Pathways Affected by Falcarinol-Type Polyacetylenes and Implications for Their Anti-Inflammatory Function and Potential in Cancer Chemoprevention
Abstract
1. Introduction
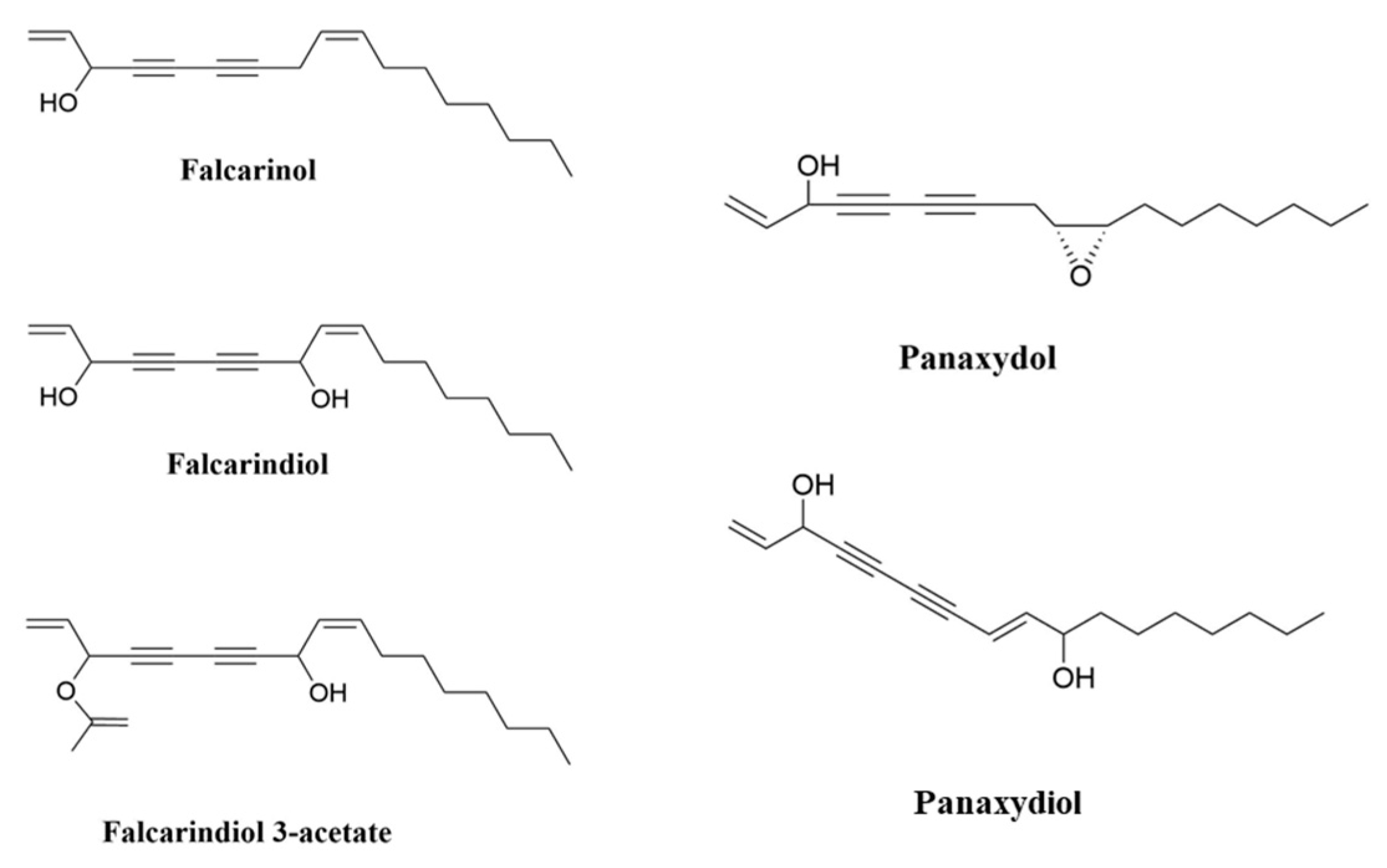
2. Polyacetylenes and Inflammation
2.1. Chronic Inflammation Disease and Cancer
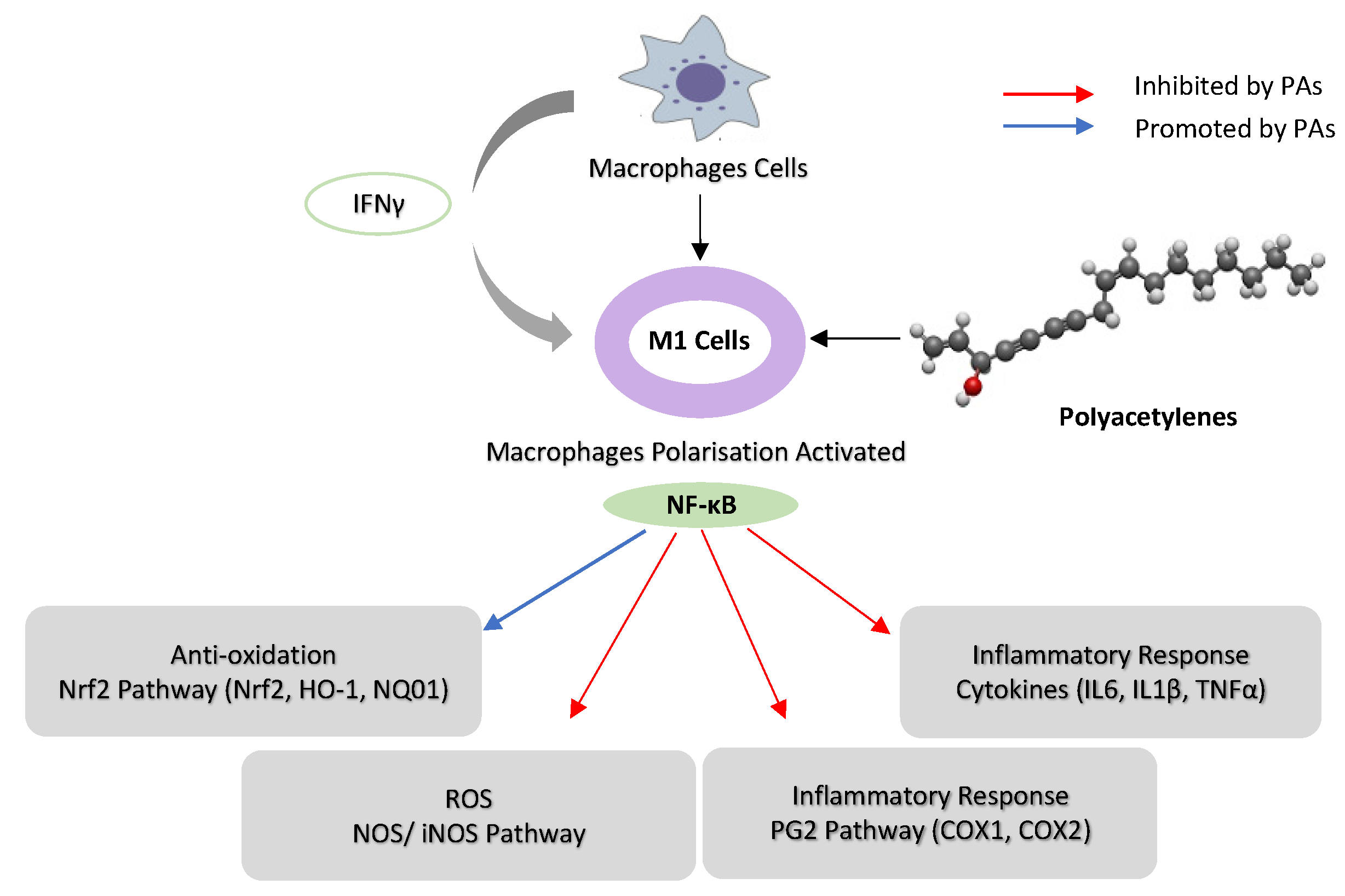
2.2. Inhibition of Nuclear Factor Kappa B (NF-κB) Pathways
2.3. Oxidative Stress
2.3.1. Inhibition of Nitric Oxide Synthase (NOS) and Pro-Inflammatory Cytokine Pathways
2.3.2. Reactive Oxygen Species (ROS) Pathways
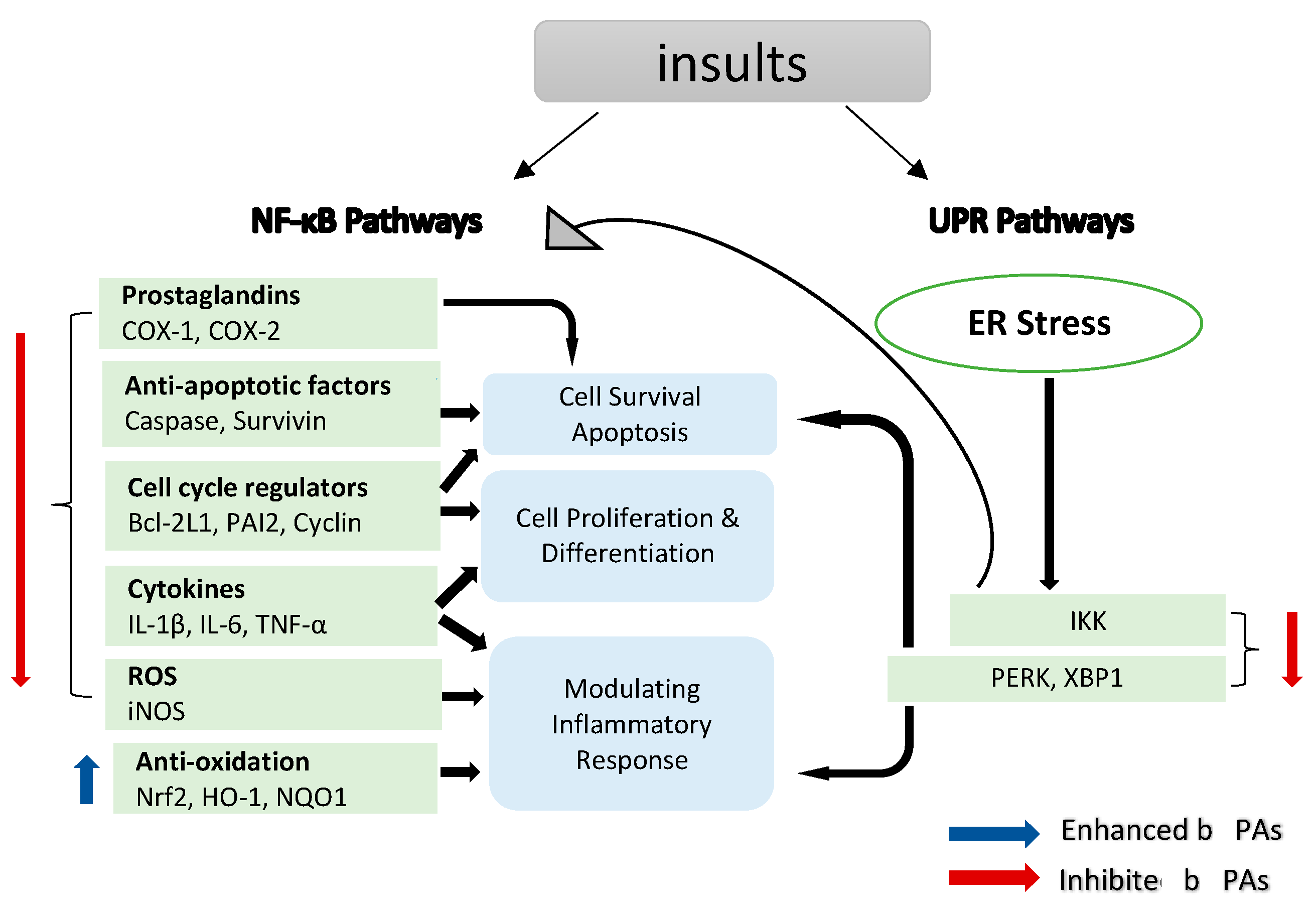
3. Unfolded Protein Response (UPR) Pathways
4. Cancer
4.1. In Vitro
4.1.1. Anti-Proliferative Activity
4.1.2. Pro-Apoptosis Activity
4.1.3. Gut Microbiota Composition
4.1.4. Other Effects
4.2. In Vivo
5. Polyacetylene Toxicology and Pharmacokinetics
5.1. Toxicology of PAs
5.2. Pharmacokinetics of PAs
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, X.; Ouyang, Y.; Liu, J.; Zhu, M.; Zhao, G.; Bao, W.; Hu, F.B. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: Systematic review and dose-response meta-analysis of prospective cohort studies. Postgrad. Med. J. 2014, 349, g4490. [Google Scholar] [CrossRef]
- Negri, R. Polyacetylenes from terrestrial plants and fungi: Recent phytochemical and biological advances. Fitoterapia 2015, 106, 92–109. [Google Scholar] [CrossRef]
- Dawid, C.; Dunemann, F.; Schwab, W.; Nothnagel, T.; Hofmann, T. Bioactive C17-polyacetylenes in carrots (Daucus carota L.): Current knowledge and future perspectives. J. Agric. Food Chem. 2015, 63, 9211–9222. [Google Scholar] [CrossRef]
- Hansen, L.; Boll, P.M. Polyacetylenes in Araliaceae: Their chemistry, biosynthesis and biological significance. Phytochemistry 1986, 25, 285–293. [Google Scholar] [CrossRef]
- Stefanson, A.; Bakovic, M. Dietary polyacetylene falcarinol upregulated intestinal heme oxygenase-1 and modified plasma cytokine profile in late phase lipopolysaccharide-induced acute inflammation in CB57BL/6 mice. Nutr. Res. 2020, 80, 89–105. [Google Scholar] [CrossRef] [PubMed]
- Moore, T. Vitamin A and carotene: The association of vitamin A activity with carotene in the carrot root. Biochem. J. 1929, 23, 803. [Google Scholar] [CrossRef] [PubMed]
- Butnariu, M. Therapeutic Properties of Vegetable. J. Bioequivalence Bioavailab. 2014, 6, e55. [Google Scholar] [CrossRef]
- Chaparala, A.; Poudyal, D.; Tashkandi, H.; Witalison, E.E.; Chumanevich, A.A.; Hofseth, J.L.; Nguyen, I.; Hardy, O.; Pittman, D.L.; Wyatt, M.D. Panaxynol, a bioactive component of American ginseng, targets macrophages and suppresses colitis in mice. Oncotarget 2020, 11, 2026. [Google Scholar] [CrossRef]
- Cambria, C.; Sabir, S.; Shorter, I.C. Ginseng; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Hong, H.; Baatar, D.; Hwang, S.G. Anticancer activities of ginsenosides, the main active components of ginseng. Evid. -Based Complement. Altern. Med. 2021, 2021, 8858006. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, F.; Lu, S.; Ren, L.; Bian, S.; Liu, M.; Zhao, D.; Wang, S.; Wang, J. Ginseng root extract attenuates inflammation by inhibiting the MAPK/NF-κB signaling pathway and activating autophagy and p62-Nrf2-Keap1 signaling in vitro and in vivo. J. Ethnopharmacol. 2022, 283, 114739. [Google Scholar] [CrossRef]
- Jeon, J.E.; Kim, J.-G.; Fischer, C.R.; Mehta, N.; Dufour-Schroif, C.; Wemmer, K.; Mudgett, M.B.; Sattely, E. A pathogen-responsive gene cluster for highly modified fatty acids in tomato. Cell 2020, 180, 176–187.e119. [Google Scholar] [CrossRef]
- Mares-Perlman, J.A.; Millen, A.E.; Ficek, T.L.; Hankinson, S.E. The body of evidence to support a protective role for lutein and zeaxanthin in delaying chronic disease. Overview. J. Nutr. 2002, 132, 518S–524S. [Google Scholar] [CrossRef] [PubMed]
- Sommer, A.; Vyas, K.S. A global clinical view on vitamin A and carotenoids. Am. J. Clin. Nutr. 2012, 96, 1204S–1206S. [Google Scholar] [CrossRef]
- Tapiero, H.; Townsend, D.M.; Tew, K.D. The role of carotenoids in the prevention of human pathologies. Biomed. Pharmacother. 2004, 58, 100–110. [Google Scholar] [CrossRef]
- Deding, U.; Baatrup, G.; Christensen, L.P.; Kobaek-Larsen, M. Carrot intake and risk of colorectal cancer: A prospective cohort study of 57,053 Danes. Nutrients 2020, 12, 332. [Google Scholar] [CrossRef] [PubMed]
- Bjelakovic, G.; Nikolova, D.; Gluud, C. Antioxidant Supplements to Prevent Mortality. JAMA-J. Am. Med. Assoc. 2013, 310, 1178–1179. [Google Scholar] [CrossRef] [PubMed]
- Kordiak, J.; Bielec, F.; Jabłoński, S.; Pastuszak-Lewandoska, D. Role of Beta-Carotene in Lung Cancer Primary Chemoprevention: A Systematic Review with Meta-Analysis and Meta-Regression. Nutrients 2022, 14, 1361. [Google Scholar] [CrossRef]
- Brandt, K.; Christensen, L.P.; Hansen-Møller, J.; Hansen, S.; Haraldsdottir, J.; Jespersen, L.; Purup, S.; Kharazmi, A.; Barkholt, V.; Frøkiær, H. Health promoting compounds in vegetables and fruits: A systematic approach for identifying plant components with impact on human health. Trends Food Sci. Technol. 2004, 15, 384–393. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef]
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Ju, T.; Hernandez, L.; Mohsin, N.; Labib, A.; Frech, F.; Nouri, K. Evaluation of risk in chronic cutaneous inflammatory conditions for malignant transformation. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 231–242. [Google Scholar] [CrossRef]
- Park, J.M.; Kim, J.; Lee, Y.J.; Bae, S.U.; Lee, H.W. Inflammatory bowel disease–associated intestinal fibrosis. J. Pathol. Transl. Med. 2023, 57, 60–66. [Google Scholar] [CrossRef]
- Lirhus, S.S. Inflammatory Bowel Disease and Health Registry Data: Estimates of Incidence, Prevalence and Regional Treatment Variation. Ph.D. Thesis, University of Oslo, Oslo, Norway, January 2022. [Google Scholar]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Raposo, T.; Beirão, B.; Pang, L.; Queiroga, F.; Argyle, D. Inflammation and cancer: Till death tears them apart. Vet. J. 2015, 205, 161–174. [Google Scholar] [CrossRef]
- Macarthur, M.; Hold, G.L.; El-Omar, E.M. Inflammation and Cancer II. Role of chronic inflammation and cytokine gene polymorphisms in the pathogenesis of gastrointestinal malignancy. Am. J. Physiol. -Gastrointest. Liver Physiol. 2004, 286, G515–G520. [Google Scholar] [CrossRef]
- De Marzo, A.M.; Marchi, V.L.; Epstein, J.I.; Nelson, W.G. Proliferative inflammatory atrophy of the prostate: Implications for prostatic carcinogenesis. Am. J. Pathol. 1999, 155, 1985–1992. [Google Scholar] [CrossRef] [PubMed]
- Kuper, H.; Adami, H.O.; Trichopoulos, D. Infections as a major preventable cause of human cancer. J. Intern. Med. 2001, 249, 61–74. [Google Scholar] [CrossRef]
- Scholl, S.; Pallud, C.; Beuvon, F.; Hacene, K.; Stanley, E.; Rohrschneider, L.; Tang, R.; Pouillart, P.; Lidereau, R. Anti-colony-stimulating factor-1 antibody staining in primary breast adenocarcinomas correlates with marked inflammatory cell infiltrates and prognosis. J. Natl. Cancer Inst. 1994, 86, 120–126. [Google Scholar] [CrossRef]
- Ernst, P.B.; Gold, B.D. The disease spectrum of Helicobacter pylori: The immunopathogenesis of gastroduodenal ulcer and gastric cancer. Annu. Rev. Microbiol. 2000, 54, 615. [Google Scholar] [CrossRef] [PubMed]
- Ness, R.B.; Cottreau, C. Possible role of ovarian epithelial inflammation in ovarian cancer. J. Natl. Cancer Inst. 1999, 91, 1459–1467. [Google Scholar] [CrossRef] [PubMed]
- Bröcker, E.; Zwadlo, G.; Holzmann, B.; Macher, E.; Sorg, C. Inflammatory cell infiltrates in human melanoma at different stages of tumor progression. Int. J. Cancer 1988, 41, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Kundu, J.K.; Surh, Y.-J. Inflammation: Gearing the journey to cancer. Mutat. Res./Rev. Mutat. Res. 2008, 659, 15–30. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Multhoff, G.; Molls, M.; Radons, J. Chronic inflammation in cancer development. Front. Immunol. 2012, 2, 98. [Google Scholar] [CrossRef]
- Christensen, L.P. Bioactive C17 and C18 acetylenic oxylipins from terrestrial plants as potential lead compounds for anticancer drug development. Molecules 2020, 25, 2568. [Google Scholar] [CrossRef]
- Xie, Q.; Wang, C. Polyacetylenes in herbal medicine: A comprehensive review of its occurrence, pharmacology, toxicology, and pharmacokinetics (2014–2021). Phytochemistry 2022, 113288. [Google Scholar] [CrossRef]
- Ahmad, T.; Cawood, M.; Iqbal, Q.; Ariño, A.; Batool, A.; Tariq, R.M.S.; Azam, M.; Akhtar, S. Phytochemicals in Daucus carota and their health benefits. Foods 2019, 8, 424. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Ju, J.; Song, J.-L.; Yang, S.-g.; Park, K.-Y. Anti-colitic effect of purple carrot on dextran sulfate sodium (DSS)-induced colitis in C57BL/6J Mice. Prev. Nutr. Food Sci. 2018, 23, 77. [Google Scholar] [CrossRef]
- Metzger, B.T.; Barnes, D.M.; Reed, J.D. Purple carrot (Daucus carota L.) polyacetylenes decrease lipopolysaccharide-induced expression of inflammatory proteins in macrophage and endothelial cells. J. Agric. Food Chem. 2008, 56, 3554–3560. [Google Scholar] [CrossRef] [PubMed]
- Kobaek-Larsen, M.; Baatrup, G.; Notabi, M.K.; El-Houri, R.B.; Pipó-Ollé, E.; Christensen Arnspang, E.; Christensen, L.P. Dietary polyacetylenic oxylipins falcarinol and falcarindiol prevent inflammation and colorectal neoplastic transformation: A mechanistic and dose-response study in a rat model. Nutrients 2019, 11, 2223. [Google Scholar] [CrossRef]
- George, B.P.; Chandran, R.; Abrahamse, H. Role of phytochemicals in cancer chemoprevention: Insights. Antioxidants 2021, 10, 1455. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.U.; Ahmed, M.B.; Ahsan, H.; Islam, M.; Shehzad, A.; Sonn, J.K.; Lee, Y.S. An update on the role of dietary phytochemicals in human skin cancer: New insights into molecular mechanisms. Antioxidants 2020, 9, 916. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Rahman, M.H.; Hossain, M.S.; Biswas, P.; Islam, R.; Uddin, M.J.; Rahman, M.H.; Rhim, H. Molecular insights into the multifunctional role of natural compounds: Autophagy modulation and cancer prevention. Biomedicines 2020, 8, 517. [Google Scholar] [CrossRef]
- Kumar, S.; Gupta, S. Dietary phytochemicals and their role in cancer chemoprevention. J. Cancer Metastasis Treat. 2021, 7, 51. [Google Scholar] [CrossRef]
- Spooner, R.; Yilmaz, Ö. The role of reactive-oxygen-species in microbial persistence and inflammation. Int. J. Mol. Sci. 2011, 12, 334–352. [Google Scholar] [CrossRef]
- Kauppinen, A.; Suuronen, T.; Ojala, J.; Kaarniranta, K.; Salminen, A. Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell. Signal. 2013, 25, 1939–1948. [Google Scholar] [CrossRef]
- Crusz, S.M.; Balkwill, F.R. Inflammation and cancer: Advances and new agents. Nat. Rev. Clin. Ocol. 2015, 12, 584–596. [Google Scholar] [CrossRef]
- Song, W.; Mazzieri, R.; Yang, T.; Gobe, G.C. Translational significance for tumor metastasis of tumor-associated macrophages and epithelial–mesenchymal transition. Front. Immunol. 2017, 8, 1106. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.-J.; Choi, G.-E.; Ryu, S.; Kwon, S.J.; Kim, S.C.; Booth, C.; Nichols, K.E.; Kim, H.S. Stepwise phosphorylation of p65 promotes NF-κB activation and NK cell responses during target cell recognition. Nat. Commun. 2016, 7, 11686. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.S.; Ghosh, S. Shared principles in NF-κB signaling. Cell 2008, 132, 344–362. [Google Scholar] [CrossRef]
- Sau, A.; Lau, R.; Cabrita, M.A.; Nolan, E.; Crooks, P.A.; Visvader, J.E.; Pratt, M.C. Persistent activation of NF-κB in BRCA1-deficient mammary progenitors drives aberrant proliferation and accumulation of DNA damage. Cell Stem Cell 2016, 19, 52–65. [Google Scholar] [CrossRef]
- Salazar, L.; Kashiwada, T.; Krejci, P.; Meyer, A.N.; Casale, M.; Hallowell, M.; Wilcox, W.R.; Donoghue, D.J.; Thompson, L.M. Fibroblast growth factor receptor 3 interacts with and activates TGFβ-activated kinase 1 tyrosine phosphorylation and NFκB signaling in multiple myeloma and bladder cancer. PLoS ONE 2014, 9, e86470. [Google Scholar] [CrossRef]
- Burstein, E.; Fearon, E.R. Colitis and cancer: A tale of inflammatory cells and their cytokines. J. Clin. Investig. 2008, 118, 464–467. [Google Scholar] [CrossRef]
- Sun, S.-C. The non-canonical NF-κB pathway in immunity and inflammation. Nat. Rev. Immunol. 2017, 17, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Sun, X.; Zhang, T.; Liu, S.; Cai, E.; Zhu, H. Study on the antidepressant effect of panaxynol through the IκB-α/NF-κB signaling pathway to inhibit the excessive activation of BV-2 microglia. Biomed. Pharmacother. 2021, 138, 111387. [Google Scholar] [CrossRef]
- Chen, T.; Mou, Y.; Tan, J.; Wei, L.; Qiao, Y.; Wei, T.; Xiang, P.; Peng, S.; Zhang, Y.; Huang, Z. The protective effect of CDDO-Me on lipopolysaccharide-induced acute lung injury in mice. Int. Immunopharmacol. 2015, 25, 55–64. [Google Scholar] [CrossRef]
- Kang, H.; Bang, T.-S.; Lee, J.-W.; Lew, J.-H.; Eom, S.H.; Lee, K.; Choi, H.-Y. Protective effect of the methanol extract from Cryptotaenia japonica Hassk. against lipopolysaccharide-induced inflammation in vitro and in vivo. BMC Complement. Altern. Med. 2012, 12, 199. [Google Scholar] [CrossRef]
- Shiao, Y.-J.; Lin, Y.-L.; Sun, Y.-H.; Chi, C.-W.; Chen, C.-F.; Wang, C.-N. Falcarindiol impairs the expression of inducible nitric oxide synthase by abrogating the activation of IKK and JAK in rat primary astrocytes. Br. J. Pharmacol. 2005, 144, 42. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.N.; Kim, J.D.; Yeo, J.H.; Son, H.-J.; Park, S.B.; Park, G.H.; Eo, H.J.; Jeong, J.B. Heracleum moellendorffii roots inhibit the production of pro-inflammatory mediators through the inhibition of NF-κB and MAPK signaling, and activation of ROS/Nrf2/HO-1 signaling in LPS-stimulated RAW264.7 cells. BMC Complement. Altern. Med. 2019, 19, 310. [Google Scholar] [CrossRef]
- Yao, C.; Narumiya, S. Prostaglandin-cytokine crosstalk in chronic inflammation. Br. J. Pharmacol. 2019, 176, 337–354. [Google Scholar] [CrossRef]
- Lee, S.; Shin, S.; Kim, H.; Han, S.; Kim, K.; Kwon, J.; Kwak, J.-H.; Lee, C.-K.; Ha, N.-J.; Yim, D. Anti-inflammatory function of arctiin by inhibiting COX-2 expression via NF-κB pathways. J. Inflamm. 2011, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Nagaraju, G.P.; El-Rayes, B.F. Cyclooxygenase-2 in gastrointestinal malignancies. Cancer 2019, 125, 1221–1227. [Google Scholar] [CrossRef]
- Ghosh, N.; Chaki, R.; Mandal, V.; Mandal, S.C. COX-2 as a target for cancer chemotherapy. Pharmacol. Rep. 2010, 62, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, U.; Kumari, N.; Vasudeva, P.; Mohanty, N.K.; Saxena, S. Overexpression of COX2 indicates poor survival in urothelial bladder cancer. Ann. Diagn. Pathol. 2018, 34, 50–55. [Google Scholar] [CrossRef]
- Harris, R.E.; Casto, B.C.; Harris, Z.M. Cyclooxygenase-2 and the inflammogenesis of breast cancer. World J. Clin. Oncol. 2014, 5, 677. [Google Scholar] [CrossRef] [PubMed]
- Petkova, D.; Clelland, C.; Ronan, J.; Pang, L.; Coulson, J.; Lewis, S.; Knox, A. Overexpression of cyclooxygenase-2 in non-small cell lung cancer. Respir. Med. 2004, 98, 164–172. [Google Scholar] [CrossRef]
- Yip-Schneider, M.T.; Barnard, D.S.; Billings, S.D.; Cheng, L.; Heilman, D.K.; Lin, A.; Marshall, S.J.; Crowell, P.L.; Marshall, M.S.; Sweeney, C.J. Cyclooxygenase-2 expression in human pancreatic adenocarcinomas. Carcinogenesis 2000, 21, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Saba, N.F.; Choi, M.; Muller, S.; Shin, H.J.C.; Tighiouart, M.; Papadimitrakopoulou, V.A.; El-Naggar, A.K.; Khuri, F.R.; Chen, Z.G.; Shin, D.M. Role of Cyclooxygenase-2 in Tumor Progression and Survival of Head and Neck Squamous Cell CarcinomaRole of COX-2 in Head and Neck Cancer. Cancer Prev. Res. 2009, 2, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Masferrer, J.L.; Leahy, K.M.; Koki, A.T.; Zweifel, B.S.; Settle, S.L.; Woerner, B.M.; Edwards, D.A.; Flickinger, A.G.; Moore, R.J.; Seibert, K. Antiangiogenic and Antitumor Activities of cyclooxygenase-2 inhibitors. Cancer Res. 2000, 60, 1306–1311. [Google Scholar] [PubMed]
- Shi, G.; Li, D.; Fu, J.; Sun, Y.; Li, Y.; Qu, R.; Jin, X.; Li, D. Upregulation of cyclooxygenase-2 is associated with activation of the alternative nuclear factor kappa B signaling pathway in colonic adenocarcinoma. Am J Transl Res 2015, 7, 1612–1620. [Google Scholar] [PubMed]
- Qualls, J.E.; Kaplan, A.M.; Van Rooijen, N.; Cohen, D.A. Suppression of experimental colitis by intestinal mononuclear phagocytes. J. Leukoc. Biol. 2006, 80, 802–815. [Google Scholar] [CrossRef]
- Xavier, R.J.; Podolsky, D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007, 448, 427–434. [Google Scholar] [CrossRef]
- Chen, S. Natural products triggering biological targets-a review of the anti-inflammatory phytochemicals targeting the arachidonic acid pathway in allergy asthma and rheumatoid arthritis. Curr. Drug Targets 2011, 12, 288–301. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, C.; Röllinghoff, M.; Diefenbach, A. The role of nitric oxide in innate immunity. Immunol. Rev. 2000, 173, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Ekmekcioglu, S.; Grimm, E.A.; Roszik, J. Targeting iNOS to increase efficacy of immunotherapies. Hum. Vaccines Immunother. 2017, 13, 1105–1108. [Google Scholar] [CrossRef] [PubMed]
- Hausel, P.; Latado, H.; Courjault-Gautier, F.; Felley-Bosco, E. Src-mediated phosphorylation regulates subcellular distribution and activity of human inducible nitric oxide synthase. Oncogene 2006, 25, 198–206. [Google Scholar] [CrossRef]
- Pautz, A.; Art, J.; Hahn, S.; Nowag, S.; Voss, C.; Kleinert, H. Regulation of the expression of inducible nitric oxide synthase. Nitric Oxide 2010, 23, 75–93. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, T.; Li, J.; Nagarkatti, P.; Nagarkatti, M.; Hofseth, L.J.; Windust, A.; Cui, T. American ginseng preferentially suppresses STAT/iNOS signaling in activated macrophages. J. Ethnopharmacol. 2009, 125, 145–150. [Google Scholar] [CrossRef]
- Qu, C.; Li, B.; Lai, Y.; Li, H.; Windust, A.; Hofseth, L.J.; Nagarkatti, M.; Nagarkatti, P.; Wang, X.L.; Tang, D. Identifying panaxynol, a natural activator of nuclear factor erythroid-2 related factor 2 (Nrf2) from American ginseng as a suppressor of inflamed macrophage-induced cardiomyocyte hypertrophy. J. Ethnopharmacol. 2015, 168, 326–336. [Google Scholar] [CrossRef]
- Ďuračková, Z. Some current insights into oxidative stress. Physiol. Res. 2010, 59, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Scialo, F.; Sanz, A. Coenzyme Q redox signalling and longevity. Free Radic. Biol. Med. 2021, 164, 187–205. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Yu, S.-J.; Oh, H.J.; Lee, J.Y.; Kim, Y.; Sohn, J. Panaxydol induces apoptosis through an increased intracellular calcium level, activation of JNK and p38 MAPK and NADPH oxidase-dependent generation of reactive oxygen species. Apoptosis 2011, 16, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Young, J.F.; Christensen, L.P.; Theil, P.K.; Oksbjerg, N. The polyacetylenes falcarinol and falcarindiol affect stress responses in myotube cultures in a biphasic manner. Dose-Response 2008, 6, 239–251. [Google Scholar] [CrossRef]
- Ohnuma, T.; Nakayama, S.; Anan, E.; Nishiyama, T.; Ogura, K.; Hiratsuka, A. Activation of the Nrf2/ARE pathway via S-alkylation of cysteine 151 in the chemopreventive agent-sensor Keap1 protein by falcarindiol, a conjugated diacetylene compound. Toxicol. Appl. Pharmacol. 2010, 244, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Chiang, S.K.; Chen, S.E.; Chang, L.C. A Dual Role of Heme Oxygenase-1 in Cancer Cells. Int J Mol Sci 2018, 20, 39. [Google Scholar] [CrossRef]
- Stefanson, A.L.; Bakovic, M. Falcarinol is a potent inducer of heme oxygenase-1 and was more effective than sulforaphane in attenuating intestinal inflammation at diet-achievable doses. Oxidative Med. Cell. Longev. 2018, 2018, 3153527. [Google Scholar] [CrossRef] [PubMed]
- Sano, R.; Reed, J.C. ER stress-induced cell death mechanisms. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 2013, 1833, 3460–3470. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, L.; Zhang, G.; Ji, G.; Xu, H. The role and therapeutic implication of endoplasmic reticulum stress in inflammatory cancer transformation. Am. J. Cancer Res. 2022, 12, 2277–2292. [Google Scholar] [PubMed]
- Jin, H.; Zhao, J.; Zhang, Z.; Liao, Y.; Wang, C.; Huang, W.; Li, S.; He, T.; Yuan, C.; Du, W. The antitumor natural compound falcarindiol promotes cancer cell death by inducing endoplasmic reticulum stress. Cell Death Dis. 2012, 3, e376. [Google Scholar] [CrossRef]
- Kim, H.S.; Lim, J.M.; Kim, J.Y.; Kim, Y.; Park, S.; Sohn, J. Panaxydol, a component of P anax ginseng, induces apoptosis in cancer cells through EGFR activation and ER stress and inhibits tumor growth in mouse models. Int. J. Cancer 2016, 138, 1432–1441. [Google Scholar] [CrossRef]
- Andersen, C.B.; Runge Walther, A.; Pipó-Ollé, E.; Notabi, M.K.; Juul, S.; Eriksen, M.H.; Lovatt, A.L.; Cowie, R.; Linnet, J.; Kobaek-Larsen, M. Falcarindiol Purified From Carrots Leads to Elevated Levels of Lipid Droplets and Upregulation of Peroxisome Proliferator-Activated Receptor-γ Gene Expression in Cellular Models. Front. Pharmacol. 2020, 11, 565524. [Google Scholar] [CrossRef]
- Cheung, S.S.; Hasman, D.; Khelifi, D.; Tai, J.; Smith, R.W.; Warnock, G.L. Devil’s Club falcarinol-type polyacetylenes inhibit pancreatic cancer cell proliferation. Nutr. Cancer 2019, 71, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Zaini, R.; Brandt, K.; Clench, M.; Maitre, C. Effects of bioactive compounds from carrots (Daucus carota L.), polyacetylenes, beta-carotene and lutein on human lymphoid leukaemia cells. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem. -Anti-Cancer Agents) 2012, 12, 640–652. [Google Scholar]
- Zidorn, C.; Jöhrer, K.; Ganzera, M.; Schubert, B.; Sigmund, E.M.; Mader, J.; Greil, R.; Ellmerer, E.P.; Stuppner, H. Polyacetylenes from the Apiaceae vegetables carrot, celery, fennel, parsley, and parsnip and their cytotoxic activities. J. Agric. Food Chem. 2005, 53, 2518–2523. [Google Scholar] [CrossRef]
- Bernart, M.W.; Cardellina, J.H.; Balaschak, M.S.; Alexander, M.R.; Shoemaker, R.H.; Boyd, M.R. Cytotoxic falcarinol oxylipins from Dendropanax arboreus. J. Nat. Prod. 1996, 59, 748–753. [Google Scholar] [CrossRef]
- Sapienza, C.; Issa, J.-P. Diet, nutrition, and cancer epigenetics. Annu. Rev. Nutr. 2016, 36, 665–681. [Google Scholar] [CrossRef]
- Le, H.T.; Nguyen, H.T.; Min, H.-Y.; Hyun, S.Y.; Kwon, S.; Lee, Y.; Van Le, T.H.; Lee, J.; Park, J.H.; Lee, H.-Y. Panaxynol, a natural Hsp90 inhibitor, effectively targets both lung cancer stem and non-stem cells. Cancer Lett. 2018, 412, 297–307. [Google Scholar] [CrossRef]
- Chatterjee, S.; Burns, T.F. Targeting heat shock proteins in cancer: A promising therapeutic approach. Int. J. Mol. Sci. 2017, 18, 1978. [Google Scholar] [CrossRef]
- Neckers, L.; Workman, P. Hsp90 molecular chaperone inhibitors: Are we there yet? Clin. Cancer Res. 2012, 18, 64–76. [Google Scholar] [CrossRef]
- Nair, A.; Morsy, M.A.; Jacob, S. Dose translation between laboratory animals and human in preclinical and clinical phases of drug development. Drug Dev. Res. 2018, 79, 373–382. [Google Scholar] [CrossRef]
- Kobaek-Larsen, M.; Nielsen, D.S.; Kot, W.; Krych, Ł.; Christensen, L.P.; Baatrup, G. Effect of the dietary polyacetylenes falcarinol and falcarindiol on the gut microbiota composition in a rat model of colorectal cancer. BMC Res. Notes 2018, 11, 411. [Google Scholar] [CrossRef]
- Hansen, S.L.; Purup, S.; Christensen, L.P. Bioactivity of falcarinol and the influenceof processing and storage on its content in carrots (Daucus carota L). J. Sci. Food Agric. 2003, 83, 1010–1017. [Google Scholar] [CrossRef]
- Purup, S.; Larsen, E.; Christensen, L.P. Differential effects of falcarinol and related aliphatic C17-polyacetylenes on intestinal cell proliferation. J. Agric. Food Chem. 2009, 57, 8290–8296. [Google Scholar] [CrossRef] [PubMed]
- Young, J.F.; Duthie, S.J.; Milne, L.; Christensen, L.P.; Duthie, G.G.; Bestwick, C.S. Biphasic effect of falcarinol on CaCo-2 cell proliferation, DNA damage, and apoptosis. J. Agric. Food Chem. 2007, 55, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Dolatpanah, M.; Rashtchizadeh, N.; Abbasi, M.M.; Nazari, S.; Mohammadian, J.; Roshangar, L.; Argani, H.; Ghorbanihaghjo, A. Falcarindiol attenuates cisplatin-induced nephrotoxicity through the modulation of NF-kB and Nrf2 signaling pathways in mice. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Tan, K.W.; Killeen, D.P.; Li, Y.; Paxton, J.W.; Birch, N.P.; Scheepens, A. Dietary polyacetylenes of the falcarinol type are inhibitors of breast cancer resistance protein (BCRP/ABCG2). Eur. J. Pharmacol. 2014, 723, 346–352. [Google Scholar] [CrossRef]
- Deding, U.; Baatrup, G.; Kaalby, L.; Kobaek-Larsen, M. Carrot Intake and Risk of Developing Cancer: A Prospective Cohort Study. Nutrients 2023, 15, 678. [Google Scholar] [CrossRef] [PubMed]
- Deding, U.; Clausen, B.H.; Al-Najami, I.; Baatrup, G.; Jensen, B.L.; Kobaek-Larsen, M. Effect of Oral Intake of Carrot Juice on Cyclooxygenases and Cytokines in Healthy Human Blood Stimulated by Lipopolysaccharide. Nutrients 2023, 15, 632. [Google Scholar] [CrossRef] [PubMed]
- Almqbel, M.; Seal, C.; Brandt, K. Effects of carrot powder intake after weaning on tumours in APCMin mice. Proc. Nutr. Soc. 2017, 76. [Google Scholar] [CrossRef]
- Saleh, H.; Garti, H.; Carroll, M.; Brandt, K. Effect of carrot feeding to APC(Min) mouse on intestinal tumours. Proc. Nutr. Soc. 2013, 72, E183. [Google Scholar] [CrossRef]
- Kobæk-Larsen, M.; Christensen, L.P.; Vach, W.; Ritskes-Hoitinga, J.; Brandt, K. Inhibitory effects of feeding with carrots or (−)-falcarinol on development of azoxymethane-induced preneoplastic lesions in the rat colon. J. Agric. Food Chem. 2005, 53, 1823–1827. [Google Scholar] [CrossRef]
- Kobaek-Larsen, M.; El-Houri, R.B.; Christensen, L.P.; Al-Najami, I.; Fretté, X.; Baatrup, G. Dietary polyacetylenes, falcarinol and falcarindiol, isolated from carrots prevents the formation of neoplastic lesions in the colon of azoxymethane-induced rats. Food Funct. 2017, 8, 964–974. [Google Scholar] [CrossRef]
- Crosby, D.; Aharonson, N. The structure of carotatoxin, a natural toxicant from carrot. Tetrahedron 1967, 23, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Uwai, K.; Ohashi, K.; Takaya, Y.; Ohta, T.; Tadano, T.; Kisara, K.; Shibusawa, K.; Sakakibara, R.; Oshima, Y. Exploring the structural basis of neurotoxicity in C17-polyacetylenes isolated from water hemlock. J. Med. Chem. 2000, 43, 4508–4515. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-J.; Kwon, H.-S.; Kang, M.; Leem, H.H.; Lee, K.-H.; Kim, D.-Y. The antitumor natural compound falcarindiol disrupts neural stem cell homeostasis by suppressing Notch pathway. Int. J. Mol. Sci. 2018, 19, 3432. [Google Scholar] [CrossRef] [PubMed]
- Czyzewska, M.M.; Chrobok, L.; Kania, A.; Jatczak, M.; Pollastro, F.; Appendino, G.; Mozrzymas, J.W. Dietary acetylenic oxylipin falcarinol differentially modulates GABAA receptors. J Nat Prod 2014, 77, 2671–2677. [Google Scholar] [CrossRef]
- Czepa, A.; Hofmann, T. Structural and sensory characterization of compounds contributing to the bitter off-taste of carrots (Daucus carota L.) and carrot puree. J. Agric. Food Chem. 2003, 51, 3865–3873. [Google Scholar] [CrossRef] [PubMed]
- Downs, C.; Phillips, J.; Ranger, A.; Farrell, L. A hemlock water dropwort curry: A case of multiple poisoning. Emerg. Med. J. 2002, 19, 472–473. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Christensen, L. Bioactivity of Polyacetylenes in Food Plants: Bioactive Foods in Promoting Health (Chapter 20); Academic Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Ozdemir, C.; Schneider, L.; Hinrichs, R.; Staib, G.; Weber, L.; Weiss, J.; Scharffetter-Kochanek, K. Allergic contact dermatitis to common ivy (Hedera helix L.). Der Hautarzt; Z. Fur Dermatol. Venerol. Und Verwandte Geb. 2003, 54, 966–969. [Google Scholar]
- Paulsen, E.; Christensen, L.; Andersen, K. Dermatitis from common ivy (Hedera helix L. subsp helix) in Europe: Past, present, and future. Contact Dermat. 2010, 62, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Machado, S.; Silva, E.; Massa, A. Occupational allergic contact dermatitis from falcarinol. Contact Dermat. 2002, 47, 113–114. [Google Scholar] [CrossRef]
- Leonti, M.; Casu, L.; Raduner, S.; Cottiglia, F.; Floris, C.; Altmann, K.; Gertsch, J. Falcarinol is a covalent cannabinoid CB1 receptor antagonist and induces pro-allergic effects in skin. Biochem. Pharmacol. 2010, 79, 1815–1826. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; Tai, W.; Yang, L.; Chen, Y.; Chen, C.; Liu, C. Challenges and solutions of pharmacokinetics for efficacy and safety of traditional Chinese medicine. Curr. Drug Metab. 2015, 16, 756–776. [Google Scholar] [CrossRef]
- Jakobsen, U.; Kobæk-Larsen, M.; Kjøller, K.D.; Antonsen, S.; Baatrup, G.; Trelle, M.B. Quantification of the anti-neoplastic polyacetylene falcarinol from carrots in human serum by LC-MS/MS. J. Chromatogr. B 2022, 1210, 123440. [Google Scholar] [CrossRef]
- Tashkandi, H.; Chaparala, A.; Peng, S.; Nagarkatti, M.; Nagarkatti, P.; Chumanevich, A.A.; Hofseth, L.J. Pharmacokinetics of Panaxynol in Mice. J. Cancer Sci. Clin. Ther. 2020, 4, 133. [Google Scholar] [CrossRef]
| Compound/Source | Dose/Route | Time | Model | Effect/Pathway Investigated | Ref. |
|---|---|---|---|---|---|
| FaOH/carrot | Orally 10 mg/kg | 1 weeks | Mice | Activate Nrf2 pathway | [5,92] |
| FaOH/P. quinquefolius | 0.5–100 µM/mL | 12 h | Primary Macrophages | Induce DNA damage/apoptosis | [8] |
| FaOH/P. quinquefolius | Orally 0.01–1 mg/kg | 2 weeks | Mice | Reduce inflammation/Induce apoptosis | [8] |
| PAs/purple carrot | 6.6 or 13.3 µg/mL | 16 h | RAW 264.7 cells | Reduce secretion of the proinflammatory cytokines (IL-6, IL-1β, TNF-α) | [45] |
| FaDOH/P. quinquefolius | 50 µM/mL | 30–60 min | Astrocytes cells | Modulate NF-κB Pathway | [64] |
| FaOH/S. divaricata | Orally 0.1–1 mg/kg | 1 weeks | Mice | Modulate NF-κB pathway/proinflammatory | [61] |
| FaOH/S. divaricata | 1–10 µM/mL | 3.5–24 h | Microglia cells | Reduce nitric oxide secretion/NF-κB pathway | [61] |
| FaOH, FaDOH/carrot | Orally 0.16–35 µg/g feed | 20 weeks | Rats | Reduce tumour growth in colon/NF-κB pathway | [46] |
| FaOH/H. moellendorffii | 6.25–50 µg/mL | 20 h | RAW 264.7 cells | Activate ROS/Nrf2/HO-1 signaling pathway/Modulate NF-κB pathway | [65] |
| FaOH/P. quinquefolius | 0.5 µM/mL | 6 h | Macrophages cell lines | Activate Nrf2 pathway/NF-κB pathway | [85] |
| Panaxydol/P. ginseng | 50 µg/mL | 6 h | MCF-7 cells | Induce ROS generation, induce apoptosis/activate Nrf2 pathway | [88] |
| FaOH, FaDOH/carrot | 6.25–50 µM/mL | 24 h | Myotube cells | Modulate ROS pathway | [89] |
| FaDOH/N. incisum | 2.5 µM/mL | 24 h | HEK293 cells | Modulate Nrf2/ARE pathway | [90] |
| FaDOH/O. horridus | 10 µM/mL | 8 h | Colon cancer cells | Modulate ER/UPR pathway | [95] |
| Panaxydol/P. ginseng | 20 µg/mL | 2–4 h | MCF-7 cells | Modulate ER/EGFR pathway/induce apoptosis | [96] |
| FaDOH/carrot | 5 µg/mL | 24 h | HT-29/hMSCs cells | Modulate PPARγ pathway | [97] |
| FaOH/O. horridus | 0.3 µg/mL | 48 h | PANC-1 cells | Inhibit human pancreatic cancer cells/Induce apoptosis | [98] |
| FaOH, FaDOH/carrot | 25–100 µM/mL | 24 h | Leukaemia cells | Induced apoptosis in leukaemia cells/arrest of cell cycle | [99] |
| FaOH/P. ginseng | Orally 50–100 mg/kg mouse | 8 weeks | Mice | Reduced lung tumorigenesis | [103] |
| FaOH/P. ginseng | 1 µM/mL | - | NSCLC cells | Induce apoptosis | [103] |
| FaOH, FaDOH/carrot | Orally 7 µg/g feed | 20 weeks | Rats | Alter gut microbiota, Lactobacillus reuteri, Turicibacter | [107] |
| FaOH/carrot | 0.5–100 μM/mL | 72 h | CaCo-2 Cell | Induce proliferation/apoptosis | [110] |
| FaDOH | IP injection/50–100 mg/kg | 4 days | Mice | Activate Nrf2 pathway/NF-κB pathway | [111] |
| FaOH, FaDOH, FaDOH3Ac/carrot | 20 µM/mL | 105 min | HEK293 cells | Modulate ABCG2 pathway | [112] |
| FaOH in purple carrot juice | Orally approx. 18 mg in 500 mL | 1 h | Human | Reduce secretion of the proinflammatory cytokines IL-1α and IL-16 | [114] |
| PAs in carrot powder | Orally approx. 20 µg/g feed | 12 weeks | Mice | Reduced tumours in intestine | [116] |
| PAs in carrot powder | Orally approx. 20 µg/g feed | 10 weeks | Mice | Reduced tumours in intestine | [115] |
| Panaxydol/P. ginseng | IP injection/50–100 mg/kg | 30 days | Mice | Reduced syngeneic and xenogeneic tumours | [96] |
| FaOH/carrot | Orally 3.5 μg/g feed | 18 weeks | Rats | Reduced tumours in colon | [117] |
| FaOH+FaDOH/carrot | Orally 7 + 7 μg/g feed | 18 weeks | Rats | Reduced tumours in colon | [118] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfurayhi, R.; Huang, L.; Brandt, K. Pathways Affected by Falcarinol-Type Polyacetylenes and Implications for Their Anti-Inflammatory Function and Potential in Cancer Chemoprevention. Foods 2023, 12, 1192. https://doi.org/10.3390/foods12061192
Alfurayhi R, Huang L, Brandt K. Pathways Affected by Falcarinol-Type Polyacetylenes and Implications for Their Anti-Inflammatory Function and Potential in Cancer Chemoprevention. Foods. 2023; 12(6):1192. https://doi.org/10.3390/foods12061192
Chicago/Turabian StyleAlfurayhi, Ruyuf, Lei Huang, and Kirsten Brandt. 2023. "Pathways Affected by Falcarinol-Type Polyacetylenes and Implications for Their Anti-Inflammatory Function and Potential in Cancer Chemoprevention" Foods 12, no. 6: 1192. https://doi.org/10.3390/foods12061192
APA StyleAlfurayhi, R., Huang, L., & Brandt, K. (2023). Pathways Affected by Falcarinol-Type Polyacetylenes and Implications for Their Anti-Inflammatory Function and Potential in Cancer Chemoprevention. Foods, 12(6), 1192. https://doi.org/10.3390/foods12061192







