Development Control and Inactivation of Byssochlamys nivea Ascospores by Hyperbaric Storage at Room Temperature
Abstract
1. Introduction
2. Materials and Methods
2.1. Culture Media and Chemicals
2.2. Ascospore Production, Harvesting and Storage
2.3. Ascospore Inoculation and Hyperbaric Storage Conditions
2.4. Pre-Activation
2.5. Hyperbaric Storage Conditions
2.6. Determination of Ascospore Germination and Inactivation
2.7. First-Order Kinetic Model of B. nivea Ascospores Inactivation
2.8. Phase-Contrast Microscopy
2.9. Statistical Analyses
3. Results
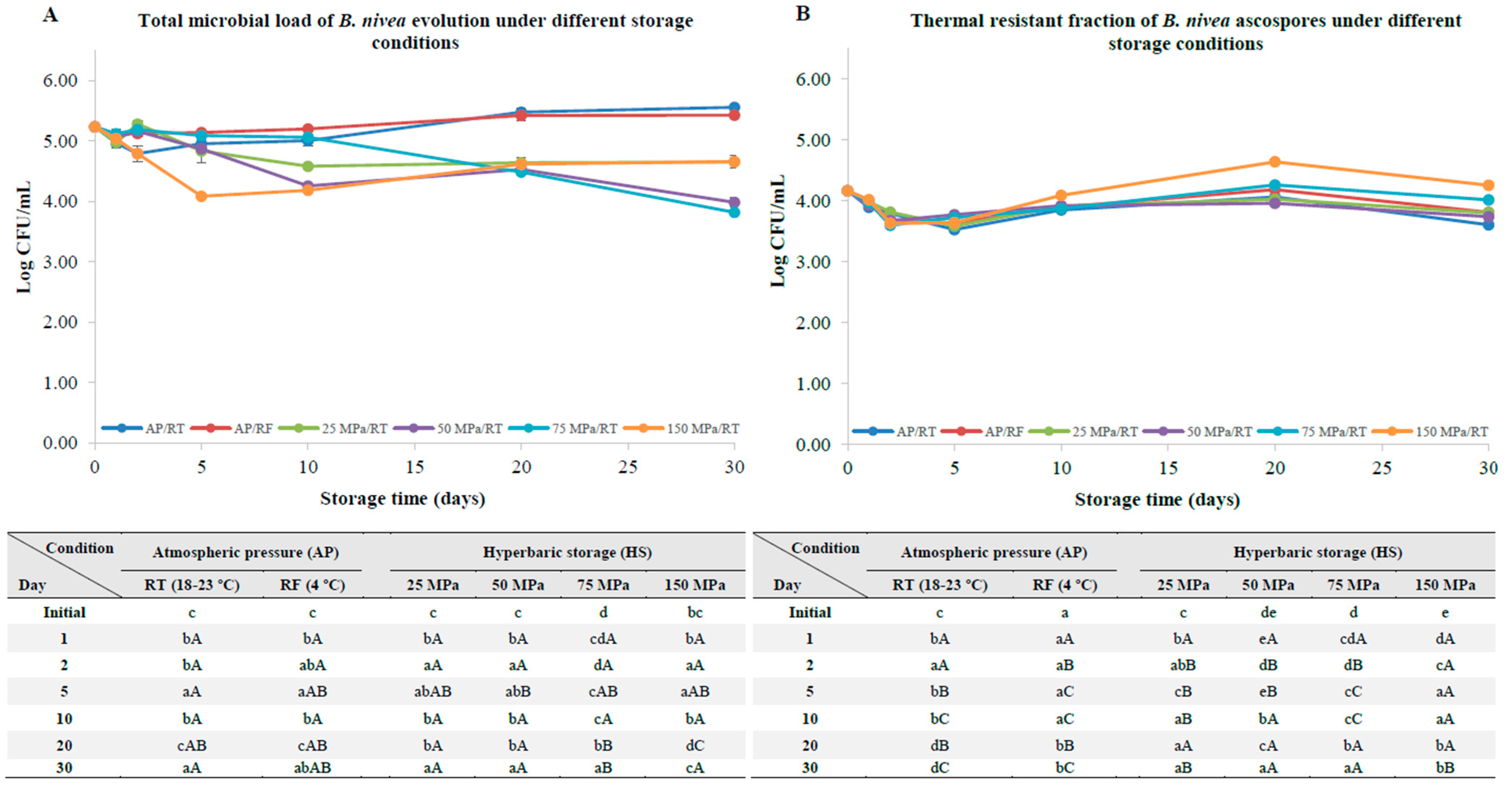
3.1. B. nivea Ascospores without a Pre-Activation Step
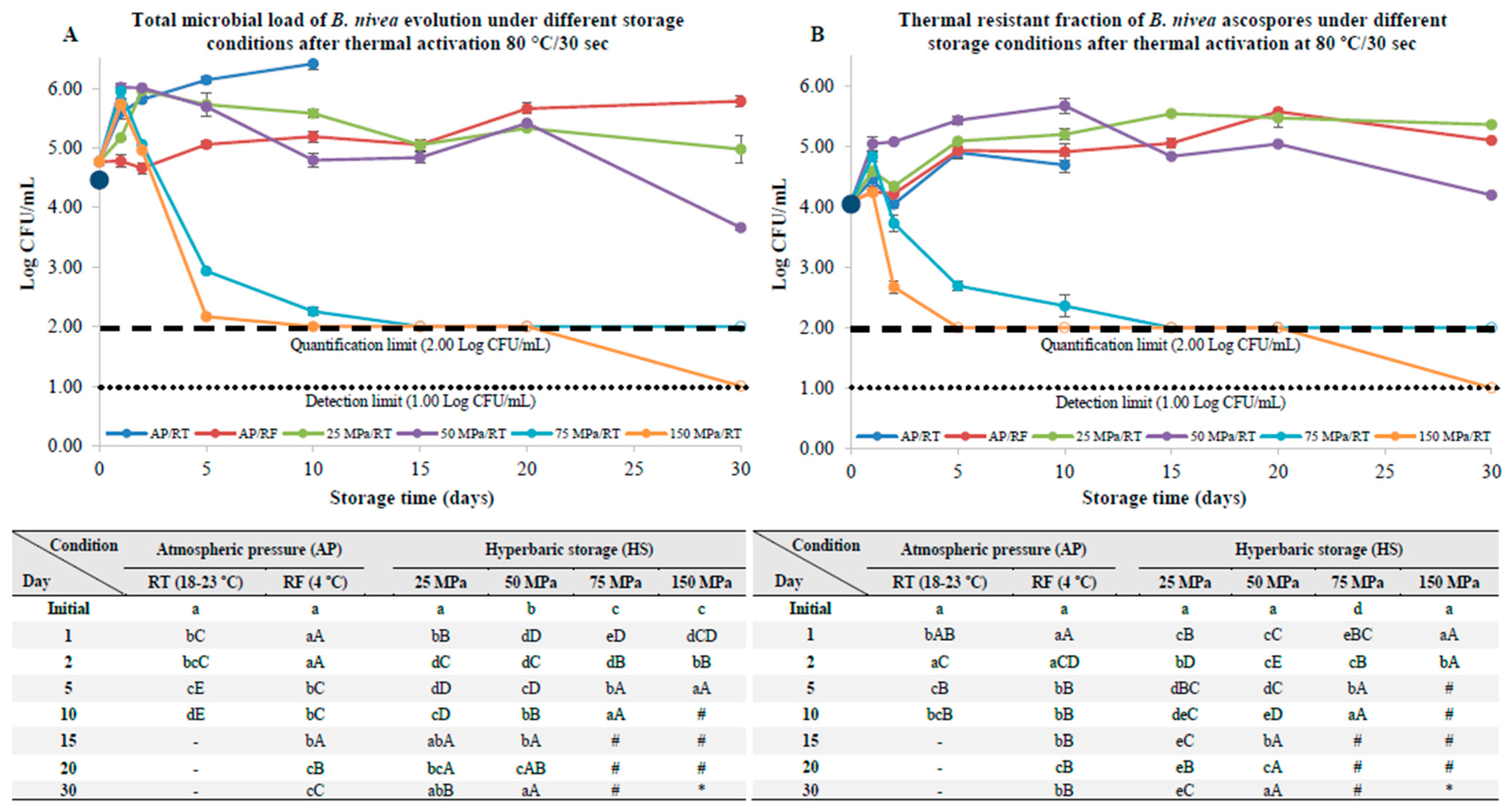
3.2. B. nivea Ascospores Pre-Activated by Thermal Pasteurization
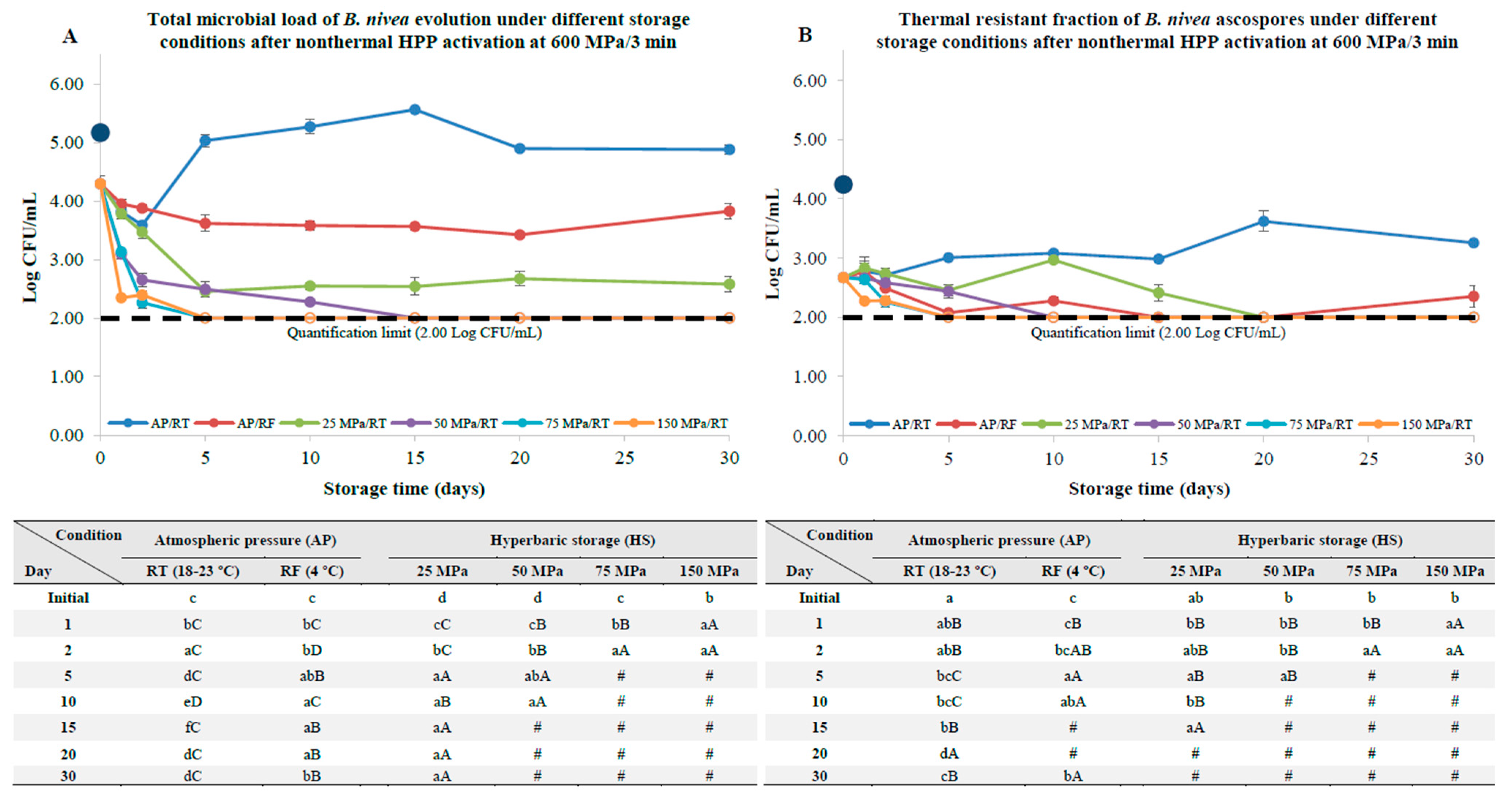
3.3. B. nivea Ascospores Pre-Activation by HPP
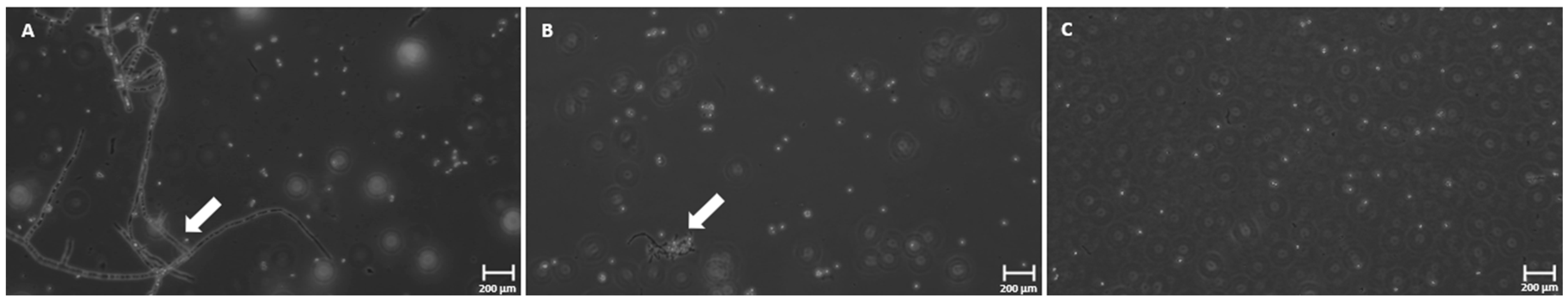
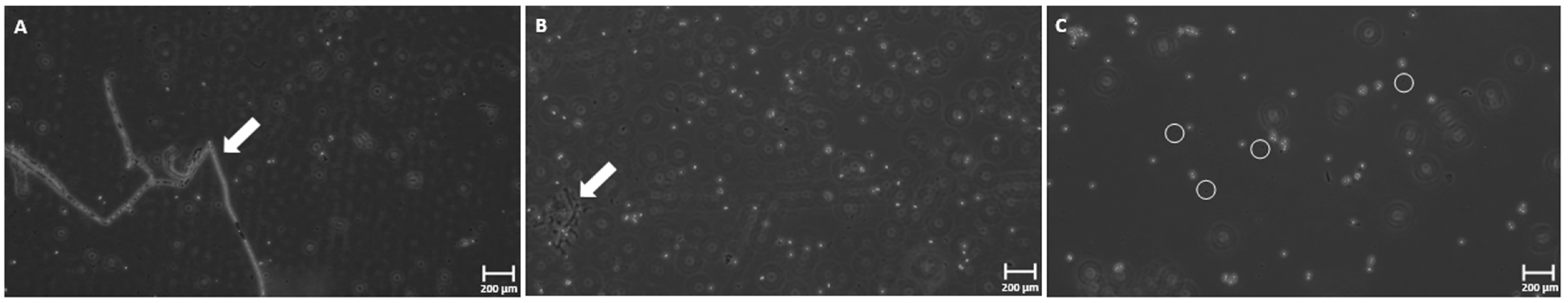
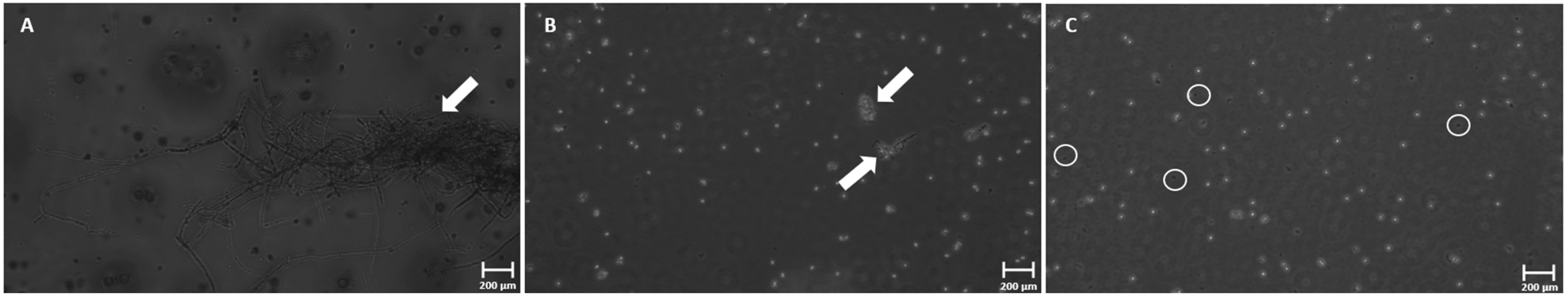
3.4. Phase-Contrast Microscopy
4. Discussion
4.1. B. nivea Ascospores without a Pre-Activation Step
4.2. B. nivea Ascospores with a Thermal and Nonthermal Pasteurization Step
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aguilar-Marcelino, L.; Mendoza-de-Gives, P.; Al-Ani, L.K.T.; López-Arellano, M.E.; Gómez-Rodríguez, O.; Villar-Luna, E.; Reyes-Guerrero, D.E. Using Molecular Techniques Applied to Beneficial Microorganisms as Biotechnological Tools for Controlling Agricultural Plant Pathogens and Pest. In Molecular Aspects of Plant Beneficial Microbes in Agriculture; Sharma, V., Salwan, R., Al-Ani, L.K.T., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 333–349. ISBN 978-0-12-818469-1. [Google Scholar]
- Trail, F. Fungal Cannons: Explosive Spore Discharge in the Ascomycota. FEMS Microbiol. Lett. 2007, 276, 12–18. [Google Scholar] [CrossRef]
- Begum, M.; Hocking, A.; Miskelly, D. Inactivation of Food Spoilage Fungi by Ultra Violet (UVC) Irradiation. Int. J. Food Microbiol. 2009, 129, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Sauceda-Gálvez, J.N.; Roca-Couso, R.; Martinez-Garcia, M.; Hernández-Herrero, M.M.; Gervilla, R.; Roig-Sagués, A.X. Inactivation of Ascospores of Talaromyces Macrosporus and Neosartorya Spinosa by UV-C, UHPH and Their Combination in Clarified Apple Juice. Food Control. 2019, 98, 120–125. [Google Scholar] [CrossRef]
- Dijksterhuis, J. Heat-Resistant Ascospores. In Food Mycology: A Multifaceted Approach to Fungi and Food; Dijksterhuis, J., Samson, R.A., Eds.; CRC Press, Taylor and Francis: Boca Raton, FL, USA, 2007; pp. 101–118. ISBN 978-0-8493-9818-6. [Google Scholar]
- Pinto, C.A.; Moreira, S.A.; Fidalgo, L.G.; Inácio, R.S.; Barba, F.J.; Saraiva, J.A. Effects of High-Pressure Processing on Fungi Spores: Factors Affecting Spore Germination and Inactivation and Impact on Ultrastructure. Compr. Rev. Food Sci. Food Saf. 2020, 19, 553–573. [Google Scholar] [CrossRef] [PubMed]
- Perdoncini, M.R.F.G.; Sereia, M.J.; Scopel, F.H.P.; Formigoni, M.; Rigobello, E.S.; Beneti, S.C.; Cardoso, F.A.R.; Marchi, L.B.; Junior, C.G.d.S.; Fernandes, P.G.M.; et al. Growth of Fungal Cells and the Production of Mycotoxins. In Cell Growth; IntechOpen Limited: London, UK, 2019; p. 23. ISBN 978-1-78984-557-0. [Google Scholar]
- Yousefi, M.; Mohammadi, M.A.; Khajavi, M.Z.; Ehsani, A.; Scholtz, V. Application of Novel Non-Thermal Physical Technologies to Degrade Mycotoxins. J. Fungi 2021, 7, 395. [Google Scholar] [CrossRef] [PubMed]
- Adeyeye, S.A.O. Fungal Mycotoxins in Foods: A Review. Cogent Food Agric. 2016, 2, 1213127. [Google Scholar] [CrossRef]
- Kotzekidou, P. Byssochlamys. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.L., Eds.; Academic Press: Oxford, UK, 2014; pp. 344–350. ISBN 978-0-12-384733-1. [Google Scholar]
- Dijksterhuis, J. Fungal Spores: Highly Variable and Stress-Resistant Vehicles for Distribution and Spoilage. Food Microbiol. 2019, 81, 2–11. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, J.L.P.; Samapundo, S.; Pimentel, G.C.; Van Impe, J.; Sant’Ana, A.S.; Devlieghere, F. Assessment of Minimum Oxygen Concentrations for the Growth of Heat-Resistant Moulds. Food Microbiol. 2019, 84, 103243. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.V.M. Evelyn Resistant Moulds as Pasteurization Target for Cold Distributed High Pressure and Heat Assisted High Pressure Processed Fruit Products. J. Food Eng. 2020, 282, 109998. [Google Scholar] [CrossRef]
- Maruyama, S.; Streletskaya, N.A.; Lim, J. Clean Label: Why This Ingredient but Not That One? Food Qual. Prefer. 2021, 87, 104062. [Google Scholar] [CrossRef]
- Pinto, C.A.; Martins, A.P.; Santos, M.D.; Fidalgo, L.G.; Delgadillo, I.; Saraiva, J.A. Growth Inhibition and Inactivation of Alicyclobacillus Acidoterrestris Endospores in Apple Juice by Hyperbaric Storage at Ambient Temperature. Innov. Food Sci. Emerg. Technol. 2019, 52, 232–236. [Google Scholar] [CrossRef]
- Patriarca, A. Fungi and Mycotoxin Problems in the Apple Industry. Curr. Opin. Food Sci. 2019, 29, 42–47. [Google Scholar] [CrossRef]
- Evelyn; Silva, F.V.M. Resistance of Byssochlamys Nivea and Neosartorya Fischeri Mould Spores of Different Age to High Pressure Thermal Processing and Thermosonication. J. Food Eng. 2017, 201, 9–16. [Google Scholar] [CrossRef]
- Chapman, B.; Winley, E.; Fong, A.S.W.; Hocking, A.D.; Stewart, C.M.; Buckle, K.A. Ascospore Inactivation and Germination by High Pressure Processing Is Affected by Ascospore Age. Innov. Food Sci. Emerg. Technol. 2007, 8, 531–534. [Google Scholar] [CrossRef]
- Erkmen, O. Practice 6-Yeasts and Molds Counting Techniques. In Microbiological Analysis of Foods and Food Processing Environments; Erkmen, O., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 43–51. ISBN 978-0-323-91651-6. [Google Scholar]
- Beuchat, L.R. Surface Decontamination of Fruits and Vegetables Eaten Raw-A Review. 1998. Available online: https://apps.who.int/iris/handle/10665/64435 (accessed on 1 February 2023).
- Samapundo, S.; Vroman, A.; Eeckhout, M.; Devlieghere, F. Effect of Heat Treatment Intensity on the Survival, Activation and Subsequent Outgrowth of Byssochlamys Nivea Ascospores. Lwt 2018, 93, 599–605. [Google Scholar] [CrossRef]
- Bigelow, W.D. The Logarithmic Nature of Thermal Death Time Curves. J. Infect. Dis. 1921, 29, 528–536. [Google Scholar] [CrossRef]
- Dijksterhuis, J.; Wyatt, T.; Hanssen, M.; Golovina, E.; Hoekstra, F.; Lugones, L. Abundant Small Protein ICARUS Inside the Cell Wall of Stress-Resistant Ascospores of Talaromyces Macrosporus Suggests a Novel Mechanism of Constitutive Dormancy. J. Fungi 2021, 7, 216. [Google Scholar] [CrossRef]
- Pinto, C.A.; Holovicova, M.; Habanova, M.; Lima, V.; Duarte, R.V.; Barba, F.J.; Saraiva, J.A. Effects of High Hydrostatic Pressure on Fungal Spores and Plant Bioactive Compounds. Encyclopedia 2022, 2, 1453–1463. [Google Scholar] [CrossRef]
- Jin, Z.; Solanki, S.; Ameen, G.; Gross, T.; Poudel, R.S.; Borowicz, P.; Brueggeman, R.S.; Schwarz, P. Expansion of Internal Hyphal Growth in Fusarium Head Blight–Infected Grains Contributes to the Elevated Mycotoxin Production During the Malting Process. Mol. Plant-Microbe Interact. 2021, 34, 793–802. [Google Scholar] [CrossRef]
- Corradini, M.G.; Normand, M.D.; Eisenberg, M.; Peleg, M. Evaluation of a Stochastic Inactivation Model for Heat-Activated Spores of Bacillus Spp. Appl. Environ. Microbiol. 2010, 76, 4402–4412. [Google Scholar] [CrossRef]
- Evelyn; Kim, H.J.; Silva, F.V.M. Modeling the Inactivation of Neosartorya Fischeri Ascospores in Apple Juice by High Pressure, Power Ultrasound and Thermal Processing. Food Control. 2016, 59, 530–537. [Google Scholar] [CrossRef]
- Wargenau, A.; Kampen, I.; Kwade, A. Linking Aggregation of Aspergillus Niger Spores to Surface Electrostatics: A Theoretical Approach. Biointerphases 2013, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Chrevatidis, A. Mycotoxins | Occurrence and Determination. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Oxford, UK, 2003; pp. 4089–4096. ISBN 978-0-12-227055-0. [Google Scholar]
- Bermejo-Prada, A.; López-Caballero, M.E.; Otero, L. Hyperbaric Storage at Room Temperature: Effect of Pressure Level and Storage Time on the Natural Microbiota of Strawberry Juice. Innov. Food Sci. Emerg. Technol. 2016, 33, 154–161. [Google Scholar] [CrossRef]
- Fidalgo, L.G.; Santos, M.D.; Queirós, R.P.; Inácio, R.S.; Mota, M.J.; Lopes, R.P.; Gonçalves, M.S.; Neto, R.F.; Saraiva, J.A. Hyperbaric Storage at and above Room Temperature of a Highly Perishable Food. Food Bioprocess Technol. 2014, 7, 2028–2037. [Google Scholar] [CrossRef]
- Eicher, R.; Ludwig, H. Influence of Activation and Germination on High Pressure Inactivation of Ascospores of the Mould Eurotium Repens. Comp. Biochem. Physiol. Part A: Mol. Integr. Physiol. 2002, 131, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Maggi, A.; Gola, S.; Spotti, E.; Rovere, P.P. Mutti Trattamenti Ad Alta Pressione Di Ascospore Di Muffe Termoresistenti e Di Patulina.in Nettare Di Albicocca e in Acqua. Ind. Conserve 1994, 69, 26–29. [Google Scholar]
- Basso, F.; Manzocco, L.; Nicoli, M.C. Hyperbaric Storage of Food: Applications, Challenges, and Perspectives. Food Eng. Rev. 2021, 14, 20–30. [Google Scholar] [CrossRef]
- Santos, M.D.; Fidalgo, L.G.; Pinto, C.A.; Duarte, R.V.; Lemos, Á.T.; Delgadillo, I.; Saraiva, J.A. Hyperbaric Storage at Room like Temperatures as a Possible Alternative to Refrigeration: Evolution and Recent Advances. Crit. Rev. Food Sci. Nutr. 2020, 61, 2078–2089. [Google Scholar] [CrossRef]
- Dijksterhuis, J.; Van Driel, K.G.; Sanders, M.G.; Molenaar, D.; Houbraken, J.A.; Samson, R.A.; Kets, E.P. Trehalose Degradation and Glucose Efflux Precede Cell Ejection during Germination of Heat-Resistant Ascospores of Talaromyces Macrosporus. Arch. Microbiol. 2002, 178, 1–7. [Google Scholar] [CrossRef]
- Reineke, K.; Mathys, A. Endospore Inactivation by Emerging Technologies: A Review of Target Structures and Inactivation Mechanisms. Annu. Rev. Food Sci. Technol. 2020, 11, 255–274. [Google Scholar] [CrossRef]
- Evelyn; Silva, F.V.M. Inactivation of Byssochlamys Nivea Ascospores in Strawberry Puree by High Pressure, Power Ultrasound and Thermal Processing. Int. J. Food Microbiol. 2015, 214, 129–136. [Google Scholar] [CrossRef]
- Ferreira, E.H.d.R.; Masson, L.M.P.; Rosenthal, A.; Souza, M.d.L.; Tashima, L.; Massaguer, P.R. de Thermoresistance of Filamentous Fungi Isolated from Asceptically Packaged Fruit Nectars. Braz. J. Food Technol. 2011, 14, 164–171. [Google Scholar] [CrossRef]
- Dijksterhuis, J.; Samson, R.A. Activation of Ascospores by Novel Food Preservation Techniques. In Advances in Food Mycology; Hocking, A.D., Pitt, J.I., Samson, R.A., Thrane, U., Eds.; Springer US: Boston, MA, USA, 2006; pp. 247–260. [Google Scholar]
- Kaya Yıldırım, F.; Hatice Ulusoy, B.; Shifamussa Hamed, N. Heat-Resistant Moulds: Assessment, Prevention and Their Consequences for Food Safety and Public Health. Czech J. Food Sci. 2022, 40, 273–280. [Google Scholar] [CrossRef]
- Ferreira, E.H.d.R.; Rosenthal, A.; Calado, V.; Saraiva, J.; Mendo, S. Byssochlamys Nivea Inactivation in Pineapple Juice and Nectar Using High Pressure Cycles. J. Food Eng. 2009, 95, 664–669. [Google Scholar] [CrossRef]
- Kotzekidou, P. Heat Resistance of Byssochlamys Nivea, Byssochlamys Fulva and Neosartorya Fischeri Isolated from Canned Tomato Paste. J. Food Sci. 1997, 62, 410–412. [Google Scholar] [CrossRef]
- Stabel, J.R.; Lambertz, A. Efficacy of Pasteurization Conditions for the Inactivation of Mycobacterium Avium Subsp. Paratuberculosis in Milk. J. Food Prot. 2004, 67, 2719–2726. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto, C.A.; Galante, D.; Espinoza-Suarez, E.; Gaspar, V.M.; Mano, J.F.; Barba, F.J.; Saraiva, J.A. Development Control and Inactivation of Byssochlamys nivea Ascospores by Hyperbaric Storage at Room Temperature. Foods 2023, 12, 978. https://doi.org/10.3390/foods12050978
Pinto CA, Galante D, Espinoza-Suarez E, Gaspar VM, Mano JF, Barba FJ, Saraiva JA. Development Control and Inactivation of Byssochlamys nivea Ascospores by Hyperbaric Storage at Room Temperature. Foods. 2023; 12(5):978. https://doi.org/10.3390/foods12050978
Chicago/Turabian StylePinto, Carlos A., Diogo Galante, Edelman Espinoza-Suarez, Vítor M. Gaspar, João F. Mano, Francisco J. Barba, and Jorge A. Saraiva. 2023. "Development Control and Inactivation of Byssochlamys nivea Ascospores by Hyperbaric Storage at Room Temperature" Foods 12, no. 5: 978. https://doi.org/10.3390/foods12050978
APA StylePinto, C. A., Galante, D., Espinoza-Suarez, E., Gaspar, V. M., Mano, J. F., Barba, F. J., & Saraiva, J. A. (2023). Development Control and Inactivation of Byssochlamys nivea Ascospores by Hyperbaric Storage at Room Temperature. Foods, 12(5), 978. https://doi.org/10.3390/foods12050978







