Optimizing Levilactobacillus brevis NPS-QW 145 Fermentation for Gamma-Aminobutyric Acid (GABA) Production in Soybean Sprout Yogurt-like Product
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Strain
2.2. Preparation of Soybean Sprouts Milk
2.3. Preparation of Fermented Yogurt-like Product
2.4. Optimization of Fermentation Conditions for the Production of GABA by Lb. brevis 145 in Soybean Sprout Yogurt-like Product
2.4.1. Single-Factor Experiments
2.4.2. Response Surface Methodology (RSM)
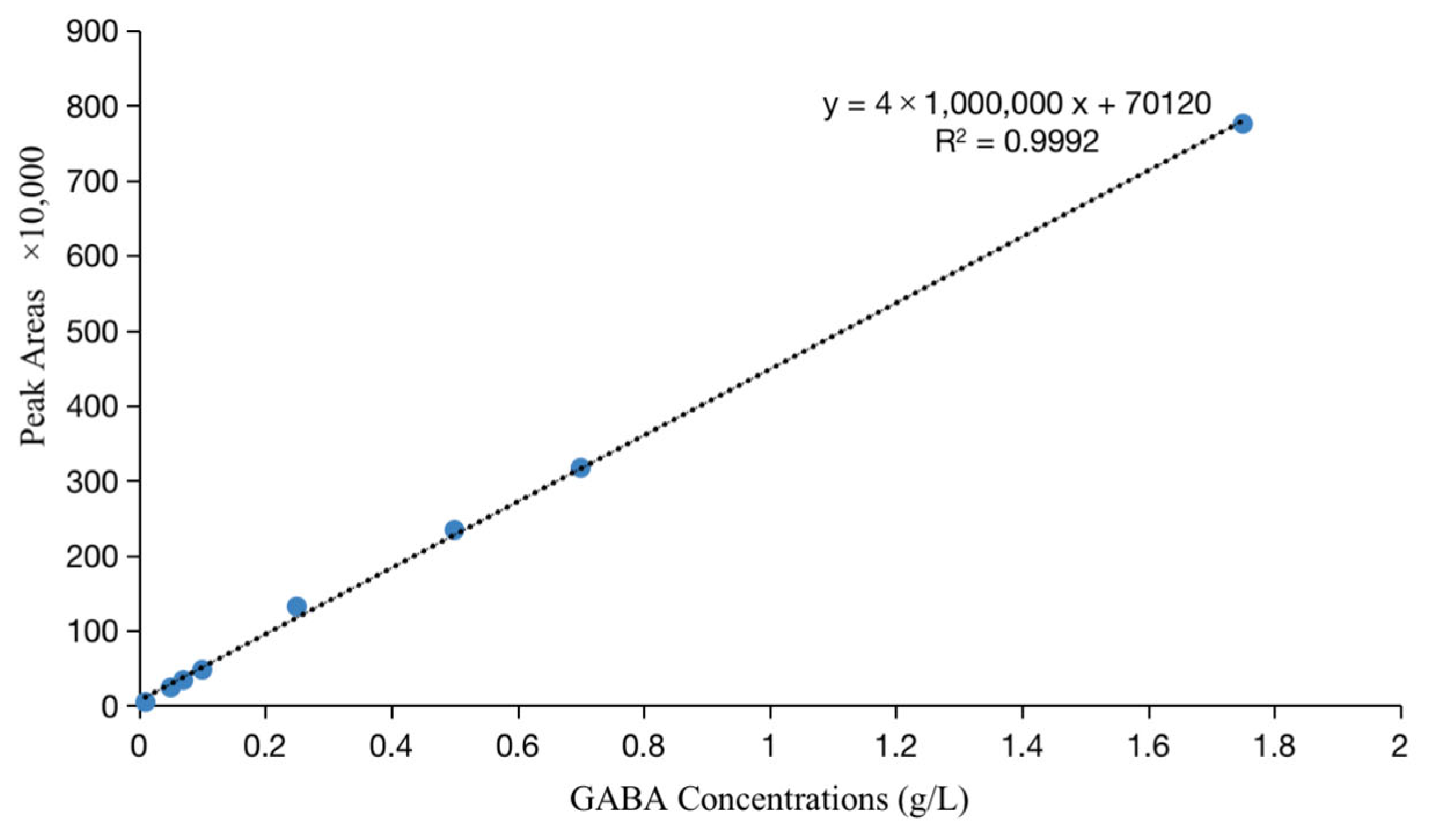
2.5. Determination of GABA Production by RP-HPLC
2.5.1. Protein and Peptide Removal from Soybean Sprout Yogurt-like Product
2.5.2. Amino acid Derivatization
2.6. Determination of pH and Viable Cell Counts in Fermented Soybean Sprout Yogurt-like Product
2.7. Protein Content of Fermented Soybean Sprout Yogurt-like Product
2.8. Texture Analysis
2.9. Sensory Analysis
2.10. Statistical Analysis
3. Results and Discussion
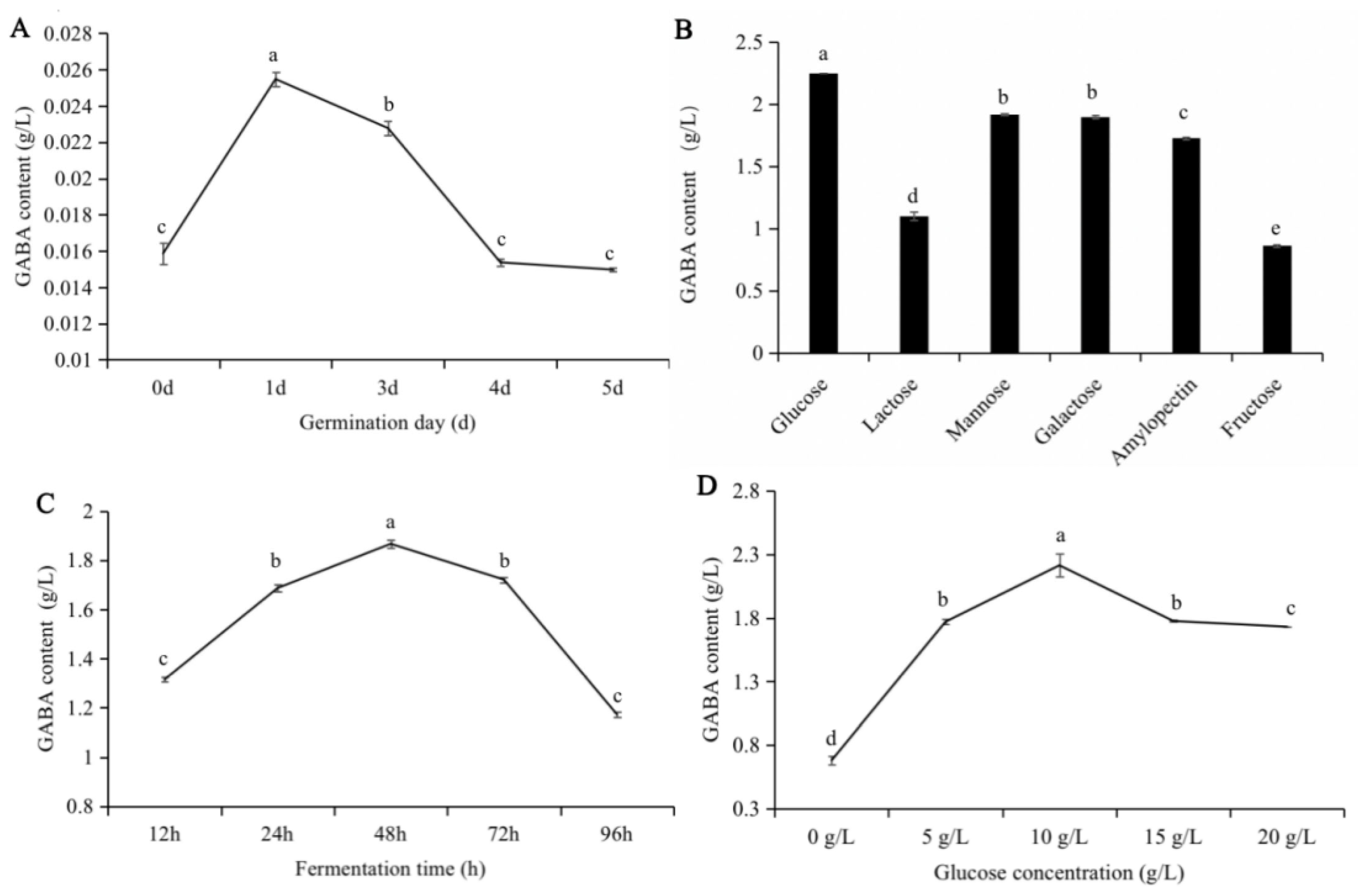
3.1. The Effect of Various Conditions on GABA Production by Lb. brevis 145 in Soybean Sprout Yogurt-like Product
3.2. Optimizing the Fermentation Process to Produce GABA by Lb. brevis 145 in Soybean Sprout Yogurt-like Product
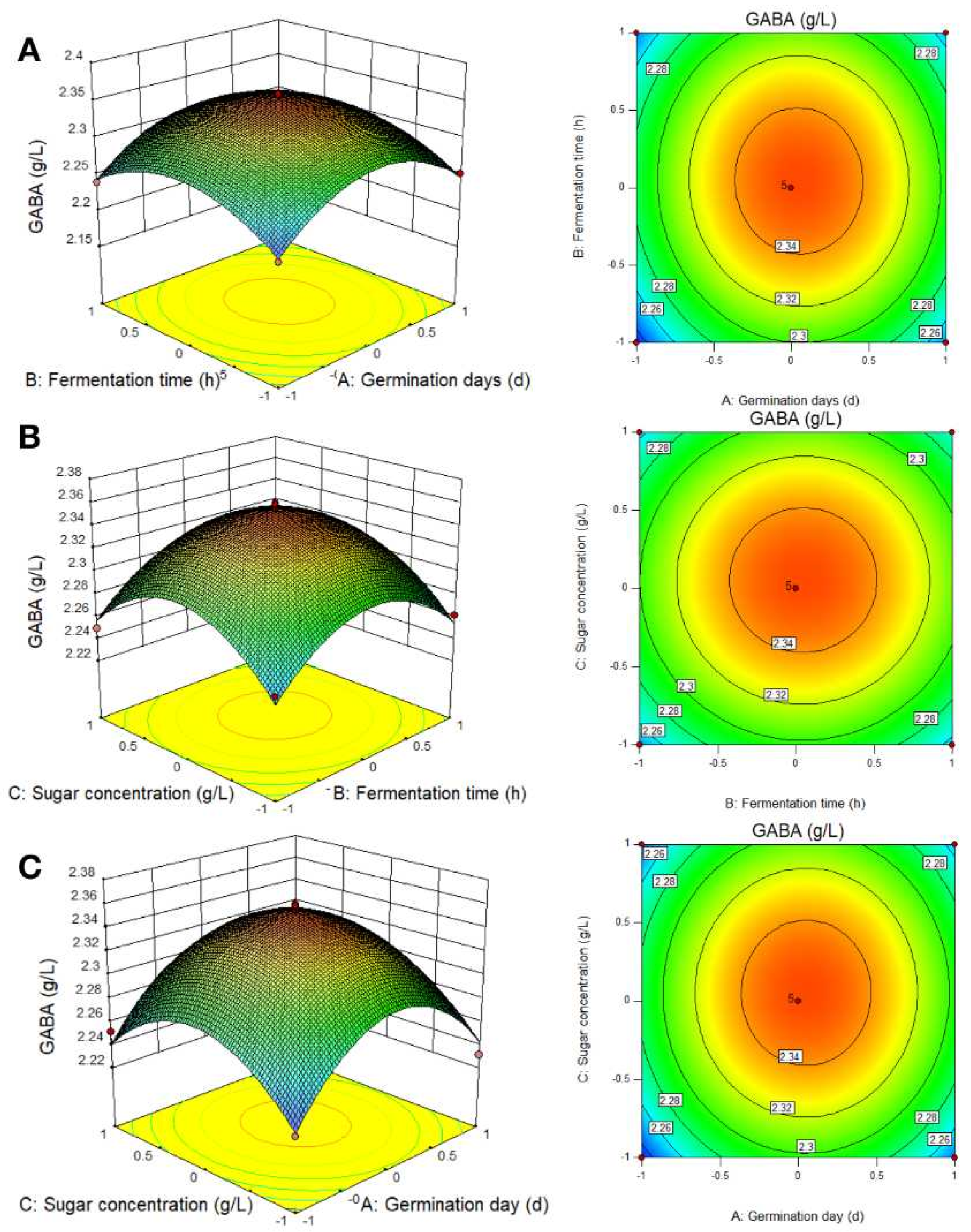
3.2.1. RSM Results
3.2.2. RSM Analysis of the Best-Fermented Parameters
3.3. Finished Product Quality Analysis
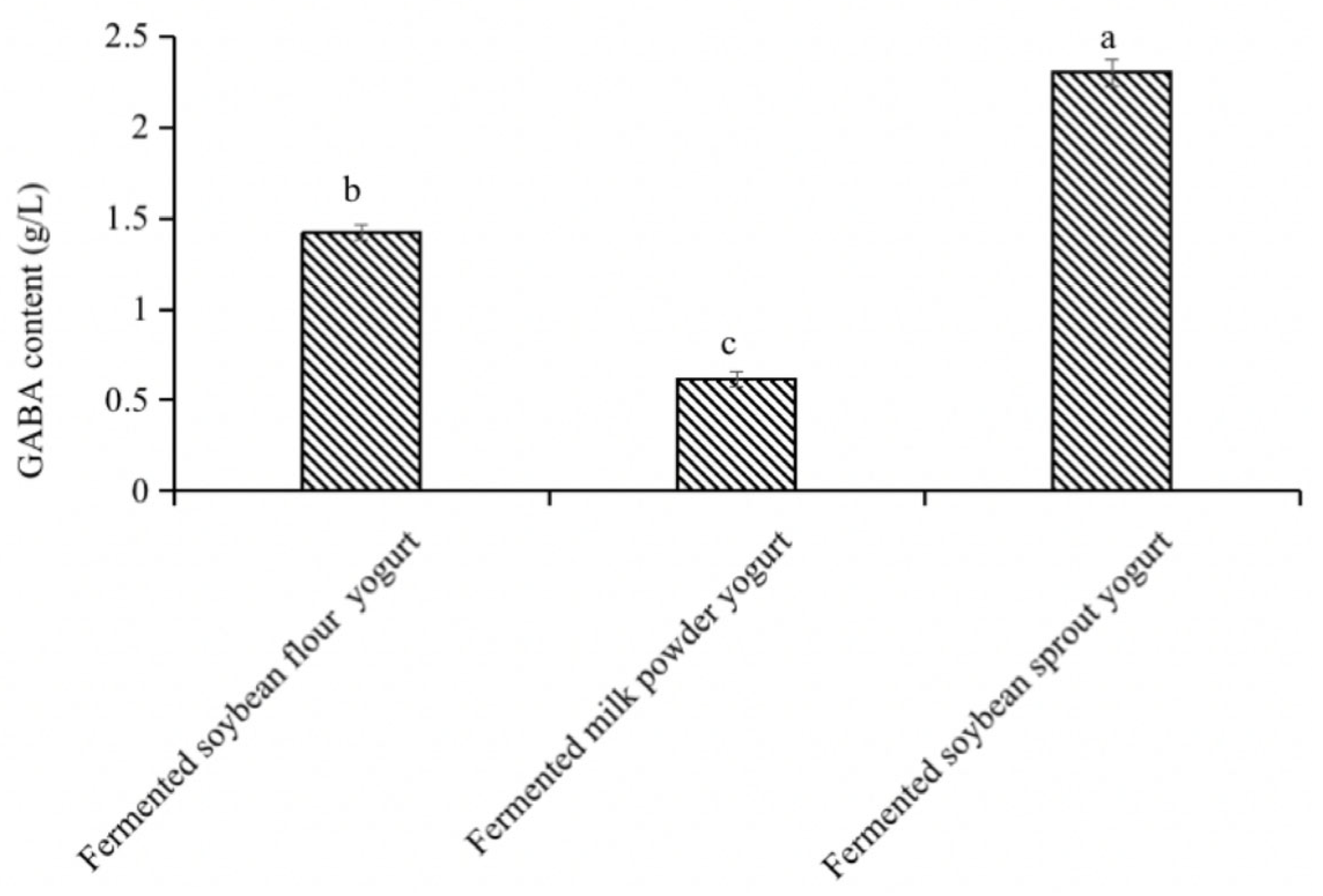
3.3.1. GABA Concentration in GABA-Rich Yogurt
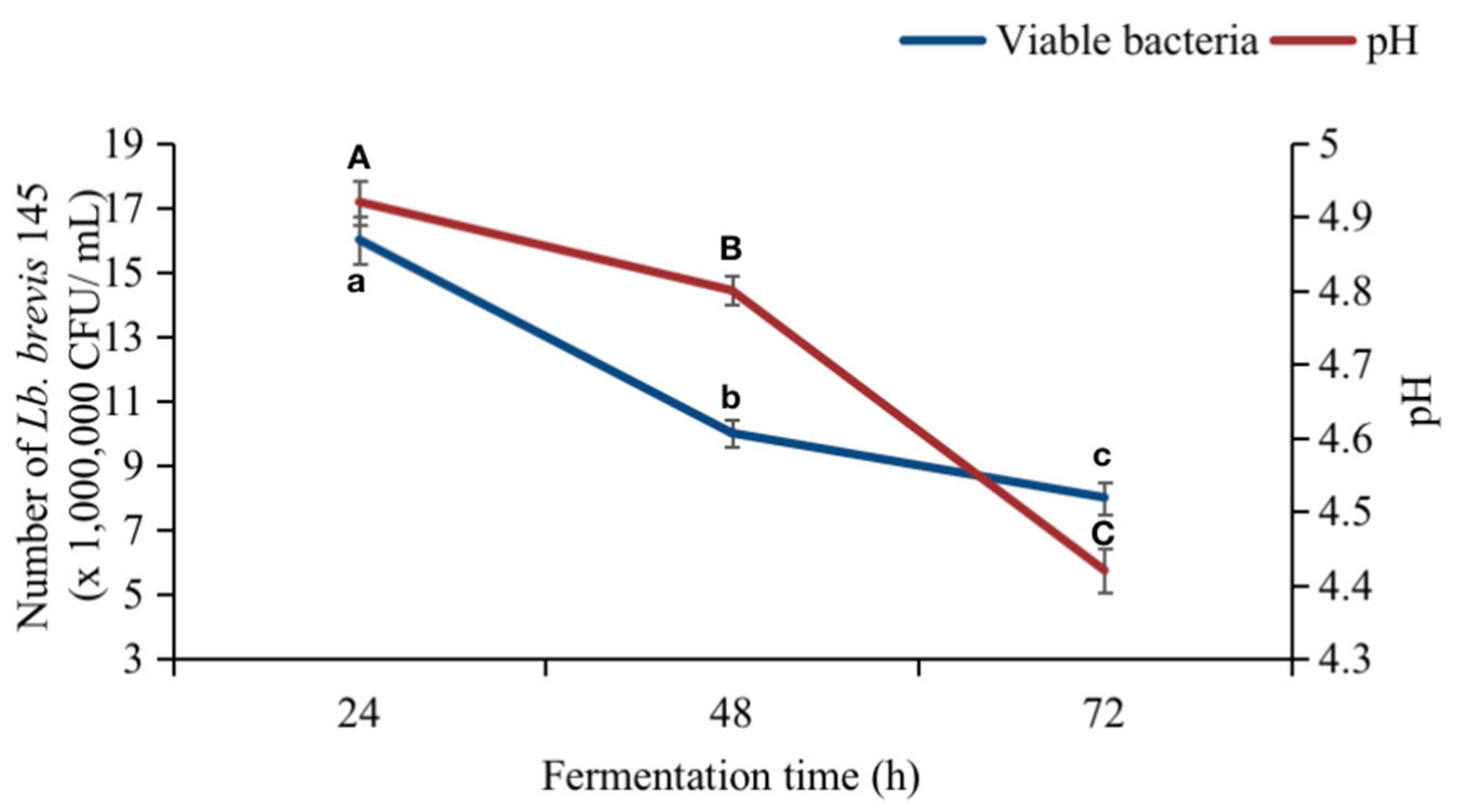
3.3.2. pH and Cell Viability in Fermented Soybean Sprout Yogurt-like Product
3.3.3. Texture Characteristic and Protein Content in GABA-Rich Yogurt-like Product
3.3.4. Sensory Evaluation of Yogurt-like Product Rich in GABA
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, Q.; Shah, N.P. Gas release-based prescreening combined with reversed-phase HPLC quantitation for efficient selection of high-γ-aminobutyric acid (GABA)-producing lactic acid bacteria. J. Dairy Sci. 2015, 98, 790–797. [Google Scholar] [CrossRef]
- Wu, Q.; Shah, N.P. Restoration of GABA production machinery in Lactobacillus brevis by accessible carbohydrates, anaerobiosis and early acidification. Food Microbiol. 2018, 69, 151–158. [Google Scholar] [CrossRef]
- Binh, T.T.T.; Ju, W.T.; Jung, W.J.; Park, R.D. Optimization of γ-amino butyric acid production in a newly isolated Lactobacillus brevis. Biotechnol. Lett. 2014, 36, 93–98. [Google Scholar] [CrossRef]
- Luo, X.; Wang, Y.; Li, Q.; Wang, D.; Xing, C.; Zhang, L.; Xu, T.; Fang, F.; Wang, F. Accumulating mechanism of γ-aminobutyric acid in soybean (Glycine max L.) during germination. Int. J. Food Sci. Technol. 2018, 53, 106–111. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, C.; Ma, T.; Zhao, J. Physicochemical and functional properties of γ-aminobutyric acid-treated soy proteins. Food Chem. 2019, 295, 267–273. [Google Scholar] [CrossRef]
- Park, K.B.; Oh, S.H. Production of yogurt with enhanced levels of gamma-aminobutyric acid and valuable nutrients using lactic acid bacteria and germinated soybean extract. Bioresour. Technol. 2007, 98, 1675–1679. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Man, C.X.; Han, X.; Li, L.; Guo, Y.; Deng, Y.; Li, T.; Zhang, L.W.; Jiang, Y.J. Evaluation of improved γ-aminobutyric acid production in yogurt using Lactobacillus plantarum NDC75017. J. Dairy Sci. 2015, 98, 2138–2149. [Google Scholar] [CrossRef] [PubMed]
- Vann, K.; Techaparin, A.; Apiraksakorn, J. Beans germination as a potential tool for GABA-enriched tofu production. J. Food Sci. Technol. 2020, 57, 3947–3954. [Google Scholar] [CrossRef] [PubMed]
- Tung, Y.T.; Lee, B.H.; Liu, C.F.; Pan, T.M. Optimization of culture condition for ACEI and GABA production by lactic acid bacteria. J. Food Sci. 2011, 76, M585–M591. [Google Scholar] [CrossRef] [PubMed]
- Aoki, H.; Furuya, Y.; Endo, Y.; Fujimoto, K. Effect of γ-aminobutyric acid-enriched tempeh-like fermented soybean (GABA-tempeh) on the blood pressure of spontaneously hypertensive rats. Biosci. Biotechnol. Biochem. 2003, 67, 1806–1808. [Google Scholar] [CrossRef]
- Geisler, C.E.; Ghimire, S.; Bruggink, S.M.; Miller, K.E.; Weninger, S.N.; Kronenfeld, J.M.; Yoshino, J.; Klein, S.; Duca, F.A.; Renquist, B.J. A critical role of hepatic GABA in the metabolic dysfunction and hyperphagia of obesity. Cell Rep. 2021, 35, 109301. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Fattah, A.; Sakr, S.; El-Dieb, S.; Elkashef, H. Developing functional yogurt rich in bioactive peptides and gamma-aminobutyric acid related to cardiovascular health. LWT 2018, 98, 390–397. [Google Scholar] [CrossRef]
- Sharma, S.; Saxena, D.C.; Riar, C.S. Changes in the GABA and polyphenols contents of foxtail millet on germination and their relationship with in vitro antioxidant activity. Food Chem. 2018, 245, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yue, Y.; Wang, X.; Dai, W.; Piao, C.; Yu, H. Optimization of fermentation for γ-aminobutyric acid (GABA) production by yeast Kluyveromyces marxianus C21 in okara (soybean residue). Bioprocess Biosyst. Eng. 2022, 45, 1111–1123. [Google Scholar] [CrossRef] [PubMed]
- Ohmori, T.; Tahara, M.; Ohshima, T. Mechanism of gamma-aminobutyric acid (GABA) production by a lactic acid bacterium in yogurt-sake. Process Biochem. 2018, 74, 21–27. [Google Scholar] [CrossRef]
- Guo, Y.; Yang, R.; Chen, H.; Song, Y.; Gu, Z. Accumulation of γ-aminobutyric acid in germinated soybean (Glycine max L.) in relation to glutamate decarboxylase and diamine oxidase activity induced by additives under hypoxia. Eur. Food Res. Technol. 2012, 234, 679–687. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, P.; Gu, Z.; Sun, M.; Yang, R. Effects of germination on physio-biochemical metabolism and phenolic acids of soybean seeds. J. Food Compos. Anal. 2022, 112, 104717. [Google Scholar] [CrossRef]
- Sęczyk, Ł.; Świeca, M.; Gawlik-Dziki, U. Soymilk enriched with green coffee phenolics—Antioxidant and nutritional properties in the light of phenolics-food matrix interactions. Food Chem. 2017, 223, 1–7. [Google Scholar] [CrossRef]
- Xiao, T.; Shah, N.P. Lactic acid produced by Streptococcus thermophilus activated glutamate decarboxylase (GadA) in Lactobacillus brevis NPS-QW 145 to improve γ-amino butyric acid production during soymilk fermentation. LWT 2021, 137, 110474. [Google Scholar] [CrossRef]
- Chan, C.L.; Gan, R.Y.; Shah, N.P.; Corke, H. Enhancing antioxidant capacity of Lactobacillus acidophilus-fermented milk fortified with pomegranate peel extracts. Food Biosci. 2018, 26, 185–192. [Google Scholar] [CrossRef]
- Wu, Q.; Law, Y.S.; Shah, N.P. Dairy Streptococcus thermophilus improves cell viability of Lactobacillus brevis NPS-QW-145 and its γ-aminobutyric acid biosynthesis ability in milk. Sci. Rep. 2015, 5, 12885. [Google Scholar] [CrossRef]
- Giri, A.; Kanawjia, S.K.; Khetra, Y. Textural and melting properties of processed cheese spread as affected by incorporation of different inulin levels. Food Bioprocess Technol. 2014, 7, 1533–1540. [Google Scholar] [CrossRef]
- Meilgaard, M.C.; Carr, B.T.; Civille, G.V. Sensory Evaluation Techniques; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar]
- Rizzello, C.G.; Cassone, A.; Di Cagno, R.; Gobbetti, M. Synthesis of angiotensin I-converting enzyme (ACE)-inhibitory peptides and γ-aminobutyric acid (GABA) during sourdough fermentation by selected lactic acid bacteria. J. Agric. Food Chem. 2008, 56, 6936–6943. [Google Scholar] [CrossRef]
- Yang, R.; Feng, L.; Wang, S.; Yu, N.; Gu, Z. Accumulation of γ-aminobutyric acid in soybean by hypoxia germination and freeze–thawing incubation. J. Sci. Food Agric. 2016, 96, 2090–2096. [Google Scholar] [CrossRef]
- Hwang, C.E.; Haque, M.; Lee, J.H.; Song, Y.H.; Lee, H.Y.; Kim, S.C.; Cho, K.M. Bioconversion of γ-aminobutyric acid and isoflavone contents during the fermentation of high-protein soy powder yogurt with Lactobacillus brevis. Appl. Biol. Chem. 2018, 61, 409–421. [Google Scholar] [CrossRef]
- Xu, J.G.; Hu, Q.P. Changes in γ-aminobutyric acid content and related enzyme activities in Jindou 25 soybean (Glycine max L.) seeds during germination. LWT 2014, 55, 341–346. [Google Scholar] [CrossRef]
- Chew, S.Y.; Than, L.T.L. Glucose Metabolism and Use of Alternative Carbon Sources in Medically-Important Fungi. Encycl. Mycol. 2021, 2021, 220–229. [Google Scholar]
- Meena, P.K.; Gupta, V.K.; Meena, G.S.; Raju, P.N.; Parmar, P.T. Application of ultrafiltration technique for the quality improvement of dahi. J. Food Sci. Technol. 2015, 52, 7974–7983. [Google Scholar] [CrossRef]
- Buriti, F.C.; Castro, I.A.; Saad, S.M. Effects of refrigeration, freezing and replacement of milk fat by inulin and whey protein concentrate on texture profile and sensory acceptance of synbiotic guava mousses. Food Chem. 2010, 123, 1190–1197. [Google Scholar] [CrossRef]
- Chen, L.; Alcazar, J.; Yang, T.; Lu, Z.; Lu, Y. Optimized cultural conditions of functional yogurt for γ-aminobutyric acid augmentation using response surface methodology. J. Dairy Sci. 2018, 101, 10685–10693. [Google Scholar] [CrossRef] [PubMed]
- Tárrega, A.; Costell, E. Effect of inulin addition on rheological and sensory properties of fat-free starch-based dairy desserts. Int. Dairy J. 2006, 16, 1104–1112. [Google Scholar] [CrossRef]
- Adhikari, B.; Howes, T.; Bhandari, B.R.; Truong, V. Stickiness in foods: A review of mechanisms and test methods. Int. J. Food Prop. 2001, 4, 1–33. [Google Scholar] [CrossRef]
- Weenen, H.; Van Gemert, L.J.; Van Doorn, J.M.; Dijksterhuis, G.B.; De Wijk, R.A. Texture and mouthfeel of semisolid foods: Commercial mayonnaises, dressings, custard desserts and warm sauces. J. Texture Stud. 2003, 34, 159–179. [Google Scholar] [CrossRef]
- Niamah, A.K.; Sahi, A.A.; Al-Sharifi, A.S. Effect of feeding soy milk fermented by probiotic bacteria on some blood criteria and weight of experimental animals. Probiotics Antimicrob. Proteins 2017, 9, 284–291. [Google Scholar] [CrossRef]
| Encoding | A: Germination Day (d) | B: Fermentation Time (h) | C: Sugar Concentration (g L−1) |
|---|---|---|---|
| −1 | 0 | 24 | 5 |
| 0 | 1 | 48 | 10 |
| 1 | 3 | 72 | 15 |
| No. | A | B | C | GABA Conc (g L−1) |
|---|---|---|---|---|
| 1 | −1 | −1 | 0 | 2.228 |
| 2 | 1 | −1 | 0 | 2.252 |
| 3 | −1 | 1 | 0 | 2.240 |
| 4 | 1 | 1 | 0 | 2.256 |
| 5 | −1 | 0 | −1 | 2.229 |
| 6 | 1 | 0 | −1 | 2.232 |
| 7 | −1 | 0 | 1 | 2.252 |
| 8 | 1 | 0 | 1 | 2.256 |
| 9 | 0 | −1 | −1 | 2.254 |
| 10 | 0 | 1 | −1 | 2.262 |
| 11 | 0 | −1 | 1 | 2.250 |
| 12 | 0 | 1 | 1 | 2.259 |
| 13 | 0 | 0 | 0 | 2.319 |
| 14 | 0 | 0 | 0 | 2.356 |
| 15 | 0 | 0 | 0 | 2.360 |
| 16 | 0 | 0 | 0 | 2.358 |
| 17 | 0 | 0 | 0 | 2.358 |
| Source of Mean Square | Sum of Squares | Degree of Freedom | Mean Square | F | Pr > F | Significance |
|---|---|---|---|---|---|---|
| Model | 0.038444824 | 9 | 0.004271647 | 17.50715543 | 0.000525758 | Significant |
| A | 0.000282031 | 1 | 0.000282031 | 1.15589251 | 0.317973848 | ns |
| B | 0.000140281 | 1 | 0.000140281 | 0.574936452 | 0.47305463 | ns |
| C | 0.000210125 | 1 | 0.000210125 | 0.861187949 | 0.384288761 | ns |
| AB | 1.225 × 10−5 | 1 | 1.225 × 10−5 | 0.050206079 | 0.829104465 | ns |
| AC | 5.625 × 10−7 | 1 | 5.625 × 10−7 | 0.002305381 | 0.963045971 | ns |
| BC | 5.625 × 10−7 | 1 | 5.625 × 10−7 | 0.002305381 | 0.963045971 | ns |
| A2 | 0.015226182 | 1 | 0.015226182 | 62.40382843 | 9.87946E−05 | ** |
| B2 | 0.008961845 | 1 | 0.008961845 | 36.72972303 | 0.000510618 | * |
| C2 | 0.009705095 | 1 | 0.009705095 | 39.77590003 | 0.000401524 | * |
| Residual | 0.001707961 | 7 | 0.000243994 | |||
| Lack of Fit | 0.000496813 | 3 | 0.000165604 | 0.546932882 | 0.67624669 | ns |
| Pure Error | 0.001211148 | 4 | 0.000302787 | |||
| Cor Total | 0.040152785 | 16 |
| Item | Texture Analysis | Protein (%) | |||
|---|---|---|---|---|---|
| Firmness | Work of Shear | Stickiness | Work of Adhesion | ||
| Fermented bean sprout yogurt-like product | 8.81 ± 0.31 a | 4.4 ± 0.21 a | −7.85 ± 0.07 b | −11.55 ± 0.13 b | 4.41 ± 0.05 a |
| Fermented soybean flour yogurt-like product | 4.55 ± 0.29 b | 4.22 ± 0.19 a | −7.97 ± 0.11 b | −11.26 ± 0.31 b | 2.93 ± 0.31 c |
| Fermented milk yogurt | 5.78 ± 0.42 c | 1.93 ± 0.07 b | −1.53 ± 0.27 a | −1.05 ± 0.31 a | 3.22 ± 0.17 b |
| Item | Soybean Sprout Yogurt-Like Product | Commercially Available Yogurt |
|---|---|---|
| Appearance | 7.75 ± 0.56 a | 7.88 ± 0.48 a |
| Odor | 5.63 ± 0.41 b | 7.75 ± 0.29 a |
| Acidity | 7.25 ± 0.25 a | 7.25 ± 0.29 a |
| Thickness | 7.63 ± 0.22 a | 7.75 ± 0.41 a |
| Fluidness | 7.15 ± 0.25 a | 7.25 ± 0.41 a |
| Taste | 6.75 ± 0.35 b | 7.13 ± 0.48 a |
| Overall acceptance | 7.75 ± 0.41 a | 7.75 ± 0.29 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhu, M.; Lu, W.; Zhang, C.; Chen, D.; Shah, N.P.; Xiao, C. Optimizing Levilactobacillus brevis NPS-QW 145 Fermentation for Gamma-Aminobutyric Acid (GABA) Production in Soybean Sprout Yogurt-like Product. Foods 2023, 12, 977. https://doi.org/10.3390/foods12050977
Zhang Y, Zhu M, Lu W, Zhang C, Chen D, Shah NP, Xiao C. Optimizing Levilactobacillus brevis NPS-QW 145 Fermentation for Gamma-Aminobutyric Acid (GABA) Production in Soybean Sprout Yogurt-like Product. Foods. 2023; 12(5):977. https://doi.org/10.3390/foods12050977
Chicago/Turabian StyleZhang, Yue, Mengjiao Zhu, Wenjing Lu, Cen Zhang, Di Chen, Nagendra P. Shah, and Chaogeng Xiao. 2023. "Optimizing Levilactobacillus brevis NPS-QW 145 Fermentation for Gamma-Aminobutyric Acid (GABA) Production in Soybean Sprout Yogurt-like Product" Foods 12, no. 5: 977. https://doi.org/10.3390/foods12050977
APA StyleZhang, Y., Zhu, M., Lu, W., Zhang, C., Chen, D., Shah, N. P., & Xiao, C. (2023). Optimizing Levilactobacillus brevis NPS-QW 145 Fermentation for Gamma-Aminobutyric Acid (GABA) Production in Soybean Sprout Yogurt-like Product. Foods, 12(5), 977. https://doi.org/10.3390/foods12050977







