trends Plant Sci. 2009, 14, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Zhao, Z.; Lv, C.; Zhang, Z.; Yuan, L.; Liu, X. Effects of Sulfur Application on Selenium Uptake and Seed Selenium Speciation in Soybean (Glycine max L.) Grown in Different Soil Types. Ecotoxicol. Environ. Saf. 2021, 209, 111790. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Long, W.; Liang, T.; Zhu, M.; Zhu, A.; Luo, X.; Fu, L.; Hu, Z.; Zhu, R.; Wu, X. Effect of Foliar Spraying of Organic and Inorganic Selenium Fertilizers during Different Growth Stages on Selenium Accumulation and Speciation in Rice. Plant Soil 2022, volume number, 1–15. [Google Scholar] [CrossRef]
- Lessa, J.H.L.; Araujo, A.M.; Silva, G.N.T.; Guilherme, L.R.G.; Lopes, G. Adsorption-Desorption Reactions of Selenium (VI) in Tropical Cultivated and Uncultivated Soils under Cerrado Biome. Chemosphere 2016, 164, 271–277. [Google Scholar] [CrossRef]
- Araujo, A.M.; de Lima Lessa, J.H.; Ferreira, L.A.; Guilherme, L.R.G.; Lopes, G. Soil Management and Ionic Strength on Selenite Retention in Oxidic Soils. Ciênc. Agrotec. 2018, 42, 395–407. [Google Scholar] [CrossRef]
- Li, Z.; Liang, D.; Peng, Q.; Cui, Z.; Huang, J.; Lin, Z. Interaction between Selenium and Soil Organic Matter and Its Impact on Soil Selenium Bioavailability: A Review. Geoderma 2017, 295, 69–79. [Google Scholar] [CrossRef]
- Schiavon, M.; Nardi, S.; dalla Vecchia, F.; Ertani, A. Selenium Biofortification in the 21st Century: Status and Challenges for Healthy Human Nutrition. Plant Soil 2020, 453, 245–270. [Google Scholar] [CrossRef]
- Premarathna, L.; McLaughlin, M.J.; Kirby, J.K.; Hettiarachchi, G.M.; Stacey, S.; Chittleborough, D.J. Selenate-Enriched Urea Granules Are a Highly Effective Fertilizer for Selenium Biofortification of Paddy Rice Grain. J. Agric. Food Chem. 2012, 60, 6037–6044. [Google Scholar] [CrossRef]
- Winkel, L.; Vriens, B.; Jones, G.; Schneider, L.; Pilon-Smits, E.; Bañuelos, G. Selenium Cycling Across Soil-Plant-Atmosphere Interfaces: A Critical Review. Nutrients 2015, 7, 4199–4239. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; He, Z.; Lin, Z.; Zhu, Y.; Yuan, L.; Liu, Y.; Yin, X. Effects of Chinese Cooking Methods on the Content and Speciation of Selenium in Selenium Bio-Fortified Cereals and Soybeans. Nutrients 2018, 10, 317. [Google Scholar] [CrossRef] [PubMed]
- Bañuelos, G.S.; Freeman, J.; Arroyo, I. Accumulation and Speciation of Selenium in Biofortified Vegetables Grown under High Boron and Saline Field Conditions. Food Chem. X 2020, 5, 100073. [Google Scholar] [CrossRef]
- Schiavon, M.; Pilon-Smits, E.A.H. The Fascinating Facets of Plant Selenium Accumulation–Biochemistry, Physiology, Evolution and Ecology. New Phytol. 2016, 213, 1582–1596. [Google Scholar] [CrossRef]
- White, P.J. Selenium Metabolism in Plants. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2018, 1862, 2333–2342. [Google Scholar] [CrossRef]
- Sors, T.G.; Ellis, D.R.; Salt, D.E. Selenium Uptake, Translocation, Assimilation and Metabolic Fate in Plants. Photosynth. Res. 2005, 86, 373–389. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Gupta, S. An Overview of Selenium Uptake, Metabolism, and Toxicity in Plants. Front. Plant Sci. 2017, 7, 2074. [Google Scholar] [CrossRef] [PubMed]
- Tapiero, H.; Townsend, D.M.; Tew, K.D. The Antioxidant Role of Selenium and Seleno-Compounds. Biomed. Pharmacother. 2003, 57, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Schiavon, M.; Lima, L.W.; Jiang, Y.; Hawkesford, M.J. Effects of Selenium on Plant Metabolism and Implications for Crops and Consumers. In Selenium in Plants; Elizabeth, A.H., Pilon-Smits, E.A.H., Winkel, Z.Q.L., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 257–275. [Google Scholar]
- Institute of Medicine (US) Panel on Dietary Antioxidants and Related Compounds. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; National Academies Press (US): Washington, DC, USA, 2000. [Google Scholar]
- Ramos, S.J.; Rutzke, M.A.; Hayes, R.J.; Faquin, V.; Guilherme, L.R.G.; Li, L. Selenium Accumulation in Lettuce Germplasm. Planta 2011, 233, 649–660. [Google Scholar] [CrossRef]
- Huang, Y.; Fan, B.; Lei, N.; Xiong, Y.; Liu, Y.; Tong, L.; Wang, F.; Maesen, P.; Blecker, C. Selenium Biofortification of Soybean Sprouts: Effects of Selenium Enrichment on Proteins, Protein Structure, and Functional Properties. Front. Nutr. 2022, 9, 849928. [Google Scholar] [CrossRef]
- Sanmartín, C.; Garmendia, I.; Romano, B.; Díaz, M.; Palop, J.A.; Goicoechea, N. Mycorrhizal Inoculation Affected Growth, Mineral Composition, Proteins and Sugars in Lettuces Biofortified with Organic or Inorganic Selenocompounds. Sci. Hortic. 2014, 180, 40–51. [Google Scholar] [CrossRef]
| Abbreviation | Genotype | Method of Application | Se Source/Vehicle | Dose (g ha−1) |
|---|---|---|---|---|
| L-Cnt | Lança | - | - | 0 |
| LF-In10 | Lança | Foliar | Inorganic 1 | 10 |
| LF-In80 | Lança | Foliar | Inorganic | 80 |
| LF-Or10 | Lança | Foliar | Organic 2 | 10 |
| LS-C80 | Lança | Soil | C-MAP 3 + Se | 80 |
| LS-E80 | Lança | Soil | E-MAP 4 + Se | 80 |
| M-Cnt | M5817 | - | - | 0 |
| MF-In10 | M5817 | Foliar | Inorganic | 10 |
| MF-In80 | M5817 | Foliar | Inorganic | 80 |
| MF-Or10 | M5817 | Foliar | Organic | 10 |
| MS-C80 | M5817 | Soil | C-MAP + Se | 80 |
| MS-E80 | M5817 | Soil | E-MAP + Se | 80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, M.A.; de Sousa, G.F.; Bañuelos, G.; Amaral, D.; Brown, P.H.; Guilherme, L.R.G. Selenium Speciation in Se-Enriched Soybean Grains from Biofortified Plants Grown under Different Methods of Selenium Application. Foods 2023, 12, 1214. https://doi.org/10.3390/foods12061214
Silva MA, de Sousa GF, Bañuelos G, Amaral D, Brown PH, Guilherme LRG. Selenium Speciation in Se-Enriched Soybean Grains from Biofortified Plants Grown under Different Methods of Selenium Application. Foods. 2023; 12(6):1214. https://doi.org/10.3390/foods12061214
Chicago/Turabian StyleSilva, Maila Adriely, Gustavo Ferreira de Sousa, Gary Bañuelos, Douglas Amaral, Patrick H. Brown, and Luiz Roberto Guimarães Guilherme. 2023. "Selenium Speciation in Se-Enriched Soybean Grains from Biofortified Plants Grown under Different Methods of Selenium Application" Foods 12, no. 6: 1214. https://doi.org/10.3390/foods12061214
APA StyleSilva, M. A., de Sousa, G. F., Bañuelos, G., Amaral, D., Brown, P. H., & Guilherme, L. R. G. (2023). Selenium Speciation in Se-Enriched Soybean Grains from Biofortified Plants Grown under Different Methods of Selenium Application. Foods, 12(6), 1214. https://doi.org/10.3390/foods12061214









