Effect of Different Cold Storage Temperatures on the Evolution of Shucking Yield and Quality Properties of Offshore Cultured Japanese Oyster (Magallana gigas) Treated by High Pressure Processing (HPP)
Abstract
1. Introduction
| Reference | Key Parameters Investigated | HPP Treatment | Storage Conditions | Main Results |
|---|---|---|---|---|
| Koo et al. (2006) [16] | V. parahaemolyticus, V. vulnificus | 207–345 MPa, 0–22 min | No storage | 379 MPa, 6.5 min: >5 log reductions in V. parahaemolyticus 241 MPa, 5 min: >5.5 log reductions in V. vulnificus |
| Kural et al. (2008) [17] | V. parahaemolyticus | 250 MPa, 5 min 300 MPa, 2 min 350 MPa, 1 min | No storage | >300–350 MPa, 2 min: >5 log reductions in V. parahaemolyticus |
| Ma et al. (2011) [18] | V. parahaemolyticus, aerobic and psychrotrophic plate count, coliforms | 293 MPa, 1.5–3.5 min | 5 °C; ice | 293 MPa, 2 min: 3.52 log reductions in V. parahaemolyticus; shelf life of 6–8 days at 5 °C or 16–18 days in ice |
| Cruz-Romero et al. (2008) [20] | Aerobic and anaerobic plate count, H2S-producing bacteria, texture, color, lipid oxidation | 260–600 MPa, 5 min | 2 °C (ice) | HPP reduced microbial counts; increased lightness, cutting strength and lipid oxidation |
| Cruz-Romero et al. (2007) [21] | Proximate analysis, shucking yield, color | 260 MPa, 3 min | No storage | 260 MPa, 3 min: higher shucking yield; small differences in color; increased moisture; reduced protein |
| He et al. (2002) [24] | Aerobic plate count, sensory analysis | 207–310 MPa, 0–2 min | 4 °C | HPP: 2–3 log reductions in microbial counts; higher sensory scores during all storage |
| Liu et al. (2022) [25] | Fatty acid profile, total free amino acids, nucleotides, organic and inorganic ions | 200–600 MPa, 3 min | 4 °C; −20 °C | 400–600 MPa, 3 min: no effect on fatty acid profile; decreased total free amino acids during storage; no changes in nucleotides, organic and inorganic ions |
| López-Caballero et al. (2000) [26] | Microbial flora (total viable count, H2S-producing bacteria, lactic acid bacteria, Brochothrix thermosphacta, coliforms), total volatile bases, texture | 400 MPa, 10 min or 2 steps of 5 min | 2 °C | 400 MPa: up to 5 log reductions in microbial flora; increased total volatile bases; higher shear strength. HPP in 2 steps: no advantages |
| Rong et al. (2018) [27] | Sensory analysis, microbiological analysis (High-throughput screening), total volatile bases, lipid oxidation | 300 MPa, 2 min | 4 °C | 300 MPa, 2 min: better sensory scores during storage; shelf life of 12 days (6–8 days for controls); increased lipid oxidation; Psychrobacter was dominant in the HPP oysters |
| Ye et al. (2015) [28] | Norovirus, sensory analysis, color, texture | 300–600 MPa, 2 min | Ice | 350 and 500 MPa, 2 min: >4 log reductions of GII.4 and GI.1 HuNoV; no changes in color and texture; higher overall sensory acceptability |
2. Materials and Methods
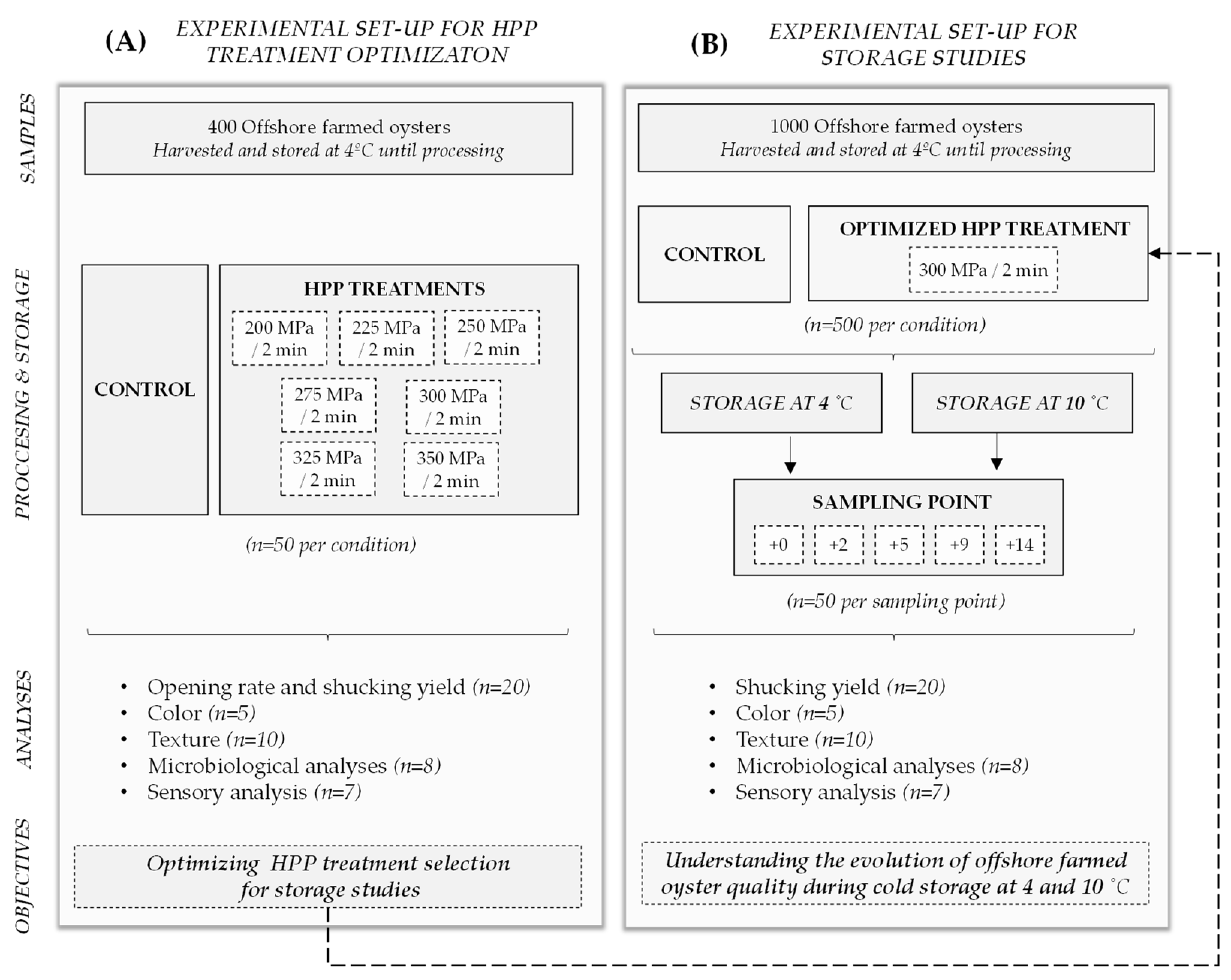
2.1. Schematic Overview of the Experimental Program
2.2. Collection of Experimental Samples
2.3. HPP Treatments
2.4. Analytical Measurements
2.4.1. Opening Rate and Shucking Yield
2.4.2. Color
2.4.3. Texture
2.4.4. Microbiological Analysis
2.4.5. Sensory Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Selection of the HPP Treatment
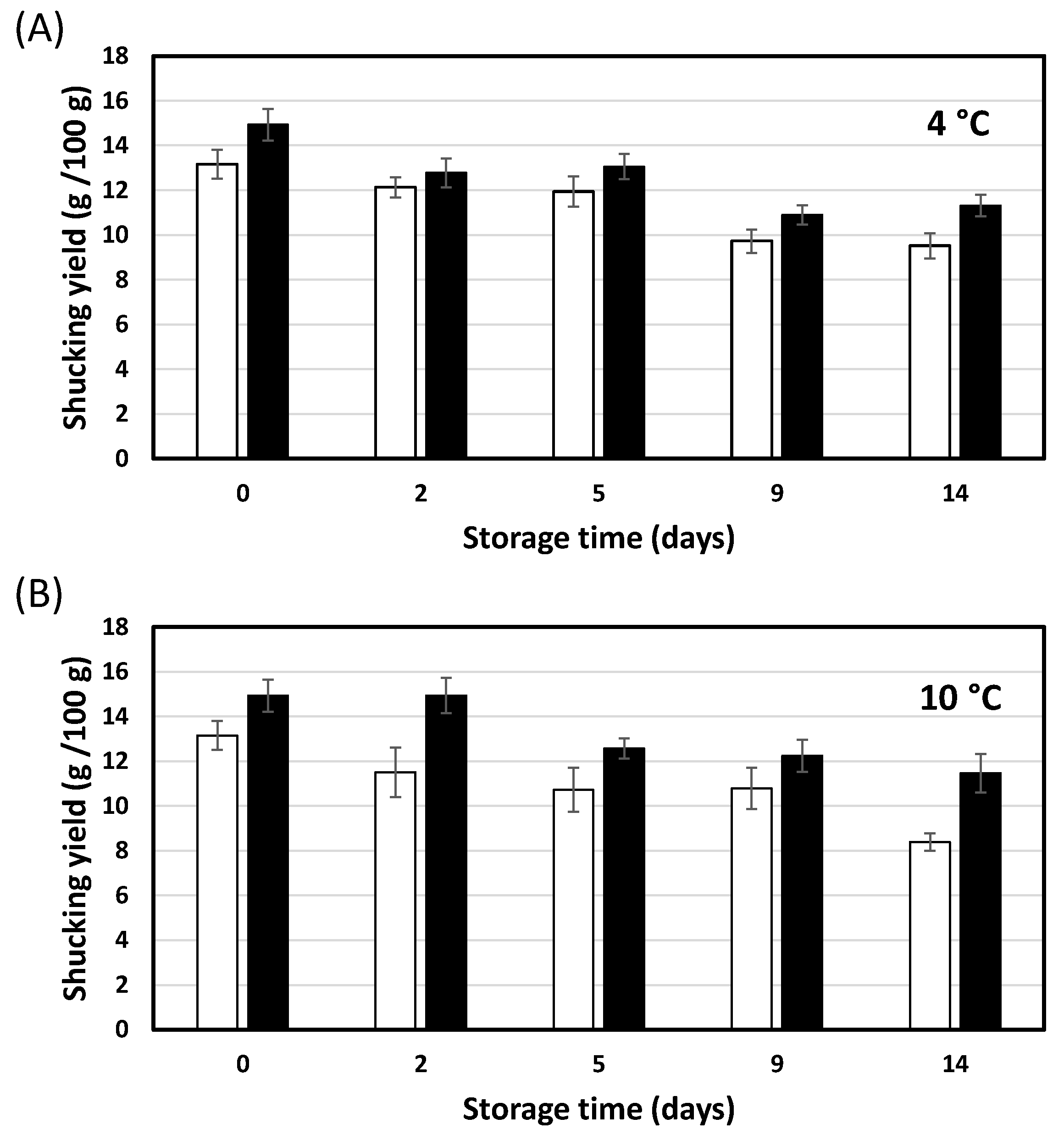
3.2. Effect of HPP and Cold Storage Temperature on Shucking Yield Evolution
3.3. Effect of HPP and Cold Storage Temperature on Color Evolution
3.4. Effect of HPP and Cold Storage Temperature on Texture Evolution
3.5. Effect of HPP and Cold Storage Temperature on Microbiological Quality Evolution
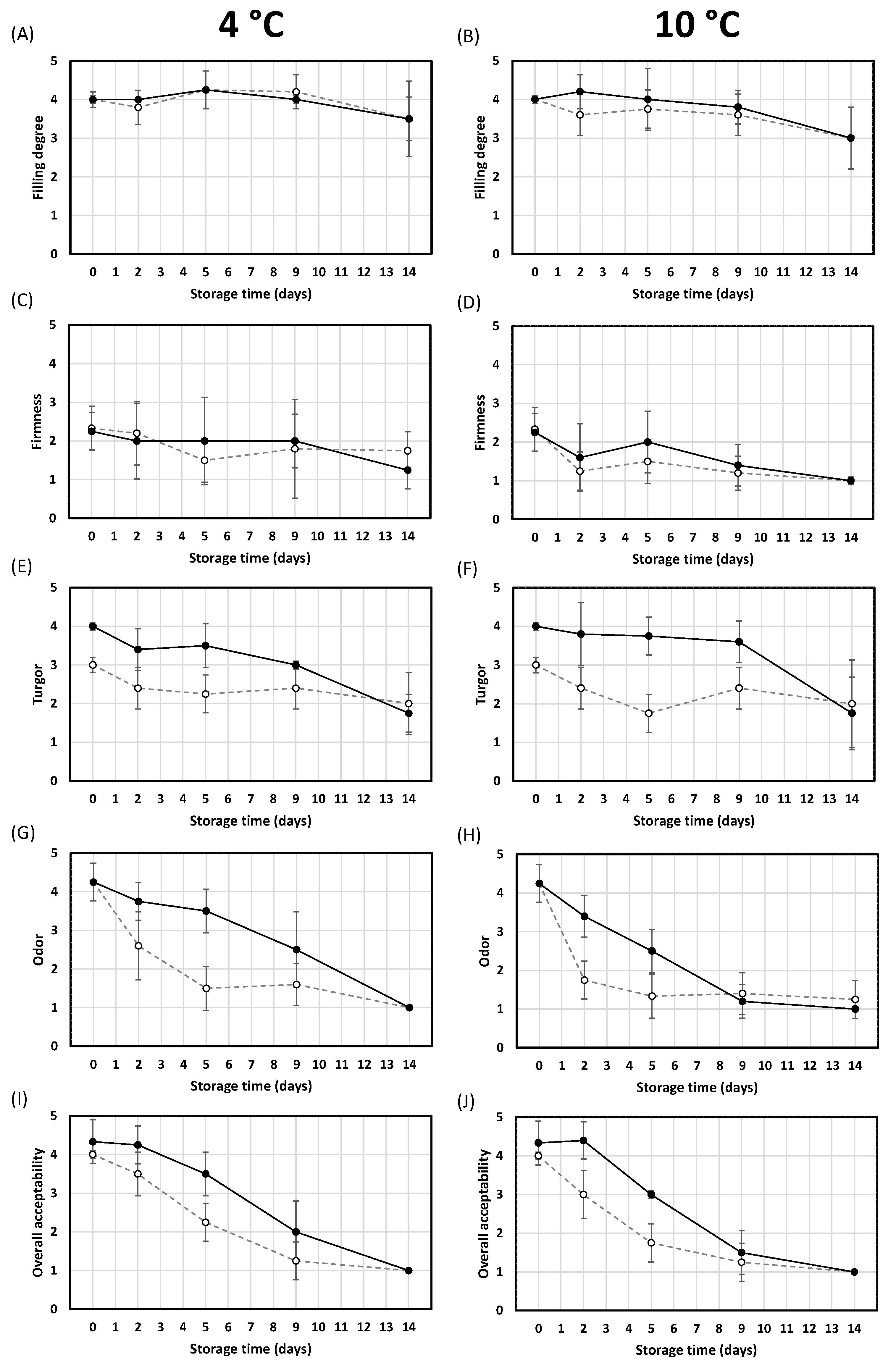
3.6. Effect of HPP and Cold Storage Temperature on Sensory Quality Evolution
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization (FAO). The State of World Fisheries and Aquaculture 2022. Towards Blue Transformation; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Pogoda, B.; Buck, B.H.; Saborowski, R.; Hagen, W. Biochemical and elemental composition of the offshore-cultivated oysters Ostrea edulis and Crassostrea gigas. Aquaculture 2013, 400–401, 53–60. [Google Scholar] [CrossRef]
- Palmer, S.C.J.; Gernez, P.M.; Thomas, Y.; Simis, S.; Miller, P.I.; Glize, P.; Barille, L. Remote Sensing-Driven Pacific Oyster (Crassostrea gigas) Growth Modeling to Inform Offshore Aquaculture Site Selection. Front. Mar. Sci. 2020, 6, 802. [Google Scholar] [CrossRef]
- Pogoda, B.; Buck, B.H.; Hagen, W. Growth performance and condition of oysters (Crassostrea gigas and Ostrea edulis) farmed in an offshore environment (North Sea, Germany). Aquaculture 2011, 319, 484–492. [Google Scholar] [CrossRef]
- Zorita, I.; Juez, A.; Solaun, O.; Muxika, I.; Rodriguez, J.G. Stocking density effect on the growth and mortality of juvenile European flat oyster (Ostrea edulis Linnaeus, 1758). Aquac. Fish Fish. 2021, 1, 60–65. [Google Scholar] [CrossRef]
- Cartagena, L.; Puértolas, E.; Martínez de Marañón, I. Evolution of quality parameters of high pressure processing (HPP) pretreated albacore (Thunnus alalunga) during long-term frozen storage. Innov. Food Sci. Emerg. Technol. 2020, 62, 102334. [Google Scholar] [CrossRef]
- Castrica, M.; Pavlovic, R.; Balzaretti, C.M.; Curone, G.; Brecchia, G.; Copelotti, E.; Panseri, S.; Pessina, D.; Arnoldi, C.; Chiesa, L.M. Effect of High-Pressure Processing on Physico-Chemical, Microbiological and Sensory Traits in Fresh Fish Fillets (Salmo salar and Pleuronectes platessa). Foods 2021, 10, 1775. [Google Scholar] [CrossRef] [PubMed]
- Ferri, G.; Lauteri, C.; Scattolini, M.; Vergara, A. Shelf Life and Safety of Vacuum Packed HPP-Treated Soaked Cod Fillets: Effects of Salt Content and Multilayer Plastic Film. Foods 2023, 12, 179. [Google Scholar] [CrossRef] [PubMed]
- Lavilla, M.; Puértolas, E.; Orcajo, J. HPP impact to reduce allergenicity of foods. In Present and Future of High Pressure Processing; Barba, F.J., Tonello-Samson, C., Puértolas, E., Lavilla, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 113–138. [Google Scholar] [CrossRef]
- Pinto de Rezende, L.; Barbosa, J.; Teixeira, P. Analysis of Alternative Shelf Life-Extending Protocols and Their Effect on the Preservation of Seafood Products. Foods 2022, 11, 1100. [Google Scholar] [CrossRef]
- Puértolas, E.; Lavilla, M. HPP in seafood products: Impact on quality and applications. In Present and Future of High Pressure Processing; Barba, F.J., Tonello-Samson, C., Puértolas, E., Lavilla, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 201–220. [Google Scholar] [CrossRef]
- Murchie, L.W.; Cruz-Romero, M.; Kerry, J.P.; Linton, M.; Patterson, M.F.; Smiddy, M.; Kelly, A.L. High pressure processing of shellfish: A review of microbiological and other quality aspects. Innov. Food Sci. Emerg. Technol. 2005, 6, 257–270. [Google Scholar] [CrossRef]
- Puértolas, E.; Álvarez-Sabatel, S.; Montes, P. Application of high-pressure assisted thermal processing (PATP) at pilot scale for replacing conventional maturation and thermal cooking in whiteleg shrimp (Litopenaeus vannamei). J. Sci. Food Agric. 2022, 102, 6464–6469. [Google Scholar] [CrossRef]
- Truong, B.Q.; Buckow, R.; Stathopoulos, E.; Nguyen, M.N. Advances in High-Pressure Processing of Fish Muscles. Food Eng. Rev. 2015, 7, 109–129. [Google Scholar] [CrossRef]
- Kingsley, D.H. High Pressure Processing of Bivalve Shellfish and HPP’s Use as a Virus Intervention. Foods 2014, 3, 336–350. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.; Jahncke, M.L.; Reno, P.W.; Hu, X.; Mallikarjunan, P. Inactivation of Vibrio parahaemolyticus and Vibrio vulnificus in Phosphate-Buffered Saline and in Inoculated Whole Oysters by High-Pressure Processing. J. Food Prot. 2006, 69, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Kural, A.G.; Shearer, E.H.; Kingsley, D.H.; Chen, H. Conditions for high pressure inactivation of Vibrio parahaemolyticus in oysters. Int. J. Food Microbiol. 2008, 127, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Su, Y.C. Validation of high pressure processing for inactivating Vibrio parahaemolyticus in Pacific oysters (Crassostrea gigas). Int. J. Food Microbiol. 2011, 144, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Sermet-Moreno, V.; Deng, K.; Wu, X.; Su, Y.C.; Fuentes, C.; Torres, J.A.; Welti-Chanes, J. Monte Carlo analysis of the product handling and high-pressure treatment effects on the Vibrio vulnificus risk to raw oysters consumers. J. Food Eng. 2015, 144, 86–92. [Google Scholar] [CrossRef]
- Cruz-Romero, M.; Kerry, J.P.; Kelly, A.L. Changes in the microbiological and physicochemical quality of high-pressure-treated oysters (Crassostrea gigas) during chilled storage. Food Control 2008, 19, 1139–1147. [Google Scholar] [CrossRef]
- Cruz-Romero, M.; Kelly, A.L.; Kerry, J.P. Effects of high-pressure and heat treatments on physical and biochemical characteristics of oysters (Crassostrea gigas). Innov. Food Sci. Emerg. Technol. 2007, 8, 30–38. [Google Scholar] [CrossRef]
- Food and Drug Administration (FDA). Quantitative Risk Assessment on the Public Health Impact of Pathogenic Vibrio Parahaemolyticus in Raw Oysters; FDA: Washington, DC, USA, 2005. Available online: https://www.fda.gov/food/cfsan-risk-safety-assessments/quantitative-risk-assessment-public-health-impact-pathogenic-vibrio-parahaemolyticus-raw-oysters (accessed on 13 January 2022).
- Love, D.C.; Kuehl, L.M.; Lane, R.M.; Fry, J.P.; Harding, J.; Davis, B.J.K.; Clancy, K.; Hudson, B. Performance of cold chains and modeled growth of Vibrio parahaemolyticus for farmed oysters distributed in the United States and internationally. Int. J. Food Microbiol. 2020, 313, 108378. [Google Scholar] [CrossRef]
- He, H.; Adams, R.M.; Farkas, D.F.; Morrissey, M.T. Use of High-pressure Processing for Oyster Shucking and Shelf-life Extension. J. Food Sci. 2002, 67, 640–645. [Google Scholar] [CrossRef]
- Liu, C.; Gu, Z.; Lin, X.; Wang, Y.; Wang, A.; Sun, Y.; Shi, Y. Effects of high hydrostatic pressure (HHP) and storage temperature on bacterial counts, color change, fatty acids and non-volatile taste active compounds of oysters (Crassostrea ariakensis). Food Chem. 2022, 372, 131247. [Google Scholar] [CrossRef] [PubMed]
- López-Caballero, M.E.; Pérez-Mateos, M.; Montero, P.; Borderías, A.J. Oyster Preservation by High-Pressure Treatment. J. Food Prot. 2000, 63, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Rong, C.; Ling, Z.; Huihui, S.; Qi, L. Characterization of microbial community in high-pressure treated oysters by high-throughput sequencing technology. Innov. Food Sci. Emerg. Technol. 2018, 45, 241–248. [Google Scholar] [CrossRef]
- Ye, M.; Lingham, T.; Huang, Y.; Ozbay, G.; Ji, L.; Karwe, M.; Chen, H. Effects of High-Hydrostatic Pressure on Inactivation of Human Norovirus and Physical and Sensory Characteristics of Oysters. J. Food Sci. 2015, 80, M1330–M1335. [Google Scholar] [CrossRef]
- Gopal, S.; Otta, S.K.; Kumar, S.; Karunasagar, I.; Nishibuchi, M.; Karunasagar, I. The occurrence of Vibrio species in tropical shrimp culture. Int. J. Food Microbiol. 2005, 102, 151–159. [Google Scholar] [CrossRef]
- ISO Standard 8586:2012; Sensory Analysis. General Guidelines for the Selection, Training and Monitoring of Selected Assessors and Expert Sensory Assessors. International Organisation of Standardisation (ISO): Geneva, Switzerland, 2012. Available online: https://www.iso.org/standard/45352.html (accessed on 10 September 2019).
- ISO Standard 8589:2007; General Guidance for the Design of Test Rooms. International Organisation of Standardisation (ISO): Geneva, Switzerland, 2007. Available online: https://www.iso.org/standard/36385.html (accessed on 10 September 2019).
- ISO Standard 4121:2003; Sensory Analysis. Guidelines for the Use of Quantitative Response Scales. International Organisation of Standardisation (ISO): Geneva, Switzerland, 2003. Available online: https://www.iso.org/standard/33817.html (accessed on 10 September 2019).
- Yuyang, M.; Wang, R.; Zhang, T.; Xu, Y.; Jiang, S.; Zhao, Y. High Hydrostatic Pressure Treatment of Oysters (Crassostrea gigas)—Impact on Physicochemical Properties, Texture Parameters, and Volatile Flavor Compounds. Molecules 2021, 26, 5731. [Google Scholar] [CrossRef]
- Liu, C.; Ji, W.; Jiang, H.; Shi, Y.; He, L.; Gu, Z.; Zhu, S. Comparison of biochemical composition and non-volatile taste active compounds in raw, high hydrostatic pressure-treated and steamed oysters Crassostrea hongkongensis. Food Chem. 2021, 344, 128632. [Google Scholar] [CrossRef]
| Control | 225 MPa | 250 MPa | 275 MPa | 300 MPa | 325 MPa | 350 MPa | |
|---|---|---|---|---|---|---|---|
| Opening rate | |||||||
| No open (%) | 100 | 30 | 0 | 0 | 0 | 0 | 0 |
| Partial open (%) | 0 | 20 | 30 | 10 | 0 | 0 | 0 |
| Full open (%) | 0 | 50 | 70 | 90 | 100 | 100 | 100 |
| Shucking yield (g/100 g) | 12.25 ± 0.49 a | 11.24 ± 1.89 a | 12.54 ± 1.38 ac | 12.11 ± 0.43 a | 15.02 ± 0.40 b | 14.44 ± 0.49 b | 15.32 ± 1.54 bc |
| Color | |||||||
| L* | 51.48 ± 3.34 a | 55.95 ± 3.81 ab | 59.05 ± 2.39 b | 57.77 ± 1.59 b | 60.40 ± 3.64 b | 59.23 ± 2.86 b | 61.84 ± 3.58 b |
| a* | −1.24 ± 0.33 a | −1.31 ± 0.29 a | −1.20 ± 0.29 a | −1.21 ± 0.34 a | −1.23 ± 0.31 a | −1.37 ± 0.20 a | −1.29 ± 0.22 a |
| b* | 10.68 ± 0.84 a | 11.03 ± 0.98 a | 10.78 ± 0.69 a | 10.46 ± 0.51 a | 11.25 ± 0.52 a | 11.43 ± 0.58 a | 12.69 ± 0.62 b |
| Texture | |||||||
| Cutting force (N) | 0.71 ± 0.26 a | 0.50 ± 0.23 a | 0.67 ± 0.24 a | 0.71 ± 0.25 a | 0.74 ± 0.26 a | 1.25 ± 0.61 a | 2.19 ± 1.63 a |
| Cutting work (N·s) | 0.36 ± 0.11 a | 0.25 ± 0.07 a | 0.30 ± 0.08 a | 0.33 ± 0.08 a | 0.36 ± 0.06 a | 0.56 ± 0.30 a | 0.56 ± 0.31 a |
| Sensory analysis | |||||||
| Filling degree | 4.13 ± 0.26 a | 4.00 ± 0.60 a | 4.14 ± 0.51 a | 4.00 ± 0.59 a | 4.43 ± 0.58 a | 4.29 ± 0.56 a | 4.43 ± 0.58 a |
| Turgor | 2.25 ± 0.34 a | 2.86 ± 0.51 a | 2.57 ± 0.40 a | 2.67 ± 0.38 a | 4.17 ± 0.56 b | 4.29 ± 0.36 b | 4.50 ± 0.41 b |
| Firmness | 2.50 ± 0.79 a | 2.86 ± 0.67 a | 2.14 ± 0.67 a | 2.00 ± 0.60 a | 2.33 ± 1.01 a | 1.71 ± 0.82 a | 3.00 ± 1.13 a |
| Odor | 4.13 ± 0.47 a | 4.14 ± 0.51 a | 4.00 ± 0.47 a | 3.86 ± 0.67 a | 3.71 ± 0.36 a | 4.00 ± 0.60 a | 4.17 ± 0.30 a |
| Overall acceptance | 4.13 ± 0.47 a | 4.00 ± 0.47 a | 4.17 ± 0.56 a | 3.83 ± 0.56 a | 4.20 ± 0.62 a | 4.00 ± 0.43 a | 4.14 ± 0.51 a |
| Microbiology | |||||||
| Aerobic plate count (log CFU/g) | 3.88 ± 0.25 a | 2.88 ± 0.25 b | 3.07 ± 0.26 b | 2.84 ± 0.26 b | 3.03 ± 0.28 b | 2.92 ± 0.26 b | 3.07 ± 0.26 b |
| Enterobacteriaceae (log CFU/g) | <1 | <1 | <1 | <1 | <1 | <1 | <1 |
| Vibrio spp. (log CFU/g) | <1 | <1 | <1 | <1 | <1 | <1 | <1 |
| Escherichia coli (log CFU/g) | <1 | <1 | <1 | <1 | <1 | <1 | <1 |
| Salmonella spp. (absence in 25 g) | - | - | - | - | - | - | - |
| Storage Time (Days) | 4 °C | 10 °C | |||
|---|---|---|---|---|---|
| Control | 300 MPa | Control | 300 MPa | ||
| L* | |||||
| 0 | 53.22 ± 3.27 aA | 61.62 ± 2.54 bAB | 53.22 ± 3.27 aA | 61.62 ± 2.54 bA | |
| 2 | 50.99 ± 2.69 aA | 63.44 ± 1.65 bA | 53.05 ± 2.45 aA | 61.67 ± 2.03 bA | |
| 5 | 51.90 ± 2.23 aA | 57.10 ± 2.22 bB | 50.05 ± 2.98 aA | 59.09 ± 2.90 bA | |
| 9 | 51.50 ± 2.24 aA | 61.65 ± 3.12 bAB | 50.60 ± 2.59 aA | 58.82 ± 1.81 bA | |
| 14 | 54.01 ± 2.48 aA | 60.36 ± 3.82 bAB | 52.76 ± 2.66 aA | 60.72 ± 2.31 bA | |
| a* | |||||
| 0 | −1.30 ± 0.51 aA | −1.27 ± 0.53 aA | −1.30 ± 0.55 aA | −1.27 ± 0.53 aA | |
| 2 | −1.20 ± 0.39 aA | −1.08 ± 0.64 aA | −1.33 ± 0.39 aA | −1.22 ± 0.66 aA | |
| 5 | −1.08 ± 0.34 aA | −1.16 ± 0.51 aA | −1.27 ± 0.32 aA | −1.17 ± 0.49 aA | |
| 9 | −1.31 ± 0.39 aA | −1.29 ± 0.39 aA | −1.38 ± 0.43 aA | −1.30 ± 0.36 aA | |
| 14 | −1.42 ± 0.32 aA | −0.92 ± 0.56 aA | −1.11 ± 0.56 aA | −1.35 ± 0.44 aA | |
| b* | |||||
| 0 | 7.31 ± 1.61 aA | 8.47 ± 1.42 aA | 7.31 ± 1.61 aA | 8.47 ± 1.42 aA | |
| 2 | 6.94 ± 0.81 aA | 8.13 ± 1.92 aA | 6.46 ± 1.50 aA | 8.55 ± 1.18 aA | |
| 5 | 6.55 ± 1.40 aA | 6.74 ± 2.04 aA | 7.36 ± 1.80 aA | 7.65 ± 1.82 aA | |
| 9 | 6.91 ± 1.59 aA | 6.53 ± 1.57 aA | 7.60 ± 1.61 aA | 6.77 ± 1.13 aA | |
| 14 | 6.25 ± 1.28 aA | 6.91 ± 0.76 aA | 7.38 ± 0.55 aA | 6.76 ± 0.75 aA |
| Storage Time (Days) | 4 °C | 10 °C | |||
|---|---|---|---|---|---|
| Control | 300 MPa | Control | 300 MPa | ||
| Cutting force (N) | |||||
| 0 | 0.72 ± 0.28 aA | 0.60 ± 0.19 aA | 0.72 ± 0.28 aA | 0.60 ± 0.19 aA | |
| 2 | 0.91 ± 0.26 aA | 0.69 ± 0.21 aA | 0.75 ± 0.28 aA | 0.64 ± 0.11 aA | |
| 5 | 0.67 ± 0.27 aA | 0.61 ± 0.15 aA | 0.76 ± 0.22 aA | 0.60 ± 0.21 aA | |
| 9 | 0.65 ± 0.25 aA | 0.60 ± 0.29 aA | 0.99 ± 0.50 aA | 0.72 ± 0.24 aA | |
| 14 | 0.62 ± 0.13 aA | 0.69 ± 0.28 aA | 0.83 ± 0.36 aA | 0.83 ± 0.36 aA | |
| Cutting work (N·s) | |||||
| 0 | 0.38 ± 0.19 aA | 0.26 ± 0.05 aA | 0.36 ± 0.19 aA | 0.26 ± 0.05 aA | |
| 2 | 0.39 ± 0.09 aA | 0.35 ± 0.09 aA | 0.36 ± 0.28 aA | 0.26 ± 0.08 aA | |
| 5 | 0.42 ± 0.18 aA | 0.32 ± 0.06 aA | 0.35 ± 0.06 aA | 0.31 ± 0.10 aA | |
| 9 | 0.30 ± 0.07 aA | 0.28 ± 0.12 aA | 0.46 ± 0.22 aA | 0.33 ± 0.10 aA | |
| 14 | 0.29 ± 0.05 aA | 0.33 ± 0.12 aA | 0.41 ± 0.13 aA | 0.33 ± 0.09 aA |
| Storage Time (Days) | 4 °C | 10 °C | |||
|---|---|---|---|---|---|
| Control | 300 MPa | Control | 300 MPa | ||
| Aerobic Plate Count (APC) | |||||
| 0 | 3.62 ± 0.33 aA | 2.85 ± 0.23 bA | 3.62 ± 0.34 aA | 2.85 ± 0.23 bA | |
| 2 | 4.22 ± 0.24 aB | 2.93 ± 0.28 bA | 5.87 ± 0.16 cB | 3.04 ± 0.22 bA | |
| 5 | 5.61 ± 0.35 aC | 3.19 ± 0.43 bA | 6.23 ± 0.20 cC | 4.78 ± 0.23 dB | |
| 9 | 6.46 ± 0.25 aD | 5.36 ± 0.26 bB | 7.04 ± 0.18 cD | 6.38 ± 0.31 aC | |
| 14 | 7.36 ± 0.19 aE | 7.29 ± 0.28 aC | 8.23 ± 0.36 bE | 8.15 ± 0.21 bD | |
| Enterobacteriaceae | |||||
| 0 | <1 | <1 | <1 | <1 | |
| 2 | 1.91 ± 0.35 aA | <1 | 1.60 ± 0.19 aA | <1 | |
| 5 | 2.60 ± 0.25 aB | <1 | 2.71 ± 0.30 aB | 1.32 ± 0.22 bA | |
| 9 | 3.20 ± 0.28 aC | 2.55 ± 0.28 bA | 4.52 ± 0.41 cC | 3.96 ± 0.30 cB | |
| 14 | 4.02 ± 0.33 aD | 4.32 ± 0.39 aB | 5.81 ± 0.37 bD | 6.20 ± 0.34 bC | |
| Vibrio spp. | |||||
| 0 | <1 | <1 | <1 | <1 | |
| 2 | <1 | <1 | <1 | <1 | |
| 5 | <1 | <1 | <1 | <1 | |
| 9 | <1 | <1 | <1 | <1 | |
| 14 | <1 | <1 | <1 | <1 | |
| Escherichia coli | |||||
| 0 | <1 | <1 | <1 | <1 | |
| 2 | <1 | <1 | <1 | <1 | |
| 5 | <1 | <1 | <1 | <1 | |
| 9 | <1 | <1 | <1 | <1 | |
| 14 | <1 | <1 | <1 | <1 | |
| Salmonella spp. | |||||
| 0 | - | - | - | - | |
| 2 | - | - | - | - | |
| 5 | - | - | - | - | |
| 9 | - | - | - | - | |
| 14 | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puértolas, E.; García-Muñoz, S.; Caro, M.; Alvarez-Sabatel, S. Effect of Different Cold Storage Temperatures on the Evolution of Shucking Yield and Quality Properties of Offshore Cultured Japanese Oyster (Magallana gigas) Treated by High Pressure Processing (HPP). Foods 2023, 12, 1156. https://doi.org/10.3390/foods12061156
Puértolas E, García-Muñoz S, Caro M, Alvarez-Sabatel S. Effect of Different Cold Storage Temperatures on the Evolution of Shucking Yield and Quality Properties of Offshore Cultured Japanese Oyster (Magallana gigas) Treated by High Pressure Processing (HPP). Foods. 2023; 12(6):1156. https://doi.org/10.3390/foods12061156
Chicago/Turabian StylePuértolas, Eduardo, Sonia García-Muñoz, Mercedes Caro, and Saioa Alvarez-Sabatel. 2023. "Effect of Different Cold Storage Temperatures on the Evolution of Shucking Yield and Quality Properties of Offshore Cultured Japanese Oyster (Magallana gigas) Treated by High Pressure Processing (HPP)" Foods 12, no. 6: 1156. https://doi.org/10.3390/foods12061156
APA StylePuértolas, E., García-Muñoz, S., Caro, M., & Alvarez-Sabatel, S. (2023). Effect of Different Cold Storage Temperatures on the Evolution of Shucking Yield and Quality Properties of Offshore Cultured Japanese Oyster (Magallana gigas) Treated by High Pressure Processing (HPP). Foods, 12(6), 1156. https://doi.org/10.3390/foods12061156






