Prunus lusitanica L. Fruits: A Promising Underexploited Source of Nutrients with Potential Economic Value
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Collection and Preparation of Plant Material
2.3. Nutritional Composition of Prunus lusitanica Fruits
2.3.1. Content of Dry Matter, Ash, and Moisture
2.3.2. Content in Total Protein, Total Fat, Carbohydrates, and Energy Value
2.3.3. Content of Dietary Fibre
2.3.4. Content in Soluble Sugars
2.4. Amino Acids Analysis
2.5. Quantification of Mineral Composition
2.6. Statistical Analysis
3. Results
3.1. Nutritional Composition of P. lusitanica Fruits Harvested from Different Locations in Each Year
3.2. Comparative Analysis of Nutritional Composition of Fruits Grown under Different Locations or Years
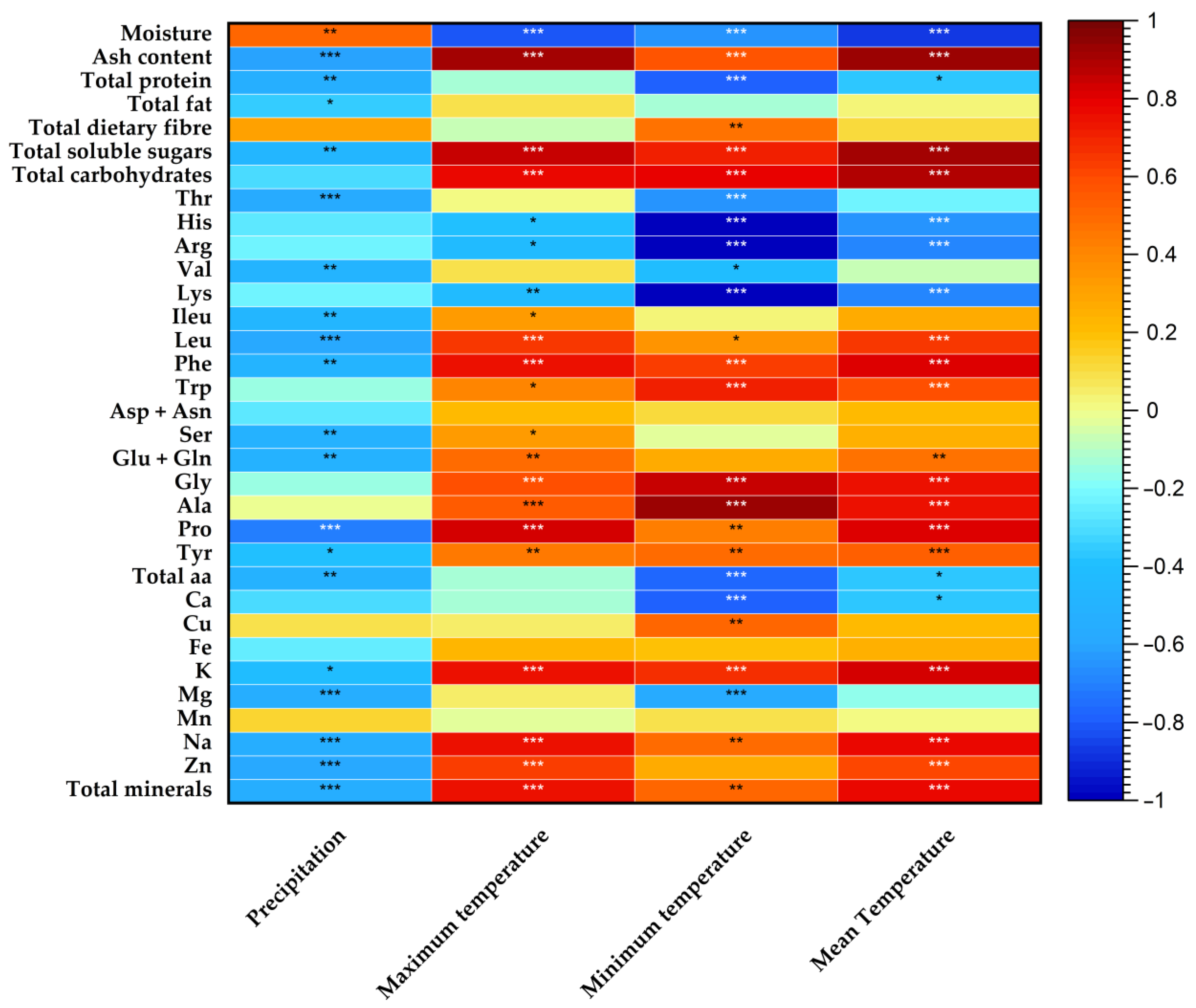
3.3. Pearson’s Correlation Analysis between Nutritional Composition and Meteorological Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luna-Vázquez, F.J.; Ibarra-alvarado, C.; Rojas-molina, A.; Rojas-molina, J.I. Prunus. In Fruit and Vegetable Phytochemicals: Chemistry and Human Health; John Wiley and Sons Ltd: West Sussex, UK, 2018; Volume II, pp. 1215–1226. [Google Scholar]
- Ullah, H.; De Filippis, A.; Khan, H.; Xiao, J.; Daglia, M. An overview of the health benefits of Prunus species with special reference to metabolic syndrome risk factors. Food Chem. Toxicol. 2020, 144, 111574. [Google Scholar] [CrossRef] [PubMed]
- Meena, N.K.; Choudhary, K. Nutritional Composition of Stone Fruits. In Production Technology of Stone Fruits; Springer: Singapore, 2021; ISBN 978-9-81158-920-1. [Google Scholar] [CrossRef]
- Bento, C.; Gonçalves, A.C.; Silva, B.; Silva, L.R. Peach (Prunus persica): Phytochemicals and Health Benefits. Food Rev. Int. 2020, 38, 1703–1734. [Google Scholar] [CrossRef]
- Komakech, R.; Kang, Y.; Lee, J.H.; Omujal, F. A review of the potential of phytochemicals from prunus Africana (Hook f.) kalkman stem bark for chemoprevention and chemotherapy of prostate cancer. Evid.-Based Complement. Altern. Med. 2017, 2017, 3014019. [Google Scholar] [CrossRef] [PubMed]
- Magiera, A.; Czerwińska, M.E.; Owczarek, A.; Marchelak, A.; Granica, S.; Olszewska, M.A. Polyphenol-Enriched Extracts of Prunus spinosa Fruits: Anti-Inflammatory and Antioxidant Effects in Human Immune Cells Ex Vivo in Relation to Phytochemical Profile. Molecules 2022, 27, 1691. [Google Scholar] [CrossRef] [PubMed]
- Piccolella, S.; Fiorentino, A.; Pacifico, S.; D’Abrosca, B.; Uzzo, P.; Monaco, P. Antioxidant properties of sour cherries (Prunus cerasus L.): Role of colorless phytochemicals from the methanolic extract of ripe fruits. J. Agric. Food Chem. 2008, 56, 1928–1935. [Google Scholar] [CrossRef] [PubMed]
- Telichowska, A.; Kobus-Cisowska, J.; Stuper-Szablewska, K.; Ligaj, M.; Tichoniuk, M.; Szymanowska, D.; Szulc, P. Exploring antimicrobial and antioxidant properties of phytocomponents from different anatomical parts of Prunus padus L. Int. J. Food Prop. 2020, 23, 2097–2109. [Google Scholar] [CrossRef]
- Fanning, K.J.; Topp, B.; Russell, D.; Stanley, R.; Netzel, M. Japanese plums (Prunus salicina Lindl.) and phytochemicals—Breeding, horticultural practice, postharvest storage, processing and bioactivity. J. Sci. Food Agric. 2014, 94, 2137–2147. [Google Scholar] [CrossRef]
- Kalpana, C.; Meena, N.K.; Prajapati, U. Orchard Factors Affecting Postharvest Quality of Stone Fruits. In Production Technology of Stone Fruits; Springer: Singapore, 2021; pp. 211–225. [Google Scholar]
- Komakech, R.; Kang, Y. Ethnopharmacological potential of African cherry [Prunus africana]. J. Herb. Med. 2019, 17, 100283. [Google Scholar] [CrossRef]
- Pignatti, S. Evolutionary Trends in Mediterranean Flora and Vegetation. In Plant Species and Plant Communities; Springer: Dordrecht, The Netherlands, 1978; pp. 157–167. [Google Scholar]
- do Céu Costa, M.; Duarte, P.; Neng, N.R.; Nogueira, J.M.F.; Costa, F.; Rosado, C. Novel insights for permeant lead structures through in vitro skin diffusion assays of Prunus lusitanica L., the Portugal Laurel. J. Mol. Struct. 2015, 1079, 327–336. [Google Scholar] [CrossRef]
- Wilson, B. Prunus lusitanica. In The IUCN Red List of Threatened Species 2021: E.T62857A64116943; International Union for Conservation of Nature and Natural Resources: Gland, Switzerland, 2021. [Google Scholar] [CrossRef]
- Carapeto, A.; Francisco, A.; Pereira, P.; Porto, M. Lista Vermelha da Flora Vascular de Portugal Continental; Sociedade Portuguesa de Botânica, Associação Portuguesa de Ciência da Vegetação—PHYTOS e Instituto da Conservação da Natureza e das Florestas (coord.); Coleção «Botânica em Português»; Imprensa Nacional: Lisboa, Portugal, 2020; Volume 7, ISBN 978-9-72272-876-8.
- Raposo, M.; Nunes, L.; Quinto-Canas, R.; del Río, S.; Pardo, F.M.V.; Galveias, A.; Pinto-Gomes, C.J. Prunus lusitanica L.: An Endangered Plant Species Relict in the Central Region of Mainland Portugal. Diversity 2021, 13, 359. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Stampar, F.; Veberic, R.; Sircelj, H.; Mikulic-Petkovsek, M.; Stampar, F.; Veberic, R.; Sircelj, H.; Mikulic-Petkovsek, M.; Stampar, F.; et al. Wild Prunus fruit species as a rich source of bioactive compounds. J. Food Sci. 2016, 81, C1928–C1937. [Google Scholar] [CrossRef] [PubMed]
- Plants for a Future Prunus lusitanica-L. Available online: https://pfaf.org/user/Plant.aspx?LatinName=Prunus+lusitanica (accessed on 7 February 2023).
- Plants, P. Prunus lusitanica Portugal Laurel a Perennial Woody Evergreen Member of the Prunus Genus in the Family Rosaceae. Available online: https://practicalplants.org/wiki/prunus_lusitanica/ (accessed on 7 February 2023).
- Magalhães, J.D.S. Exploring Plant Extracts for Cosmetic and Textile Industry. Ph.D. Thesis, Universidade do Minho, Braga, Portugal, 2021. [Google Scholar]
- Abraão, A.S.; Fernandes, N.; Silva, A.M.; Domínguez-Perles, R.; Barros, A. Prunus lusitanica L. Fruits as a Novel Source of Bioactive Compounds with Antioxidant Potential: Exploring the Unknown. Antioxidants 2022, 11, 1738. [Google Scholar] [CrossRef] [PubMed]
- Cornes, R.C.; van der Schrier, G.; van den Besselaar, E.J.M.; Jones, P.D. An Ensemble Version of the E-OBS Temperature and Precipitation Data Sets. J. Geophys. Res. Atmos. 2018, 123, 9391–9409. [Google Scholar] [CrossRef]
- AOAC. AOAC Official Methods of Analysis (2019), 21st ed.; AOAC International: Gaithersburg, MD, USA, 2019. [Google Scholar]
- Brusewitz, G.H. Density of Rewetted High Moisture Grains. Transit. ASAE 1975, 18, 935–938. [Google Scholar] [CrossRef]
- Crisan, V.; Sands, A. Nutritional Value of Edible Mushroom. In The Biology and Cultivation of Edible Mushrooms; Chang, S.T., Haye, W.T., Eds.; Academic Press: New York, NY, USA, 1978; pp. 137–168. [Google Scholar]
- Andrade, E.; Mendes-Ferreira, A.; Botelho, S.; Marques, G.; Cone, J.W.; Rodrigues, M.; Ferreira, L. Preservation of Fungal-Treated Cowpea Straw in Association with Discarded Apple by Ensilage Process. Waste Biomass Valorization 2021, 12, 5533–5543. [Google Scholar] [CrossRef]
- Savych, A.; Polonets, O.; Morozova, L.; Syrovatko, K.; Recun, T. HPLC-FLD analysis of amino acids content in Chrysanthemum morifolium. Pharmacia 2022, 69, 337–343. [Google Scholar] [CrossRef]
- Baltazar, M.; Oppolzer, D.; Carvalho, A.; Gouvinhas, I.; Ferreira, L.; Barros, A.; Lima-brito, J. Hydropriming and Nutripriming of Bread Wheat Seeds Improved the Flour’s Nutritional Value of the First Unprimed Offspring. Plants 2023, 12, 240. [Google Scholar] [CrossRef]
- Machado, N.; Oppolzer, D.; Ramos, A.; Ferreira, L.; Rosa, E.A.S.; Rodrigues, M.; Domínguez-Perles, R.; Barros, A.I.R.N.A. Evaluating the freezing impact on the proximate composition of immature cowpea (Vigna unguiculata L.) pods: Classical versus spectroscopic approaches. J. Sci. Food Agric. 2017, 97, 4295–4305. [Google Scholar] [CrossRef]
- Gouvinhas, I.; Santos, R.A.; Queiroz, M.; Leal, C.; Saavedra, M.J.; Domínguez-Perles, R.; Rodrigues, M.; Barros, A.I.R.N.A. Monitoring the antioxidant and antimicrobial power of grape (Vitis vinifera L.) stems phenolics over long-term storage. Ind. Crops Prod. 2018, 126, 83–91. [Google Scholar] [CrossRef]
- Rop, O.; Jurikova, T.; Mlcek, J.; Kramarova, D.; Sengee, Z. Antioxidant activity and selected nutritional values of plums (Prunus domestica L.) typical of the White Carpathian Mountains. Sci. Hortic. 2009, 122, 545–549. [Google Scholar] [CrossRef]
- Sánchez-Moreno, C.; Pascual-Teresa, S.; De Ancos, B.; Cano, P. Nutritional Values of Fruits. In Handbook of Fruits and Fruit Processing; Hui, Y.H., Ed.; Blackwell Publishing: Ames, IA, USA, 2006; pp. 29–43. [Google Scholar]
- Rejman, K.; Górska-Warsewicz, H.; Kaczorowska, J.; Laskowski, W. Nutritional significance of fruit and fruit products in the average polish diet. Nutrients 2021, 13, 2079. [Google Scholar] [CrossRef] [PubMed]
- Bastos, C.; Barros, L.; Dueñas, M.; Calhelha, R.C.; Queiroz, M.J.R.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Chemical characterisation and bioactive properties of Prunus avium L.: The widely studied fruits and the unexplored stems. Food Chem. 2015, 173, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Mihaylova, D.; Popova, A.; Desseva, I.; Petkova, N.; Stoyanova, M.; Vrancheva, R.; Slavov, A.; Slavchev, A.; Lante, A. Comparative Study of Early-and Mid-Ripening Peach (Prunus persica L.) Varieties: Biological Activity, Macro-, and Micro-Nutrient Profile. Foods 2021, 10, 164. [Google Scholar] [CrossRef] [PubMed]
- Ibourki, M.; Bouzid, H.A.; Bijla, L.; Aissa, R.; Sakar, E.H.; Ainane, T.; Gharby, S.; Hammadi, A. El Physical fruit traits, proximate composition, fatty acid and elemental profiling of almond [Prunus dulcis Mill. DA Webb] kernels from ten genotypes grown in southern Morocco. OCL-Oilseeds Fats Crop. Lipids 2022, 29, 9. [Google Scholar] [CrossRef]
- Smanalieva, J.; Iskakova, J.; Oskonbaeva, Z.; Wichern, F.; Darr, D. Determination of physicochemical parameters, phenolic content, and antioxidant capacity of wild cherry plum (Prunus divaricata Ledeb.) from the walnut-fruit forests of Kyrgyzstan. Eur. Food Res. Technol. 2019, 245, 2293–2301. [Google Scholar] [CrossRef]
- Al-Shehri, M.A.; El-Sheikh, M.A.; Al-Farhan, A.H.; Arif, I.A.; Rajakrishnan, R.; Alatar, A.A.; Faisal, M.; Basahi, R.A.; Al-Abbadi, G.A. Ecology of endangered Prunus korshinskyi Hand.-Mazz. in Jabal Al-Lauz, Saudi Arabia: Plant associations, size structure, and nutritional screening. Saudi J. Biol. Sci. 2020, 27, 147–156. [Google Scholar] [CrossRef]
- Haciseferoǧullari, H.; Gezer, I.; Özcan, M.M.; MuratAsma, B. Post-harvest chemical and physical-mechanical properties of some apricot varieties cultivated in Turkey. J. Food Eng. 2007, 79, 364–373. [Google Scholar] [CrossRef]
- Nisar, H.; Ahmed, M.; Anjum, M.A.; Hussain, S. Genetic Diversity in Fruit Nutritional Composition, Anthocyanins, Phenolics and Antioxidant Capacity of Plum (Prunus domestica) Genotypes. Acta Sci. Pol. Hortorum Cultus 2015, 14, 45–61. [Google Scholar]
- Jayarajan, S.; Sharma, R.R.; Sethi, S.; Saha, S.; Sharma, V.K.; Singh, S. Chemical and nutritional evaluation of major genotypes of nectarine (Prunus persica var nectarina) grown in North-Western Himalayas. J. Food Sci. Technol. 2019, 56, 4266–4273. [Google Scholar] [CrossRef]
- Measham, P.F.; Wilson, S.J.; Gracie, A.J.; Bound, S.A. Tree water relations: Flow and fruit. Agric. Water Manag. 2014, 137, 59–67. [Google Scholar] [CrossRef]
- Peschel, S.; Beyer, M.; Knoche, M. Surface characteristics of sweet cherry fruit: Stomata-number, distribution, functionality and surface wetting. Sci. Hortic. 2003, 97, 265–278. [Google Scholar] [CrossRef]
- Serradilla, M.J.; Akšic, M.F.; Manganaris, G.A.; Ercisli, S.; González-Gómez, D.; Valero, D. Fruit chemistry, nutritional benefits and social aspects of cherries. Cherries Bot. Prod. Uses 2017, 420–441. [Google Scholar] [CrossRef]
- Alasalvar, C.; Al-Farsi, M.; Shahidi, F.; Ar, A.R.; Ohamed, M.O.; Ohamed, O.; Arsi, F.F.F. Compositional characteristics and antioxidant components of cherry laurel varieties and pekmez. J. Food Sci. 2005, 70, S47–S52. [Google Scholar] [CrossRef]
- Vivek, K.; Mishra, S.; Pradhan, R.C. Physicochemical characterization and mass modelling of Sohiong (Prunus nepalensis L.) fruit. J. Food Meas. Charact. 2018, 12, 923–936. [Google Scholar] [CrossRef]
- Andronie, L.; Holonec, L.; Pop, I.; Truta, A.M.; Odagiu, A.; Sălăgean, T.; Sobolu, R.; Coroian, A.; Balta, I.; Şuba, E.E. Antioxidant capacity of several romanian forest fruits (Rosa canina L., Prunus spinosa L., Vaccium vitis-idaea L. and Cornus mas L.). Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 1178–1184. [Google Scholar] [CrossRef]
- Iordanescu, O.A.; Alexa, E.; Lalescu, D.; Berbecea, A.; Camen, D.; Poiana, M.A.; Moigradean, D.; Bala, M. Chemical composition and antioxidant activity of some apricot varieties at different ripening stages. Chil. J. Agric. Res. 2018, 78, 266–275. [Google Scholar] [CrossRef]
- Telichowska, A.; Kobus-Cisowska, J.; Szulc, P. Phytopharmacological Possibilities of Bird Cherry Prunus padus L. and Prunus serotina L. Species and Their Bioactive Phytochemicals. Nutrients 2020, 12, 1966. [Google Scholar] [CrossRef]
- Chyne, D.A.L.; Ananthan, R.; Longvah, T. Food compositional analysis of Indigenous foods consumed by the Khasi of Meghalaya, North-East India. J. Food Compos. Anal. 2019, 77, 91–100. [Google Scholar] [CrossRef]
- Shariatifar, N.; Pourfard, I.M.; Khaniki, G.J.; Nabizadeh, R.; Akbarzadeh, A.; Nejad, A.S.M. Mineral composition, physico-chemical properties and fatty acids profile of Prunus armeniaca apricot seed oil. Asian J. Chem. 2017, 29, 2011–2015. [Google Scholar] [CrossRef]
- Heldt, H.-W.; Piechulla, B. Lipids Are Membrane Constituents and Function as Carbon Stores. In Plant Biochemistry; Elsevier Inc.: London, UK, 2011; pp. 359–398. [Google Scholar]
- Sakar, E.H.; El Yamani, M.; Boussakouran, A.; Ainane, A.; Ainane, T.; Gharby, S.; Rharrabti, Y. Variability of oil content and its physicochemical traits from the main almond [Prunus dulcis Mill. DA Webb] cultivars grown under contrasting environments in north-eastern Morocco. Biocatal. Agric. Biotechnol. 2021, 32, 101952. [Google Scholar] [CrossRef]
- Askin, M.A.; Balta, M.F.; Tekintas, F.E.; Kazankaya, A.; Balta, F. Fatty acid composition affected by kernel weight in almond [Prunus dulcis (Mill.) D.A. Webb.] genetic resources. J. Food Compos. Anal. 2007, 20, 7–12. [Google Scholar] [CrossRef]
- Asp, N.G. Dietary carbohydrates: Classification by chemistry and physiology. Food Chem. 1996, 57, 9–14. [Google Scholar] [CrossRef]
- Blando, F.; Oomah, B.D. Sweet and sour cherries: Origin, distribution, nutritional composition and health benefits. Trends Food Sci. Technol. 2019, 86, 517–529. [Google Scholar] [CrossRef]
- Neenu, S.; Biswas, A.K.; Subba Rao, A. Impact of Climatic Factors on Crop Production—A Review. Agric. Rev. 2013, 34, 97–106. [Google Scholar]
- Pacifico, S.; Di Maro, A.; Petriccione, M.; Galasso, S.; Piccolella, S.; Di Giuseppe, A.M.A.; Scortichini, M.; Monaco, P. Chemical composition, nutritional value and antioxidant properties of autochthonous Prunus avium cultivars from Campania Region. Food Res. Int. 2014, 64, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Saidani, F.; Giménez, R.; Aubert, C.; Chalot, G.; Betrán, J.A.; Gogorcena, Y. Phenolic, sugar and acid profiles and the antioxidant composition in the peel and pulp of peach fruits. J. Food Compos. Anal. 2017, 62, 126–133. [Google Scholar] [CrossRef]
- Guo, C.; Bi, J.; Li, X.; Lyu, J.; Zhou, M.; Wu, X. Antioxidant profile of thinned young and ripe fruits of Chinese peach and nectarine varieties. Int. J. Food Prop. 2020, 23, 1272–1286. [Google Scholar] [CrossRef]
- Tamura, M.; Ohnishi, Y.; Kotani, T.; Gato, N. Effects of new dietary fiber from Japanese apricot (Prunus mume Sieb. et Zucc.) on gut function and intestinal microflora in adult mice. Int. J. Mol. Sci. 2011, 12, 2088–2099. [Google Scholar] [CrossRef]
- European Parliament & Council Regulation (EC). No 1924/2006 of the European Par-liament and the of the Council on nutrition and health claims made on foods. Off. J. Eur. Union 2006, 404, 9–25. [Google Scholar]
- Gill, S.K.; Lever, E.; Emery, P.W.; Whelan, K. Nutrient, fibre, sorbitol and chlorogenic acid content of prunes (Prunus domestica): An updated analysis and comparison of different countries of origin and database values. Int. J. Food Sci. Nutr. 2019, 70, 924–931. [Google Scholar] [CrossRef]
- Elsadr, H.T.; Sherif, S. Peaches and Nectarines, 1st ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; ISBN 978-0-12384-953-3. [Google Scholar]
- McCune, L.M.; Kubota, C.; Stendell-Hollis, N.R.; Thomson, C.A. Cherries and health: A review. Crit. Rev. Food Sci. Nutr. 2011, 51, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sochor, J.; Skutkova, H.; Babula, P.; Zitka, O.; Cernei, N.; Rop, O.; Krska, B.; Adam, V.; Provazník, I.; Kizek, R. Mathematical evaluation of the amino acid and polyphenol content and antioxidant activities of fruits from different apricot cultivars. Molecules 2011, 16, 7428–7457. [Google Scholar] [CrossRef] [PubMed]
- Iordănescu, O.A.; Alexa, E.; Radulov, I.; Costea, A.; Dobrei, A.; Dobrei, A. Minerals and Amino Acids in Peach (Prunus persica L.) Cultivars and Hybrids Belonging to World Germoplasm Collection in the Conditions of West Romania. Agric. Agric. Sci. Procedia 2015, 6, 145–150. [Google Scholar] [CrossRef]
- Botoran, O.R.; Ionete, R.E.; Miricioiu, M.G.; Costinel, D.; Radu, G.L.; Popescu, R. Amino acid profile of fruits as potential fingerprints of varietal origin. Molecules 2019, 24, 4500. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhao, D.; Liu, Q. Connections Between Amino Acid Metabolisms in Plants: Lysine as an Example. Front. Plant Sci. 2020, 11, 928. [Google Scholar] [CrossRef] [PubMed]
- Dukhi, N. Global Prevalence of Malnutrition: Evidence from Literature. Malnutrition 2020, 1, 1–16. [Google Scholar] [CrossRef]
- Salami, S.O.; Adegbaju, O.D.; Idris, O.A.; Jimoh, M.O.; Olatunji, T.L.; Omonona, S.; Orimoloye, I.R.; Adetunji, A.E.; Olusola, A.; Maboeta, M.S.; et al. South African wild fruits and vegetables under a changing climate: The implications on health and economy. S. Afr. J. Bot. 2022, 145, 13–27. [Google Scholar] [CrossRef]
- Wojdyło, A.; Nowicka, P.; Turkiewicz, I.P.; Tkacz, K. Profiling of polyphenols by LC-QTOF/ESI-MS, characteristics of nutritional compounds and in vitro effect on pancreatic lipase, α-glucosidase, α-amylase, cholinesterase and cyclooxygenase activities of sweet (Prunus avium) and sour (P. cerasus) cherries le. Ind. Crops Prod. 2021, 174, 114214. [Google Scholar] [CrossRef]
- Alajil, O.; Sagar, V.R.; Kaur, C.; Rudra, S.G.; Sharma, R.R.; Kaushik, R.; Verma, M.K.; Tomar, M.; Kumar, M.; Mekhemar, M. Nutritional and phytochemical traits of apricots (Prunus armeniaca L.) for application in nutraceutical and health industry. Foods 2021, 10, 1344. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Jafari, S.M. The importance of minerals in human nutrition: Bioavailability, food fortification, processing effects and nanoencapsulation. Trends Food Sci. Technol. 2017, 62, 119–132. [Google Scholar] [CrossRef]
- Jurikova, T.; Sochor, J.; Rop, O.; Mlček, J.; Balla, Š.; Szekeres, L.; Žitný, R.; Zitka, O.; Adam, V.; Kizek, R. Evaluation of polyphenolic profile and nutritional value of non-traditional fruit species in the Czech Republic—A comparative study. Molecules 2012, 17, 8968–8981. [Google Scholar] [CrossRef]
- Motyleva, S.; Upadysheva, G.; Tumaeva, T. The Mineral Composition Of Cherry (Prunus Cerasus Mill.) Fruits Depending On The Scion-Stock Combination. Potravin. Slovak J. Food Sci. 2021, 15, 74–82. [Google Scholar] [CrossRef]
- Telichowska, A.; Kobus-Cisowska, J.; Ligaj, M.; Stuper-Szablewska, K.; Szymanowska, D.; Tichoniuk, M.; Szulc, P. Polyphenol content and antioxidant activities of Prunus padus L. and Prunus serotina L. leaves: Electrochemical and spectrophotometric approach and their antimicrobial properties. Open Chem. 2020, 18, 1125–1135. [Google Scholar] [CrossRef]
- Cosmulescu, S.; Trandafir, I.; Nour, V. Phenolic acids and flavonoids profiles of extracts from edible wild fruits and their antioxidant properties. Int. J. Food Prop. 2017, 20, 3124–3134. [Google Scholar] [CrossRef]
- Soetan, K.O.; Olaiya, C.O.; Oyewole, O.E. The importance of mineral elements for humans, domestic animals and plants: A review. Afr. J. Food Sci. 2010, 4, 200–222. [Google Scholar]
- Thouraya, A.-G.; Ali, A.; Antonio Campoy, J.; Majid, M.; Hela, B.A.; Youssef, A. Effect of soil mineralogical composition on fruit quality of sweet cherry cultivars. Int. J. Agron. Agric. Res. 2016, 9, 45–56. [Google Scholar]
- Sun, H.; Huang, X.; Chen, T.; Zhou, P.; Huang, X.; Jin, W.; Liu, D.; Zhang, H.; Zhou, J.; Wang, Z.; et al. Fruit quality prediction based on soil mineral element content in peach orchard. Food Sci. Nutr. 2022, 10, 1756–1767. [Google Scholar] [CrossRef] [PubMed]
- Babicki, S.; Arndt, D.; Marcu, A.; Liang, Y.; Grant, J.R.; Maciejewski, A.; Wishart, D.S. Heatmapper: Web-enabled heat mapping for all. Nucleic Acids Res. 2016, 44, W147–W153. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.M. Global Climate Change and Agriculture: An Economic Perspective. Am. J. Agric. Econ. 1989, 71, 1272–1279. [Google Scholar] [CrossRef]
- Beach, R.H.; Sulser, T.B.; Crimmins, A.; Cenacchi, N.; Cole, J.; Fukagawa, N.K.; Mason-D’Croz, D.; Myers, S.; Sarofim, M.C.; Smith, M.; et al. Combining the effects of increased atmospheric carbon dioxide on protein, iron, and zinc availability and projected climate change on global diets: A modelling study. Lancet Planet. Health 2019, 3, e307–e317. [Google Scholar] [CrossRef]
- Bisbis, M.B.; Gruda, N.; Blanke, M. Potential impacts of climate change on vegetable production and product quality—A review. J. Clean. Prod. 2018, 170, 1602–1620. [Google Scholar] [CrossRef]
- Giulia, S.; Lea, B.F.; Carol, Z.C.; Lisa, M.; Harper, S.L.; Elizabeth, C.J. The effect of climatic factors on nutrients in foods: Evidence from a systematic map. Environ. Res. Lett. 2020, 15, 113002. [Google Scholar] [CrossRef]
- Téllez-Pérez, C.; Cardador-Martínez, A.; Tejada-Ortigoza, V.; Soria-Mejía, M.C.; Balderas-León, I.; Alonzo-Macías, M. Antioxidant content of frozen, convective air-dried, freeze-dried, and swell-dried chokecherries (Prunus virginiana L.). Molecules 2020, 25, 1190. [Google Scholar] [CrossRef]
- Khammayom, N.; Maruyama, N.; Chaichana, C. The Effect of Climatic Parameters on Strawberry Production in a Small Walk-In Greenhouse. Agriengineering 2022, 4, 104–121. [Google Scholar] [CrossRef]
- Scheelbeek, P.F.D.; Bird, F.A.; Tuomisto, H.L.; Green, R.; Harris, F.B.; Joy, E.J.M.; Chalabi, Z.; Allen, E.; Haines, A.; Dangour, A.D. Effect of environmental changes on vegetable and legume yields and nutritional quality. Proc. Natl. Acad. Sci. USA 2018, 115, 6804–6809. [Google Scholar] [CrossRef]
- Dong, J.; Gruda, N.; Lam, S.K.; Li, X.; Duan, Z. Effects of elevated CO2 on nutritional quality of vegetables: A review. Front. Plant Sci. 2018, 9, 924. [Google Scholar] [CrossRef]
- Christopoulos, M.; Ouzounidou, G. Climate Change Effects on the Perceived and Nutritional Quality of Fruit and Vegetables. J. Innov. Econ. Manag. 2020, 34, 79–99. [Google Scholar] [CrossRef]
- Karagiannis, E.; Tanou, G.; Samiotaki, M.; Michailidis, M.; Diamantidis, G.; Minas, I.S.; Molassiotis, A. Comparative physiological and proteomic analysis reveal distinct regulation of peach skin quality traits by altitude. Front. Plant Sci. 2016, 7, 1689. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, I.; Giné-Bordonaba, J.; Garanto, X.; Reig, G. Rootstock affects quality and phytochemical composition of ‘Big Top’nectarine fruits grown under hot climatic conditions. Sci. Hortic. 2019, 256, 108586. [Google Scholar] [CrossRef]
- López-Ortiz, C.M.; Prats-Moya, S.; Beltrán Sanahuja, A.; Maestre-Pérez, S.E.; Grané-Teruel, N.; Martín-Carratalá, M.L. Comparative study of tocopherol homologue content in four almond oil cultivars during two consecutive years. J. Food Compos. Anal. 2008, 21, 144–151. [Google Scholar] [CrossRef]
- Čolić, S.D.; Bakić, I.V.; Dabić Zagorac, D.; Natić, M.M.; Smailagić, A.T.; Pergal, M.V.; Pešić, M.B.; Milinčić, D.D.; Rabrenović, B.B.; Fotirić Akšić, M.M. Chemical Fingerprint and Kernel Quality Assessment in Different Grafting Combinations of Almond Under Stress Condition. Sci. Hortic. 2021, 275, 109705. [Google Scholar] [CrossRef]
- Lakatos, L.; Szabó, T.; Szabó, Z.; Soltész, M.; Nyéki, J.; Sun, Z.; Dussi, M.C. The influence of meteorological variables on sour cherry quality parameters. Acta Hortic. 2014, 1020, 287–292. [Google Scholar] [CrossRef]

| Nutritional Composition | Location | p-Value | ||
|---|---|---|---|---|
| Location 1 | Location 2 | Location 3 | ||
| Energy value (kcal/100 g) | 159.37 ± 0.35 b | 160.2 ± 0.09 b | 133.9 ± 0.77 a | <0.001 |
| Basic nutrients (g/100 g fw) | ||||
| Ash | 1.62 ± 0.00 c | 1.51 ± 0.00 b | 1.36 ± 0.00 a | <0.001 |
| Moisture | 62.51 ± 0.00 a | 63.28 ± 0.00 b | 69.96 ± 0.00 c | <0.001 |
| Protein | 3.26 ±0.12 b | 3.01 ±0.13 b | 2.73 ± 0.01 a | 0.002 |
| Fat | 2.33 ± 0.01 a | 3.11 ± 0.12 b | 3.32 ± 0.19 b | <0.001 |
| Carbohydrates | 30.29 ±0.13 c | 29.10 ± 0.24 b | 22.63 ± 0.20 a | <0.001 |
| Dietary Fibre | 9.80 ± 0.31 b | 10.26 ± 0.06 b | 8.22 ± 0.14 a | <0.001 |
| Soluble Sugar | 17.89 ± 0.15 b | 17.67 ± 0.10 b | 14.40 ± 0.02 a | <0.001 |
| Amino acids (mg/100 g fw) | ||||
| Essential amino acids | ||||
| His | 34.98 ± 2.32 b | 36.28 ± 1.88 b | 30.24 ± 0.78 a | 0.014 |
| Arg | 134.28 ± 7.55 a | 172.56 ± 20.61 b | 130.86 ± 4.88 a | 0.014 |
| Thr | 47.82 ± 1.11 bc | 53.23 ± 4.46 c | 43.14 ± 3.78 a | 0.032 |
| Val | 74.40 ± 2.93 bc | 87.09 ± 10.38 c | 70.34 ± 3.57 a | 0.047 |
| Lys | 78.70 ± 7.22 b | 128.25 ± 7.62 c | 60.77 ± 5.97 a | <0.001 |
| Ileu | 59.85 ± 0.65 bc | 67.24 ± 6.22 c | 55.53 ± 3.76 a | 0.038 |
| Leu | 146.71 ± 4.31 bc | 159.51 ± 10.17 c | 134.78 ± 4.28 a | 0.013 |
| Phe | 101.28 ± 6.92 bc | 108.21 ± 2.33 c | 90.32 ± 3.00 a | 0.008 |
| Trp | 13.92 ± 1.03 a | 13.67 ± 0.12 a | 12.62 ± 0.52 a | N.s. |
| Non-essential amino acids | ||||
| Asp + Asn | 580.63 ± 37.10 b | 700.49 ± 54.32 c | 467.84 ± 25.95 a | 0.001 |
| Ser | 55.32 ± 2.12 a | 71.65 ± 8.21 b | 52.04 ± 3.86 a | 0.009 |
| Glu + Gln | 720.28 ± 34.24 a | 828.28 ± 89.28 a | 691.79 ± 16.81 a | N.s. |
| Gly | 66.46 ± 8.97 a | 81.43 ± 2.66 c | 71.22 ± 2.74 bc | 0.043 |
| Ala | 83.94 ± 2.25 a | 95.80 ± 11.78 a | 83.06 ± 8.67 a | N.s. |
| Pro | 118.38 ± 4.74 b | 122.65 ± 7.74 b | 77.45 ± 3.47 a | <0.001 |
| Tyr | 828.70 ± 24.38 b | 814.62 ± 0.47 b | 730.01 ± 17.03 a | <0.001 |
| Total aa | 3145.66 ± 60.86 b | 3540.96 ± 129.65 c | 2802.00 ± 66.82 a | <0.001 |
| Minerals (mg/100 g fw) | ||||
| Ca | 0.72 ± 0.01 a | 0.78 ± 0.01 b | 0.88 ± 0.03 c | <0.001 |
| Cu | 0.40 ± 0.01 b | 0.41 ± 0.01 b | 0.27 ± 0.02 a | <0.001 |
| Fe | 1.29 ± 0.15 a | 1.36 ± 0.03 a | 1.36 ± 0.09 a | N.s. |
| K | 4.89 ± 0.04 b | 4.81 ± 0.04 b | 3.49 ± 0.10 a | <0.001 |
| Mg | 0.88 ± 0.03 b | 0.93 ± 0.01 b | 0.79 ± 0.03 a | 0.001 |
| Mn | 0.16 ± 0.00 a | 0.18 ± 0.00 a | 0.19 ± 0.02 a | 0.057 |
| Na | 2.36 ± 0.15 b | 2.36 ± 0.05 b | 2.03 ± 0.03 a | 0.006 |
| Zn | 0.51 ± 0.00 b | 0.48 ± 0.05 b | 0.40 ± 0.02 a | 0.013 |
| Total minerals | 11.22 ± 0.39 b | 11.31 ± 0.05 b | 9.40 ± 0.16 a | <0.001 |
| Nutritional Composition | Location | p-Value | ||
|---|---|---|---|---|
| Location 1 | Location 2 | Location 3 | ||
| Energy value (kcal/100 g) | 194.73 ± 1.8 b | 190.72 ± 0.17 a | 189.43 ± 1.94 a | 0.013 |
| Basic nutrients (g/100 g fw) | ||||
| Ash | 2.00 ± 0.00 a | 2.00 ± 0.00 a | 2.09 ± 0.00 b | 0.021 |
| Moisture | 55.87 ± 0.00 b | 55.73 ± 0.00 a | 56.22 ± 0.00 c | <0.001 |
| Protein | 4.82 ± 0.02 c | 3.79 ± 0.04 a | 4.22 ± 0.15 b | <0.001 |
| Fat | 5.32 ± 0.41 b | 3.43 ± 0.07 a | 3.94 ± 0.35 a | <0.001 |
| Carbohydrates | 31.99 ± 0.39 a | 35.05 ± 0.12 c | 33.53 ± 0.19 b | <0.001 |
| Dietary Fibre | 8.76 ± 0.64 a | 8.81 ± 0.61 a | 9.34 ± 0.15 a | N.s. |
| Soluble Sugar | 21.24 ± 0.12 a | 21.24 ± 0.10 a | 21.56 ± 0.18 a | N.s. |
| Amino acids (mg/100 g fw) | ||||
| Essential aminoacids | ||||
| His | 47.22 ± 5.25 a | 72.46 ± 1.68 b | 45.31 ± 5.80 a | <0.001 |
| Arg | 208.91 ± 10.83 ab | 173.02 ±9.04 a | 220.54 ± 25.55 b | 0.032 |
| Thr | 71.49 ± 0.53 a | 98.41 ± 7.05 b | 74.38 ± 8.34 a | 0.004 |
| Val | 117.20 ± 5.58 a | 126.67 ± 13.04 a | 117.11 ± 5.49 a | N.s. |
| Lys | 104.66 ± 5.05 a | 141.78 ± 19.91 b | 94.06 ± 12.88 a | 0.013 |
| Ileu | 97.15 ± 14.02 a | 80.48 ± 7.10 a | 96.96 ± 10.26 a | N.s. |
| Leu | 215.17 ± 14.64 a | 204.90 ± 22.41 a | 216.13 ± 8.05 a | N.s. |
| Phe | 142.85 ± 9.56 a | 132.31 ± 6.02 a | 149.15 ± 6.93 a | N.s. |
| Trp | 20.33 ± 1.65 b | 18.37 ± 0.49 ab | 16.90 ± 0.84 a | 0.025 |
| Non-essential aminoacids | ||||
| Asp + Asn | 830.27 ± 50.80 a | 731.35 ± 108.70 a | 701.44 ± 77.79 a | N.s. |
| Ser | 103.32 ± 10.71 a | 88.74 ± 11.52 a | 82.22 ± 4.74 a | N.s. |
| Glu + Gln | 1170.26 ± 59.03 a | 968.91 ± 42.41 a | 996.72 ± 135.70 a | N.s. |
| Gly | 124.85 ± 13.64 a | 170.93 ± 9.93 b | 107.30 ± 3.14 a | <0.001 |
| Ala | 136.04 ± 8.50 a | 119.08 ± 13.60 a | 116.13 ± 9.67 a | N.s. |
| Pro | 214.97 ± 14.03 a | 253.87 ± 19.68 a | 238.20 ± 22.36 a | N.s. |
| Tyr | 1124.83 ± 67.64 a | 1033.12 ± 32.84 a | 1002.93 ± 38.09 a | N.s. |
| Total aa | 472.53 ± 111.60 b | 4414.43 ± 225.90 ab | 4275.47 ± 185.82 a | 0.035 |
| Minerals (mg/100 g fw) | ||||
| Ca | 0.83 ± 0.01 b | 0.82 ± 0.00 b | 0.76 ± 0.04 a | 0.013 |
| Cu | 0.44 ± 0.00 a | 0.38 ± 0.04 a | 0.40 ± 0.00 a | N.s. |
| Fe | 1.83 ± 0.06 b | 1.82 ± 0.00 b | 1.67 ± 0.03 a | 0.006 |
| K | 6.03 ± 0.02 c | 5.85 ± 0.08 b | 5.71 ± 0.01 a | <0.001 |
| Mg | 1.06 ± 0.02 b | 0.99 ± 0.01 a | 0.99 ± 0.01 a | <0.001 |
| Mn | 0.17 ± 0.00 a | 0.17 ± 0.02 a | 0.15 ± 0.00 a | N.s. |
| Na | 2.79 ± 0.08 a | 2.98 ± 0.07 a | 3.94 ±0.08 b | <0.001 |
| Zn | 0.89 ± 0.07 b | 0.61 ± 0.05 a | 0.73 ± 0.02 a | 0.002 |
| Total minerals | 14.04 ± 0.13 b | 13.62 ± 0.12 a | 14.35 ± 0.02 c | <0.001 |
| Nutritional Composition | Location | p-Value | ||
|---|---|---|---|---|
| Location 1 | Location 2 | Location 3 | ||
| Energy value (kcal/100 g) | 181.78 ± 0.22 c | 165.72 ± 0.68 b | 157.93 ± 0.18 a | <0.001 |
| Basic nutrients (g/100 g fw) | ||||
| Ash | 1.75 ± 0.04 b | 1.48 ± 0.03 a | 1.50 ± 0.04 a | <0.001 |
| Moisture | 60.08 ± 0.04 a | 62.06 ± 0.04 b | 63.00 ± 0.25 c | <0.001 |
| Protein | 4.14 ± 0.03 c | 4.04 ± 0.02 b | 3.86 ± 0.01 a | <0.001 |
| Total Fat | 5.93 ± 0.08 c | 4.00 ± 0.18 b | 2.94 ± 0.05 a | <0.001 |
| Carbohydrates | 28.09 ± 0.11 a | 28.42 ± 0.19 b | 28.71 ± 0.06 b | 0.004 |
| Dietary Fibre | 10.81 ± 0.07 b | 8.60 ± 0.28 a | 12.08 ± 0.15 c | <0.001 |
| Soluble Sugar | 17.89 ± 0.05 b | 17.51 ± 0.10 a | 18.25 ± 0.03 c | <0.001 |
| Amino acids (mg/100 g fw) | ||||
| Essential | ||||
| His | 45.34 ± 0.67 b | 42.39 ± 0.38 ab | 37.07 ± 3.69 a | 0.010 |
| Arg | 223.72 ± 28.29 b | 207.40 ± 8.38 b | 145.15 ± 8.95 a | 0.004 |
| Thr | 93.28 ± 7.19 c | 66.29 ± 1.83 b | 52.58 ± 5.38 a | <0.001 |
| Val | 121.46 ± 3.10 b | 119.00 ± 6.61 b | 87.36 ± 3.89 a | <0.001 |
| Lys | 103.97 ± 4.03 c | 78.28 ± 3.66 b | 66.16 ± 0.06 a | <0.001 |
| Ileu | 101.31 ± 8.89 c | 81.94 ± 5.98 b | 65.05 ± 0.64 a | 0.001 |
| Leu | 212.56 ± 2.05 c | 178.81 ± 1.75 b | 153.07 ± 0.67 a | <0.001 |
| Phe | 135.65 ± 0.70 c | 123.52 ± 0.21 b | 104.93 ± 2.83 a | <0.001 |
| Trp | 20.50 ± 1.21 a | 20.33 ± 1.36 a | 19.21 ± 1.10 a | N.s. |
| Non-essential | ||||
| Asp + Asn | 821.32 ± 23.68 b | 825.12 ± 36.33 b | 572.06 ± 37.09 a | <0.001 |
| Ser | 110.29 ± 3.89 c | 72.23 ± 3.50 b | 56.75 ± 1.56 a | <0.001 |
| Glu + Gln | 1108.10 ± 14.06 b | 930.47 ± 6.99 a | 870.82 ± 77.59 a | 0.002 |
| Gly | 137.84 ± 7.27 b | 146.23 ± 10.99 b | 82.22 ± 6.47 a | <0.001 |
| Ala | 134.73 ± 2.94 b | 130.94 ± 1.19 b | 90.78 ± 4.69 a | <0.001 |
| Pro | 165.59 ± 4.11 a | 160.01 ± 4.97 a | 164.35 ± 0.11 a | N.s. |
| Tyr | 1101.32 ± 30.20 b | 1099.26 ± 56.64 b | 983.24 ± 51.08 a | 0.036 |
| Total aa | 4637.00 ± 39.94 c | 4282.22 ± 73.00 b | 3550.80 ± 121.30 a | <0.001 |
| Minerals (mg/100 g fw) | ||||
| Ca | 0.70 ± 0.04 b | 0.62 ± 0.01 a | 0.65 ± 0.00 ab | 0.013 |
| Cu | 0.58 ± 0.02 b | 0.38 ± 0.05 a | 0.57 ± 0.00 b | <0.001 |
| Fe | 2.09 ± 0.01 c | 1.77 ± 0.06 b | 1.50 ± 0.05 a | <0.001 |
| K | 5.62 ± 0.01 c | 4.83 ± 0.04 b | 4.36 ± 0.29 a | <0.001 |
| Mg | 1.09 ± 0.02 b | 0.88 ± 0.03 a | 0.91 ± 0.03 a | <0.001 |
| Mn | 0.21 ± 0.01 b | 0.10 ± 0.00 a | 0.20 ± 0.02 b | <0.001 |
| Na | 2.68 ± 0.01 b | 2.86 ± 0.06 c | 2.28 ± 0.06 a | <0.001 |
| Zn | 0.75 ± 0.03 c | 0.59 ± 0.00 b | 0.42 ± 0.02 a | <0.001 |
| Total minerals | 13.71 ± 0.01 c | 12.03 ± 0.15 b | 10.89 ± 0.42 a | <0.001 |
| Nutritional Composition | Location | p-Value | ||
|---|---|---|---|---|
| Location 1 | Location 2 | Location 3 | ||
| Energy value (kcal/100 g) | 137.64 ± 0.68 b | 145.89 ± 1.11 c | 120.79 ± 0.63 a | <0.001 |
| Basic nutrients (g/100 g fw) | ||||
| Ash | 1.34 ± 0.04 a | 1.37 ± 0.04 a | 1.32 ± 0.01 a | N.s. |
| Moisture | 68.86 ± 0.04 b | 66.50 ± 0.02 a | 69.71 ± 0.03 c | <0.001 |
| Protein | 4.99 ± 0.02 a | 5.64 ± 0.12 b | 6.45 ± 0.13 b | <0.001 |
| Total Fat | 4.84 ± 0.15 b | 4.95 ± 0.20 b | 2.78 ± 0.13 a | <0.001 |
| Carbohydrates | 19.97 ± 0.15 a | 21.54 ± 0.17 b | 19.73 ± 0.18 a | <0.001 |
| Dietary Fibre | 7.76 ± 0.16 b | 6.68 ± 0.18 a | 9.97 ± 0.10 c | <0.001 |
| Soluble Sugar | 14.16 ± 0.10 b | 15.08 ± 0.03 c | 13.32 ± 0.11 a | <0.001 |
| Amino acids (mg/100 g fw) | ||||
| Essential aminoacids | ||||
| His | 252.79 ± 16.27 a | 272.93 ± 25.55 ab | 321.65 ± 15.86 b | 0.013 |
| Arg | 1189.02 ± 20.02 a | 1292.62 ± 41.84 a | 1500.39 ± 74.59 b | <0.001 |
| Thr | 87.22 ± 12.54 a | 108.32 ± 15.33 a | 114.33 ± 4.31 a | N.s. |
| Val | 70.83 ± 9.04 a | 158.03 ± 0.36 b | 170.86 ± 6.77 b | <0.001 |
| Lys | 1256.57 ± 152.30 a | 1245.17 ± 126.50 a | 1323.38 ± 77.04 a | N.s. |
| Ileu | 58.38 ± 1.28 a | 62.72 ± 2.90 a | 112.35 ± 3.34 b | <0.001 |
| Leu | 135.71 ± 14.57 a | 147.72 ± 16.19 ab | 183.15 ± 17.22 b | 0.026 |
| Phe | 86.27 ± 4.73 a | 86.92 ± 9.14 a | 96.58 ± 8.09 a | N.s. |
| Trp | 8.58 ± 0.22 a | 9.66 ± 1.51 a | 14.15 ± 0.28 b | <0.001 |
| Non-essential aminoacids | ||||
| Asp + Asn | 460.08 ± 58.13 a | 547.31 ± 23.94 a | 973.62 ± 31.42 b | <0.001 |
| Ser | 56.97 ± 4.13 a | 101.13 ± 3.75 c | 79.23 ± 7.74 b | <0.001 |
| Glu + Gln | 727.64 ± 17.18 a | 771.24 ± 73.26 a | 998.65 ± 70.34 b | 0.003 |
| Gly | N.d. | 1.91 ± 0.06 b | 0.87 ± 0.08 a | <0.001 |
| Ala | 2.57 ± 0.30 a | 2.99 ± 0.11 a | 5.70 ± 0.77 b | <0.001 |
| Pro | 93.46 ± 4.41 a | 122.05 ± 3.99 b | 131.02 ± 5.20 b | <0.001 |
| Tyr | 714.63 ± 11.71 a | 820.28 ± 28.47 b | 923.00 ± 47.14 c | <0.001 |
| Total aa | 5200.74 ± 195.91 a | 5751.00 ± 204.15 b | 6948.93 ± 125.01 c | <0.001 |
| Minerals (mg/100 g fw) | ||||
| Ca | 1.03 ± 0.02 b | 1.17 ± 0.07 c | 0.89 ± 0.01 a | <0.001 |
| Cu | 0.31 ± 0.00 a | 0.32 ± 0.02 ab | 0.34 ± 0.01 b | 0.036 |
| Fe | 0.98 ± 0.01 a | 1.13 ± 0.05 a | 2.35 ± 0.12 b | <0.001 |
| K | 3.37 ± 0.03 a | 3.72 ± 0.07 b | 3.81 ± 0.21 b | 0.013 |
| Mg | 1.03 ± 0.03 a | 1.11 ± 0.05 ab | 1.13 ± 0.00 b | 0.017 |
| Mn | 0.17 ± 0.02 a | 0.15 ± 0.01 a | 0.17 ± 0.00 a | N.s. |
| Na | 2.04 ± 0.09 a | 2.23 ± 0.04 b | 2.11 ± 0.00 ab | 0.010 |
| Zn | 0.44 ± 0.01 a | 0.53 ± 0.01 b | 0.56 ± 0.01 c | <0.001 |
| Total minerals | 9.36 ± 0.10 a | 10.36 ± 0.30 b | 11.35 ± 0.33 c | <0.001 |
| Nutritional Composition | Location | Year | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Location 1 | Location 2 | Location 3 | p-Value | Y2016 | Y2017 | Y2018 | Y2019 | p-Value | |
| Energy value (kcal/100 g) | 168.38 ± 22.78 a | 165.63 ± 16.92 a | 150.51 ± 27.3 a | N.s. | 151.16 ± 12.95 b | 191.63 ± 2.74 d | 168.48 ± 10.54 c | 134.77 ± 11.1 a | <0.001 |
| Basic nutrients (g/100 g fw) | |||||||||
| Ash | 1.68 ± 0.25 a | 1.59 ± 0.25 a | 1.57 ± 0.32 a | N.s. | 1.50 ± 0.11 b | 2.03 ± 0.05 c | 1.58 ± 0.13 b | 1.34 ± 0.02 a | <0.001 |
| Moisture | 61.83 ± 4.91 a | 61.89 ± 4.08 a | 64.72 ± 5.90 a | N.s. | 65.25 ± 3.55 c | 55.94 ± 0.22 a | 61.71 ± 1.29 b | 68.36 ± 1.44 d | <0.001 |
| Protein | 4.30 ± 0.71 a | 4.12 ± 1.00 a | 4.31 ± 1.41 a | N.s. | 3.00 ± 0.24 a | 4.28 ± 0.46 b | 4.01 ± 0.13 b | 5.69 ± 0.64 c | <0.001 |
| Fat | 4.61 ± 1.44 b | 3.87 ± 0.74 ab | 3.24 ± 0.50 a | 0.007 | 2.92 ± 0.47 a | 4.23 ± 0.89 b | 4.29 ± 1.32 b | 4.19 ± 1.07 b | 0.015 |
| Carbohydrates | 27.58 ± 4.82 a | 28.53 ± 5.00 a | 26.15 ± 5.59 a | N.s. | 27.34 ± 3.57 b | 33.52 ± 1.35 c | 28.41 ± 0.29 b | 20.42 ± 0.86 a | <0.001 |
| Dietary Fibre | 9.28 ± 1.23 a | 8.59 ± 1.37 a | 9.90 ± 1.47 a | N.s. | 9.43 ± 0.94 ab | 8.97 ± 0.53 a | 10.50 ± 1.53 b | 8.14 ± 1.46 c | 0.002 |
| Soluble Sugar | 17.79 ± 2.62 a | 17.87 ± 2.29 a | 16.88 ± 3.41 a | N.s. | 16.65 ± 1.69 b | 21.34 ± 0.20 d | 17.88 ± 0.33 c | 14.18 ± 0.77 a | <0.001 |
| Amino acids (mg/100 g fw) | |||||||||
| Essential aminoacids | |||||||||
| His | 95.08 ± 95.51 a | 106.02 ± 102.25 a | 108.57 ± 128.82 a | N.s. | 33.83 ± 3.15 a | 55.00 ± 13.72 a | 41.60 ± 4.09 a | 282.46 ± 35.10 b | <0.001 |
| Arg | 438.98 ± 453.95 a | 461.40 ± 501.88 a | 499.23 ± 605.71 a | N.s. | 145.90 ± 22.99 a | 200.82 ± 25.94 a | 192.09 ± 39.08 a | 1327.30 ± 144.17 b | <0.001 |
| Thr | 74.96 ± 19.36 a | 81.56 ± 24.72 a | 71.11 ± 29.04 a | N.s. | 48.06 ± 5.29 a | 81.43 ± 13.92 b | 70.72 ± 18.51 b | 103.29 ± 15.96 c | <0.001 |
| Val | 95.97 ± 24.96 a | 122.70 ± 27.44 a | 111.42 ± 40.12 a | N.s. | 77.28 ± 9.46 a | 120.33 ± 8.97 b | 109.27 ± 16.98 ab | 133.24 ± 47.47 b | <0.001 |
| Lys | 385.97 ± 529.12 a | 398.37 ± 514.16 a | 386.09 ± 566.35 a | N.s. | 89.24 ± 30.87 a | 113.50 ± 24.86 a | 82.80 ± 16.94 a | 1275.00 ± 112.33 b | <0.001 |
| Ileu | 79.17 ± 22.18 a | 73.10 ± 9.96 a | 82.47 ± 24.60 a | N.s. | 60.87 ± 6.30 a | 91.53 ± 12.52 b | 82.77 ± 16.60 b | 77.82 ± 26.07 ab | 0.005 |
| Leu | 177.54 ± 39.23 a | 172.74 ± 25.86 a | 171.78 ± 33.31 a | N.s. | 147.00 ± 12.24 a | 212.07 ± 14.98 c | 181.48 ± 25.88 b | 155.53 ± 25.47 ab | <0.001 |
| Phe | 116.51 ± 25.12 a | 112.74 ± 18.61 a | 110.24 ± 24.56 a | N.s. | 99.94 ± 8.76 a | 141.43 ± 9.91 c | 121.37 ± 13.48 b | 89.93 ± 8.24 a | <0.001 |
| Trp | 15.83 ± 5.27 a | 15.51 ± 4.43 a | 15.72 ± 2.72 a | N.s. | 13.40 ± 0.83 b | 18.54 ± 1.77 c | 20.01 ± 1.22 c | 10.80 ± 2.67 a | <0.001 |
| Non-essential aminoacids | |||||||||
| Asp + Asn | 673.08 ± 169.92 a | 701.07 ± 118.00 a | 678.74 ± 201.85 a | N.s. | 582.99 ± 106.78 a | 754.35 ± 92.32 a | 739.50 ± 128.79 a | 660.34 ± 240.56 a | N.s. |
| Ser | 81.48 ± 27.10 a | 83.44 ± 14.36 a | 67.56 ± 14.54 a | N.s. | 59.67 ± 10.22 a | 91.43 ± 12.45 b | 79.75 ± 24.02 ab | 79.11 ± 19.71 ab | 0.005 |
| Glu + Gln | 931.57 ± 220.21 a | 874.73 ± 97.66 a | 889.50 ± 150.12 a | N.s. | 746.78 ± 79.02 a | 1045.3 ± 121.88 c | 969.80 ± 113.99 bc | 832.5 ± 136.14 ab | <0.001 |
| Gly | 82.29 ± 57.52 a | 100.13 ± 68.66 a | 65.40 ± 41.37 a | N.s. | 73.04 ± 8.22 b | 134.36 ± 29.73 c | 122.10 ± 31.01 c | 0.93 ± 0.83 a | <0.001 |
| Ala | 89.32 ± 56.86 a | 87.20 ± 53.03 a | 73.92 ± 43.47 a | N.s. | 87.60 ± 9.63 b | 123.75 ± 13.20 c | 118.82 ± 21.28 c | 3.76 ± 1.53 a | <0.001 |
| Pro | 148.10 ± 49.04 a | 164.65 ± 56.94 a | 152.75 ± 61.65 a | N.s. | 106.16 ± 22.15 a | 235.68 ± 23.63 c | 163.32 ± 4.10 b | 115.51 ± 17.44 a | <0.001 |
| Tyr | 942.37 ± 186.46 a | 941.82 ± 135.65 a | 909.80 ± 117.88 a | N.s. | 791.11 ± 48.56 a | 1053.60 ± 69.27 b | 1061.30 ± 71.47 b | 819.3 ± 94.52 a | <0.001 |
| Total aa | 4428.23 ± 889.97 a | 4497.15 ± 920.05 a | 4394.3 ± 1806.21 a | N.s. | 3162.87 ± 369.78 a | 4473.14 ± 232.65 b | 4156.67 ± 553.88 b | 5966.89 ± 893.87 c | <0.001 |
| Minerals (mg/100 g fw) | |||||||||
| Ca | 0.82 ± 0.14 a | 0.85 ± 0.21 a | 0.80 ± 0.10 a | N.s. | 0.79 ± 0.07 b | 0.81 ± 0.04 b | 0.66 ± 0.04 a | 1.03 ± 0.13 c | <0.001 |
| Cu | 0.43 ± 0.10 a | 0.38 ± 0.04 a | 0.39 ± 0.12 a | N.s. | 0.36 ± 0.07 ab | 0.41 ± 0.04 b | 0.51 ± 0.10 c | 0.32 ± 0.01 a | <0.001 |
| Fe | 1.55 ± 0.46 a | 1.52 ± 0.30 a | 1.72 ± 0.40 a | N.s. | 1.34 ± 0.10 a | 1.77 ± 0.09 b | 1.78 ± 0.26 b | 1.48 ± 0.65 a | 0.030 |
| K | 4.98 ± 1.06 a | 4.80 ± 0.79 a | 4.32 ± 0.91 a | N.s. | 4.40 ± 0.69 b | 5.86 ± 0.14 c | 4.94 ± 0.57 b | 3.63 ± 0.23 a | <0.001 |
| Mg | 1.01 ± 0.09 a | 0.98 ± 0.09 a | 0.96 ± 0.13 a | N.s. | 0.87 ± 0.06 a | 1.01 ± 0.03 bc | 0.96 ± 0.10 b | 1.09 ± 0.05 c | <0.001 |
| Mn | 0.18 ± 0.02 b | 0.15 ± 0.03 a | 0.18 ± 0.02 ab | 0.024 | 0.18 ± 0.01 a | 0.17 ± 0.01 a | 0.17 ± 0.05 a | 0.16 ± 0.01 a | N.s. |
| Na | 2.47 ± 0.32 a | 2.61 ± 0.33 a | 2.59 ± 0.82 a | N.s. | 2.25 ± 0.18 ab | 3.24 ± 0.54 c | 2.61 ± 0.26 b | 2.13 ± 0.10 a | <0.001 |
| Zn | 0.65 ± 0.19 a | 0.55 ± 0.06 a | 0.53 ± 0.14 a | N.s. | 0.46 ± 0.06 a | 0.74 ± 0.13 b | 0.59 ± 0.14 a | 0.51 ± 0.06 a | <0.001 |
| Total minerals | 12.08 ± 2.21 a | 11.83 ± 1.38 a | 11.5 ± 2.08 a | N.s. | 10.64 ± 1.08 a | 14.00 ± 0.37 c | 12.21 ± 1.42 b | 10.36 ± 1.00 a | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abraão, A.; Yu, M.; Gouvinhas, I.; Ferreira, L.; Silva, A.M.; Domínguez-Perles, R.; Barros, A. Prunus lusitanica L. Fruits: A Promising Underexploited Source of Nutrients with Potential Economic Value. Foods 2023, 12, 973. https://doi.org/10.3390/foods12050973
Abraão A, Yu M, Gouvinhas I, Ferreira L, Silva AM, Domínguez-Perles R, Barros A. Prunus lusitanica L. Fruits: A Promising Underexploited Source of Nutrients with Potential Economic Value. Foods. 2023; 12(5):973. https://doi.org/10.3390/foods12050973
Chicago/Turabian StyleAbraão, Ana, Manyou Yu, Irene Gouvinhas, Luís Ferreira, Amélia M. Silva, Raúl Domínguez-Perles, and Ana Barros. 2023. "Prunus lusitanica L. Fruits: A Promising Underexploited Source of Nutrients with Potential Economic Value" Foods 12, no. 5: 973. https://doi.org/10.3390/foods12050973
APA StyleAbraão, A., Yu, M., Gouvinhas, I., Ferreira, L., Silva, A. M., Domínguez-Perles, R., & Barros, A. (2023). Prunus lusitanica L. Fruits: A Promising Underexploited Source of Nutrients with Potential Economic Value. Foods, 12(5), 973. https://doi.org/10.3390/foods12050973








