HPLC/HRMS and GC/MS for Triacylglycerols Characterization of Tuna Fish Oils Obtained from Green Extraction
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- de Oliveira, D.A.S.B.; Licodiedoff, S.; Furigo, A.; Ninow, J.L.; Bork, J.A.; Podestá, R.; Block, J.M.; Waszczynskyj, N. Enzymatic extraction of oil from yellowfin tuna (Thunnus albacares) by-products: A comparison with other extraction methods. Int. J. Food Sci. Technol. 2017, 52, 699–705. [Google Scholar] [CrossRef]
- Rubio-Rodríguez, N.; de Diego, S.M.; Beltrán, S.; Jaime, I.; Sanz, M.T.; Rovira, J. Supercritical fluid extraction of fish oil from fish by-products: A comparison with other extraction methods. J. Food Eng. 2012, 109, 238–248. [Google Scholar] [CrossRef]
- Babbitt, J.K. Intrinsic quality and species of North Pacific fish. Making profits out of seafood wastes. In Proceedings of the International Conference on Fish By-Products, Anchorage, Alaska, 25–27 April 1990. [Google Scholar]
- Rubio-Rodríguez, N.; Beltrán, S.; Jaime, I.; de Diego, S.M.; Sanz, M.T.; Carballido, J.R. Production of omega-3 polyunsaturated fatty acid concentrates: A review. Innov. Food Sci. Emerg. Technol. 2010, 11, 1–12. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Q.; Liu, S.; Wei, S.; Xia, Q.; Ji, H.; Deng, C.; Hao, J. Extraction of fish oil from fish heads using ultra-high pressure pre-treatment prior to enzymatic hydrolysis. Innov. Food Sci. Emerg. Technol. 2021, 70, 102670. [Google Scholar] [CrossRef]
- Patel, A.; Desai, S.S.; Mane, V.K.; Enman, J.; Rova, U.; Christakopoulos, P.; Matsakas, L. Futuristic food fortification with a balanced ratio of dietary ω-3/ω-6 omega fatty acids for the prevention of lifestyle diseases. Trends Food Sci. Technol. 2022, 120, 140–153. [Google Scholar] [CrossRef]
- Wijesundera, C. Synthesis of regioisomerically pure triacylglycerols containing n -3 very long-chain polyunsaturated fatty acids. Eur. J. Lipid Sci. Technol. 2005, 107, 824–832. [Google Scholar] [CrossRef]
- Liu, Z.; Cocks, B.G.; Rochfort, S. Comparison of Molecular Species Distribution of DHA-Containing Triacylglycerols in Milk and Different Infant Formulas by Liquid Chromatography–Mass Spectrometry. J. Agric. Food Chem. 2016, 64, 2134–2144. [Google Scholar] [CrossRef]
- Heird, W.C. Biological effects and safety issues related to long-chain polyunsaturated fatty acids in infants. Lipids 1999, 34, 207–214. [Google Scholar] [CrossRef]
- García-Moreno, P.J.; Batista, I.; Pires, C.; Bandarra, N.M.; Espejo-Carpio, F.J.; Guadix, A.; Guadix, E.M. Antioxidant activity of protein hydrolysates obtained from discarded Mediterranean fish species. Food Res. Int. 2014, 65, 469–476. [Google Scholar] [CrossRef]
- Das, U.N. Essential fatty acids and their metabolites in the context of hypertension. Hypertens. Res. 2010, 33, 782–785. [Google Scholar] [CrossRef]
- Patel, A.; Rova, U.; Christakopoulos, P.; Matsakas, L. Introduction to Essential Fatty Acids. In Nutraceutical Fatty Acids from Oleaginous Microalgae; Patel, A., Matsakas, L., Eds.; Wiley: Hoboken, NJ, USA, 2020; pp. 1–22. [Google Scholar] [CrossRef]
- Leonard, A.E.; Pereira, S.L.; Sprecher, H.; Huang, Y.-S. Elongation of long-chain fatty acids. Prog. Lipid Res. 2004, 43, 36–54. [Google Scholar] [CrossRef] [PubMed]
- Burns-Whitmore, B.; Froyen, E.; Heskey, C.; Parker, T.; Pablo, G.S. Alpha-Linolenic and Linoleic Fatty Acids in the Vegan Diet: Do They Require Dietary Reference Intake/Adequate Intake Special Consideration? Nutrients 2019, 11, 2365. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Karageorgou, D.; Katapodis, P.; Sharma, A.; Rova, U.; Christakopoulos, P.; Matsakas, L. Bioprospecting of thraustochytrids for omega-3 fatty acids: A sustainable approach to reduce dependency on animal sources. Trends Food Sci. Technol. 2021, 115, 433–444. [Google Scholar] [CrossRef]
- Pauly, D.; Alder, J.; Bennett, E.; Christensen, V.; Tyedmers, P.; Watson, R. The Future for Fisheries. Science 2003, 302, 1359–1361. [Google Scholar] [CrossRef]
- Robert, S.S.; Singh, S.P.; Zhou, X.-R.; Petrie, J.R.; Blackburn, S.I.; Mansour, P.M.; Nichols, P.D.; Liu, Q.; Green, A.G. Metabolic engineering of Arabidopsis to produce nutritionally important DHA in seed oil. Funct. Plant Biol. 2005, 32, 473–479. [Google Scholar] [CrossRef]
- Napier, J.A.; Beaudoin, F.; Sayanova, O. Reverse engineering of long-chain polyunsaturated fatty acid biosynthesis into transgenic plants. Eur. J. Lipid Sci. Technol. 2005, 107, 249–255. [Google Scholar] [CrossRef]
- Domergue, F.; Abbadi, A.; Heinz, E. Relief for fish stocks: Oceanic fatty acids in transgenic oilseeds. Trends Plant Sci. 2005, 10, 112–116. [Google Scholar] [CrossRef]
- Qi, B.; Fraser, T.; Mugford, S.; Dobson, G.; Sayanova, O.; Butler, J.; Napier, J.A.; Stobart, A.K.; Lazarus, C.M. Production of very long chain polyunsaturated omega-3 and omega-6 fatty acids in plants. Nat. Biotechnol. 2004, 22, 739–745. [Google Scholar] [CrossRef]
- Abbadi, A.; Domergue, F.; Bauer, J.; Napier, J.A.; Welti, R.; Zähringer, U.; Cirpus, P.; Heinz, E. Biosynthesis of Very-Long-Chain Polyunsaturated Fatty Acids in Transgenic Oilseeds: Constraints on Their Accumulation. Plant Cell 2004, 16, 2734–2748. [Google Scholar] [CrossRef]
- Napier, J.A.; Sayanova, O.; Qi, B.; Lazarus, C.M. Progress toward the production of long-chain polyunsaturated fatty acids in transgenic plants. Lipids 2004, 39, 1067–1075. [Google Scholar] [CrossRef]
- Green, A.G. From alpha to omega-producing essential fatty acids in plants. Nat. Biotechnol. 2004, 22, 680–682. [Google Scholar] [CrossRef]
- Singh, S.P.; Zhou, X.-R.; Liu, Q.; Stymne, S.; Green, A.G. Metabolic engineering of new fatty acids in plants. Curr. Opin. Plant Biol. 2005, 8, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Monsiváis-Alonso, R.; Mansouri, S.S.; Román-Martínez, A. Life cycle assessment of intensified processes towards circular economy: Omega-3 production from waste fish oil. Chem. Eng. Process. 2020, 158, 108171. [Google Scholar] [CrossRef]
- Belarbi, E.H.; Molina, E.; Chisti, Y. A process for high yield and scaleable recovery of high purity eicosapentaenoic acid esters from microalgae and fish oil. Enzym. Microb. Technol. 2000, 26, 516–529. [Google Scholar] [CrossRef]
- Fouda, T. Using Green Cold Pressing to Produce High Quality Fish Oil from Industrial Salmon Waste. J. Zoöl. Res. 2020, 2, 1. [Google Scholar] [CrossRef]
- Adeoti, I.A.; Hawboldt, K. A review of lipid extraction from fish processing by-product for use as a biofuel. Biomass Bioenergy 2014, 63, 330–340. [Google Scholar] [CrossRef]
- Bonilla-Méndez, J.R.; Hoyos-Concha, J.L. Methods of extraction refining and concentration of fish oil as a source of omega-3 fatty acids. Cienc. Y Tecnol. Agropecu. 2018, 19, 645–668. [Google Scholar]
- Ferdosh, S.; Sarker, Z.I.; Norulaini, N.; Oliveira, A.; Yunus, K.; Chowdury, A.J.; Akanda, J.; Omar, M. Quality of Tuna Fish Oils Extracted Using SC-CO2. J. Food Process. Preserv. 2015, 39, 432–441. [Google Scholar] [CrossRef]
- Fournier, V.; Destaillats, F.; Juanéda, P.; Dionisi, F.; Lambelet, P.; Sébédio, J.; Berdeaux, O. Thermal degradation of long-chain polyunsaturated fatty acids during deodorization of fish oil. Eur. J. Lipid Sci. Technol. 2006, 108, 33–42. [Google Scholar] [CrossRef]
- Ivanovs, K.; Blumberga, D. Extraction of fish oil using green extraction methods: A short review. Energy Procedia 2017, 128, 477–483. [Google Scholar] [CrossRef]
- Mjøs, S.A. Properties of trans isomers of eicosapentaenoic acid and docosahexaenoic acid methyl esters on cyanopropyl stationary phases. J. Chromatogr. A 2005, 1100, 185–192. [Google Scholar] [CrossRef]
- Dijkstra, A.J.; Opstal, M.V. Process for Producing Degummed Vegetable Oils and Gums of High Phosphatidic Acid Content. US Patent 4698185, 6 October 1987. [Google Scholar]
- Šimat, V.; Vlahović, J.; Soldo, B.; Skroza, D.; Ljubenkov, I.; Mekinić, I.G. Production and Refinement of Omega-3 Rich Oils from Processing By-Products of Farmed Fish Species. Foods 2019, 8, 125. [Google Scholar] [CrossRef]
- Taati, M.M.; Bahareh, S.; Mehdi, O. Extraction of oil from tuna by-product by supercritical fluid extraction (SFE) and comparison with wet reduction method. Aquacult. Aquarium Conserv. Legis. 2017, 10, 1546–1553. [Google Scholar]
- Čmolík, J.; Pokorný, J. Physical refining of edible oils. Eur. J. Lipid Sci. Technol. 2000, 102, 472–486. [Google Scholar] [CrossRef]
- Ferreri, C.; Chatgilialoglu, C. Geometrical trans Lipid Isomers: A New Target for Lipidomics. Chembiochem 2005, 6, 1722–1734. [Google Scholar] [CrossRef] [PubMed]
- Bhosle, B.; Subramanian, R. New approaches in deacidification of edible oils––A review. J. Food Eng. 2005, 69, 481–494. [Google Scholar] [CrossRef]
- Chang, S.S.; Bao, Y.; Pelura, T.J. Purification of Fish Oil. U.S. Patent 4.874.629, 17 October 1989. [Google Scholar]
- Sugasini, D.; Yalagala, P.C.; Goggin, A.; Tai, L.M.; Subbaiah, P.V. Enrichment of brain docosahexaenoic acid (DHA) is highly dependent upon the molecular carrier of dietary DHA: Lysophosphatidylcholine is more efficient than either phosphatidylcholine or triacylglycerol. J. Nutr. Biochem. 2019, 74, 108231. [Google Scholar] [CrossRef] [PubMed]
- Fard, G.S.; Wang, F.; Sinclair, A.J.; Elliott, G.; Turchini, G.M. How does high DHA fish oil affect health? A systematic review of evidence. Crit. Rev. Food Sci. Nutr. 2019, 59, 1684–1727. [Google Scholar] [CrossRef]
- Sahena, F.; Zaidul, I.S.M.; Norulaini, N.N.A.; Jinap, S.; Jahurul, M.H.A.; Omar, M.A.K. Storage stability and quality of polyunsaturated fatty acid rich oil fraction from Longtail tuna (Thunnus tonggol) head using supercritical extraction. CYTA-J. Food 2014, 12, 183–188. [Google Scholar] [CrossRef]
- Ciriminna, R.; Bongiorno, D.; Scurria, A.; Danzì, C.; Timpanaro, G.; Delisi, R.; Avellone, G.; Pagliaro, M. Sicilian Opuntia ficus-indica seed oil: Fatty acid composition and bio-economical aspects. Eur. J. Lipid Sci. Technol. 2017, 119, 1700232. [Google Scholar] [CrossRef]
- Agozzino, P.; Avellone, G.; Bongiorno, D.; Ceraulo, L.; Indelicato, S.; Indelicato, S.; Vèkey, K. Determination of the cultivar and aging of Sicilian olive oils using HPLC-MS and linear discriminant analysis. J. Mass Spectrom. 2010, 45, 989–995. [Google Scholar] [CrossRef]
- Byrdwell, W.C.; Emken, E.A.; Neff, W.E.; Adlof, R.O. Quantitative analysis of triglycerides using atmospheric pressure chemical ionization-mass spectrometry. Lipids 1996, 31, 919–935. [Google Scholar] [CrossRef]
- Yin, H.; Solval, K.M.; Huang, J.; Bechtel, P.J.; Sathivel, S. Effects of Oil Extraction Methods on Physical and Chemical Properties of Red Salmon Oils (Oncorhynchus nerka). J. Am. Oil Chem. Soc. 2011, 88, 1641–1648. [Google Scholar] [CrossRef]
- Inguglia, L.; Chiaramonte, M.; Di Stefano, V.; Schillaci, D.; Cammilleri, G.; Pantano, L.; Mauro, M.; Vazzana, M.; Ferrantelli, V.; Nicolosi, R.; et al. Salmo salar fish waste oil: Fatty acids composition and antibacterial activity. PeerJ 2020, 8, e9299. [Google Scholar] [CrossRef]
- IOC-International Olive Council. Determination of Fatty Acid Methyl esters by Gas Chromatography. COI/T 20/Doc No 33 Rev 1. Available online: https://www.internationaloliveoil.org/wp-content/uploads/2019/11/COI-T.20-Doc.-No-33-Rev.-1-2017.pdf (accessed on 6 March 2023).
- Indelicato, S.; Bongiorno, D.; Ceraulo, L.; Emmanuello, C.; Mazzotti, F.; Siciliano, C.; Piazzese, D. One-Pot Analysis: A New Integrated Methodology for Determination of TAG and FA Determination through LC/MS and in-silico Saponification. Food Anal. Methods 2018, 11, 873–882. [Google Scholar] [CrossRef]
- Suseno, S.H.; Hayati, S.; Izaki, A.F.; Hayati, S. Fatty Acid Composition of Some Potential Fish Oil from Production Centers in Indonesia. Orient. J. Chem. 2014, 30, 975. [Google Scholar] [CrossRef]
- Khoddami, A.; Ariffin, A.A.; Bakar, J.; Ghazali, H.M. Fatty acid profile of the oil extracted from fish waste (head, intestine and liver) (Sardinella lemuru). World Appl. Sci. J. 2009, 7, 127–131. [Google Scholar] [CrossRef]
- Ahmed, R.; Haq, M.; Cho, Y.J.; Chun, B.S. Quality evaluation of oil recovered from by-products of bigeye tuna using supercritical carbon dioxide extraction. Turkish J. Fish. Aquat. Sci. 2017, 17, 663–672. [Google Scholar] [CrossRef]
- Neff, W.E.; Byrdwell, W.; List, G.R. Triacylglycerol structures of food fats high in saturated acids by hplc and mass spectrometry. J. Liq. Chromatogr. Relat. Technol. 2001, 24, 837–854. [Google Scholar] [CrossRef]
- Neff, W.E.; Byrdwell, W.; Steidley, K.R.; List, G.R.; Snowder, G. Triacylglycerol structure of animal tallows, potential food formulation fats, by high performance liquid chromatography coupled with mass spectrometry. J. Liq. Chromatogr. Relat. Technol. 2002, 25, 985–998. [Google Scholar] [CrossRef]
- Bongiorno, D.; Ceraulo, L.; Giorgi, G.; Indelicato, S.; Liveri, V.T. Do electrospray mass spectra of surfactants mirror their aggregation state in solution? J. Mass Spectrom. 2011, 46, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Byrdwell, W.C.; Neff, W.E. Dual parallel electrospray ionization and atmospheric pressure chemical ionization mass spectrometry (MS), MS/MS and MS/MS/MS for the analysis of triacylglycerols and triacylglycerol oxidation products. Rapid Commun. Mass Spectrom. 2002, 16, 300–319. [Google Scholar] [CrossRef]
- Holčapek, M.; Jandera, P.; Zderadička, P.; Hruba, L. Characterization of triacylglycerol and diacylglycerol composition of plant oils using high-performance liquid chromatography-atmospheric pressure chemical ionization mass spectrometry. J. Chromatogr. A 2003, 1010, 195–215. [Google Scholar] [CrossRef]
- Holčapek, M.; Lísa, M.; Jandera, P.; Kabátová, N. Quantitation of triacylglycerols in plant oils using HPLC with APCI-MS, evaporative light-scattering, and UV detection. J. Sep. Sci. 2005, 28, 1315–1333. [Google Scholar] [CrossRef]
- Di Stefano, V.; Bongiorno, D.; Buzzanca, C.; Indelicato, S.; Santini, A.; Lucarini, M.; Fabbrizio, A.; Mauro, M.; Vazzana, M.; Arizza, V.; et al. Fatty Acids and Triacylglycerols Profiles from Sicilian (Cold Pressed vs. Soxhlet) Grape Seed Oils. Sustainability 2021, 13, 13038. [Google Scholar] [CrossRef]
- Baiocchi, C.; Medana, C.; Bello, F.D.; Giancotti, V.; Aigotti, R.; Gastaldi, D. Analysis of regioisomers of polyunsaturated triacylglycerols in marine matrices by HPLC/HRMS. Food Chem. 2015, 166, 551–560. [Google Scholar] [CrossRef]
- Indelicato, S.; Bongiorno, D.; Pitonzo, R.; Di Stefano, V.; Calabrese, V.; Indelicato, S.; Avellone, G. Triacylglycerols in edible oils: Determination, characterization, quantitation, chemometric approach and evaluation of adulterations. J. Chromatogr. A 2017, 1515, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Shen, Y.; Zhang, Y.; Li, L.; Wang, X. Regiospecific Analysis of Fatty Acids and Calculation of Triglyceride Molecular Species in Marine Fish Oils. BioMed. Res. Int. 2018, 2018, 9016840. [Google Scholar] [CrossRef]
- Oliveira, D.; Bernardi, D.; Drummond, F.; Dieterich, F.; Boscolo, W.; Leivas, C.; Kiatkoski, E.; Waszczynskyj, N. Potential Use of Tuna (Thunnus albacares) by-product: Production of Antioxidant Peptides and Recovery of Unsaturated Fatty Acids from Tuna Head. Int. J. Food Eng. 2017, 13, 20150365. [Google Scholar] [CrossRef]
- Truzzi, C.; Annibaldi, A.; Illuminati, S.; Antonucci, M.; Api, M.; Scarponi, G.; Lombardo, F.; Pignalosa, P.; Carnevali, O. Characterization of the fatty acid composition in cultivated atlanticbluefin tuna (Thunnusthynnus L.) Muscle by gas chromatography-mass spectrometry. Anal. Lett. 2018, 51, 2981–2993. [Google Scholar] [CrossRef]
- Chung, K.-H.; Lee, K.-Y. Removal of trimethylamine by adsorption over zeolite catalysts and deodorization of fish oil. J. Hazard. Mater. 2009, 172, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Chew, S.C.; Kar, L.N. Refining of edible oils. In Lipids and Edible Oils; Academic Press: Cambridge, MA, USA, 2020; pp. 213–241. [Google Scholar]

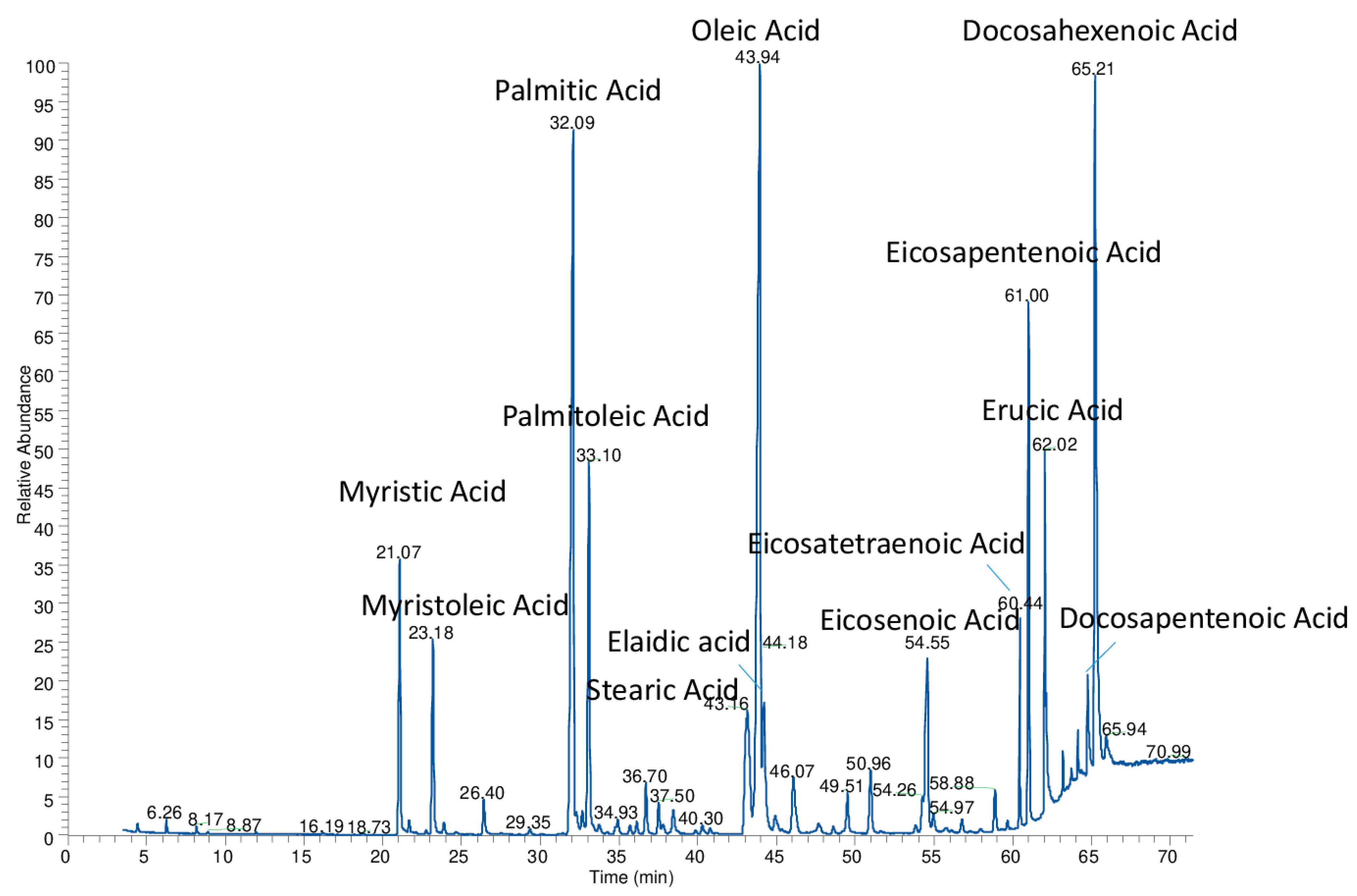
| Fatty Acids * | M | Po | P | Ml | Ln | L | O + El | S | Ec | EPA | Er | DHA | Dp | Et |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C14:0 | C16:1 | C16:0 | C14:1 | C18:3 | C18:2 | C18:1 | C18:0 | C20:1 | C18:5 | C22:1 | C22:6 | C22:5 | C20:4 | |
| ISS (%) | 5.2 | 6.6 | 20.2 | 0.8 | 0.8 | 1.2 | 22.8 | 4.3 | 1.5 | 11.4 | 8.3 | 12.1 | 2.4 | 2.2 |
| FAMEs (GC/MS) (%) | 5.7 | 7.6 | 18.1 | 0.6 | 1.2 | 1.5 | 18.3 | 4.2 | 3.1 | 11.1 | 11.4 | 12.4 | 1.3 | 3.5 |
| Rel. Variation (%) | −9 | −13 | +12 | +44 | −38 | −20 | +24 | +2 | −52 | +3 | −27 | −2 | +88 | −36 |
| TAGs * | TAG+ AM | # DAG1+ AM | DAG2+ AM | DAG3+ AM | TAGs | TAG+ AM | DAG1+ AM | DAG2+ AM | DAG3+ AM |
|---|---|---|---|---|---|---|---|---|---|
| EcErDHA | 1015.86 | 687.61 | 677.53 | 1015.86 | OErP | 915.83 | 659.59 | 577.51 | 915.83 |
| DHALgS | 1019.89 | 735.62 | 651.52 | 1019.89 | SPEr | 917.84 | 579.53 | 661.60 | 917.84 |
| DHADHADHA | 1023.73 | 695.49 | 695.49 | 1023.73 | PoEPADHA | 923.70 | 595.46 | 621.48 | 923.70 |
| DHADHADp | 1025.75 | 695.49 | 697.50 | 1025.75 | DHADHAM | 923.70 | 695.49 | 595.46 | 923.70 |
| ErErEc | 1025.93 | 715.64 | 687.61 | 1025.93 | DHAStO | 925.54 | 643.28 | 649.51 | 925.54 |
| MMEPA | 797.66 | 495.43 | 569.44 | 797.66 | PEPADHA | 925.72 | 597.48 | 623.49 | 925.72 |
| PPoP | 805.72 | 549.48 | 551.49 | 805.72 | PDpEPA | 927.73 | 625.51 | 597.48 | 927.73 |
| MOP | 805.72 | 549.48 | 523.46 | 805.72 | PEtDHA | 927.73 | 599.49 | 623.49 | 927.73 |
| PoPoO | 829.72 | 547.46 | 575.49 | 829.72 | DHASO | 933.78 | 651.52 | 649.51 | 933.78 |
| OOM | 831.74 | 603.53 | 549.48 | 831.74 | PEcD | 943.86 | 605.54 | 633.57 | 943.86 |
| PPO | 833.75 | 551.49 | 577.51 | 833.75 | DpOS | 935.80 | 651.52 | 653.54 | 935.80 |
| LnMlEPA | 845.65 | 543.43 | 619.46 | 845.65 | DHASS | 935.80 | 651.52 | 651.52 | 935.80 |
| OEPAM | 851.70 | 623.49 | 549.48 | 851.70 | ErOO | 941.84 | 659.59 | 659.59 | 941.84 |
| ErDpP | 963.83 | 707.58 | 633.57 | 963.83 | MErEr | 943.86 | 605.54 | 605.54 | 943.86 |
| PPEPA | 853.72 | 551.49 | 597.48 | 853.72 | DHADHAPo | 949.72 | 695.49 | 621.48 | 949.72 |
| PPEt | 855.73 | 551.49 | 599.49 | 855.73 | EcEeEPA | 951.73 | 649.50 | 651.52 | 951.73 |
| MlEcO | 857.75 | 575.49 | 547.46 | 857.75 | OEPADHA | 951.73 | 623.49 | 649.51 | 951.73 |
| POPo | 831.74 | 577.51 | 549.48 | 831.74 | DHADHAP | 951.73 | 695.49 | 623.49 | 951.73 |
| DHAOO | 931.77 | 649.51 | 649.51 | 931.77 | PDpDHA | 953.75 | 625.51 | 623.49 | 953.75 |
| POS | 861.79 | 577.51 | 579.53 | 861.79 | DpEPAS | 955.76 | 671.49 | 653.54 | 955.76 |
| PoLnEPA | 873.69 | 571.46 | 595.46 | 873.69 | DHASG | 961.81 | 651.52 | 677.54 | 961.81 |
| EcMlEPA | 877.71 | 575.49 | 651.52 | 877.71 | OErEPA | 961.81 | 659.59 | 623.49 | 961.81 |
| LnOLn | 877.72 | 599.49 | 595.46 | 877.72 | SErEPA | 963.83 | 661.60 | 625.51 | 963.83 |
| DHAPPo | 877.72 | 623.49 | 621.48 | 877.72 | DHAEPAEPA | 971.70 | 669.47 | 669.47 | 971.70 |
| EPAOP | 879.73 | 623.49 | 597.48 | 879.73 | ErErP | 971.89 | 715.64 | 633.57 | 971.89 |
| DHAPP | 879.73 | 623.49 | 623.49 | 879.73 | DHADHAO | 977.75 | 695.49 | 649.51 | 977.75 |
| POEt | 881.75 | 577.51 | 599.49 | 881.75 | SErDHA | 989.84 | 661.60 | 651.52 | 989.84 |
| OLO | 883.77 | 601.51 | 603.53 | 883.77 | DHAEPADHA | 997.71 | 669.47 | 695.49 | 997.71 |
| PoPEr | 887.80 | 549.48 | 631.55 | 887.80 | DHADpEPA | 999.73 | 697.50 | 669.47 | 999.73 |
| EPAEPAPo | 897.68 | 643.46 | 595.46 | 897.68 | DHAEtDHA | 999.73 | 671.49 | 695.49 | 999.73 |
| MEPADHA | 897.68 | 569.44 | 595.46 | 897.68 | ErErS | 999.92 | 715.64 | 661.60 | 999.92 |
| PEPAEPA | 899.70 | 597.48 | 597.48 | 899.70 | MEPAEPA | 871.67 | 569.44 | 569.44 | 871.67 |
| MaEcO | 901.81 | 619.55 | 591.53 | 901.81 | PoErEr | 969.87 | 631.55 | 631.55 | 969.87 |
| OLEPA | 903.73 | 601.51 | 623.49 | 903.73 | ErErEr | 1053.96 | 715.64 | 715.64 | 1053.96 |
| EPAOO | 905.75 | 623.49 | 623.49 | 905.75 | PoPoEr | 885.78 | 547.46 | 631.55 | 885.78 |
| DHAPO | 905.75 | 623.49 | 649.51 | 905.75 | ErErO | 997.90 | 715.64 | 659.59 | 997.90 |
| DpOP | 907.77 | 651.52 | 625.51 | 907.77 | DHAErEr | 1043.88 | 705.57 | 705.57 | 1043.88 |
| DHASP | 907.77 | 651.52 | 623.49 | 907.77 | DHADHAEr | 1033.81 | 695.49 | 705.57 | 1033.81 |
| EtOS | 909.78 | 625.51 | 627.52 | 909.78 | DHAMEr | 933.78 | 595.46 | 705.57 | 933.78 |
| PoLEr | 911.80 | 573.48 | 631.55 | 911.80 | DHAErEc | 1015.85 | 705.57 | 677.53 | 1015.85 |
| PoErO | 913.81 | 631.55 | 575.49 | 913.81 |
| TAG | Tuna-1 | Tuna-2 | Tuna-3 | Tuna-4 | Average | %RSD | |
|---|---|---|---|---|---|---|---|
| EcErDHA | 0.50 | 0.14 | 0.32 | 0.31 | 0.32 | ± | 47.3 |
| DHADHADHA | 0.04 | 0.02 | 0.03 | 0.03 | 0.03 | ± | 20.7 |
| DHADHADp | 0.02 | 0.01 | 0.02 | 0.02 | 0.02 | ± | 27.4 |
| ErErEc | 0.13 | 0.00 | 0.07 | 0.07 | 0.07 | ± | 80.7 |
| MMEPA | 0.34 | 0.50 | 0.43 | 0.42 | 0.42 | ± | 15.5 |
| PPoP | 0.41 | 0.62 | 0.51 | 0.52 | 0.52 | ± | 16.9 |
| MOP | 6.56 | 9.65 | 8.38 | 7.98 | 8.14 | ± | 15.6 |
| PoPoO | 1.43 | 2.03 | 1.73 | 1.70 | 1.72 | ± | 14.2 |
| OOM | 1.05 | 1.48 | 1.31 | 1.21 | 1.26 | ± | 14.3 |
| PPO | 3.43 | 4.22 | 3.76 | 3.81 | 3.80 | ± | 8.5 |
| LnMlEPA | 0.76 | 0.94 | 0.86 | 0.87 | 0.86 | ± | 8.4 |
| OEPAM | 4.21 | 5.01 | 4.36 | 4.77 | 4.59 | ± | 8.0 |
| DDpP | 2.61 | 2.58 | 2.66 | 2.46 | 2.58 | ± | 3.3 |
| PPEPA | 1.88 | 2.58 | 2.18 | 2.28 | 2.23 | ± | 13.0 |
| PPEt | 1.93 | 2.06 | 1.98 | 1.94 | 1.98 | ± | 2.9 |
| MlEcO | 1.19 | 1.56 | 1.40 | 1.43 | 1.40 | ± | 11.0 |
| POPo | 2.38 | 2.81 | 2.57 | 2.63 | 2.60 | ± | 6.8 |
| DHAOO | 3.71 | 4.56 | 4.10 | 4.20 | 4.14 | ± | 8.4 |
| POS | 1.39 | 1.44 | 1.48 | 1.45 | 1.44 | ± | 2.4 |
| PoLnEPA | 0.08 | 0.12 | 0.10 | 0.10 | 0.10 | ± | 15.3 |
| EcMlEPA | 0.87 | 0.47 | 0.63 | 0.80 | 0.69 | ± | 26.5 |
| LnOLn | 0.53 | 0.74 | 0.60 | 0.66 | 0.63 | ± | 14.1 |
| DHAPPo | 2.47 | 3.43 | 2.81 | 3.06 | 2.94 | ± | 13.8 |
| EPAOP | 4.10 | 3.72 | 3.42 | 4.39 | 3.91 | ± | 10.9 |
| DHAPP | 1.29 | 1.77 | 1.74 | 1.36 | 1.54 | ± | 16.2 |
| POEt | 2.22 | 2.48 | 2.58 | 2.32 | 2.40 | ± | 7.0 |
| OLO | 1.46 | 1.12 | 1.36 | 1.30 | 1.31 | ± | 10.8 |
| PoPEr | 3.41 | 3.47 | 3.69 | 3.38 | 3.49 | ± | 4.9 |
| EPAEPAPo | 0.87 | 0.90 | 0.91 | 0.88 | 0.89 | ± | 3.9 |
| MEPADHA | 0.42 | 0.58 | 0.52 | 0.48 | 0.50 | ± | 13.5 |
| PEPAEPA | 1.67 | 2.06 | 2.00 | 1.88 | 1.90 | ± | 8.7 |
| MaEcO | 0.31 | 0.07 | 0.22 | 0.16 | 0.19 | ± | 51.3 |
| OLEPA | 1.94 | 2.47 | 2.29 | 2.19 | 2.22 | ± | 9.5 |
| EPAOO | 1.83 | 2.02 | 1.92 | 1.99 | 1.94 | ± | 5.0 |
| DHAPO | 3.53 | 4.43 | 4.08 | 3.84 | 3.97 | ± | 9.5 |
| DpOP | 1.54 | 1.59 | 1.65 | 1.54 | 1.58 | ± | 3.6 |
| DHASP | 2.58 | 2.45 | 2.75 | 2.26 | 2.51 | ± | 9.4 |
| EtOS | 1.92 | 1.09 | 1.62 | 1.46 | 1.52 | ± | 21.6 |
| PoLEr | 0.77 | 0.54 | 0.87 | 0.86 | 0.76 | ± | 19.2 |
| PoErO | 1.73 | 1.21 | 1.84 | 1.86 | 1.66 | ± | 17.3 |
| OErP | 2.86 | 2.03 | 4.61 | 4.47 | 3.49 | ± | 33.3 |
| SPEr | 0.28 | 0.49 | 0.40 | 0.37 | 0.38 | ± | 20.8 |
| PoEPADHA | 0.71 | 0.24 | 0.50 | 0.47 | 0.48 | ± | 36.7 |
| DHADHAM | 0.20 | 0.26 | 0.22 | 0.23 | 0.23 | ± | 12.5 |
| DHAStO | 1.23 | 1.48 | 1.40 | 1.35 | 1.37 | ± | 7.1 |
| PEPADHA | 1.41 | 1.70 | 1.59 | 1.53 | 1.56 | ± | 8.0 |
| PDpEPA | 0.93 | 1.07 | 1.09 | 0.95 | 1.01 | ± | 8.7 |
| PEtDHA | 0.47 | 0.46 | 0.48 | 0.44 | 0.46 | ± | 4.5 |
| DHASO | 1.49 | 1.08 | 1.41 | 1.16 | 1.29 | ± | 16.3 |
| PEcDe | 0.00 | 0.01 | 0.01 | 0.01 | 0.01 | ± | 56.0 |
| DpOS | 0.29 | 0.23 | 0.28 | 0.24 | 0.26 | ± | 12.8 |
| DHASS | 1.84 | 1.51 | 1.73 | 1.60 | 1.67 | ± | 8.4 |
| ErOO | 1.15 | 0.57 | 1.27 | 1.26 | 1.04 | ± | 45.5 |
| MErEr | 1.77 | 0.67 | 2.30 | 2.22 | 1.74 | ± | 40.0 |
| DHADHAPo | 0.44 | 0.48 | 0.43 | 0.50 | 0.46 | ± | 7.9 |
| EcEeEPA | 1.22 | 1.23 | 1.31 | 1.15 | 1.23 | ± | 6.6 |
| OEPADHA | 1.22 | 0.86 | 1.12 | 1.00 | 1.05 | ± | 14.8 |
| DHADHAP | 0.78 | 0.86 | 0.93 | 0.76 | 0.83 | ± | 11.1 |
| PDpDHA | 1.20 | 0.41 | 0.84 | 0.78 | 0.81 | ± | 37.7 |
| DpEPAS | 1.20 | 0.41 | 0.86 | 0.80 | 0.82 | ± | 37.2 |
| DHASG | 1.95 | 1.29 | 1.72 | 1.57 | 1.63 | ± | 15.9 |
| OErEPA | 0.75 | 0.38 | 0.76 | 0.79 | 0.67 | ± | 27.2 |
| SErEPA | 0.45 | 0.35 | 0.81 | 0.81 | 0.60 | ± | 37.0 |
| DHAEPAEPA | 0.28 | 0.22 | 0.26 | 0.24 | 0.25 | ± | 8.9 |
| ErErP | 0.92 | 0.23 | 1.14 | 1.17 | 0.87 | ± | 46.8 |
| DHADHAO | 0.69 | 0.68 | 0.70 | 0.65 | 0.68 | ± | 3.8 |
| SErDHA | 0.57 | 0.10 | 0.63 | 0.64 | 0.48 | ± | 49.3 |
| DHAEPADHA | 0.09 | 0.07 | 0.12 | 0.03 | 0.08 | ± | 73.4 |
| DHADpEPA | 0.09 | 0.04 | 0.06 | 0.06 | 0.06 | ± | 31.6 |
| DHAEtDHA | 0.05 | 0.13 | 0.10 | 0.08 | 0.09 | ± | 35.1 |
| ErErS | 0.17 | 0.00 | 0.18 | 0.19 | 0.13 | ± | 61.4 |
| MEPAEPA | 0.82 | 0.86 | 0.87 | 0.80 | 0.84 | ± | 6.9 |
| ErErEr | 0.06 | 0.00 | 0.06 | 0.06 | 0.04 | ± | 61.9 |
| PoPoEr | 2.29 | 2.30 | 2.58 | 2.56 | 2.43 | ± | 6.4 |
| ErErO | 0.37 | 0.09 | 0.44 | 0.43 | 0.33 | ± | 45.8 |
| DHAErEr | 0.22 | 0.03 | 0.22 | 0.23 | 0.18 | ± | 51.8 |
| DHADHAEr | 0.72 | 0.05 | 0.79 | 0.79 | 0.58 | ± | 56.9 |
| DHAMEr | 0.35 | 0.31 | 0.39 | 0.39 | 0.36 | ± | 10.6 |
| DHAErEc | 0.51 | 0.15 | 0.50 | 0.59 | 0.44 | ± | 42.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Indelicato, S.; Di Stefano, V.; Avellone, G.; Piazzese, D.; Vazzana, M.; Mauro, M.; Arizza, V.; Bongiorno, D. HPLC/HRMS and GC/MS for Triacylglycerols Characterization of Tuna Fish Oils Obtained from Green Extraction. Foods 2023, 12, 1193. https://doi.org/10.3390/foods12061193
Indelicato S, Di Stefano V, Avellone G, Piazzese D, Vazzana M, Mauro M, Arizza V, Bongiorno D. HPLC/HRMS and GC/MS for Triacylglycerols Characterization of Tuna Fish Oils Obtained from Green Extraction. Foods. 2023; 12(6):1193. https://doi.org/10.3390/foods12061193
Chicago/Turabian StyleIndelicato, Serena, Vita Di Stefano, Giuseppe Avellone, Daniela Piazzese, Mirella Vazzana, Manuela Mauro, Vincenzo Arizza, and David Bongiorno. 2023. "HPLC/HRMS and GC/MS for Triacylglycerols Characterization of Tuna Fish Oils Obtained from Green Extraction" Foods 12, no. 6: 1193. https://doi.org/10.3390/foods12061193
APA StyleIndelicato, S., Di Stefano, V., Avellone, G., Piazzese, D., Vazzana, M., Mauro, M., Arizza, V., & Bongiorno, D. (2023). HPLC/HRMS and GC/MS for Triacylglycerols Characterization of Tuna Fish Oils Obtained from Green Extraction. Foods, 12(6), 1193. https://doi.org/10.3390/foods12061193







