From Foxtail Millet Husk (Waste) to Bioactive Phenolic Extracts Using Deep Eutectic Solvent Extraction and Evaluation of Antioxidant, Acetylcholinesterase, and α-Glucosidase Inhibitory Activities
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material and Reagents
2.2. Preparation of DES
2.3. Extractions of Phenolic Compounds
2.4. Experimental Design of Optimizing the Extraction Process
2.4.1. Single-Factor Experiments
2.4.2. Response Surface Methodology (RSM)
2.5. Determination of TPC and TFC
2.6. Measurement of Antioxidant Activity
2.6.1. DPPH Radical Scavenging Activity
2.6.2. ABTS+ Scavenging Activity
2.6.3. Ferric Reducing Antioxidant Power (FRAP)
2.7. Inhibition of Acetylcholinesterase (AChE) Activity
2.8. Inhibition of α-Glucosidase Activity
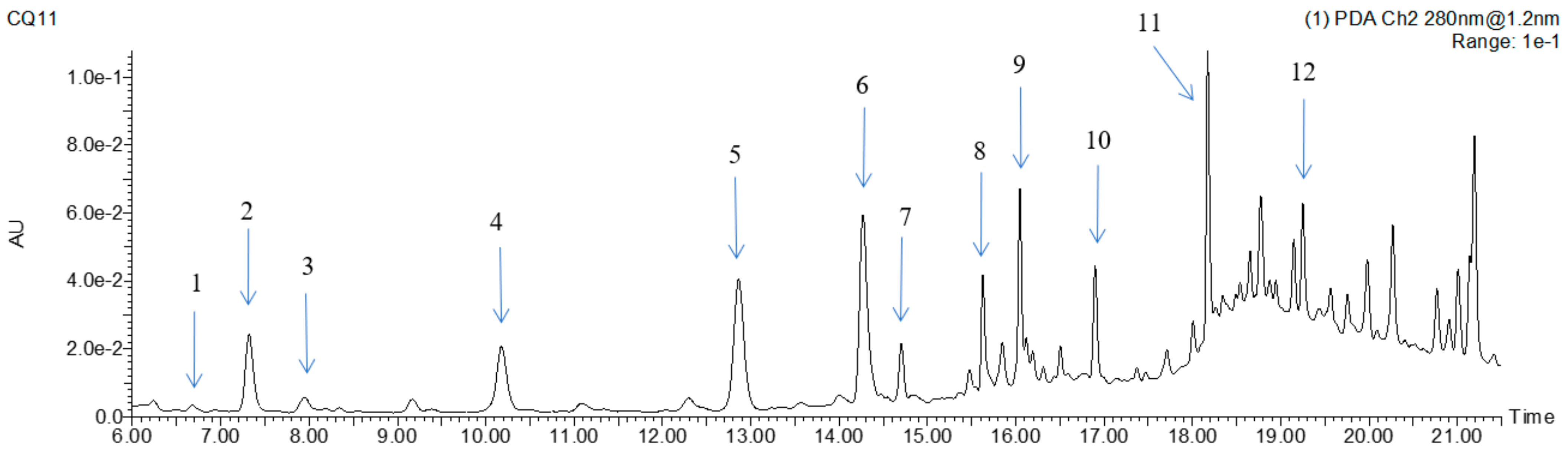
2.9. Identification and Quantification of Phenols by UPLC–MS/MS
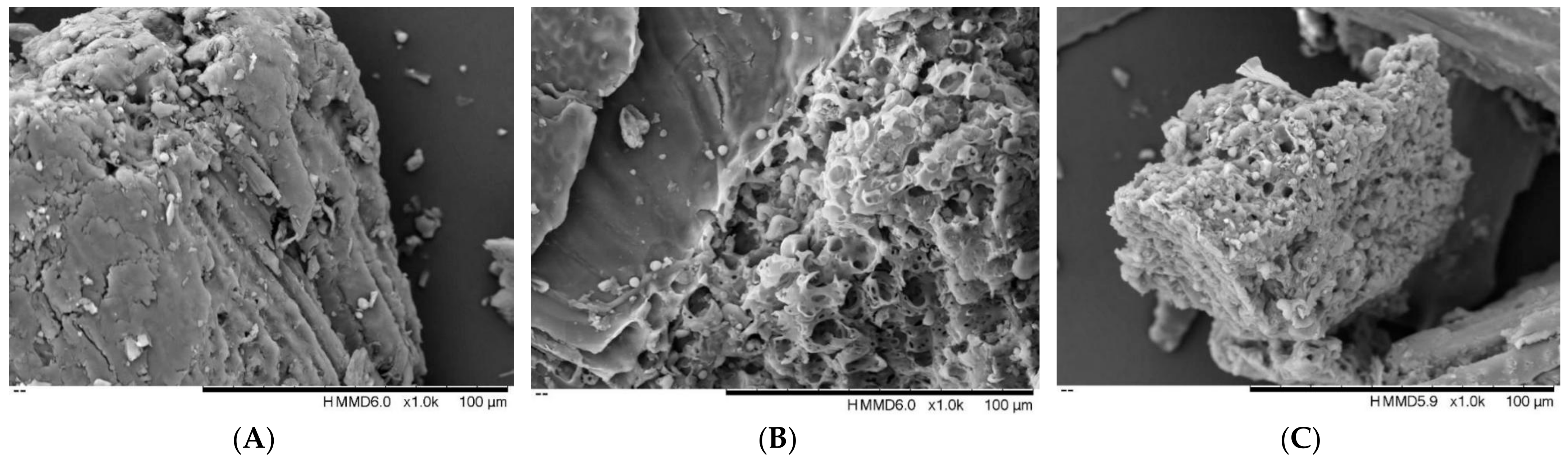
2.10. Surface Morphology Analysis
2.11. Statistical Analysis
3. Results
3.1. Selection of DES
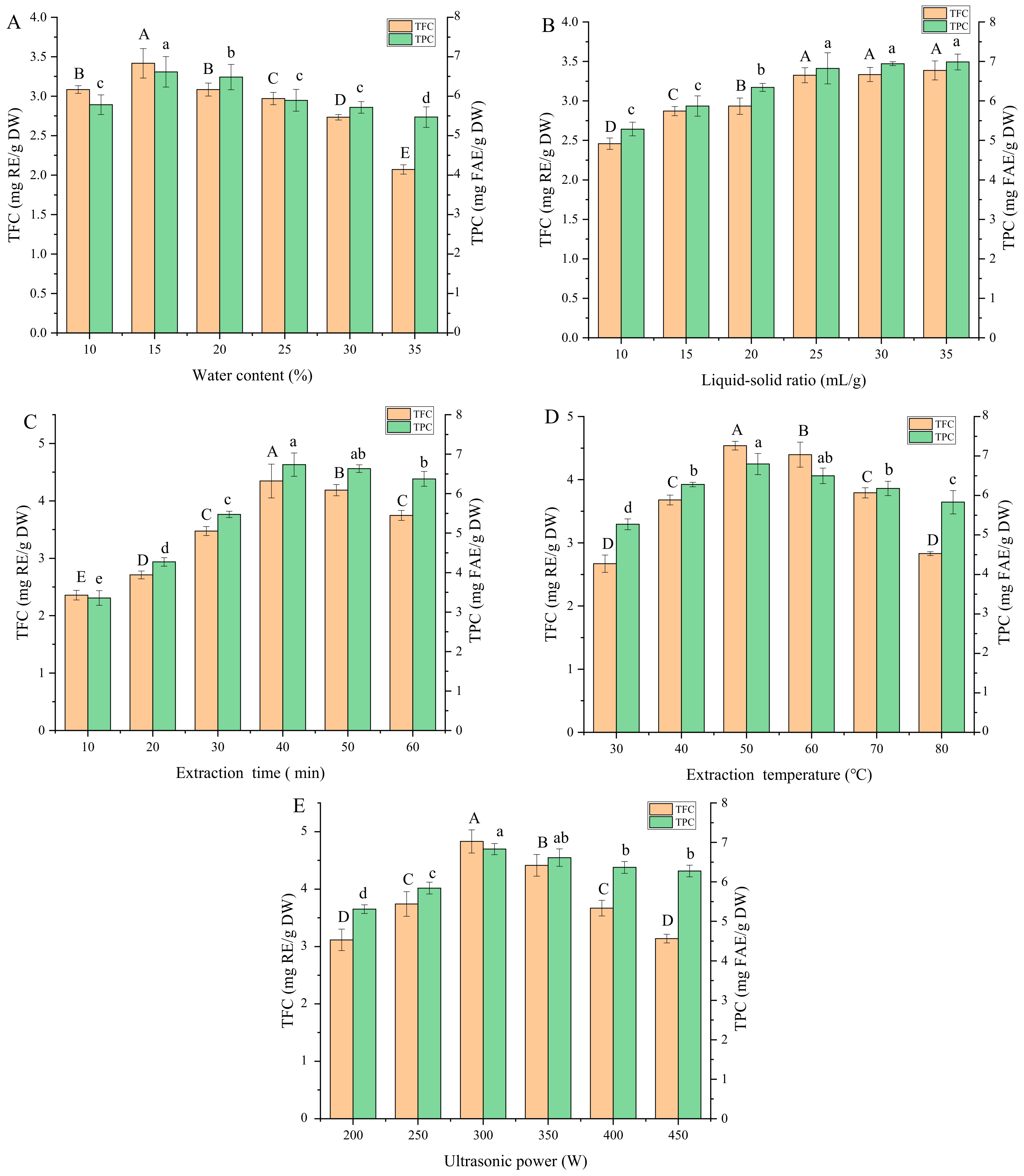
3.2. Results of Single-Factor Experiments
3.2.1. Water Content of DES
3.2.2. Liquid-to-Solid Ratio
3.2.3. Extraction Time
3.2.4. Extraction Temperature
3.2.5. Ultrasonic Power
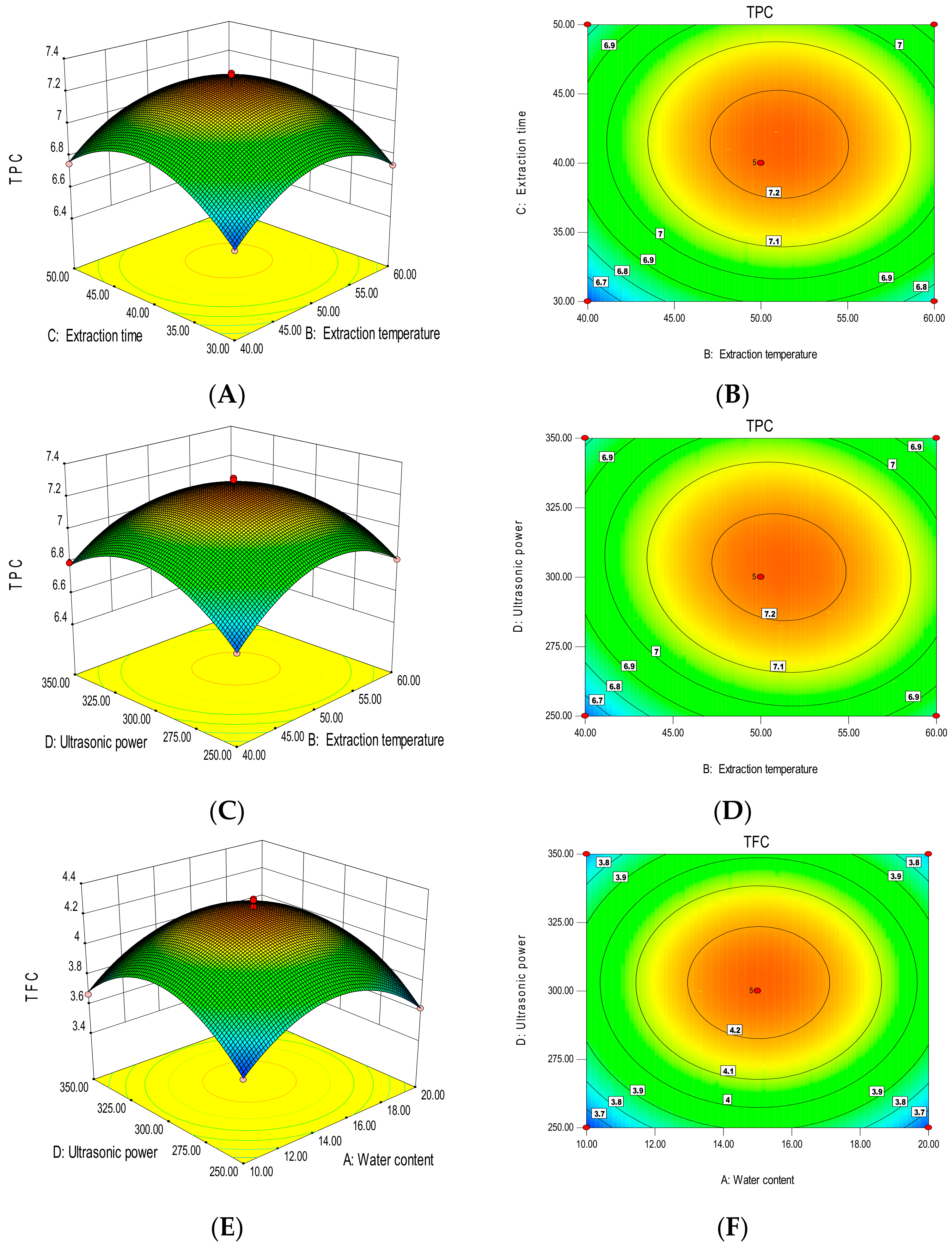
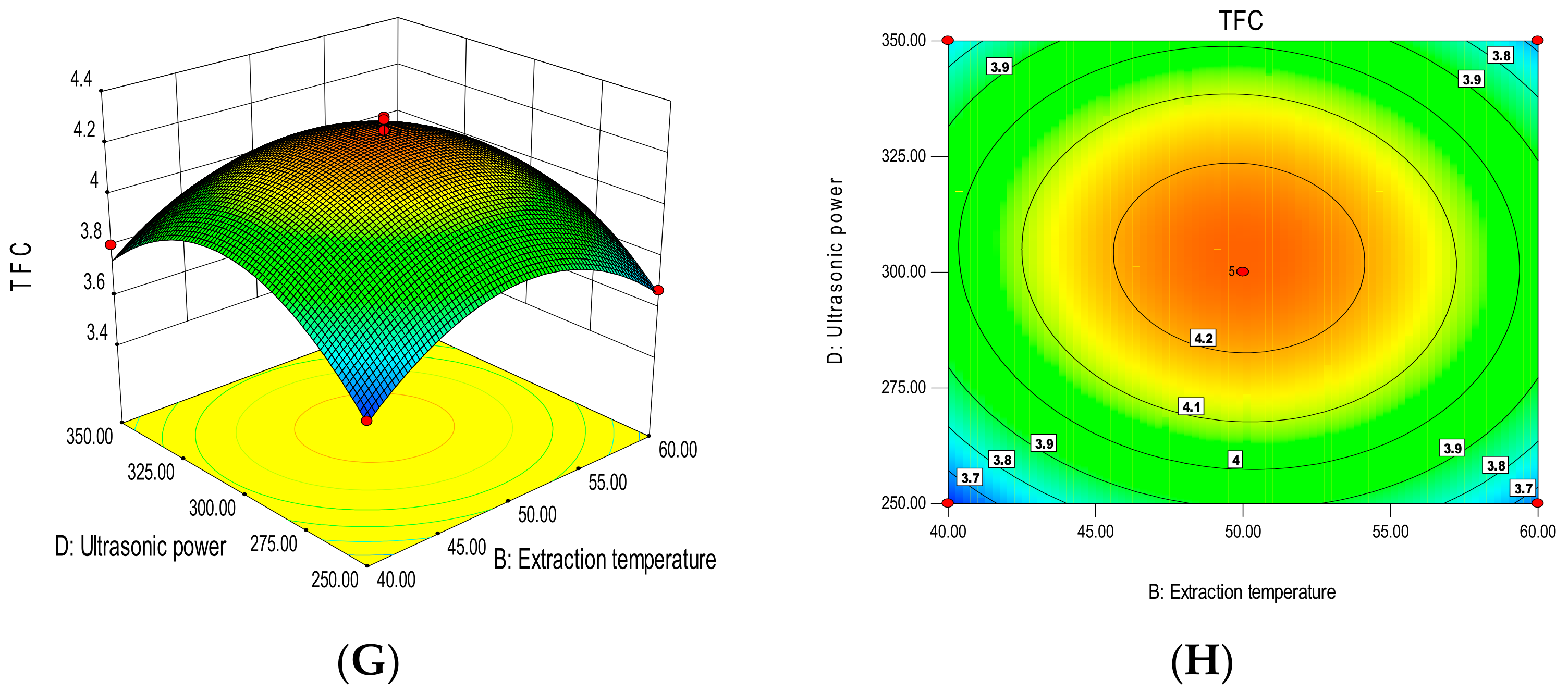
3.3. Fitting the Model
3.3.1. Total Polyphenol Content
3.3.2. Total Flavonoid Content
3.3.3. Experimental Validation of the Model
3.4. TPC, TFC, and Biological Activities In Vitro
3.5. Identification and Quantification of Phenolic Components
3.6. Scanning Electron Microscope Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABTS | 2,2-azinobis-(3-ethylbenzothiazoline-6-sulfonate) |
| AChE | acetylcholinesterase |
| DESs | deep eutectic solvents |
| DPPH | 2,2′-diphenyl-1-picrylhydrazyl |
| FAE | ferulic acid equivalents |
| FMH | foxtail millet husk |
| RE | rutin equivalents |
| TE | Trolox equivalents |
| TFC | total flavonoid content |
| TPC | total phenolic content |
| TPTZ | 2,4,6-tris(2-pyridyl)-s-triazine |
| Trolox | 6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid |
| UAE | ultrasonic-assisted extraction |
References
- Panzella, L.; Moccia, F.; Nasti, R.; Marzorati, S.; Verotta, L.; Napolitano, A. Bioactive Phenolic Compounds From Agri-Food Wastes: An Update on Green and Sustainable Extraction Methodologies. Front. Nutr. 2020, 7, 60. [Google Scholar] [CrossRef]
- Banerjee, J.; Singh, R.; Vijayaraghavan, R.; MacFarlane, D.; Patti, A.F.; Arora, A. Bioactives from fruit processing wastes: Green approaches to valuable chemicals. Food Chem. 2017, 225, 10–22. [Google Scholar] [CrossRef]
- Vandermeersch, T.; Alvarenga, R.; Ragaert, P.; Dewulf, J. Environmental sustainability assessment of food waste valorization options. Resour. Conserv. Recycl. 2014, 87, 57–64. [Google Scholar] [CrossRef]
- Mouratoglou, E.; Malliou, V.; Makris, D.P. Novel Glycerol-Based Natural Eutectic Mixtures and Their Efficiency in the Ultrasound-Assisted Extraction of Antioxidant Polyphenols from Agri-Food Waste Biomass. Waste Biomass Valoriz. 2016, 7, 1377–1387. [Google Scholar] [CrossRef]
- Jones, M.K.; Liu, X. Origins of Agriculture in East Asia. Science 2009, 324, 730–731. [Google Scholar] [CrossRef]
- Yang, Y.-B.; Jia, G.-Q.; Deng, L.-G.; Qin, L.; Chen, E.-Y.; Cong, X.-J.; Zou, R.-F.; Wang, H.-L.; Zhang, H.-W.; Liu, B.; et al. Genetic variation of yellow pigment and its components in foxtail millet (Setaria italica (L.) P. Beauv.) from different eco-regions in China. J. Integr. Agric. 2017, 16, 2459–2469. [Google Scholar] [CrossRef]
- Seo, M.-C.; Ko, J.-Y.; Song, S.-B.; Lee, J.-S.; Kang, J.-R.; Kwak, D.-Y.; Oh, B.-G.; Yoon, Y.-N.; Nam, M.-H.; Jeong, H.-S.; et al. Antioxidant Compounds and Activities of Foxtail Millet, Proso Millet and Sorghum with Different Pulverizing Methods. J. Korean Soc. Food Sci. Nutr. 2011, 40, 790–797. [Google Scholar] [CrossRef]
- Sharma, N.; Niranjan, K. Foxtail millet: Properties, processing, health benefits, and uses. Food Rev. Int. 2017, 34, 329–363. [Google Scholar] [CrossRef]
- Zhang, L.Z.; Liu, R.H. Phenolic and carotenoid profiles and antiproliferative activity of foxtail millet. Food Chem. 2015, 174, 495–501. [Google Scholar] [CrossRef]
- Naidu, B.V.K.; Venkataram, B.; Sankar, S.S.; Kumar, A.S. Synthesis of Silver Nanoparticles Using Setaria italica (Foxtail Millets) Husk and Its Antimicrobial Activity. Res. J. Nanosci. Nanotechnol. 2015, 5, 6–15. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, Y.; Xiang, J.; Zheng, B.; Yuan, Y.; Luo, D.; Fan, J. Comparative evaluation on phenolic profiles, antioxidant properties and α-glucosidase inhibitory effects of different milling fractions of foxtail millet. J. Cereal Sci. 2021, 99, 103217–103224. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 39, 70–71. [Google Scholar] [CrossRef]
- Duan, L.; Dou, L.-L.; Guo, L.; Li, P.; Liu, E.-H. Comprehensive Evaluation of Deep Eutectic Solvents in Extraction of Bioactive Natural Products. ACS Sustain. Chem. Eng. 2016, 4, 2405–2411. [Google Scholar] [CrossRef]
- Skarpalezos, D.; Detsi, A. Deep Eutectic Solvents as Extraction Media for Valuable Flavonoids from Natural Sources. Appl. Sci. 2019, 9, 4169. [Google Scholar] [CrossRef]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural Deep Eutectic Solvents–Solvents for the 21st Century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Hayyan, M.; Hashim, M.A.; Hayyan, A.; Al-Saadi, M.A.; AlNashef, I.M.; Mirghani, M.E.; Saheed, O.K. Are deep eutectic solvents benign or toxic? Chemosphere 2013, 90, 2193–2195. [Google Scholar] [CrossRef]
- Zeng, J.; Dou, Y.; Yan, N.; Li, N.; Zhang, H.; Tan, J.-N. Optimizing Ultrasound-Assisted Deep Eutectic Solvent Extraction of Bioactive Compounds from Chinese Wild Rice. Molecules 2019, 24, 2718. [Google Scholar] [CrossRef]
- Zheng, B.; Yuan, Y.; Xiang, J.; Jin, W.; Johnson, J.B.; Li, Z.; Wang, C.; Luo, D. Green extraction of phenolic compounds from foxtail millet bran by ultrasonic-assisted deep eutectic solvent extraction: Optimization, comparison and bioactivities. LWT 2022, 154, 112740. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef]
- Xiang, J.; Li, W.; Ndolo, V.U.; Beta, T. A comparative study of the phenolic compounds and in vitro antioxidant capacity of finger millets from different growing regions in Malawi. J. Cereal Sci. 2019, 87, 143–149. [Google Scholar]
- Xiang, J.; Apea-Bah, F.B.; Ndolo, V.U.; Katundu, M.C.; Beta, T. Profile of phenolic compounds and antioxidant activity of finger millet varieties. Food Chem. 2019, 275, 361–368. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Y.; Xiang, J.; Wang, C.; Johnson, J.B.; Beta, T. Diverse polyphenol components contribute to antioxidant activity and hypoglycemic potential of mulberry varieties. LWT 2023, 173, 114308. [Google Scholar] [CrossRef]
- Zheng, B.; Ding, Y.; Johnson, J.B.; Xiang, J.; Li, Z.; Zhang, Y.; Luo, D. Enrichment and bioactivities of polyphenols of crude extract by deep eutectic solvent extraction from foxtail millet bran. Int. J. Food Sci. Technol. 2022, 57, 7974–7983. [Google Scholar] [CrossRef]
- Yuan, Y.; Xiang, J.; Zheng, B.; Sun, J.; Luo, D.; Li, P.; Fan, J. Diversity of phenolics including hydroxycinnamic acid amide derivatives, phenolic acids contribute to antioxidant properties of proso millet. LWT 2022, 154, 112611. [Google Scholar] [CrossRef]
- Husanu, E.; Mero, A.; Rivera, J.G.; Mezzetta, A.; Ruiz, J.C.; D’Andrea, F.; Pomelli, C.S.; Guazzelli, L. Exploiting Deep Eutectic Solvents and Ionic Liquids for the Valorization of Chestnut Shell Waste. ACS Sustain. Chem. Eng. 2020, 8, 18386–18399. [Google Scholar] [CrossRef]
- Dai, Y.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural Deep Eutectic Solvents as a New Extraction Media for Phenolic Metabolites in Carthamus tinctorius L. Anal. Chem. 2013, 85, 6272–6278. [Google Scholar] [CrossRef]
- Jha, P.; Das, A.J.; Deka, S.C. Optimization of ultrasound and microwave assisted extractions of polyphenols from black rice (Oryza sativa cv. Poireton) husk. J. Food Sci. Technol. 2017, 54, 3847–3858. [Google Scholar] [CrossRef]
- Li, F.; Zhao, H.; Xu, R.; Zhang, X.; Zhang, W.; Du, M.; Liu, X.; Fan, L. Simultaneous optimization of the acidified water extraction for total anthocyanin content, total phenolic content, and antioxidant activity of blue honeysuckle berries (Lonicera caerulea L.) using response surface methodology. Food Sci. Nutr. 2019, 7, 2968–2976. [Google Scholar] [CrossRef]
- Da Silva, D.T.; Pauletto, R.; da Silva Cavalheiro, S.; Bochi, V.C.; Rodrigues, E.; Weber, J.; da Silva, C.d.B.; Morisso, F.D.P.; Barcia, M.T.; Emanuelli, T. Natural deep eutectic solvents as a biocompatible tool for the extraction of blueberry anthocyanins. J. Food Compos. Anal. 2020, 89, 103470–103504. [Google Scholar] [CrossRef]
- Bajkacz, S.; Adamek, J. Evaluation of new natural deep eutectic solvents for the extraction of isoflavones from soy products. Talanta 2017, 168, 329–335. [Google Scholar] [CrossRef]
- Hang, N.T.; Uyen, T.T.T.; Van Phuong, N. Green extraction of apigenin and luteolin from celery seed using deep eutectic solvent. J. Pharm. Biomed. Anal. 2021, 207, 114406–114415. [Google Scholar] [CrossRef]
- Chandrasekara, A.; Shahidi, F. Content of Insoluble Bound Phenolics in Millets and Their Contribution to Antioxidant Capacity. J. Agric. Food Chem. 2010, 58, 6706–6714. [Google Scholar] [CrossRef]
- Wu, L.; Li, L.; Chen, S.; Wang, L.; Lin, X. Deep eutectic solvent-based ultrasonic-assisted extraction of phenolic compounds from Moringa oleifera L. leaves: Optimization, comparison and antioxidant activity. Sep. Purif. Technol. 2020, 247, 117014–117025. [Google Scholar] [CrossRef]
- Kobus-Cisowska, J.; Szymanowska, D.; Maciejewska, P.; Kmiecik, D.; Gramza-Michałowska, A.; Kulczyński, B.; Cielecka-Piontek, J. In vitro screening for acetylcholinesterase and butyrylcholinesterase inhibition and antimicrobial activity of chia seeds (Salvia hispanica). Electron. J. Biotechnol. 2018, 37, 1–10. [Google Scholar] [CrossRef]
- Jo, Y.-H.; Yuk, H.-G.; Lee, J.-H.; Kim, J.-C.; Kim, R.; Lee, S.-C. Antioxidant, tyrosinase inhibitory, and acetylcholinesterase inhibitory activities of green tea (Camellia sinensis L.) seed and its pericarp. Food Sci. Biotechnol. 2012, 21, 761–768. [Google Scholar] [CrossRef]
- Choi, J.S.; Islam, M.N.; Ali, M.Y.; Kim, E.J.; Kim, Y.M.; Jung, H.A. Effects of C-glycosylation on anti-diabetic, anti-Alzheimer’s disease and anti-inflammatory potential of apigenin. Food Chem. Toxicol. 2014, 64, 27–33. [Google Scholar] [CrossRef]
- Cheng, A.Y.; Fantus, I.G. Oral antihyperglycemic therapy for type 2 diabetes mellitus. Can. Med. Assoc. J. 2005, 172, 213–226. [Google Scholar] [CrossRef]
- Yao, Y.; Sang, W.; Zhou, M.; Ren, G. Antioxidant and α-Glucosidase Inhibitory Activity of Colored Grains in China. J. Agric. Food Chem. 2009, 58, 770–774. [Google Scholar] [CrossRef]
- Xiong, Y.; Ng, K.; Zhang, P.; Warner, R.D.; Shen, S.; Tang, H.-Y.; Liang, Z.; Fang, Z. In Vitro α-Glucosidase and α-Amylase Inhibitory Activities of Free and Bound Phenolic Extracts from the Bran and Kernel Fractions of Five Sorghum Grain Genotypes. Foods 2020, 9, 1301–1318. [Google Scholar] [CrossRef]
- Liu, M.; Li, X.; Liu, Q.; Xie, S.; Chen, M.; Wang, L.; Feng, Y.; Chen, X. Comprehensive profiling of α-glucosidase inhibitors from the leaves of Rubus suavissimus using an off-line hyphenation of HSCCC, ultrafiltration HPLC-UV-MS and prep-HPLC. J. Food Compos. Anal. 2020, 85, 103336–103369. [Google Scholar] [CrossRef]
- Zeng, L.; Zhang, G.; Lin, S.; Gong, D. Inhibitory Mechanism of Apigenin on α-Glucosidase and Synergy Analysis of Flavonoids. J. Agric. Food Chem. 2016, 64, 6939–6949. [Google Scholar] [CrossRef]
- Qin, P.; Wu, L.; Yao, Y.; Ren, G. Changes in phytochemical compositions, antioxidant and α-glucosidase inhibitory activities during the processing of tartary buckwheat tea. Food Res. Int. 2013, 50, 562–567. [Google Scholar] [CrossRef]
- Chai, Y.-S.; Lei, F.; Tian, Y.; Yuan, Z.-Y.; Lu, X.; Zhao, S.; Wang, X.-P.; Xing, D.-M.; Du, L.-J. Comprehensive study of the intestinal absorption of four phenolic compounds after oral administration of Ananas comosus leaf extract in vivo and in vitro. Afr. J. Pharm. Pharmacol. 2013, 7, 1781–1792. [Google Scholar] [CrossRef]
- Murray, A.F.; Palatini, K.; Komarnytsky, S.; Gianfagna, T.J.; Munafo, J.P. Phenylpropanoid Glycerol Glucosides Attenuate Glucose Production in Hepatocytes. ACS Omega 2019, 4, 10670–10676. [Google Scholar] [CrossRef]
- Xiang, J.; Zhang, M.; Apea-Bah, F.B.; Beta, T. Hydroxycinnamic acid amide (HCAA) derivatives, flavonoid C-glycosides, phenolic acids and antioxidant properties of foxtail millet. Food Chem. 2019, 295, 214–223. [Google Scholar] [CrossRef]
- Sobolev, V.S.; Sy, A.A.; Gloer, J.B. Spermidine and Flavonoid Conjugates from Peanut (Arachis hypogaea) Flowers. J. Agric. Food Chem. 2008, 56, 2960–2969. [Google Scholar] [CrossRef]
- Luo, J.; Li, L.; Kong, L. Preparative separation of phenylpropenoid glycerides from the bulbs of Lilium lancifolium by high-speed counter-current chromatography and evaluation of their antioxidant activities. Food Chem. 2012, 131, 1056–1062. [Google Scholar] [CrossRef]
- Ma, C.; Xiao, S.-Y.; Li, Z.-G.; Wang, W.; Du, L.-J. Characterization of active phenolic components in the ethanolic extract of Ananas comosus L. leaves using high-performance liquid chromatography with diode array detection and tandem mass spectrometry. J. Chromatogr. A 2007, 1165, 39–44. [Google Scholar] [CrossRef]
- Zhong, B.; Robinson, N.A.; Warner, R.D.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A. LC-ESI-QTOF-MS/MS Characterization of Seaweed Phenolics and Their Antioxidant Potential. Mar. Drugs 2020, 18, 101–109. [Google Scholar] [CrossRef]
- Sharayei, P.; Azarpazhooh, E.; Zomorodi, S.; Ramaswamy, H.S. Ultrasound assisted extraction of bioactive compounds from pomegranate (Punica granatum L.) peel. LWT 2018, 101, 342–350. [Google Scholar] [CrossRef]
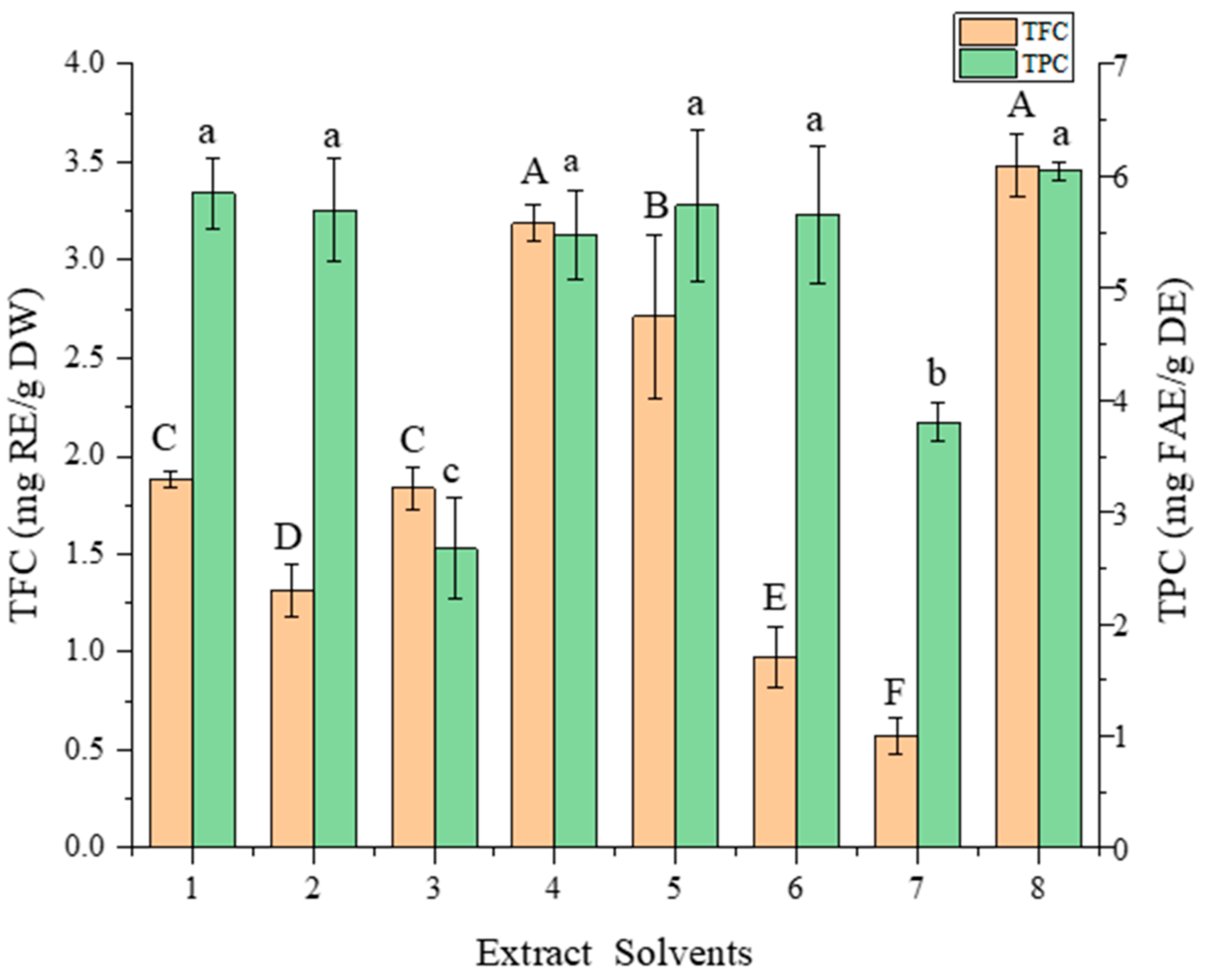
| No. | HBAs | HBDs | Molar Ratio |
|---|---|---|---|
| 1 | Betaine | Glycol | 1:4 |
| 2 | Betaine | Glycerol | 1:4 |
| 3 | Betaine | L-lactic acid | 1:2 |
| 4 | L-lactic acid | Glycol | 1:2 |
| 5 | L-lactic acid | Glycerol | 1:2 |
| 6 | Sodium acetate | Glycol | 1:2 |
| 7 | Sodium acetate | Glycerol | 1:2 |
| 8 | Sodium acetate | L-lactic acid | 1:2 |
| Run | Variables | TPC (Y1) | TFC (Y2) | |||
|---|---|---|---|---|---|---|
| X1 (%) | X2 (°C) | X3 (min) | X4 (W) | (mg FAE/g DW) | (mg RE/g DW) | |
| 1 | 10 | 40 | 40 | 300 | 6.65 ± 0.04 | 3.60 ± 0.03 |
| 2 | 20 | 40 | 40 | 300 | 6.70 ± 0.05 | 3.66 ± 0.02 |
| 3 | 10 | 60 | 40 | 300 | 6.76 ± 0.01 | 3.68 ± 0.02 |
| 4 | 20 | 60 | 40 | 300 | 6.80 ± 0.02 | 3.67 ± 0.01 |
| 5 | 15 | 50 | 30 | 250 | 6.66 ± 0.03 | 3.57 ± 0.05 |
| 6 | 15 | 50 | 50 | 250 | 6.78 ± 0.02 | 3.66 ± 0.01 |
| 7 | 15 | 50 | 30 | 350 | 6.72 ± 0.01 | 3.58 ± 0.04 |
| 8 | 15 | 50 | 50 | 350 | 6.80 ± 0.03 | 3.79 ± 0.02 |
| 9 | 10 | 50 | 40 | 250 | 6.63 ± 0.03 | 3.60 ± 0.01 |
| 10 | 20 | 50 | 40 | 250 | 6.70 ± 0.01 | 3.62 ± 0.03 |
| 11 | 10 | 50 | 40 | 350 | 6.73 ± 0.05 | 3.66 ± 0.03 |
| 12 | 20 | 50 | 40 | 350 | 6.80 ± 0.02 | 3.68 ± 0.01 |
| 13 | 15 | 40 | 30 | 300 | 6.59 ± 0.01 | 3.58 ± 0.02 |
| 14 | 15 | 60 | 30 | 300 | 6.73 ± 0.06 | 3.61 ± 0.01 |
| 15 | 15 | 40 | 50 | 300 | 6.75 ± 0.04 | 3.66 ± 0.01 |
| 16 | 15 | 60 | 50 | 300 | 6.83 ± 0.02 | 3.73 ± 0.02 |
| 17 | 10 | 50 | 30 | 300 | 6.71 ± 0.03 | 3.63 ± 0.01 |
| 18 | 20 | 50 | 30 | 300 | 6.53 ± 0.02 | 3.69 ± 0.03 |
| 19 | 10 | 50 | 50 | 300 | 6.76 ± 0.01 | 3.72 ± 0.01 |
| 20 | 20 | 50 | 50 | 300 | 6.86 ± 0.04 | 3.78 ± 0.06 |
| 21 | 15 | 40 | 40 | 250 | 6.61 ± 0.01 | 3.57 ± 0.02 |
| 22 | 15 | 60 | 40 | 250 | 6.80 ± 0.03 | 3.67 ± 0.01 |
| 23 | 15 | 40 | 40 | 350 | 6.79 ± 0.10 | 3.70 ± 0.03 |
| 24 | 15 | 60 | 40 | 350 | 6.81 ± 0.01 | 3.74 ± 0.02 |
| 25 | 15 | 50 | 40 | 300 | 7.30 ± 0.01 | 4.11 ± 0.01 |
| 26 | 15 | 50 | 40 | 300 | 7.29 ± 0.02 | 4.23 ± 0.03 |
| 27 | 15 | 50 | 40 | 300 | 7.21 ± 0.05 | 3.92 ± 0.02 |
| 28 | 15 | 50 | 40 | 300 | 7.21 ± 0.01 | 4.32 ± 0.02 |
| 29 | 15 | 50 | 40 | 300 | 7.11 ± 0.01 | 4.31 ± 0.02 |
| Source | Response Variables | |||
|---|---|---|---|---|
| TPC | TFC | |||
| F-Value | p-Value | F-Value | p-Value | |
| Model | 31.75 | <0.0001 *** | 9.50 | <0.0001 *** |
| X1 | 0.85 | 0.3730 | 14.37 | 0.0085 ** |
| X2 | 12.69 | 0.0031 ** | 5.00 | 0.3332 |
| X3 | 22.44 | 0.0003 *** | 9.46 | 0.0440 * |
| X4 | 6.66 * | 0.0218 * | 5.38 | 0.2598 |
| X1X2 | 7.39 | 0.9311 * | 3.13 | 0.7266 |
| X1X3 | 7.748 × 10−34 | 0.0166 | 2.836 × 10−5 | 0.9958 |
| X1X4 | 3.826 × 10−4 | 0.9847 | 7.089 * | 0.0491 * |
| X2X3 | 15.54 | 0.0077 ** | 5.63 | 0.8060 |
| X2X4 | 12.04 | 0.0069 ** | 8.9 * | 0.0369 * |
| X3X4 | 4.11 | 0.7015 | 2.63 | 0.8060 |
| X12 | 152.84 | <0.0001 *** | 44.42 | <0.0001 *** |
| X22 | 111.14 | <0.0001 *** | 50.64 | <0.0001 *** |
| X32 | 122.38 | <0.0001 *** | 67.73 | <0.0001 *** |
| X42 | 124.89 | <0.0001 *** | 55.17 | <0.0001 *** |
| Residual | 0.044 | 0.12 | ||
| Lack of Fit | 0.021 | 0.22 | 0.015 | 0.056 |
| Pure Error | 0.024 | 0.11 | ||
| Cor Total | 1.22 | 1.30 | ||
| R2 | 0.9695 | 0.9248 | ||
| Adj R2 | 0.9389 | 0.9196 | ||
| Pred R2 | 0.9074 | 0.8025 | ||
| Parameter | UAE-DES | UAE |
|---|---|---|
| TPC (mg FAE/g DW b) | 7.38 ± 0.18 a | 4.40 ± 0.15 b |
| TFC (mg RE/g DW) | 4.30 ± 0.17 a | 2.63 ± 0.05 b |
| ABTS·+ (μmoL TE/g DW) | 13.99 ± 0.47 a | 7.59 ± 0.28 b |
| DPPH (μmoL TE/g DW) | 12.18 ± 0.14 a | 8.37 ± 0.33 b |
| FRAP (μmoL TE/g DW) | 18.36 ± 0.65 a | 9.88 ± 0.18 b |
| IC50 of AChE (μg FAE/mL) | 295.53 ± 1.56 a | 403.51 ± 2.42 b |
| IC50 of α-glucosidase (μg FAE/mL) | 190.12 ± 1.37 a | 280.22 ± 1.49 b |
| No. | Retention Time | [M-H]− (m/z) | UV λ Max (nm) | Formula | m/z of Main Fragments (Relative Intensity, %), MS/MS | Identified Phenolic Compounds | Contents (μg/g DW b) | |
|---|---|---|---|---|---|---|---|---|
| UAE-DES | UAE | |||||||
| 1 | 6.69 | 137 | 280 | C7H5O3 | p-hydroxybenzoic acid c | 45.32 ± 0.12 a | 30.22 ± 0.08 b | |
| 2 | 7.32 | 121 | 284 | C7H5O2 | p-hydroxybenzaldehyde c | 146.21 ± 0.73 a | 87.32 ± 0.06 b | |
| 3 | 7.96 | 167 | 287 | C8H7O4 | vanillic acid c | 68.32 ± 0.04 a | 42.13 ± 0.12 b | |
| 4 | 10.19 | 353 | 320 | C16H18O9 | chlorogenic acid c | 110.00 ± 1.13 a | 63.10 ± 0.17 b | |
| 5 | 12.92 | 163 | 308 | C9H7O3 | 119(100) | p-coumaric acid c | 240.00 ± 1.23 a | 132.45 ± 1.36 b |
| 6 | 14.27 | 237 | 310 | C12H14O5 | 237(5), 163(20), 145(100), 119(50) | 1-O-p-coumaroylglycerol | 395.75 ± 2.12 a | 245.01 ± 1.37 b |
| 7 | 14.70 | 593 | 271/328 | C27H29O15 | 593(100), 503(10), 473(10), 353(10) | apigenin-C-dihexoside | 176.95 ± 1.5 a | 92.01 ± 0.56 b |
| 8 | 15.63 | 436 | 270/331 | C25H30N3O4 | 436(20), 316(55), 274(20), 195(25), 193(50), 145(20), 119(20) | N′, N″-di-p-coumaroyl spermidine | 219.25 ± 1.13 a | 107.3 ± 1.30 b |
| 9 | 16.05 | 563 | 270/330 | C26H27O14 | 563(100), 473(10), 443(10) | apigenin-C-pentosyl-C-hexoside | 566.75 ± 3.38 a | 265.44 ± 1.22 b |
| 10 | 16.93 | 193 | 323 | C10H9O4 | 193(20), 178(50), 134(100) | ferulic acid c | 55.35 ± 0.48 a | 38.17 ± 0.14 b |
| 11 | 18.17 | 413 | 313 | C22H22O8 | 413(50), 397(10), 292(5), 267(10), 237(15), 193(100), 163(100), 119(30) | 1-O-feruloyl-3-O-p-coumaroylglycerol | 890.27 ± 6.14 a | 508.20 ± 4.45 b |
| 12 | 19.28 | 329 | 340 | C17H14O7 | 329(28), 314(100), 299(60), 285(5), 271(20), 227(5) | 3,7-dimethylquercetin | 371.75 ± 1.86 a | 191.31 ± 1.33 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Li, Z.; Xiang, J.; Johnson, J.B.; Zheng, B.; Luo, L.; Beta, T. From Foxtail Millet Husk (Waste) to Bioactive Phenolic Extracts Using Deep Eutectic Solvent Extraction and Evaluation of Antioxidant, Acetylcholinesterase, and α-Glucosidase Inhibitory Activities. Foods 2023, 12, 1144. https://doi.org/10.3390/foods12061144
Wang C, Li Z, Xiang J, Johnson JB, Zheng B, Luo L, Beta T. From Foxtail Millet Husk (Waste) to Bioactive Phenolic Extracts Using Deep Eutectic Solvent Extraction and Evaluation of Antioxidant, Acetylcholinesterase, and α-Glucosidase Inhibitory Activities. Foods. 2023; 12(6):1144. https://doi.org/10.3390/foods12061144
Chicago/Turabian StyleWang, Chunqing, Zhenzhen Li, Jinle Xiang, Joel B. Johnson, Bailiang Zheng, Lei Luo, and Trust Beta. 2023. "From Foxtail Millet Husk (Waste) to Bioactive Phenolic Extracts Using Deep Eutectic Solvent Extraction and Evaluation of Antioxidant, Acetylcholinesterase, and α-Glucosidase Inhibitory Activities" Foods 12, no. 6: 1144. https://doi.org/10.3390/foods12061144
APA StyleWang, C., Li, Z., Xiang, J., Johnson, J. B., Zheng, B., Luo, L., & Beta, T. (2023). From Foxtail Millet Husk (Waste) to Bioactive Phenolic Extracts Using Deep Eutectic Solvent Extraction and Evaluation of Antioxidant, Acetylcholinesterase, and α-Glucosidase Inhibitory Activities. Foods, 12(6), 1144. https://doi.org/10.3390/foods12061144









