Ginsenoside Rg2 Promotes the Proliferation and Stemness Maintenance of Porcine Mesenchymal Stem Cells through Autophagy Induction
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Isolation and Culture of pMSCs
2.3. Flow Cytometry
2.4. Adipogenic and Osteogenic Differentiation of MSCs
2.5. Senescence-Associated β-Galactosidase (SA-β-Gal) Staining
2.6. Cell Proliferation Assay
2.7. Quantitative Real-Time PCR (qRT-PCR)
2.8. Drug Administration
2.9. Western Blot
2.10. Cell Viability Assay
2.11. Measurement of Malondialdehyde (MDA) Contents and Superoxide Dismutase (SOD) Activities
2.12. Statistical Analysis
3. Results
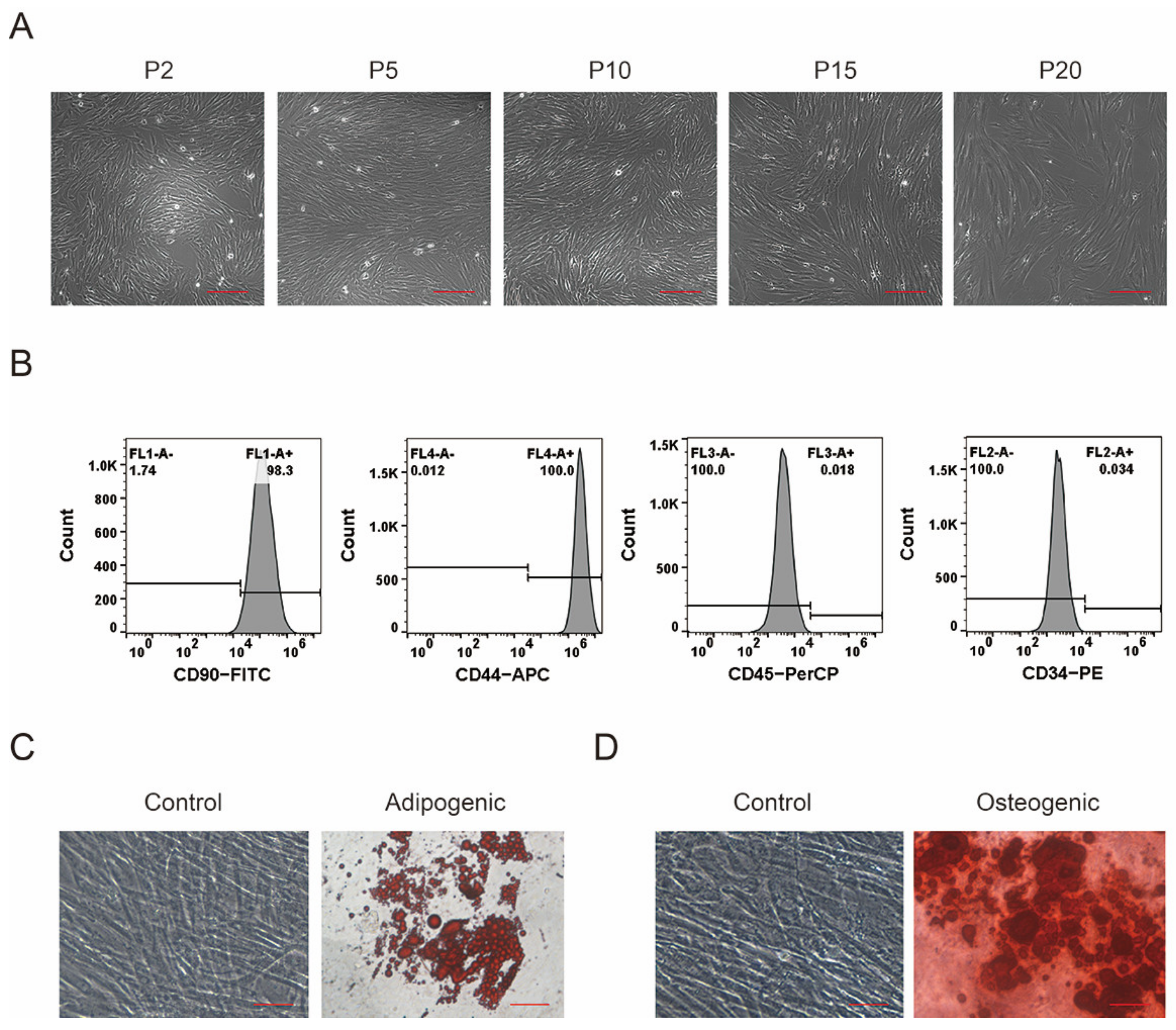
3.1. Isolation, Culture, and Identification of pMSCs
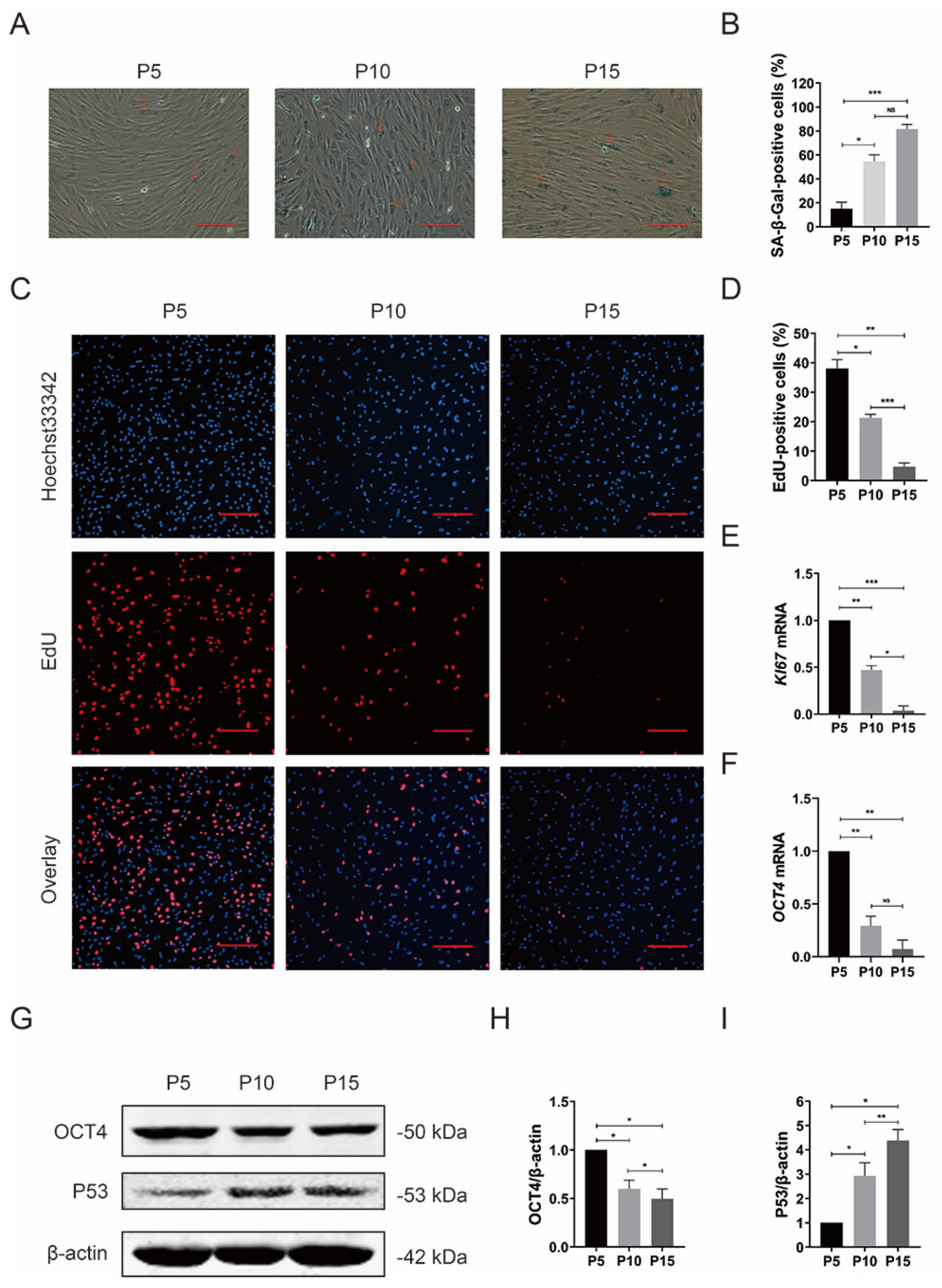
3.2. Reduced Proliferation Potential and Stemness in pMSCs after Long-Time Culture
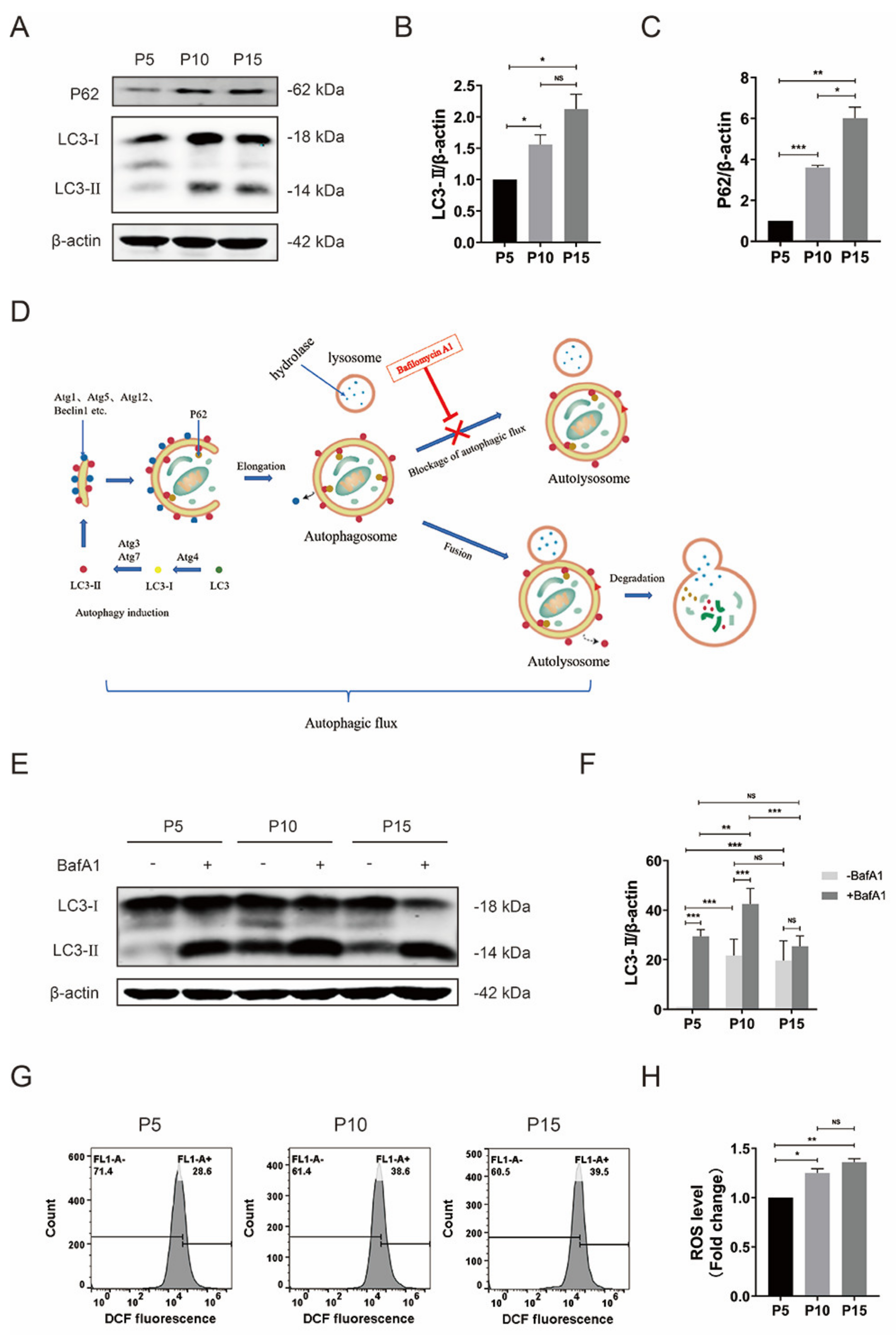
3.3. Impaired Autophagic Flux and Elevated ROS in pMSCs after Long-Time Culture
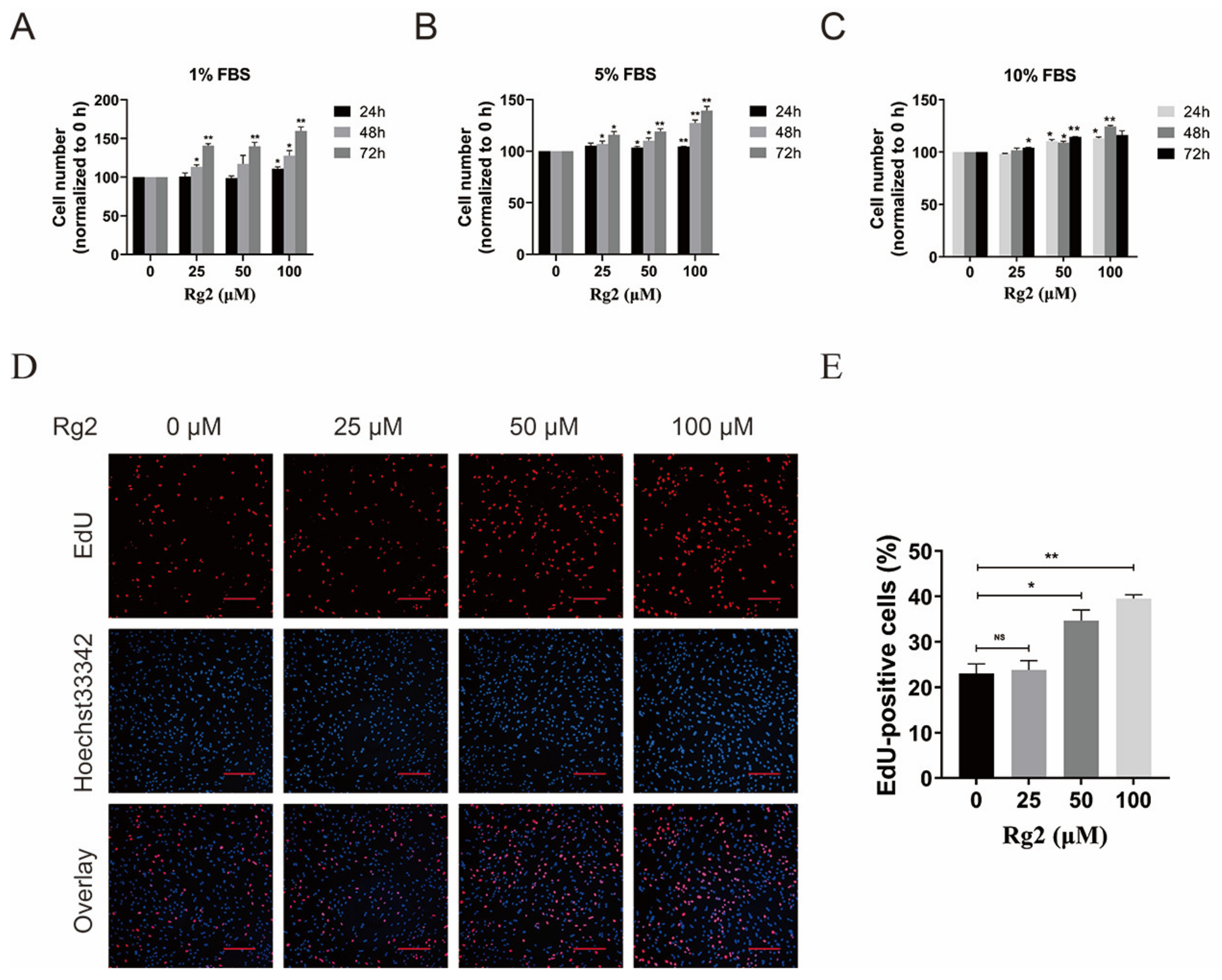
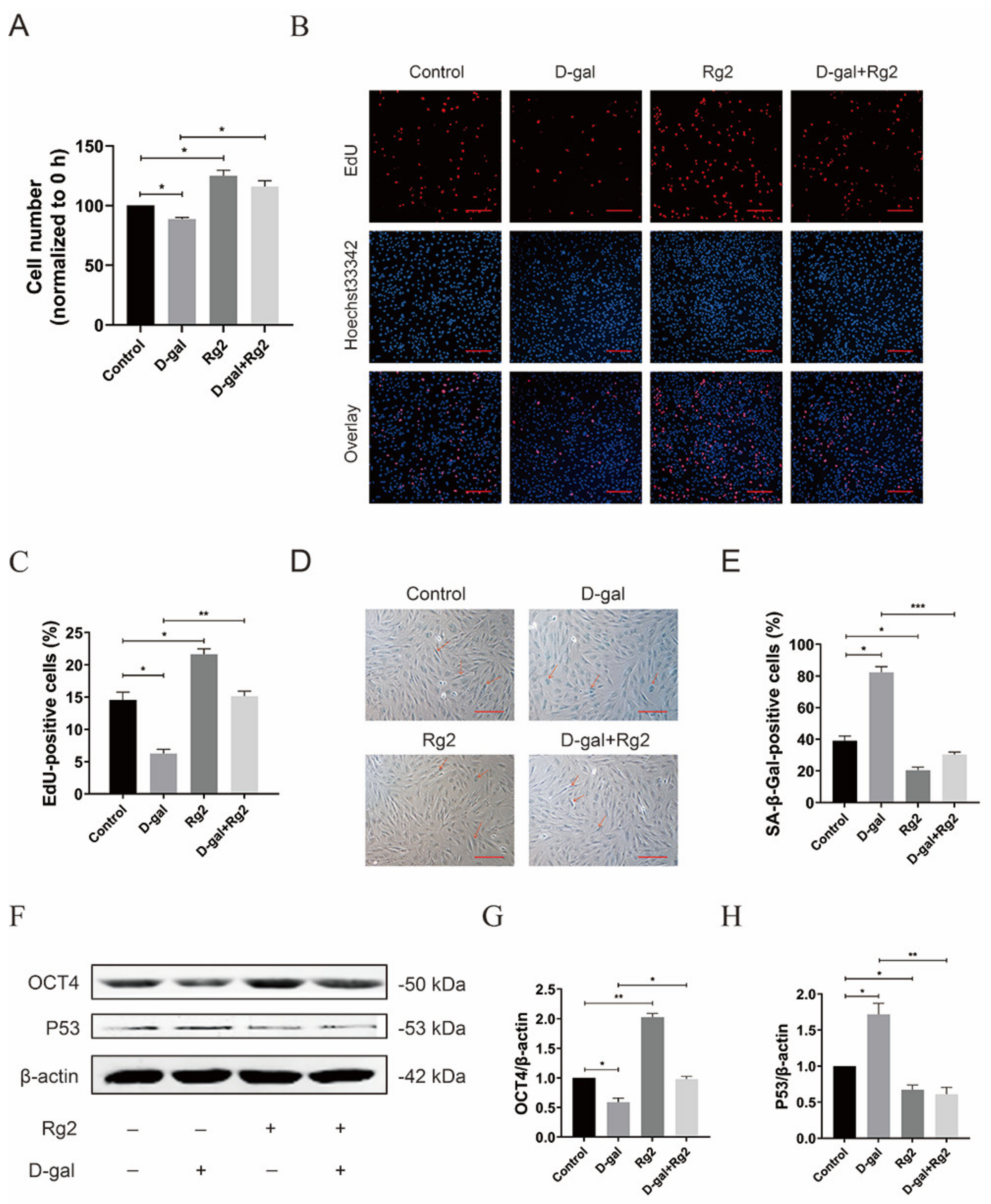
3.4. Ginsenoside Rg2 Promoted the Proliferation of pMSCs
3.5. Ginsenoside Rg2 Reversed D-Gal-Induced Senescence and Maintained Stemness in pMSCs
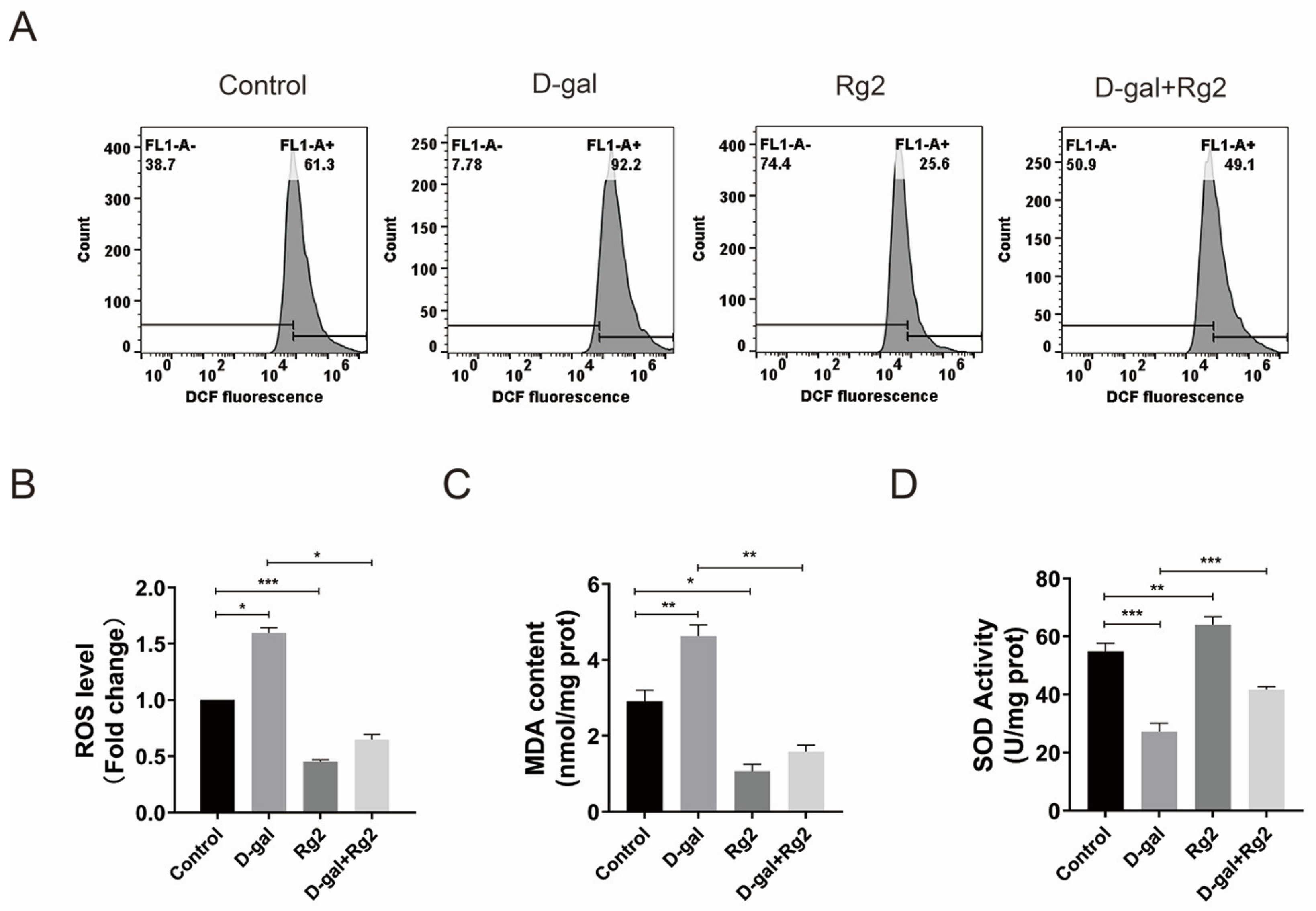
3.6. Ginsenoside Rg2 Protected pMSCs against Oxidative Stress
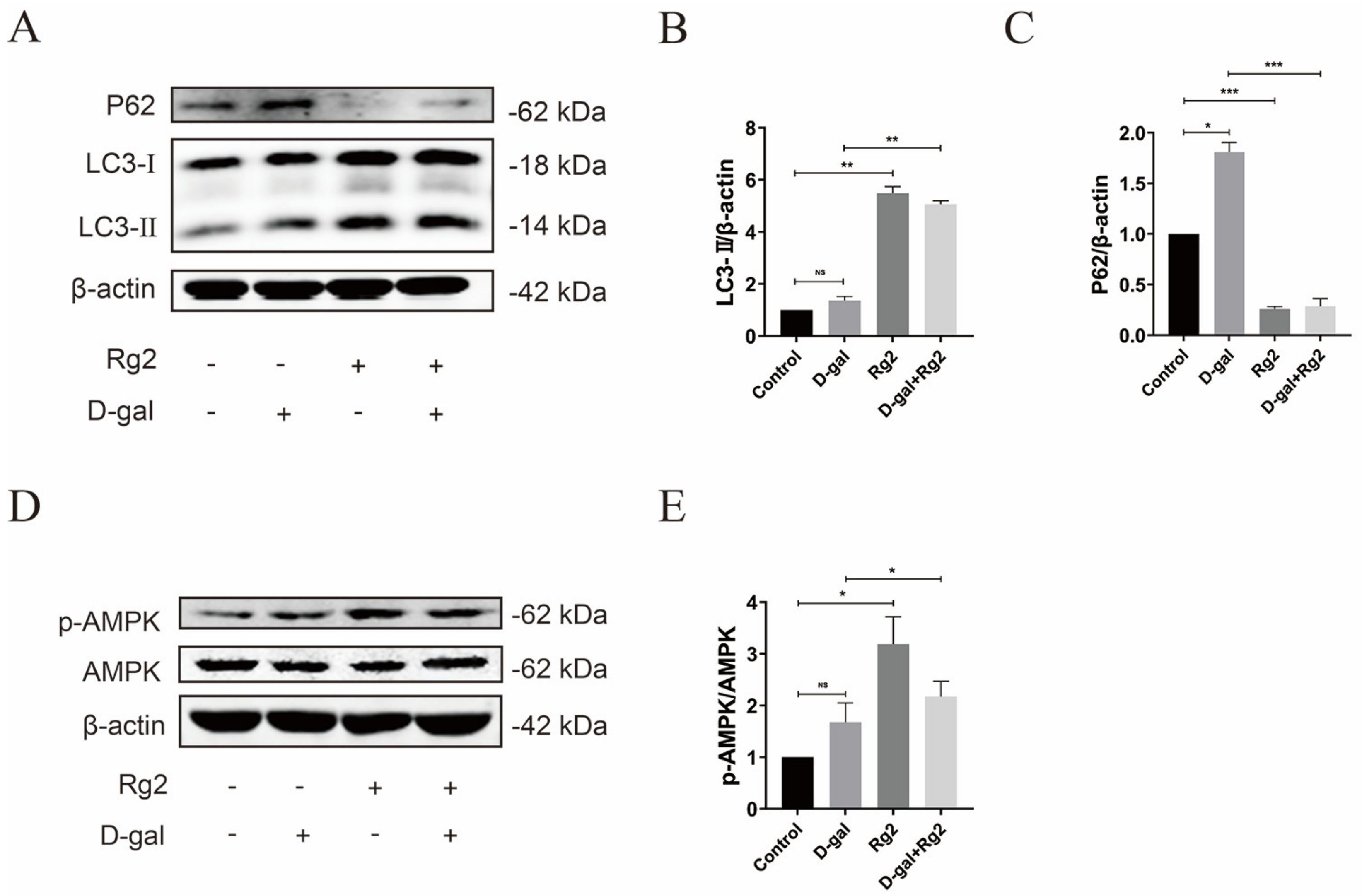
3.7. Ginsenoside Rg2 Induced Autophagy in pMSCs via the AMPK Signaling Pathway
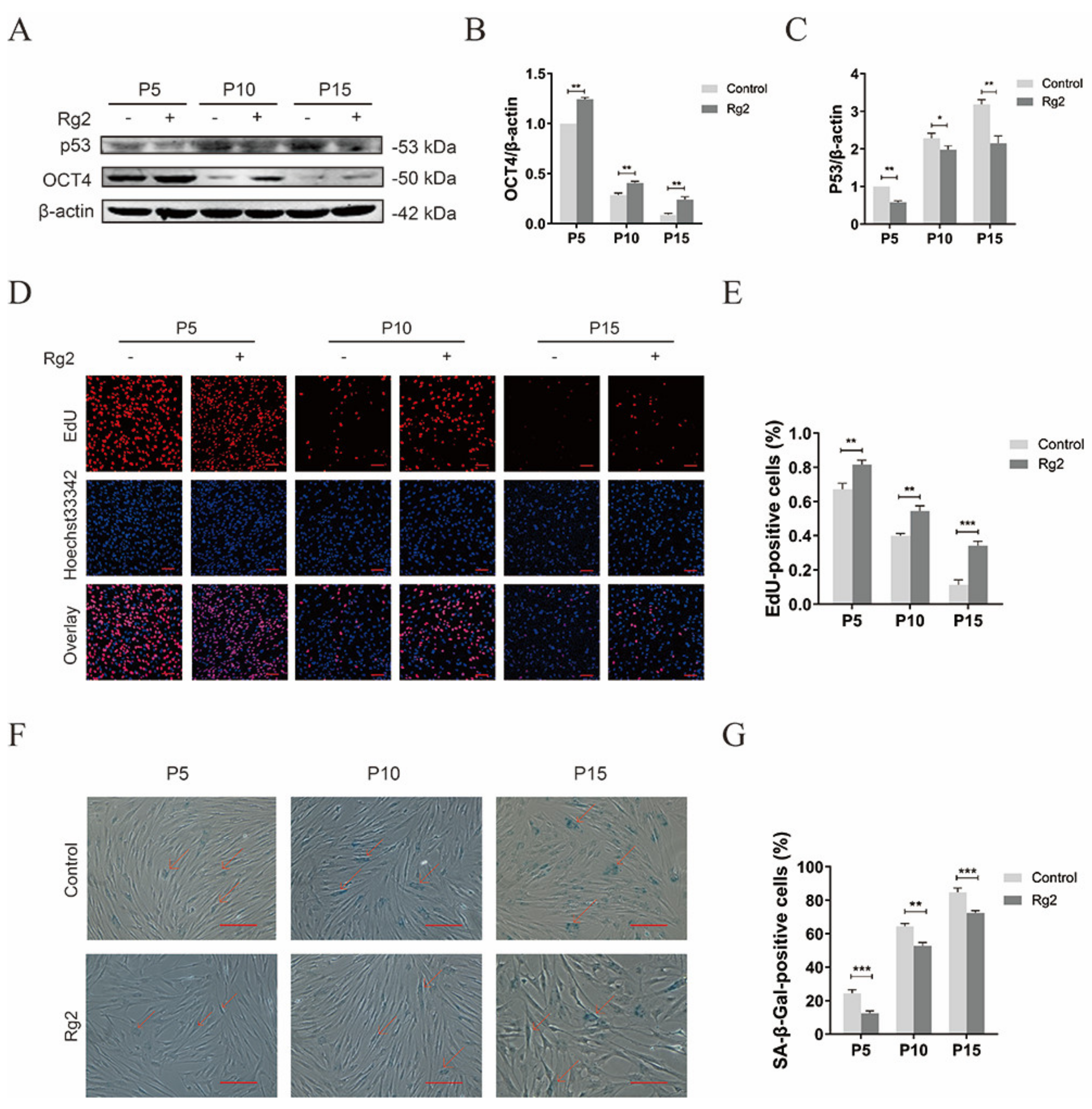
3.8. Ginsenoside Rg2 Improved Longevity of pMSCs during Long-Term Culture
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Arshad, M.S.; Javaid, M.; Sohaib, M.; Saeed, F.; Imran, A.; Yildiz, F. Tissue engineering approaches to develop cultured meat from cells: A mini review. Cogent Food Agric. 2017, 3, 1320814. [Google Scholar] [CrossRef]
- Gerbens-Leenes, P.W.; Nonhebel, S.; Krol, M.S. Food consumption patterns and economic growth. Increasing affluence and the use of natural resources. Appetite 2010, 55, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Stephens, D.N.; Dunsford, I.; Silvio, L.D.; Ellis, D.M.; Glencross, A.; Sexton, D.A. Bringing cultured meat to market: Technical, socio-political, and regulatory challenges in Cellular Agriculture. Trends Food Sci. Technol. 2018, 78, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.J.; Johnston, J.; Datar, I.; Sewalt, V.; Shatkin, J.A. Food Safety Considerations and Research Priorities for the Cultured Meat and Seafood Industry. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5421–5448. [Google Scholar] [CrossRef]
- Warner, R.D. Review: Analysis of the process and drivers for cellular meat production. Animal 2019, 13, 3041–3058. [Google Scholar] [CrossRef]
- Reiss, J.; Robertson, S.; Suzuki, M. Cell Sources for Cultivated Meat: Applications and Considerations throughout the Production Workflow. Int. J. Mol. Sci. 2021, 22, 7513. [Google Scholar] [CrossRef]
- Baldermann, S.; Wikandari, R.; Manikharda; Ningrum, A.; Taherzadeh, M.J. Application of cell culture technology and genetic engineering for production of future foods and crop improvement to strengthen food security. Bioengineered 2021, 12, 11305–11330. [Google Scholar] [CrossRef]
- Sinke, P.; Swartz, E.; Sanctorum, H.; van der Giesen, C.; Odegard, I. Ex-ante life cycle assessment of commercial-scale cultivated meat production in 2030. Int. J. Life Cycle Assess. 2023, 28, 234–254. [Google Scholar] [CrossRef]
- Ozhava, D.; Bhatia, M.; Freman, J.; Mao, Y. Sustainable Cell Sources for Cultivated Meat. J. Biomed. Res. Environ. Sci. 2022, 3, 1382–1388. [Google Scholar] [CrossRef]
- Knezic, T.; Janjusevic, L.; Djisalov, M.; Yodmuang, S.; Gadjanski, I. Using Vertebrate Stem and Progenitor Cells for Cellular Agriculture, State-of-the-Art, Challenges, and Future Perspectives. Biomolecules 2022, 12, 699. [Google Scholar] [CrossRef]
- Lee, A.Y.; Lee, J.; Kim, C.L.; Lee, K.S.; Lee, S.H.; Gu, N.Y.; Kim, J.M.; Lee, B.C.; Koo, O.J.; Song, J.Y.; et al. Comparative studies on proliferation, molecular markers and differentiation potential of mesenchymal stem cells from various tissues (adipose, bone marrow, ear skin, abdominal skin, and lung) and maintenance of multipotency during serial passages in miniature pig. Res. Vet. Sci. 2015, 100, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Serrano, R.; González-Dávalos, M.; Lozano-Flores, C.; Shimada, A.; Antaramian, A.; Varela-Echavarría, A.; Mora, O. PPAR Agonists Promote the Differentiation of Porcine Bone Marrow Mesenchymal Stem Cells into the Adipogenic and Myogenic Lineages. Cells Tissues Organs 2016, 203, 153–172. [Google Scholar] [CrossRef]
- Ramírez-Espinosa, J.J.; González-Dávalos, L.; Shimada, A.; Piña, E.; Varela-Echavarria, A. Bovine (Bos taurus) Bone Marrow Mesenchymal Cell Differentiation to Adipogenic and Myogenic Lineages. Cells Tissues Organs 2015, 201, 51–64. [Google Scholar] [CrossRef]
- Zagury, Y.; Ianovici, I.; Landau, S.; Lavon, N.; Levenberg, S. Engineered marble-like bovine fat tissue for cultured meat. Commun. Biol. 2022, 5, 927. [Google Scholar] [CrossRef]
- Machour, M.; Hen, N.; Goldfracht, I.; Safina, D.; Davidovich-Pinhas, M.; Bianco-Peled, H.; Levenberg, S. Print-and-Grow within a Novel Support Material for 3D Bioprinting and Post-Printing Tissue Growth. Adv. Sci. 2022, 9, e2200882. [Google Scholar] [CrossRef] [PubMed]
- Hanga, M.P.; Ali, J.; Moutsatsou, P.; de la Raga, F.A.; Hewitt, C.J.; Nienow, A.; Wall, I. Bioprocess development for scalable production of cultivated meat. Biotechnol. Bioeng. 2020, 117, 3029–3039. [Google Scholar] [CrossRef]
- Jiang, T.; Xu, G.; Wang, Q.; Yang, L.; Zheng, L.; Zhao, J.; Zhang, X. In vitro expansion impaired the stemness of early passage mesenchymal stem cells for treatment of cartilage defects. Cell Death Dis. 2017, 8, e2851. [Google Scholar] [CrossRef]
- Kim, J.; Kim, Y.; Choi, H.; Kwon, A.; Jekarl, D.W.; Lee, S.; Jang, W.; Chae, H.; Kim, J.R.; Kim, J.M.; et al. Ubiquitin C decrement plays a pivotal role in replicative senescence of bone marrow mesenchymal stromal cells. Cell Death Dis. 2018, 9, 139. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Shi, J.; Zhang, Y.; Zhang, Y.; Huang, Y.; Chen, Z.; Yang, J. The replicative senescent mesenchymal stem / stromal cells defect in DNA damage response and anti-oxidative capacity. Int. J. Med. Sci. 2018, 15, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Turinetto, V.; Vitale, E.; Giachino, C. Senescence in Human Mesenchymal Stem Cells: Functional Changes and Implications in Stem Cell-Based Therapy. Int. J. Mol. Sci. 2016, 17, 1164. [Google Scholar] [CrossRef] [PubMed]
- Estrada, J.C.; Torres, Y.; Benguria, A.; Dopazo, A.; Roche, E.; Carrera-Quintanar, L.; Perez, R.A.; Enriquez, J.A.; Torres, R.; Ramirez, J.C.; et al. Human mesenchymal stem cell-replicative senescence and oxidative stress are closely linked to aneuploidy. Cell Death Dis. 2013, 4, e691. [Google Scholar] [CrossRef]
- Zhang, J.; Yao, H.; Wu, M.; Li, Y.; Yang, K. Nrf2 modulates immunosuppressive ability and cellular senescence of human umbilical cord mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2020, 526, 1021–1027. [Google Scholar] [CrossRef]
- Vacanti, V.; Kong, E.; Suzuki, G.; Sato, K.; Lee, T. Phenotypic changes of adult porcine mesenchymal stem cells induced by prolonged passaging in culture. J. Cell. Physiol. 2010, 205, 194–201. [Google Scholar] [CrossRef]
- Pokrywczynska, M.; Maj, M.; Kloskowski, T.; Buhl, M.; Balcerczyk, D.; Jundzill, A.; Szeliski, K.; Rasmus, M.; Drewa, T. Molecular Aspects of Adipose-Derived Stromal Cell Senescence in a Long-Term Culture: A Potential Role of Inflammatory Pathways. Cell Transpl. 2020, 29, 963689720917341. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Komatsu, M. Autophagy: Renovation of cells and tissues. Cell 2013, 147, 728–741. [Google Scholar] [CrossRef]
- Sbrana, F.V.; Cortini, M.; Avnet, S.; Perut, F.; Columbaro, M.; Milito, A.D.; Baldini, N. The Role of Autophagy in the Maintenance of Stemness and Differentiation of Mesenchymal Stem Cells. Stem Cell Rev. 2016, 12, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Weng, Z.; Wang, Y.; Ouchi, T.; Liu, H.; Qiao, X.; Wu, C.; Zhao, Z.; Li, L.; Li, B. Mesenchymal Stem/Stromal Cell Senescence: Hallmarks, Mechanisms, and Combating Strategies. Stem Cells Transl. Med. 2022, 11, 356–371. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Han, Z.p.; Jing, Y.y.; Yang, X.; Zhang, S.s.; Sun, K.; Hao, C.; Meng, Y.; Yu, F.h.; Liu, X.Q.; et al. Autophagy prevents irradiation injury and maintains stemness through decreasing ROS generation in mesenchymal stem cells. Cell Death Dis. 2013, 4, e844. [Google Scholar] [CrossRef] [PubMed]
- García-Prat, L.; Martínez-Vicente, M.; Perdiguero, E.; Ortet, L.; Rodríguez-Ubreva, J.; Rebollo, E.; Ruiz-Bonilla, V.; Gutarra, S.; Ballestar, E.; Serrano, A.L.; et al. Autophagy maintains stemness by preventing senescence. Nature 2016, 534, S3–S4. [Google Scholar] [CrossRef]
- Zheng, Y.; Hu, C.J.; Zhuo, R.H.; Lei, Y.S.; Han, N.N.; He, L. Inhibition of autophagy alleviates the senescent state of rat mesenchymal stem cells during long-term culture. Mol. Med. Rep. 2014, 10, 3003–3008. [Google Scholar] [CrossRef]
- Yang, M.; Wen, T.; Chen, H.; Deng, J.; Yang, C.; Zhang, Z. Knockdown of insulin-like growth factor 1 exerts a protective effect on hypoxic injury of aged BM-MSCs: Role of autophagy. Stem Cell Res. Ther. 2018, 9, 284. [Google Scholar] [CrossRef]
- Yang, M.; Meng, Q.; Ying, A.; Zhang, L.; Rui, Y.; Doro, D.H.; Liu, W.; Yan, J. Autophagy controls mesenchymal stem cell properties and senescence during bone aging. Aging Cell 2018, 17, e12709. [Google Scholar] [CrossRef]
- Liu, Z.Z.; Hong, C.G.; Hu, W.B.; Chen, M.L.; Duan, R.; Li, H.M.; Yue, T.; Cao, J.; Wang, Z.X.; Chen, C.Y.; et al. Autophagy receptor OPTN (optineurin) regulates mesenchymal stem cell fate and bone-fat balance during aging by clearing FABP3. Autophagy 2021, 17, 2766–2782. [Google Scholar] [CrossRef] [PubMed]
- Kang, O.J.; Kim, J.S. Comparison of Ginsenoside Contents in Different Parts of Korean Ginseng (Panax ginseng C.A. Meyer). Prev. Nutr. Food Sci. 2016, 21, 389–392. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Park, J.S.; Ma, Y.; Ma, H.; Lee, Y.J.; Lee, G.R.; Yoo, H.S.; Hong, J.T.; Roh, Y.S. Ginseng Saponin Enriched in Rh1 and Rg2 Ameliorates Nonalcoholic Fatty Liver Disease by Inhibiting Inflammasome Activation. Nutrients 2021, 13, 856. [Google Scholar] [CrossRef]
- Lee, G.; Nguyen, T.T.H.; Lim, T.Y.; Lim, J.; Park, B.; Lee, S.; Mok, I.K.; Pal, K.; Lim, S.; Kim, D. Fermented Wild Ginseng by Rhizopus oligosporus Improved l-Carnitine and Ginsenoside Contents. Molecules 2020, 25, 2111. [Google Scholar] [CrossRef]
- Bak, M.J.; Jeong, W.S.; Kim, K.B. Detoxifying effect of fermented black ginseng on H2O2-induced oxidative stress in HepG2 cells. Int. J. Mol. Med. 2014, 34, 1516–1522. [Google Scholar] [CrossRef]
- Nguyen, T.; Huynh, D.; Jin, Y.; Jeon, H.; Heo, K.S. Protective effects of ginsenoside-Rg2 and -Rh1 on liver function through inhibiting TAK1 and STAT3-mediated inflammatory activity and Nrf2/ARE-mediated antioxidant signaling pathway. Arch. Pharmacal Res. 2021, 44, 241–252. [Google Scholar] [CrossRef]
- Hj, A.; Dtnha, B.; Nb, A.; Tlln, A.; Ksh, A. Ginsenoside-Rg2 affects cell growth via regulating ROS-mediated AMPK activation and cell cycle in MCF-7 cells. Phytomedicine 2021, 85, 153549. [Google Scholar] [CrossRef]
- Gou, D.; Pei, X.; Wang, J.; Wang, Y.; Hu, C.; Song, C.; Cui, S.; Zhou, Y. Antiarrhythmic effects of ginsenoside Rg2 on calcium chloride–induced arrhythmias without oral toxicity. J. Ginseng Res. 2020, 44, 717–724. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, N.; Rocchi, A.; Zhang, W.; Vassar, R.; Zhou, Y.; He, C. Identification of natural products with neuronal and metabolic benefits through autophagy induction. Landes Biosci. 2017, 13, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Chen, K.C.; Zhou, Y.; Wei, H.; Qi, M.H.; Wang, Z.; Zheng, Y.N.; Chen, R.X.; Liu, S.; Li, W. Evaluating the effects of mitochondrial autophagy flux on ginsenoside Rg2 for delaying D-galactose induced brain aging in mice. Phytomedicine 2022, 104, 154341. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, L.; Jiang, R.; Li, C.; Wang, Y. Ginsenoside Rg1 prevents bone marrow mesenchymal stem cell senescence via NRF2 and PI3K/Akt signaling. Free Radic. Biol. Med. 2021, 174, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.; Kim, M.Y.; Da Ly, D.; Park, S.J.; Eom, Y.W.; Park, K.S.; Baik, S.K. Ca(2+)-activated mitochondrial biogenesis and functions improve stem cell fate in Rg3-treated human mesenchymal stem cells. Stem Cell Res. Ther. 2020, 11, 467. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Yao, G. Ginsenoside Rg1 as a Potential Regulator of Hematopoietic Stem/Progenitor Cells. Stem Cells Int. 2021, 17, 849. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.C.; Li, Q.; Xie, C.E.; Niu, X.; Yu, C.Y. Chinese herbs and their active ingredients for activating xue (blood) promote the proliferation and differentiation of neural stem cells and mesenchymal stem cells. Chin. Med. 2014, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, M.; Nguyen, L.; Watanabe, N.; Fujita, Y.; Sawamoto, O.; Matsumoto, S. Development and characterization of novel clinical grade neonatal porcine bone marrow-derived mesenchymal stem cells. Xenotransplantation 2019, 26, e12501. [Google Scholar] [CrossRef] [PubMed]
- Wagner, W.; Horn, P.; Castoldi, M.; Diehlmann, A.; Bork, S.; Saffrich, R.; Benes, V.; Blake, J.; Pfister, S.; Eckstein, V.; et al. Replicative senescence of mesenchymal stem cells: A continuous and organized process. PLoS ONE 2008, 3, e2213. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Naguro, I.; Ichijo, H. Iron homeostasis and iron-regulated ROS in cell death, senescence and human diseases. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 1398–1409. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, L.; Ding, Z.; Luo, Q.; Ju, Y.; Song, G. Exogenous NAD+ Postpones the D-Gal-Induced Senescence of Bone Marrow-Derived Mesenchymal Stem Cells via Sirt1 Signaling. Antioxidants 2021, 10, 254. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, M.; Parham, A.; Dehghani, H.; Kazemi Mehrjerdi, H. Equine bone marrow-derived mesenchymal stem cells: Optimization of cell density in primary culture. Stem Cell Investig. 2018, 5, 31. [Google Scholar] [CrossRef]
- Rink, B.E.; Amilon, K.R.; Esteves, C.L.; French, H.M.; Watson, E.; Aurich, C.; Donadeu, F.X. Isolation and characterization of equine endometrial mesenchymal stromal cells. Stem Cell Res. Ther. 2017, 8, 166. [Google Scholar] [CrossRef] [PubMed]
- Tjempakasari, A.; Suroto, H.; Santoso, D. Mesenchymal Stem Cell Senescence and Osteogenesis. Medicina 2021, 58, 61. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Zhang, W.X.; Wang, K.F.; Zhang, S.; Han, X.; Guan, W.J.; Ma, Y.H. Isolation and biological characteristics of multipotent mesenchymal stromal cells derived from chick embryo intestine. Br. Poult. Sci. 2018, 59, 521–530. [Google Scholar] [CrossRef]
- Zhang, G.; Zhao, X.; Li, X.; Sun, X.; Zhou, J.; Du, G.; Chen, J. Application of cell culture techniques in cultured meat-a review. Sheng Wu Gong Cheng Xue Bao 2019, 35, 1374–1381. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, G.; Zhao, X.; Sun, X.; Zhou, J.; Du, G.; Chen, J. Prospects of process and bioreactors for large scale cultured meat production. Chin. J. Process Eng. 2020, 20, 3–11. [Google Scholar] [CrossRef]
- Oliver, L.; Hue, E.; Priault, M.; Vallette, F.M. Basal autophagy decreased during the differentiation of human adult mesenchymal stem cells. Stem Cells Dev. 2012, 21, 2779–2788. [Google Scholar] [CrossRef]
- Salemi, S.; Yousefi, S.; Constantinescu, M.A.; Fey, M.F.; Simon, H.U. Autophagy is required for self-renewal and differentiation of adult human stem cells. Cell Res. 2012, 22, 432–435. [Google Scholar] [CrossRef]
- Mortensen, M.; Watson, A.S.; Simon, A.K. Lack of autophagy in the hematopoietic system leads to loss of hematopoietic stem cell function and dysregulated myeloid proliferation. Autophagy 2011, 7, 1069–1070. [Google Scholar] [CrossRef]
- Bu, W.; Hao, X.; Yang, T.; Wang, J.; Liu, Q.; Zhang, X.; Li, X.; Gong, Y.; Shao, C. Autophagy Contributes to the Maintenance of Genomic Integrity by Reducing Oxidative Stress. Oxid. Med. Cell Longev. 2020, 2020, 2015920. [Google Scholar] [CrossRef]
- Khorraminejad-Shirazi, M.; Sani, M.; Talaei-Khozani, T.; Dorvash, M.; Attar, A. AICAR and nicotinamide treatment synergistically augment the proliferation and attenuate senescence-associated changes in mesenchymal stromal cells. Stem Cell Res. Ther. 2020, 11, 45. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Ouyang, P.; Li, W.; Zhong, L.; Gu, C.; Shen, L.; Cao, S.; Yin, L.; Ren, Z.; Zuo, Z.; et al. Curcumin Alleviates the Senescence of Canine Bone Marrow Mesenchymal Stem Cells during In Vitro Expansion by Activating the Autophagy Pathway. Int J. Mol. Sci. 2021, 22, 11356. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Jung, J.; Cho, K.J.; Lee, S.K.; Park, J.W.; Oh, I.H.; Kim, G.J. Increased SCF/c-kit by hypoxia promotes autophagy of human placental chorionic plate-derived mesenchymal stem cells via regulating the phosphorylation of mTOR. J. Cell. Biochem. 2012, 114, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, L.; Zhang, Z.; Jiang, Z. Hypoxia promotes bone marrow-derived mesenchymal stem cell proliferation through apelin/APJ/autophagy pathway. Acta Biochim. Biophys. Sin. 2015, 47, 362–367. [Google Scholar] [CrossRef]
- Dka, B.; Aea, B.; Rtb, C.; Tm, C.; Tb, C.; Neab, K.; Gka, B.; Ps, C.; Apcd, E.; Xmsfg, H. Kynurenine inhibits autophagy and promotes senescence in aged bone marrow mesenchymal stem cells through the aryl hydrocarbon receptor pathway—ScienceDirect. Exp. Gerontol. 2020, 130, 110805. [Google Scholar] [CrossRef]
- Maharajan, N.; Ganesan, C.D.; Moon, C.; Jang, C.H.; Oh, W.K.; Cho, G.W. Licochalcone D Ameliorates Oxidative Stress-Induced Senescence via AMPK Activation. Multidiscip. Digit. Publ. Inst. 2021, 22, 7324. [Google Scholar] [CrossRef]
- Maharajan, N.; Cho, G.W. Camphorquinone Promotes the Antisenescence Effect via Activating AMPK/SIRT1 in Stem Cells and D-Galactose-Induced Aging Mice. Antioxidants 2021, 10, 1916. [Google Scholar] [CrossRef]
- Feng, X.; Xing, J.; Feng, G.; Huang, D.; Lu, X.; Liu, S.; Tan, W.; Li, L.; Gu, Z. p16(INK4A) mediates age-related changes in mesenchymal stem cells derived from human dental pulp through the DNA damage and stress response. Mech. Ageing Dev. 2014, 141, 46–55. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Pan, Y.; Zhang, C.; Yan, B.X.; Yu, S.S.; Wu, D.L.; Shi, M.M.; Shi, K.; Cai, X.X.; Zhou, S.S.; et al. Wnt/beta-catenin signaling induces the aging of mesenchymal stem cells through promoting the ROS production. Mol. Cell Biochem. 2013, 374, 13–20. [Google Scholar] [CrossRef]
- Wu, J.; Niu, J.; Li, X.; Wang, X.; Guo, Z.; Zhang, F. TGF-β1 induces senescence of bone marrow mesenchymal stem cells via increase of mitochondrial ROS production. BMC Dev. Biol. 2014, 14, 21. [Google Scholar] [CrossRef]
- Basciano, L.; Nemos, C.; Foliguet, B.; de Isla, N.; de Carvalho, M.; Tran, N.; Dalloul, A. Long term culture of mesenchymal stem cells in hypoxia promotes a genetic program maintaining their undifferentiated and multipotent status. BMC Cell Biol. 2011, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Dong, Z.; Peng, Z.; Lu, F. Anti-aging effect of adipose-derived stem cells in a mouse model of skin aging induced by D-galactose. PLoS ONE 2014, 9, e97573. [Google Scholar] [CrossRef] [PubMed]
- He, Z.H.; Li, M.; Fang, Q.J.; Liao, F.L.; Zou, S.Y.; Wu, X.; Sun, H.Y.; Zhao, X.Y.; Hu, Y.J.; Xu, X.X.; et al. FOXG1 promotes aging inner ear hair cell survival through activation of the autophagy pathway. Autophagy 2021, 17, 4341–4362. [Google Scholar] [CrossRef]
- Zhang, D.; Yan, B.; Yu, S.; Zhang, C.; Wang, B.; Wang, Y.; Wang, J.; Yuan, Z.; Zhang, L.; Pan, J. Coenzyme Q10 inhibits the aging of mesenchymal stem cells induced by D-galactose through Akt/mTOR signaling. Oxid. Med. Cell Longev. 2015, 2015, 867293. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yao, H.; Chen, X.; Wang, Z.; Xiang, Y.; Xia, J.; Liu, Y.; Wang, Y. Ginsenoside Rg1 Decreases Oxidative Stress and Down-Regulates Akt/mTOR Signalling to Attenuate Cognitive Impairment in Mice and Senescence of Neural Stem Cells Induced by d-Galactose. Neurochem. Res. 2018, 43, 430–440. [Google Scholar] [CrossRef]
- Li, J.; Cai, D.; Yao, X.; Zhang, Y.; Chen, L.; Jing, P.; Wang, L.; Wang, Y. Protective Effect of Ginsenoside Rg1 on Hematopoietic Stem/Progenitor Cells through Attenuating Oxidative Stress and the Wnt/beta-Catenin Signaling Pathway in a Mouse Model of d-Galactose-induced Aging. Int. J. Mol. Sci. 2016, 17, 849. [Google Scholar] [CrossRef]
- Hou, J.; Ma, R.; Zhu, S.; Wang, Y. Revealing the Therapeutic Targets and Mechanism of Ginsenoside Rg1 for Liver Damage Related to Anti-Oxidative Stress Using Proteomic Analysis. Int. J. Mol. Sci. 2022, 23, 10045. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, Y.; Xu, X.; Xiang, H.; Shi, Y.; Gao, Y.; Wang, X.; Jiang, X.; Li, N.; Pan, J. Autophagy inhibits the mesenchymal stem cell aging induced by D-galactose through ROS/JNK/p38 signalling. Clin. Exp. Pharmacol. Physiol. 2019, 47, 466–477. [Google Scholar] [CrossRef]
- Cheng, B.; Gao, W.; Wu, X.; Zheng, M.; Gao, Y. Ginsenoside Rg2 Ameliorates High-Fat Diet-Induced Metabolic Disease through SIRT1. J. Agric. Food Chem. 2020, 68, 4215–4226. [Google Scholar] [CrossRef]
- Han, D.; Jiang, L.; Gu, X.; Huang, S.; Pang, J.; Wu, Y.; Yin, J.; Wang, J. SIRT3 deficiency is resistant to autophagy-dependent ferroptosis by inhibiting the AMPK/mTOR pathway and promoting GPX4 levels. J. Cell Physiol. 2020, 235, 8839–8851. [Google Scholar] [CrossRef]
- Gwinn, D.M.; Shackelford, D.B.; Egan, D.F.; Mihaylova, M.M.; Shaw, R.J. AMPK Phosphorylation of Raptor Mediates a Metabolic Checkpoint. Mol. Cell 2008, 30, 214–226. [Google Scholar] [CrossRef]
- Lin, M.; Hua, R.; Ma, J.; Zhou, Y.; Quan, S. Bisphenol A promotes autophagy in ovarian granulosa cells by inducing AMPK/mTOR/ULK1 signalling pathway. Environ. Int. 2021, 147, 106298. [Google Scholar] [CrossRef]
- Mao, K.; Klionsky, D.J. AMPK activates autophagy by phosphorylating ULK1. Circ. Res. 2011, 108, 787–788. [Google Scholar] [CrossRef]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Ameen, O.; Samaka, R.M.; Abo-Elsoud, R.A.A. Metformin alleviates neurocognitive impairment in aging via activation of AMPK/BDNF/PI3K pathway. Sci. Rep. 2022, 12, 17084. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Zhou, J.; Yang, C.; Zhang, X.; Shi, Y.; Liu, J.; Yan, X.; Liang, J.; Liu, X.; Luo, L.; et al. gamma-Glutamylcysteine ameliorates D-gal-induced senescence in PC12 cells and mice via activating AMPK and SIRT1. Food Funct. 2022, 13, 7560–7571. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Shen, W.; Li, J.; Jia, N.; Xiong, Y.; Miao, J.; Xie, C.; Chen, Q.; Shen, K.; Meng, P.; et al. AMPK Activator O304 Protects Against Kidney Aging Through Promoting Energy Metabolism and Autophagy. Front. Pharm. 2022, 13, 836496. [Google Scholar] [CrossRef]
- Kou, X.; Li, J.; Liu, X.; Yang, X.; Fan, J.; Chen, N. Ampelopsin attenuates the atrophy of skeletal muscle from d-gal-induced aging rats through activating AMPK/SIRT1/PGC-1α signaling cascade. Biomed. Pharmacother. Biomed. Pharmacother. 2017, 90, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, M.; Jin, Z.; Yaqoob, S.; Zheng, M.; Cai, D.; Liu, J.; Guo, S. Ginsenoside Rg2 inhibits adipogenesis in 3T3-L1 preadipocytes and suppresses obesity in high-fat-diet-induced obese mice through the AMPK pathway. Food Funct. 2019, 10, 3603–3614. [Google Scholar] [CrossRef]
- Yuan, H.D.; Kim, D.Y.; Quan, H.Y.; Su, J.K.; Mi, S.J.; Chung, S.H. Ginsenoside Rg2 induces orphan nuclear receptor SHP gene expression and inactivates GSK3β via AMP-activated protein kinase to inhibit hepatic glucose production in HepG2 cells. Chem. Biol. Interact. 2012, 195, 35–42. [Google Scholar] [CrossRef]
- Hekman, K.E.; Koss, K.M.; Ivancic, D.Z.; He, C.; Wertheim, J.A. Autophagy Enhances Longevity of Induced Pluripotent Stem Cell-Derived Endothelium via mTOR-Independent ULK1 Kinase. Stem Cells Transl. Med. 2022, 11, 1151–1164. [Google Scholar] [CrossRef] [PubMed]
| Gene | Primer Sequence |
|---|---|
| OCT4 | F:5′-GTCGCCAGAAGGGCAAAC-3′ |
| R:5′-CAGGGTGGTGAAGTGAGGG-3′ | |
| KI67 | F:5′-TTCATTCACTGGTCCTCG-3′ |
| R:5′-TTAGCCACTTCTGACTTTC-3′ | |
| GAPDH | F:5′-TGAAGGTCGGAGTGAACG-3′ |
| R:5′-CGTGGGTGGAATCATACTGG-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Che, L.; Zhu, C.; Huang, L.; Xu, H.; Ma, X.; Luo, X.; He, H.; Zhang, T.; Wang, N. Ginsenoside Rg2 Promotes the Proliferation and Stemness Maintenance of Porcine Mesenchymal Stem Cells through Autophagy Induction. Foods 2023, 12, 1075. https://doi.org/10.3390/foods12051075
Che L, Zhu C, Huang L, Xu H, Ma X, Luo X, He H, Zhang T, Wang N. Ginsenoside Rg2 Promotes the Proliferation and Stemness Maintenance of Porcine Mesenchymal Stem Cells through Autophagy Induction. Foods. 2023; 12(5):1075. https://doi.org/10.3390/foods12051075
Chicago/Turabian StyleChe, Lina, Caixia Zhu, Lei Huang, Hui Xu, Xinmiao Ma, Xuegang Luo, Hongpeng He, Tongcun Zhang, and Nan Wang. 2023. "Ginsenoside Rg2 Promotes the Proliferation and Stemness Maintenance of Porcine Mesenchymal Stem Cells through Autophagy Induction" Foods 12, no. 5: 1075. https://doi.org/10.3390/foods12051075
APA StyleChe, L., Zhu, C., Huang, L., Xu, H., Ma, X., Luo, X., He, H., Zhang, T., & Wang, N. (2023). Ginsenoside Rg2 Promotes the Proliferation and Stemness Maintenance of Porcine Mesenchymal Stem Cells through Autophagy Induction. Foods, 12(5), 1075. https://doi.org/10.3390/foods12051075






