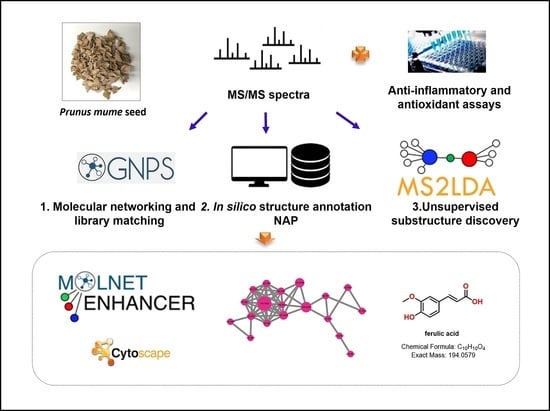
Molecular Networking-Guided Isolation of a Phenolic Constituent from Prunus mume Seed and Its Antioxidant and Anti-Inflammatory Activities
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Extraction and Isolation
2.3. DPPH Radical Scavenging Assay
2.4. ABTS Radical Scavenging Assay
2.5. Cell Culture
2.6. Cell Viability Assay
2.7. Measurement of NO Production
2.8. Western Blot Analysis
2.9. Statistical Analysis
2.10. Molecular Networking Analysis
2.10.1. Sample Preparation
2.10.2. LC-MS/MS Analysis Conditions
2.10.3. LC-MS/MS Data Analysis
MZmine 2.53 Data-Preprocessing Parameters
Feature-Based Molecular Networking
NAP
MS2LDA Substructure Discovery
MolNetEnhancer
2.11. Molecular Docking
3. Results
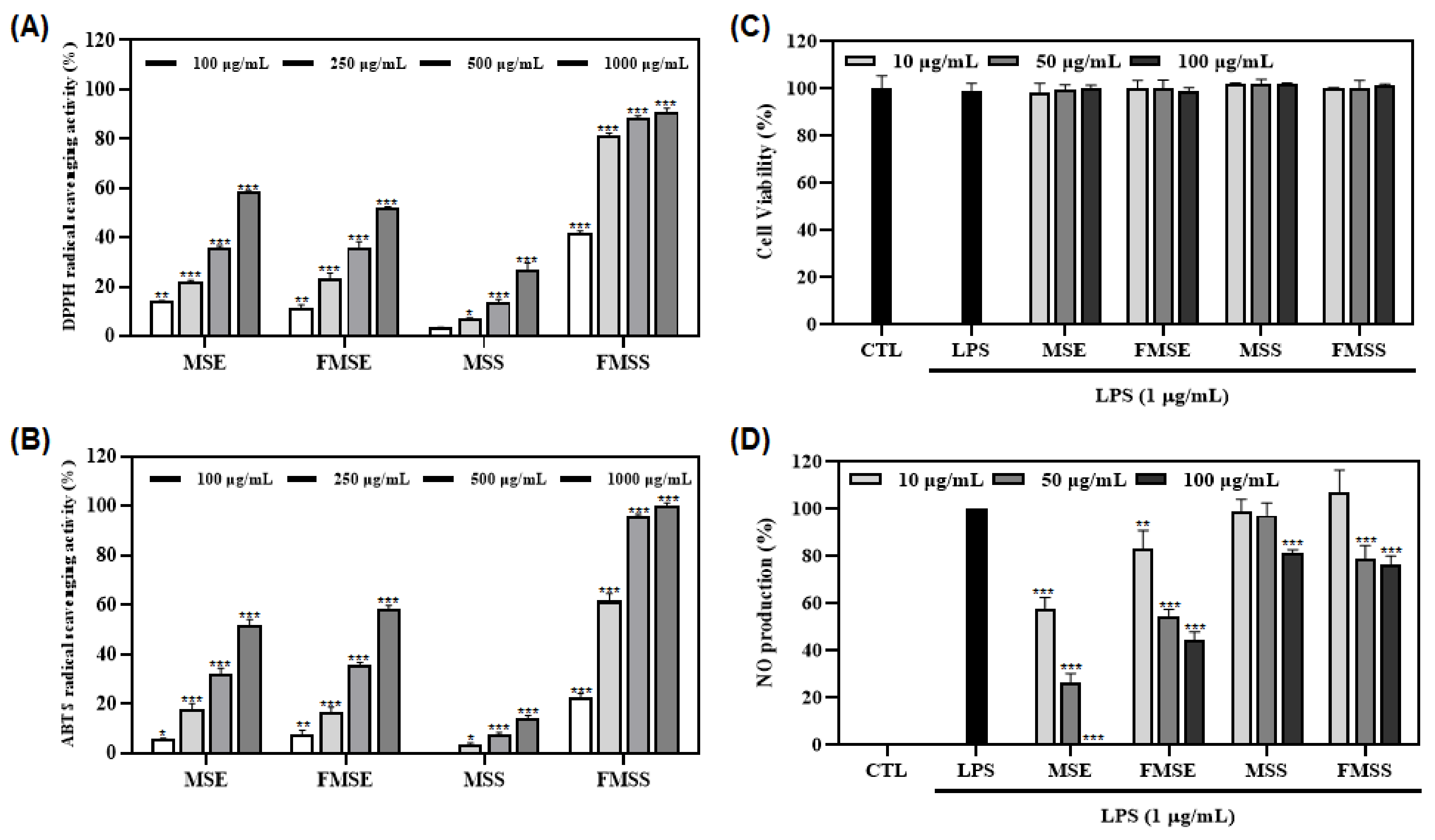
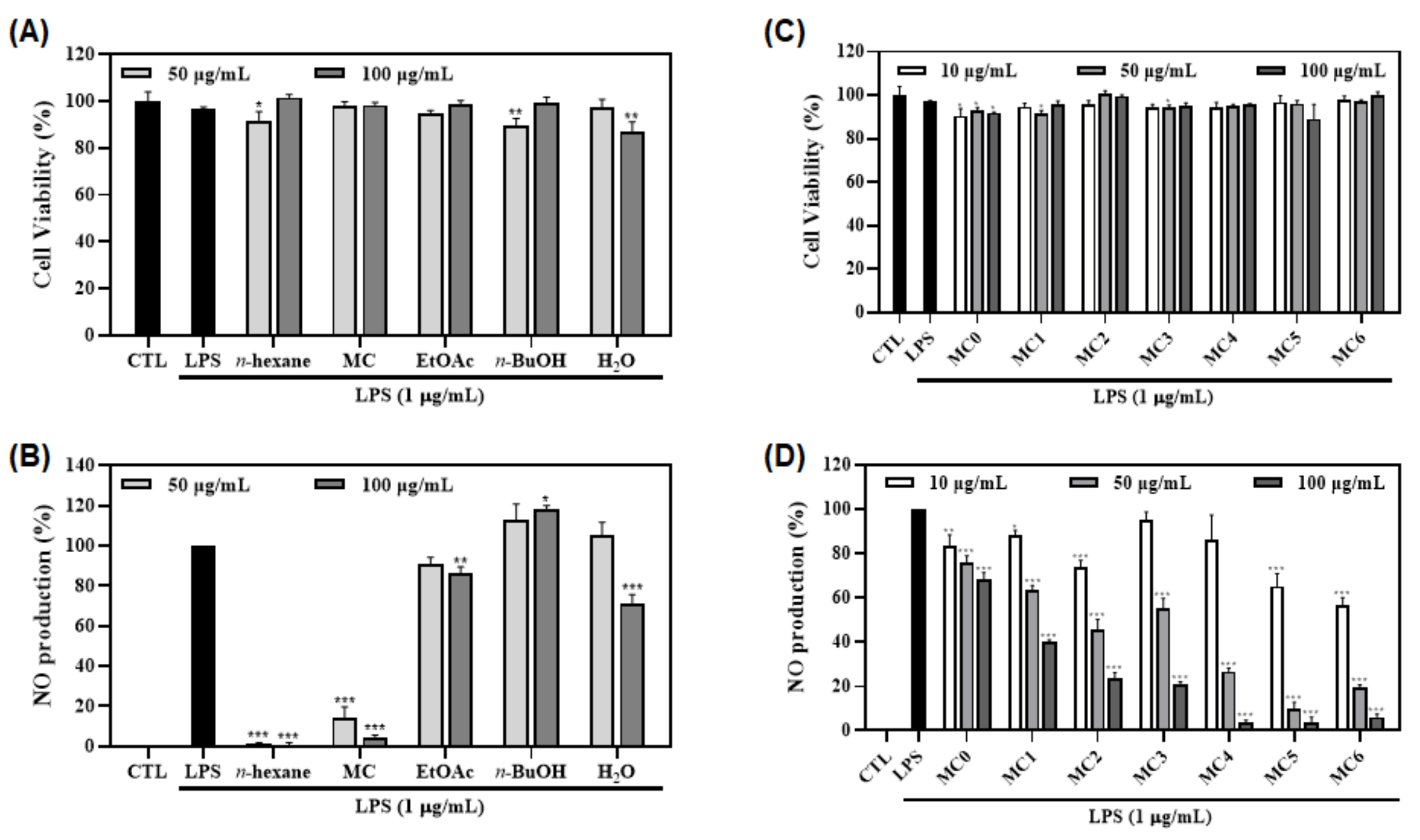
3.1. Antioxidant and Anti-Inflammatory Effects of Various P. mume Extracts
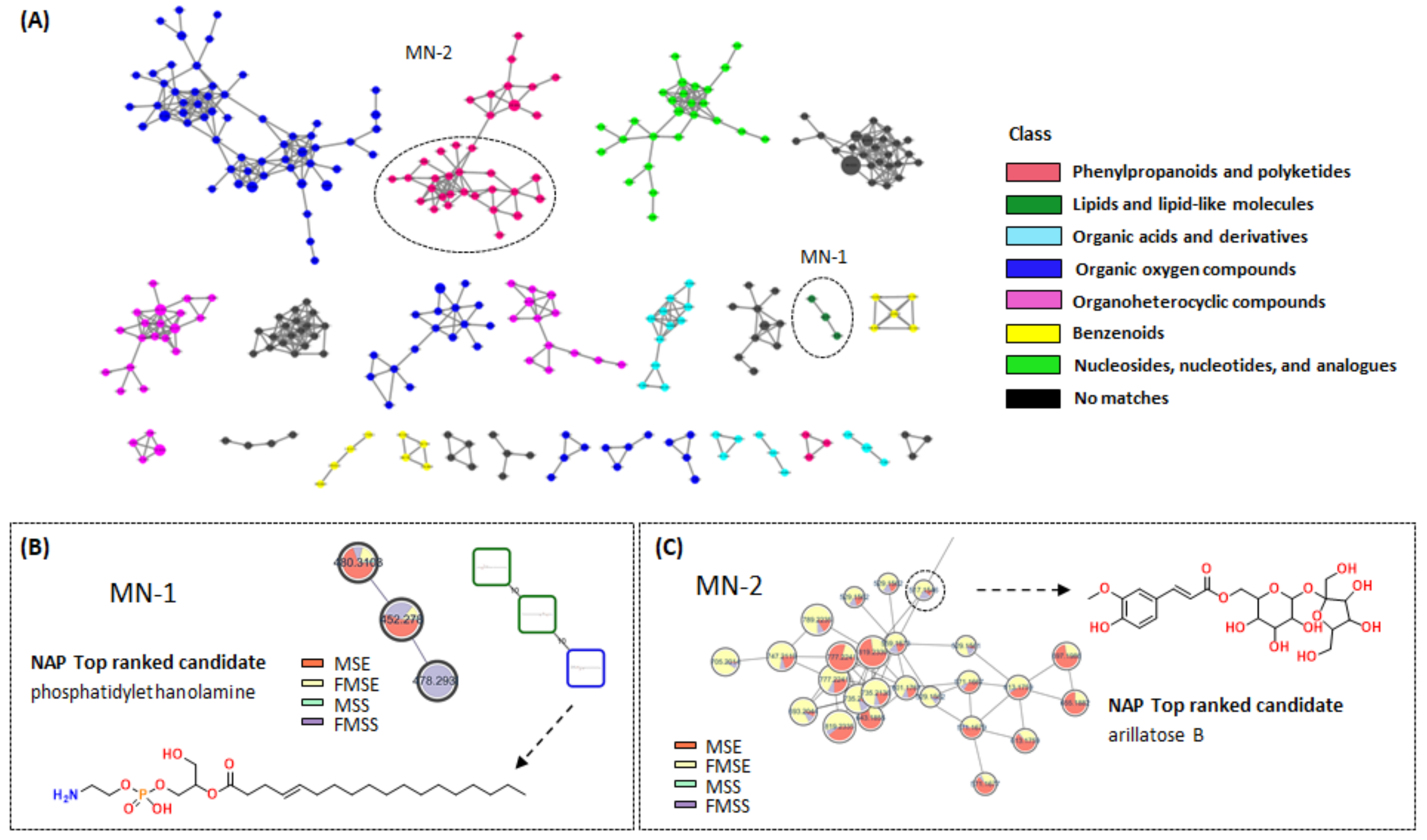
3.2. Molecular Networking Analysis of Various P. mume Extracts
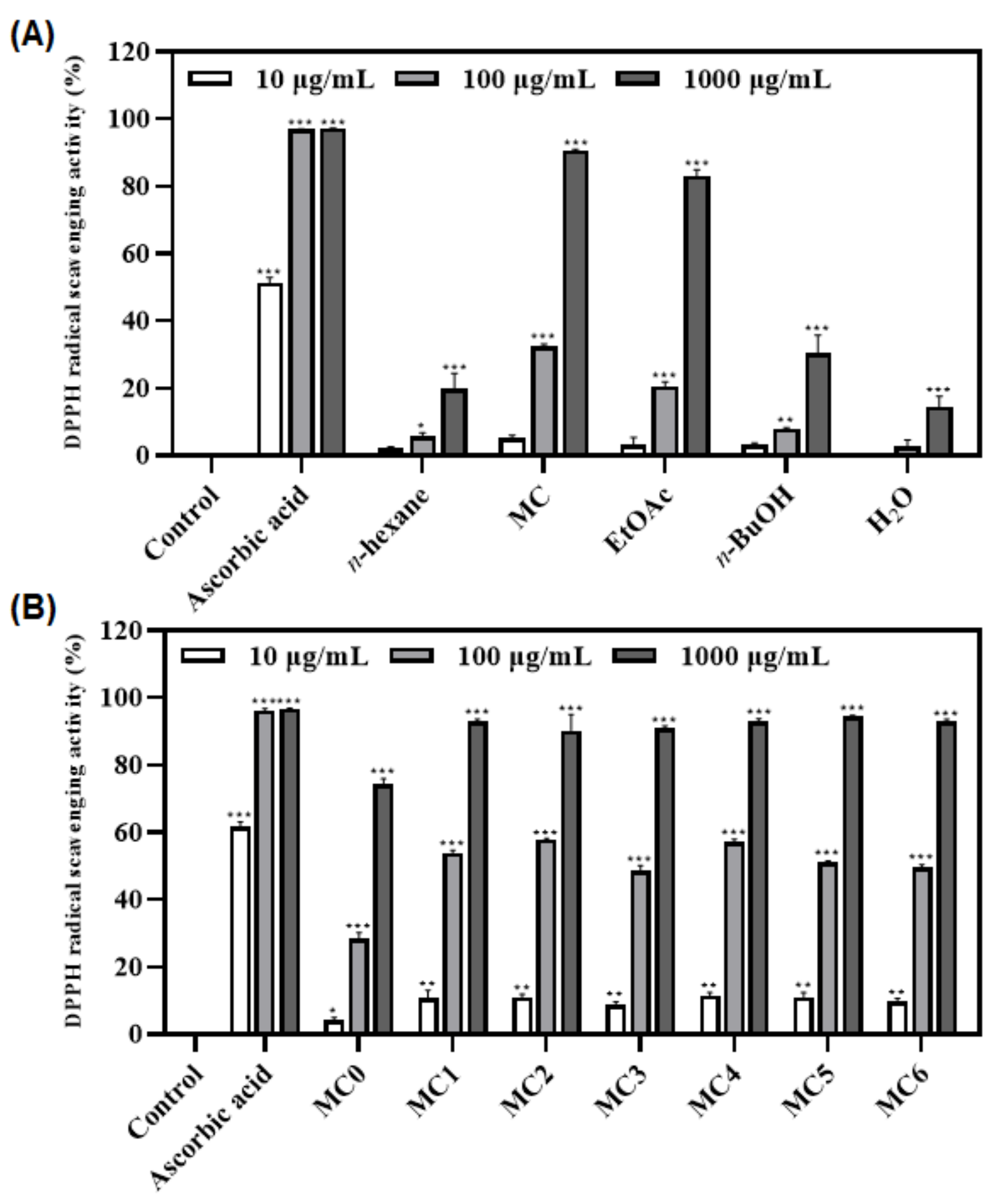
3.3. Antioxidant Activities of Fractions and Sub-Fractions of FMSE Extract
3.4. Anti-Inflammatory Activities of Fractions and Sub-Fractions of FMSE Extract
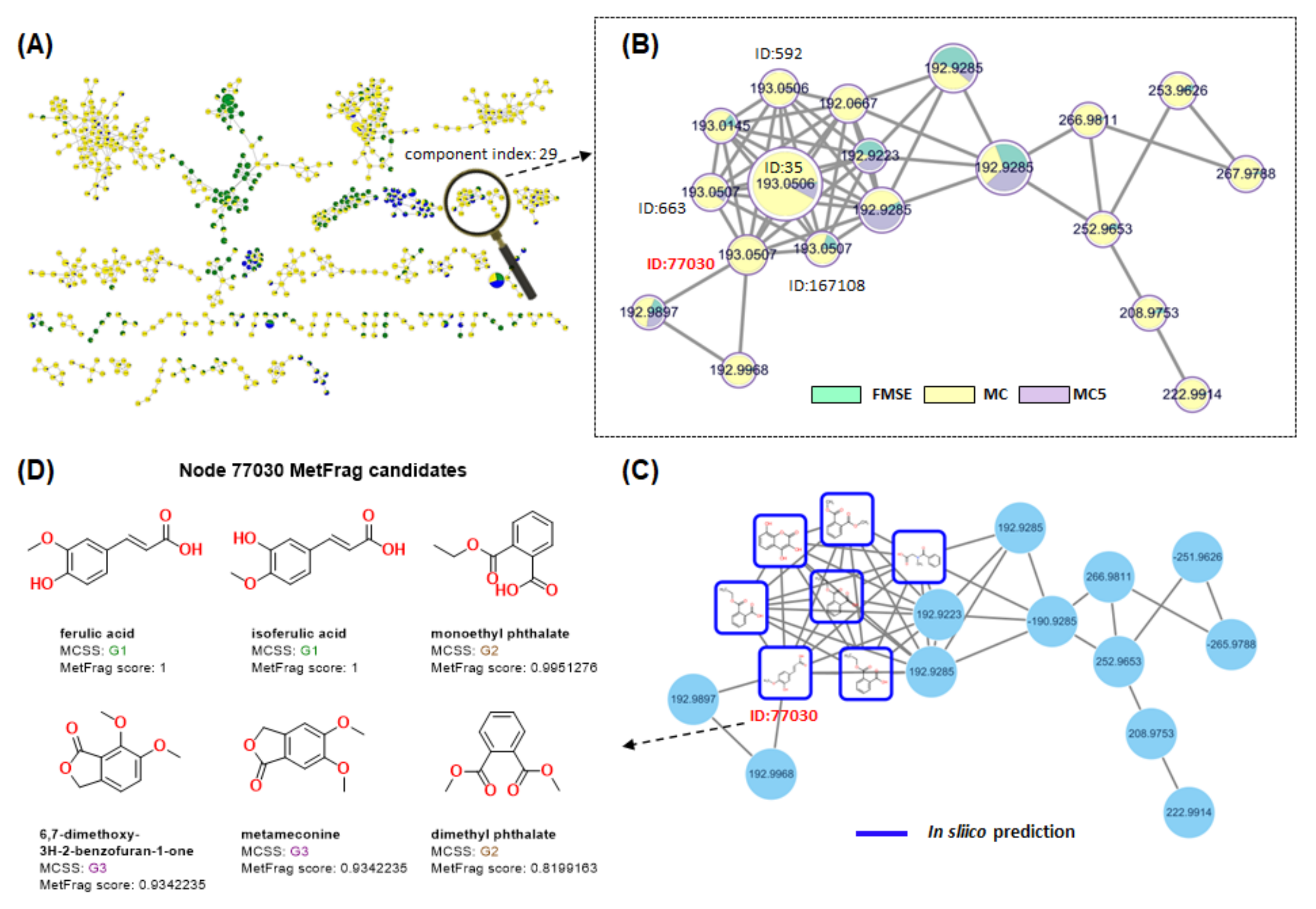
3.5. Molecular Networking Analysis of FMSE Extract, MC Fraction, and MC5 Subfraction
3.5.1. Feature-Based Molecular Networking Analysis of FMSE Extract, MC Fraction, and MC5 Subfraction
3.5.2. NAP Analysis of FMSE Extract, MC Fraction, and MC5 Subfraction
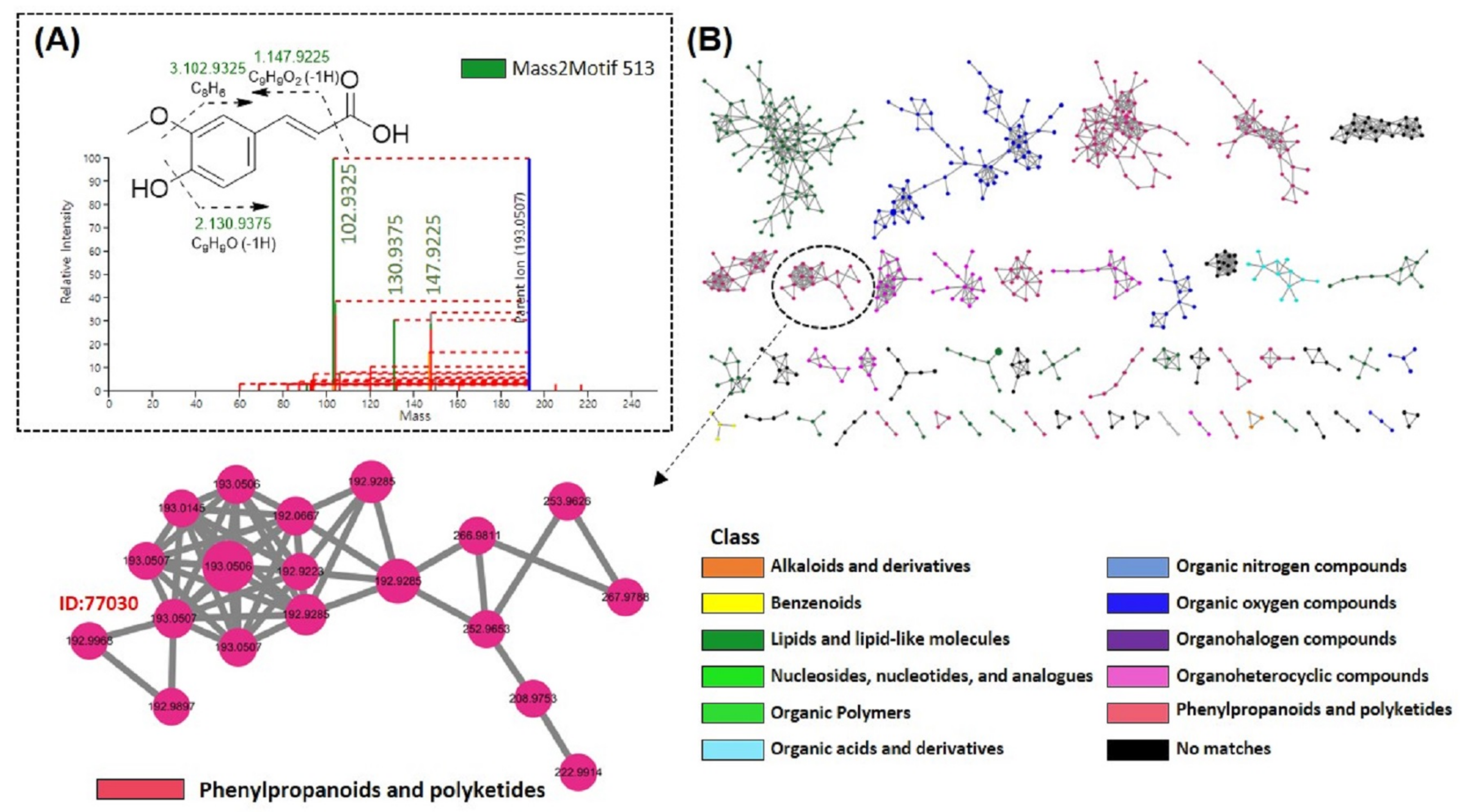
3.5.3. MS2LDA and MolNetEnhancer Analysis of FMSE Extract, MC Fraction, and MC5 Subfraction
3.6. Structure Identification of Ferulic Acid by NMR Analysis
Spectroscopic Data of Ferulic Acid
3.7. Antioxidant and Anti-Inflammatory Effects of Ferulic Acid (1)
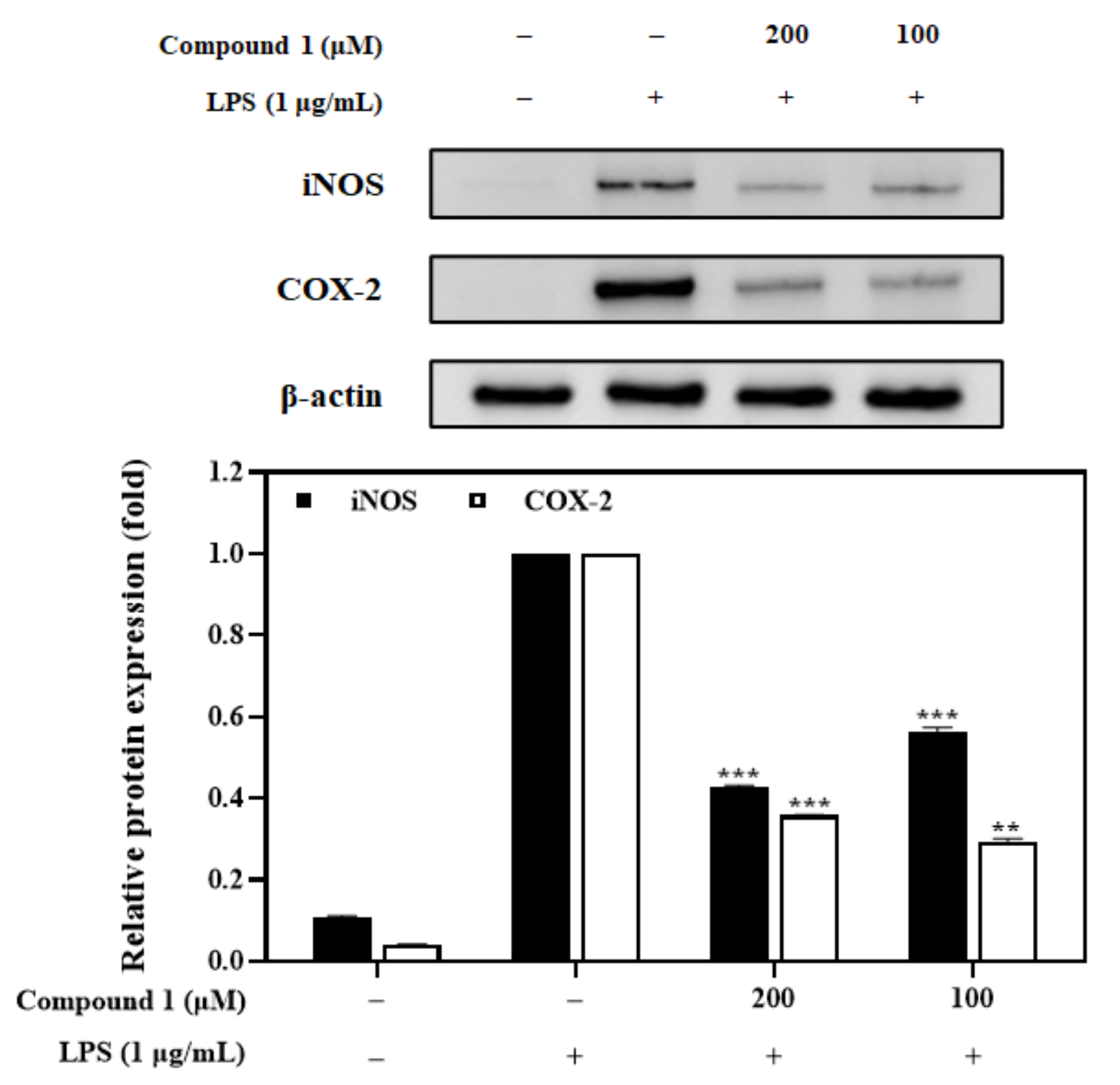
3.8. Effects of the Compound 1 on iNOS and COX-2 Expression
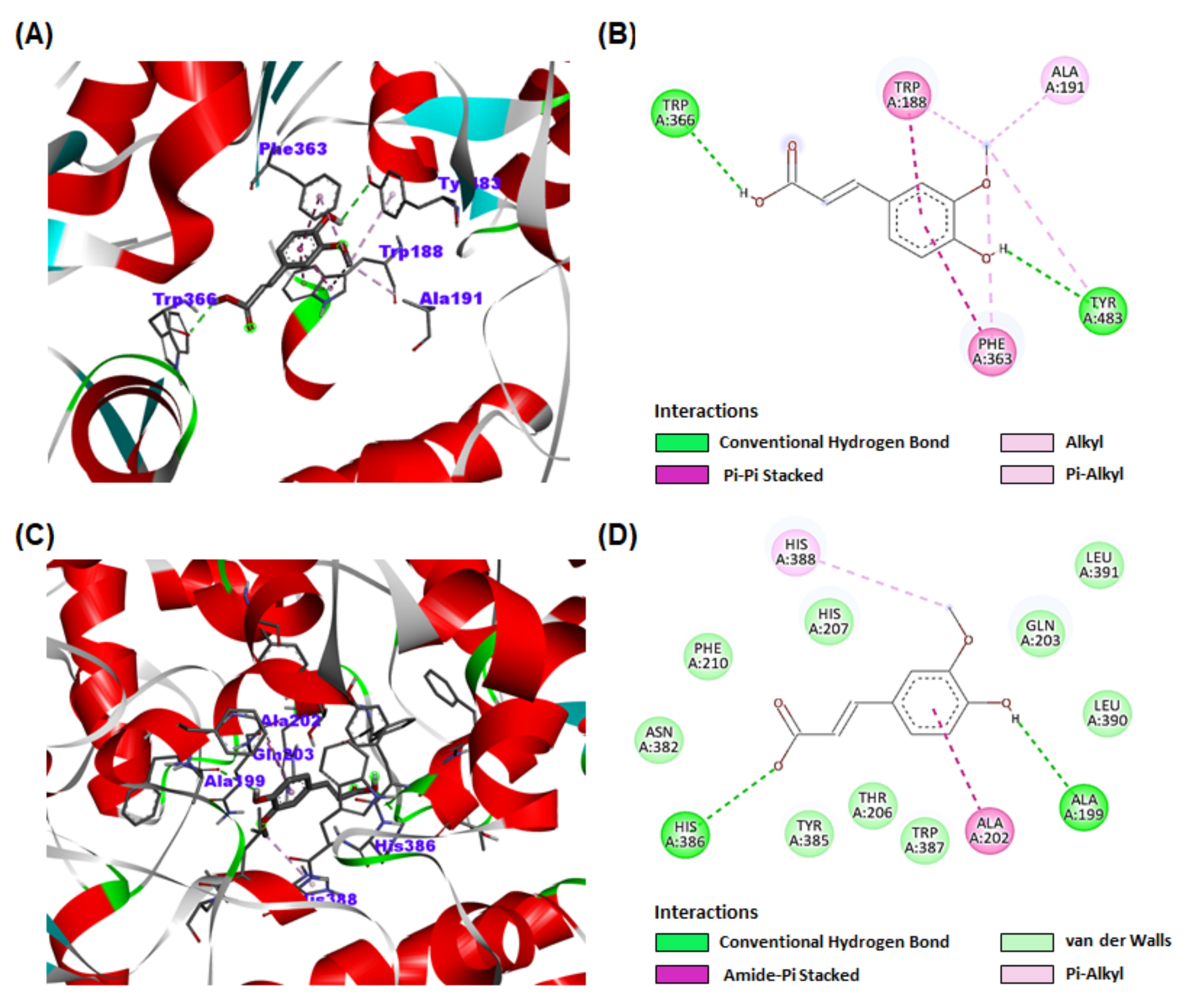
3.9. Molecular Docking
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, J.-H.; Won, Y.-S.; Cho, H.-D.; Hong, S.-M.; Moon, K.-D.; Seo, K.-I. Protective Effect of Prunus mume Fermented with Mixed Lactic Acid Bacteria in Dextran Sodium Sulfate-Induced Colitis. Foods 2021, 10, 58. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.P.; Tang, Y.; Song, Y.Y.; Du, G.; Li, J. Comprehensive Review of Phytochemical Constituents, Pharmacological Properties, and Clinical Applications of Prunus mume. Front. Pharmacol. 2021, 12, 679378. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.J.; Hur, H.J.; Sung, M.J.; Park, J.H.; Yang, H.J.; Kim, M.S.; Kwon, D.Y.; Hwang, J.T. Ethanol extract of the Prunus mume fruits stimulates glucose uptake by regulating PPAR-γ in C2C12 myotubes and ameliorates glucose intolerance and fat accumulation in mice fed a high-fat diet. Food Chem. 2013, 141, 4115–4121. [Google Scholar] [CrossRef] [PubMed]
- Jang, A.J.; Lee, J.H.; Yotsu-Yamashita, M.; Park, J.; Kye, S.; Benza, R.L.; Passineau, M.J.; Jeon, Y.J.; Nyunoya, T. A Novel Compound, “FA-1” Isolated from Prunus mume, Protects Human Bronchial Epithelial Cells and Keratinocytes from Cigarette Smoke Extract-Induced Damage. Sci. Rep. 2018, 8, 11504. [Google Scholar] [CrossRef]
- Beretta, A.; Accinni, R.; Dellanoce, C.; Tonini, A.; Cardot, J.M.; Bussière, A. Efficacy of a Standardized Extract of Prunus mume in Liver Protection and Redox Homeostasis: A Randomized, Double-Blind, Placebo-Controlled Study. Phytother. Res. 2016, 30, 949–955. [Google Scholar] [CrossRef]
- Mitani, T.; Ota, K.; Inaba, N.; Kishida, K.; Koyama, H.A. Antimicrobial Activity of the Phenolic Compounds of Prunus mume against Enterobacteria. Biol. Pharm. Bull. 2018, 41, 208–212. [Google Scholar] [CrossRef]
- Yan, X.T.; Li, W.; Sun, Y.N.; Yang, S.Y.; Lee, S.H.; Chen, J.B.; Jang, H.D.; Kim, Y.H. Identification and biological evaluation of flavonoids from the fruits of Prunus mume. Bioorg. Med. Chem. Lett. 2014, 24, 1397–1402. [Google Scholar] [CrossRef]
- Bailly, C. Anticancer properties of Prunus mume extracts (Chinese plum, Japanese apricot). J. Ethnopharmacol. 2020, 246, 112215. [Google Scholar] [CrossRef]
- Mitani, T.; Horinishi, A.; Kishida, K.; Kawabata, T.; Yano, F.; Mimura, H.; Inaba, N.; Yamanishi, H.; Oe, T.; Negoro, K.; et al. Phenolics profile of mume, Japanese apricot (Prunus mume Sieb. et Zucc.) fruit. Biosci. Biotechnol. Biochem. 2013, 77, 1623–1627. [Google Scholar] [CrossRef]
- Fox Ramos, A.E.; Evanno, L.; Poupon, E.; Champy, P.; Beniddir, M.A. Natural products targeting strategies involving molecular networking: Different manners, one goal. Nat. Prod. Rep. 2019, 36, 960–980. [Google Scholar] [CrossRef]
- Nothias, L.F.; Petras, D.; Schmid, R.; Dührkop, K.; Rainer, J.; Sarvepalli, A.; Protsyuk, I.; Ernst, M.; Tsugawa, H.; Fleischauer, M.; et al. Feature-based molecular networking in the GNPS analysis environment. Nat. Methods 2020, 17, 905–908. [Google Scholar] [CrossRef]
- da Silva, R.R.; Wang, M.; Nothias, L.F.; van der Hooft, J.J.J.; Caraballo-Rodríguez, A.M.; Fox, E.; Balunas, M.J.; Klassen, J.L.; Lopes, N.P.; Dorrestein, P.C. Propagating annotations of molecular networks using in silico fragmentation. PLoS Comput. Biol. 2018, 14, e1006089. [Google Scholar] [CrossRef]
- van der Hooft, J.J.J.; Wandy, J.; Barrett, M.P.; Burgess, K.E.; Rogers, S. Topic modeling for untargeted substructure exploration in metabolomics. Proc. Natl. Acad. Sci. USA 2016, 113, 13738–13743. [Google Scholar] [CrossRef]
- Rogers, S.; Ong, C.W.; Wandy, J.; Ernst, M.; Ridder, L.; van der Hooft, J.J.J. Deciphering complex metabolite mixtures by unsupervised and supervised substructure discovery and semi-automated annotation from MS/MS spectra. Faraday Discuss. 2019, 218, 284–302. [Google Scholar] [CrossRef]
- Ernst, M.; Kang, K.B.; Caraballo-Rodríguez, A.M.; Nothias, L.F.; Wandy, J.; Chen, C.; Wang, M.; Rogers, S.; Medema, M.H.; Dorrestein, P.C.; et al. MolNetEnhancer: Enhanced Molecular Networks by Integrating Metabolome Mining and Annotation Tools. Metabolites 2019, 9, 144. [Google Scholar] [CrossRef]
- Kang, K.B.; Woo, S.; Ernst, M.; van der Hooft, J.J.J.; Nothias, L.F.; da Silva, R.R.; Dorrestein, P.C.; Sung, S.H.; Lee, M. Assessing specialized metabolite diversity of Alnus species by a digitized LC-MS/MS data analysis workflow. Phytochemistry 2020, 173, 112292. [Google Scholar] [CrossRef]
- Choi, S.Y.; Park, J.; Kim, J.; Lee, J.; Yang, H. Investigation of Chemical Profiles of Different Parts of Morus alba Using a Combination of Molecular Networking Methods with Mass Spectral Data from Two Ionization Modes of LC/MS. Plants 2021, 10, 1711. [Google Scholar] [CrossRef]
- Kim, J.G.; Le, T.P.L.; Han, J.S.; Cho, Y.B.; Lee, D.; Lee, M.K.; Hwang, B.Y. Molecular Networking-Guided Isolation of Cycloartane-type Triterpenoids from Curculigo orchioides and Their Inhibitory Effect on Nitric Oxide Production. ACS Omega 2022, 7, 26853–26862. [Google Scholar] [CrossRef]
- Di Ottavio, F.; Gauglitz, J.M.; Ernst, M.; Panitchpakdi, M.W.; Fanti, F.; Compagnone, D.; Dorrestein, P.C.; Sergi, M. A UHPLC-HRMS based metabolomics and chemoinformatics approach to chemically distinguish ‘super foods’ from a variety of plant-based foods. Food Chem. 2020, 313, 126071. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Yan, H. In situ net fishing of α-glucosidase inhibitors from evening primrose (Oenothera biennis) defatted seeds by combination of LC-MS/MS, molecular networking, affinity-based ultrafiltration, and molecular docking. Food Funct. 2022, 13, 2545–2558. [Google Scholar] [CrossRef]
- Abdel Shakour, Z.T.; El-Akad, R.H.; Elshamy, A.I.; El Gendy, A.E.G.; Wessjohann, L.A.; Farag, M.A. Dissection of Moringa oleifera leaf metabolome in context of its different extracts, origin and in relationship to its biological effects as analysed using molecular networking and chemometrics. Food Chem. 2023, 399, 133948. [Google Scholar] [CrossRef] [PubMed]
- Ruttkies, C.; Neumann, S.; Posch, S. Improving MetFrag with statistical learning of fragment annotations. BMC Bioinform. 2019, 20, 376. [Google Scholar] [CrossRef] [PubMed]
- Shim, S.Y.; Lee, Y.E.; Song, H.Y.; Lee, M. p-Hydroxybenzoic Acid β-d-Glucosyl Ester and Cimidahurinine with Antimelanogenesis and Antioxidant Effects from Pyracantha angustifolia via Bioactivity-Guided Fractionation. Antioxidants 2020, 9, 258. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.K.; Ahn, J.; Yu, J.; Le, D.; Han, S.; Lee, M. Analysis of Antioxidant Constituents from Ilex rotunda and Evaluation of Their Blood-Brain Barrier Permeability. Antioxidants 2022, 11, 1989. [Google Scholar] [CrossRef] [PubMed]
- Pluskal, T.; Castillo, S.; Villar-Briones, A.; Oresic, M. MZmine 2: Modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinform. 2010, 11, 395. [Google Scholar] [CrossRef]
- Wang, M.; Carver, J.J.; Phelan, V.; Sanchez, L.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Wandy, J.; Zhu, Y.; van der Hooft, J.J.J.; Daly, R.; Barrett, M.P.; Rogers, S. Ms2lda.org: Web-based topic modelling for substructure discovery in mass spectrometry. Bioinformatics 2018, 34, 317–318. [Google Scholar] [CrossRef]
- Müller-Maatsch, J.; Caligiani, A.; Tedeschi, T.; Elst, K.; Sforza, S. Simple and validated quantitative ¹H NMR method for the determination of methylation, acetylation, and feruloylation degree of pectin. J. Agric. Food Chem. 2014, 62, 9081–9087. [Google Scholar] [CrossRef]
- Aron, A.T.; Gentry, E.C.; McPhail, K.L.; Nothias, L.F.; Nothias-Esposito, M.; Bouslimani, A.; Petras, D.; Gauglitz, J.M.; Sikora, N.; Vargas, F.; et al. Reproducible molecular networking of untargeted mass spectrometry data using GNPS. Nat. Protoc. 2020, 5, 1954–1991. [Google Scholar] [CrossRef]
- Astiti, M.A.; Jittmittraphap, A.; Leaungwutiwong, P.; Chutiwitoonchai, N.; Pripdeevech, P.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. LC-QTOF-MS/MS Based Molecular Networking Approach for the Isolation of α-Glucosidase Inhibitors and Virucidal Agents from Coccinia grandis (L.) Voigt. Foods 2021, 10, 3041. [Google Scholar] [CrossRef]
- Tinte, M.M.; Masike, K.; Steenkamp, P.A.; Huyser, J.; van der Hooft, J.J.J.; Tugizimana, F. Computational Metabolomics Tools Reveal Metabolic Reconfigurations Underlying the Effects of Biostimulant Seaweed Extracts on Maize Plants under Drought Stress Conditions. Metabolites 2022, 12, 487. [Google Scholar] [CrossRef]
- Lee, H.-R.; Park, Y.; Kim, H.J.; Lee, A.; Choi, J.; Pyee, J.; Park, H.; Kim, J. Extract from Prunus mume Sieb. et Zucc. Fruit Prevents LPS-induced Homotypic Aggregation of Monocytic THP-1 Cells via Suppression of Nitric Oxide Production and NF-κB Activation. J. Life Sci. 2015, 25, 801–809. [Google Scholar] [CrossRef]
- Xia, D.; Shi, J.; Gong, J.; Wu, X.; Yang, Q.; Zhang, Y. Antioxidant activity of Chinese mei (Prunus mume) and its active phytochemicals. J. Med. Plant Res. 2010, 4, 1156–1160. [Google Scholar]
- Seo, J.I.; Yu, J.S.; Lee, E.K.; Park, K.B.; Yoo, H.H. Molecular networking-guided strategy for the pharmacokinetic study of herbal medicines: Cudrania tricuspidata leaf extracts. Biomed. Pharmacother. 2022, 149, 112895. [Google Scholar] [CrossRef]
- Xie, H.F.; Kong, Y.S.; Li, R.Z.; Nothias, L.F.; Melnik, A.V.; Zhang, H.; Liu, L.L.; An, T.T.; Liu, R.; Yang, Z.; et al. Feature-Based Molecular Networking Analysis of the Metabolites Produced by In Vitro Solid-State Fermentation Reveals Pathways for the Bioconversion of Epigallocatechin Gallate. J. Agric. Food Chem. 2020, 68, 7995–8007. [Google Scholar] [CrossRef]
- Dong, S.H.; Duan, Z.K.; Ai, Y.F.; Zhou, X.F.; Zhang, X.; Lian, M.Y.; Huang, X.X.; Bai, M.; Song, S.J. Guaiane-type sesquiterpenoids with various ring skeletons from Daphne bholua uncovered by molecular networking and structural revisions of previously reported analogues. Bioorg. Chem. 2022, 129, 106208. [Google Scholar] [CrossRef]
- Cauchie, G.; N’Nang, E.O.; van der Hooft, J.J.J.; Le Pogam, P.; Bernadat, G.; Gallard, J.F.; Kumulungui, B.; Champy, P.; Poupon, E.; Beniddir, M.A. Phenylpropane as an Alternative Dearomatizing Unit of Indoles: Discovery of Inaequalisines A and B Using Substructure-Informed Molecular Networking. Org. Lett. 2020, 22, 6077–6081. [Google Scholar] [CrossRef]
- Kim, N.-H.; Park, H.-J.; Lee, E.-H.; Cho, E.-B.; Kang, I.-K.; Cho, Y.-J. The inflammatory activity of purified-ferulic acid from Tetragonia tetragonioides. J. Appl. Biol. Chem. 2019, 62, 239–246. [Google Scholar] [CrossRef]
- Xu, W.; Wang, X.-B.; Wang, Z.-M.; Wu, J.-J.; Li, F.; Wang, J.; Kong, L.-Y. Synthesis and evaluation of donepezil–ferulic acid hybrids as multi-target-directed ligands against Alzheimer’s disease. Med. Chem. Commun. 2016, 7, 990. [Google Scholar] [CrossRef]
- Zduńska, K.; Dana, A.; Kolodziejczak, A.; Rotsztejn, H. Antioxidant Properties of Ferulic Acid and Its Possible Application. Skin Pharmacol. Physiol. 2018, 31, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Alam, A. Anti-hypertensive Effect of Cereal Antioxidant Ferulic Acid and Its Mechanism of Action. Front. Nutr. 2019, 6, 121. [Google Scholar] [CrossRef] [PubMed]
- Caesar, L.K.; Kellogg, J.J.; Kvalheim, O.M.; Cech, R.A.; Cech, N.B. Integration of Biochemometrics and Molecular Networking to Identify Antimicrobials in Angelica keiskei. Planta Med. 2018, 84, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.F.; Zhang, X.; Zhu, F.; Huo, Z.Q.; Yao, Q.Q.; Feng, Q.; Liu, Z.; Zhang, G.M.; Yao, J.C.; Liang, H.B. MS/MS-Based Molecular Networking: An Efficient Approach for Natural Products Dereplication. Molecules 2022, 28, 157. [Google Scholar] [CrossRef]

| Abbreviation | Definition | Abbreviation | Definition |
|---|---|---|---|
| P. mume | Prunus mume | FMSE | Fermented Maesil Seed Extract |
| GNPS | Global Natural Products Social | MSE | Maesil Seed Extract |
| MN | Molecular Networking | MSS | Maesil Seed Shell extract without oil |
| MS2LDA | Mass2Motifs LDA parameter | FMSS | Fermented Maesil Seed Shell extract without oil |
| MolNetEnhancer | Enhanced Molecular Networks | UPLC | Ultra Performance Liquid Chromatography |
| NAP | Network Annotation Propagation | HMDB | Human Metabolome Database |
| LPS | Lipopolysaccharide | PDB | Protein Data Bank |
| DPPH | 1,1-diphenyl-β-picrylhydrazine | MCSS | Maximum Common Substructure |
| ABTS | 3-ethylbenzothiazoline-6-sulfonic aciddiammonium salt | COX-2 | Cyclooxygenase-2 |
| NO | Nitric Oxide | iNOS | Inducible Nitric Oxide Synthase |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, C.-K.; Yu, J.; Lee, M. Molecular Networking-Guided Isolation of a Phenolic Constituent from Prunus mume Seed and Its Antioxidant and Anti-Inflammatory Activities. Foods 2023, 12, 1146. https://doi.org/10.3390/foods12061146
Kim C-K, Yu J, Lee M. Molecular Networking-Guided Isolation of a Phenolic Constituent from Prunus mume Seed and Its Antioxidant and Anti-Inflammatory Activities. Foods. 2023; 12(6):1146. https://doi.org/10.3390/foods12061146
Chicago/Turabian StyleKim, Chang-Kwon, Jayeon Yu, and Mina Lee. 2023. "Molecular Networking-Guided Isolation of a Phenolic Constituent from Prunus mume Seed and Its Antioxidant and Anti-Inflammatory Activities" Foods 12, no. 6: 1146. https://doi.org/10.3390/foods12061146
APA StyleKim, C.-K., Yu, J., & Lee, M. (2023). Molecular Networking-Guided Isolation of a Phenolic Constituent from Prunus mume Seed and Its Antioxidant and Anti-Inflammatory Activities. Foods, 12(6), 1146. https://doi.org/10.3390/foods12061146