The Effect of Ginger (Zingiber officinale Roscoe) Aqueous Extract on Postprandial Glycemia in Nondiabetic Adults: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Consideration
2.2. Participants and Study Design
2.3. Ginger Extract Preparation
2.4. Intervention
2.5. Data Collection
2.6. Chemical Analysis
2.7. Total Phenolic Content Determination
2.8. Flavonoid Content Determination
2.9. Radical Inhibition Assay
2.10. Statistical Analysis
3. Results
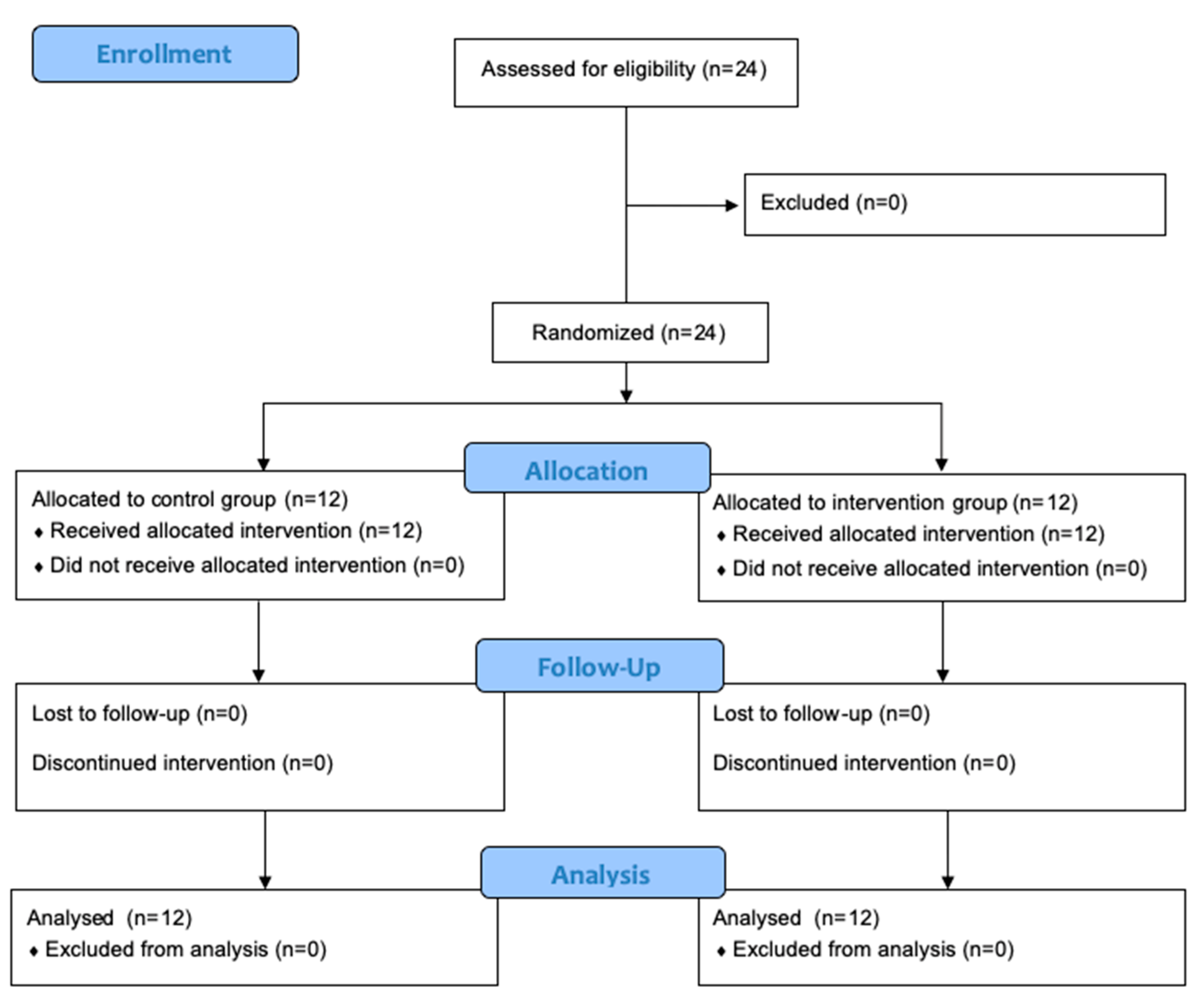
3.1. Participant Enrollment
3.2. Participant Characteristics
3.3. Glycemic Response
3.4. Total Phenols, Flavonoid, and Antioxidant Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Diabetes Association. Postprandial Blood Glucose. Diabetes Care 2001, 24, 775–778. [Google Scholar] [CrossRef] [PubMed]
- Chien, K.L.; Lee, B.C.; Lin, H.J.; Hsu, H.C.; Chen, M.F. Association of Fasting and Post-Prandial Hyperglycemia on the Risk of Cardiovascular and All-Cause Death among Non-Diabetic Chinese. Diabetes Res. Clin. Pract. 2009, 83, e47–e50. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.I.; Bracco, P.A.; Yudkin, J.S.; Bensenor, I.M.; Griep, R.H.; Barreto, S.M.; Castilhos, C.D.; Duncan, B.B. Intermediate Hyperglycaemia to Predict Progression to Type 2 Diabetes (ELSA-Brasil): An Occupational Cohort Study in Brazil. Lancet Diabetes Endocrinol. 2019, 7, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Papachristoforou, E.; Lambadiari, V.; Maratou, E.; Makrilakis, K. Association of Glycemic Indices (Hyperglycemia, Glucose Variability, and Hypoglycemia) with Oxidative Stress and Diabetic Complications. J. Diabetes Res. 2020, 2020, 7489795. [Google Scholar] [CrossRef]
- Anh, N.H.; Kim, S.J.; Long, N.P.; Min, J.E.; Yoon, Y.C.; Lee, E.G.; Kim, M.; Kim, T.J.; Yang, Y.Y.; Son, E.Y.; et al. Ginger on Human Health: A Comprehensive Systematic Review of 109 Randomized Controlled Trials. Nutrients 2020, 12, 157. [Google Scholar] [CrossRef]
- Chang, W.P.; Peng, Y.X. Does the Oral Administration of Ginger Reduce Chemotherapy-Induced Nausea and Vomiting?: A Meta-Analysis of 10 Randomized Controlled Trials. Cancer Nurs. 2019, 42, E14–E23. [Google Scholar] [CrossRef]
- Terry, R.; Posadzki, P.; Watson, L.K.; Ernst, E. The Use of Ginger (Zingiber Officinale) for the Treatment of Pain: A Systematic Review of Clinical Trials. Pain Med. 2011, 12, 1808–1818. [Google Scholar] [CrossRef]
- Ebrahimzadeh Attari, V.; Malek Mahdavi, A.; Javadivala, Z.; Mahluji, S.; Zununi Vahed, S.; Ostadrahimi, A. A Systematic Review of the Anti-Obesity and Weight Lowering Effect of Ginger (Zingiber Officinale Roscoe) and Its Mechanisms of Action. Phytother. Res. 2018, 32, 577–585. [Google Scholar] [CrossRef]
- Leach, M.J.; Kumar, S. The Clinical Effectiveness of Ginger (Zingiber Officinale) in Adults with Osteoarthritis. Int. J. Evid. Based Healthc. 2008, 6, 311–320. [Google Scholar] [CrossRef]
- Wang, J.; Ke, W.; Bao, R.; Hu, X.; Chen, F. Beneficial Effects of Ginger Zingiber Officinale Roscoe on Obesity and Metabolic Syndrome: A Review. Ann. N. Y. Acad. Sci. 2017, 1398, 83–98. [Google Scholar] [CrossRef]
- Abdi, T.; Mahmoudabady, M.; Marzouni, H.Z.; Niazmand, S.; Khazaei, M. Ginger (Zingiber Officinale Roscoe) Extract Protects the Heart Against Inflammation and Fibrosis in Diabetic Rats. Can. J. Diabetes 2021, 45, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Mahluji, S.; Ostadrahimi, A.; Mobasseri, M.; Attari, V.E.; Payahoo, L. Anti-Inflammatory Effects of Zingiber Officinale in Type 2 Diabetic Patients. Adv. Pharm. Bull. 2013, 3, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, J.; Zhang, Y. Research Progress on Chemical Constituents of Zingiber Officinale Roscoe. Biomed. Res. Int. 2019, 2019, 5370823. [Google Scholar] [CrossRef] [PubMed]
- Butt, M.S.; Sultan, M.T. Ginger and Its Health Claims: Molecular Aspects. Crit. Rev. Food Sci. Nutr. 2011, 51, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Nam, Y.H.; Hong, B.N.; Rodriguez, I.; Park, M.S.; Jeong, S.Y.; Lee, Y.G.; Shim, J.H.; Yasmin, T.; Kim, N.W.; Koo, Y.T.; et al. Steamed Ginger May Enhance Insulin Secretion through Katp Channel Closure in Pancreatic β-Cells Potentially by Increasing 1-Dehydro-6-Gingerdione Content. Nutrients 2020, 12, 324. [Google Scholar] [CrossRef] [PubMed]
- Samad, M.; Mohsin, M.N.A.; Razu, B.A.; Hossain, M.T.; Mahzabeen, S.; Unnoor, N.; Muna, I.A.; Akhter, F.; Kabir, A.U.; Hannan, J.M.A. [6]-Gingerol, from Zingiber Officinale, Potentiates GLP-1 Mediated Glucose-Stimulated Insulin Secretion Pathway in Pancreatic β-Cells and Increases RAB8/RAB10-Regulated Membrane Presentation of GLUT4 Transporters in Skeletal Muscle to Improve Hyperglycemia in Leprdb/Db Type 2 Diabetic Mice. BMC Complement Altern Med. 2017, 17, 395. [Google Scholar] [CrossRef]
- Bhandari, U.; Kanojia, R.; Pillai, K.K. Effect of Ethanolic Extract of Zingiber Officinale on Dyslipidaemia in Diabetic Rats. J. Ethnopharmacol. 2005, 97, 227–230. [Google Scholar] [CrossRef]
- Jafri, S.A.; Abass, S.; Qasim, M. Pakistan Veterinary Journal Hypoglycemic Effect of Ginger (Zingiber Officinale) in Alloxan Induced Diabetic Rats (Rattus Norvagicus). Pak. Vet. J. 2010, 31, 160–162. [Google Scholar]
- Huang, F.Y.; Deng, T.; Meng, L.X.; Ma, X.L. Dietary Ginger as a Traditional Therapy for Blood Sugar Control in Patients with Type 2 Diabetes Mellitus. Medicine 2019, 98, e15054. [Google Scholar] [CrossRef]
- Karimi, N.; Roshan, V.D.; Bayatiyani, Z.F. Individually and Combined Water-Based Exercise with Ginger Supplement, on Systemic Inflammation and Metabolic Syndrome Indices, among the Obese Women with Breast Neoplasms. Int. J. Cancer Manag. 2015, 8, e3856. [Google Scholar] [CrossRef]
- Imani, H.; Tabibi, H.; Najafi, I.; Atabak, S.; Hedayati, M.; Rahmani, L. Effects of Ginger on Serum Glucose, Advanced Glycation End Products, and Inflammation in Peritoneal Dialysis Patients. Nutrition 2015, 31, 703–707. [Google Scholar] [CrossRef] [PubMed]
- Bordia, A.; Verma, S.K.; Srivastava, K.C. Effect of Ginger (Zingiber Officinale Rosc.) and Fenugreek (Trigonella Foenumgraecum L.) on Blood Lipids, Blood Sugar and Platelet Aggregation in Patients with Coronary Artery Disease. Prostaglandins Leukot Essent Fat. Acids 1997, 56, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Tsai, M.F.; Thorat, R.S.; Xiao, D.; Zhang, X.; Sandhu, A.K.; Edirisinghe, I.; Burton-Freeman, B.M. Endothelial Function and Postprandial Glucose Control in Response to Test-Meals Containing Herbs and Spices in Adults with Overweight/Obesity. Front. Nutr. 2022, 9, 811433. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, J.M. Effect of Ginger Tea on the Fetal Development of Sprague-Dawley Rats. Reprod. Toxicol. 2000, 14, 507–512. [Google Scholar] [CrossRef]
- American Diabetes Association Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2010, 33, S62–S69. [CrossRef]
- Prabha, M.R.; Vasantha, K. Antioxidant, Cytotoxicity and Polyphenolic Content of Calotropis Procera (Ait.) R. Br. Flowers. J. Appl. Pharm. Sci. 2011, 1, 136–140. [Google Scholar]
- Khan, M.A.; Gannon, M.C.; Nuttall, F.Q. Glucose Appearance Rate Following Protein Ingestion in Normal Subjects. J. Am. Coll. Nutr. 1992, 11, 701–706. [Google Scholar] [CrossRef]
- King, D.G.; Walker, M.; Campbell, M.D.; Breen, L.; Stevenson, E.J.; West, D.J. A Small Dose of Whey Protein Co-Ingested with Mixed-Macronutrient Breakfast and Lunch Meals Improves Postprandial Glycemia and Suppresses Appetite in Men with Type 2 Diabetes: A Randomized Controlled Trial. Am. J. Clin. Nutr. 2018, 107, 550–557. [Google Scholar] [CrossRef]
- El Khoury, D.; Hwalla, N. Metabolic and Appetite Hormone Responses of Hyperinsulinemic Normoglycemic Males to Meals with Varied Macronutrient Compositions. Ann. Nutr. Metab. 2010, 57, 59–67. [Google Scholar] [CrossRef]
- Kung, B.; Anderson, G.H.; Paré, S.; Tucker, A.J.; Vien, S.; Wright, A.J.; Goff, H.D. Effect of Milk Protein Intake and Casein-to-Whey Ratio in Breakfast Meals on Postprandial Glucose, Satiety Ratings, and Subsequent Meal Intake. J. Dairy Sci. 2018, 101, 8688–8701. [Google Scholar] [CrossRef]
- Paterson, M.; Bell, K.J.; O’Connell, S.M.; Smart, C.E.; Shafat, A.; King, B. The Role of Dietary Protein and Fat in Glycaemic Control in Type 1 Diabetes: Implications for Intensive Diabetes Management. Curr. Diab. Rep. 2015, 15, 61. [Google Scholar] [CrossRef] [PubMed]
- Shidfar, F.; Rajab, A.; Rahideh, T.; Khandouzi, N.; Hosseini, S.; Shidfar, S. The Effect of Ginger (Zingiber Officinale) on Glycemic Markers in Patients with Type 2 Diabetes. J. Complement. Integr. Med. 2015, 12, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Arablou, T.; Aryaeian, N.; Valizadeh, M.; Sharifi, F.; Hosseini, A.; Djalali, M. The Effect of Ginger Consumption on Glycemic Status, Lipid Profile and Some Inflammatory Markers in Patients with Type 2 Diabetes Mellitus. Int. J. Food Sci. Nutr. 2014, 65, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Khandouzi, N.; Shidfar, F.; Rajab, A.; Rahideh, T.; Hosseini, P.; Taheri, M.M. The Effects of Ginger on Fasting Blood Sugar, Hemoglobin A1c, Apolipoprotein B, Apolipoprotein A-I and Malondialdehyde in Type 2 Diabetic Patients. Iran. J. Pharm. Res. 2015, 14, 131–140. [Google Scholar] [PubMed]
- Mozaffari-Khosravi, H.; Talaei, B.; Jalali, B.A.; Najarzadeh, A.; Mozayan, M.R. The Effect of Ginger Powder Supplementation on Insulin Resistance and Glycemic Indices in Patients with Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled Trial. Complement Ther. Med. 2014, 22, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Mahluji, S.; Attari, V.E.; Mobasseri, M.; Payahoo, L.; Ostadrahimi, A.; Golzari, S.E. Effects of Ginger (Zingiber Officinale) on Plasma Glucose Level, HbA1c and Insulin Sensitivity in Type 2 Diabetic Patients. Int. J. Food Sci. Nutr. 2013, 64, 682–686. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Luo, D.; Ma, Y.; Zhang, J.; Li, M.; Yao, L.; Shi, X.; Liu, X.; Yang, K. Ginger for Health Care: An Overview of Systematic Reviews. Complement Ther. Med. 2019, 45, 114–123. [Google Scholar] [CrossRef]
- Crichton, M.; Davidson, A.R.; Innerarity, C.; Marx, W.; Lohning, A.; Isenring, E.; Marshall, S. Orally Consumed Ginger and Human Health: An Umbrella Review. Am. J. Clin. Nutr. 2022, 115, 1511–1527. [Google Scholar] [CrossRef]
- Venkateswaran, M.; Jayabal, S.; Hemaiswarya, S.; Murugesan, S.; Enkateswara, S.; Doble, M.; Periyasamy, S. Polyphenol-Rich Indian Ginger Cultivars Ameliorate GLUT4 Activity in C2C12 Cells, Inhibit Diabetes-Related Enzymes and LPS-Induced Inflammation: An in Vitro Study. J. Food Biochem. 2021, 45, e13600. [Google Scholar] [CrossRef]
- Li, Y.; Tran, V.H.; Duke, C.C.; Roufogalis, B.D. Gingerols of Zingiber Officinale Enhance Glucose Uptake by Increasing Cell Surface GLUT4 in Cultured L6 Myotubes. Planta Med. 2012, 78, 1549–1555. [Google Scholar] [CrossRef]
- Dudonné, S.; Vitrac, X.; Coutiére, P.; Woillez, M.; Mérillon, J.M. Comparative Study of Antioxidant Properties and Total Phenolic Content of 30 Plant Extracts of Industrial Interest Using DPPH, ABTS, FRAP, SOD, and ORAC Assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Manjunathan, T.; Guru, A.; Arokiaraj, J.; Gopinath, P. 6-Gingerol and Semisynthetic 6-Gingerdione Counteract Oxidative Stress Induced by ROS in Zebrafish-PubMed. Chem. Biodivers 2021, 18, e2100650. [Google Scholar] [CrossRef] [PubMed]
- Fathi, R.; Akbari, A.; Nasiri, K.; Chardahcherik, M. Ginger (Zingiber Officinale Roscoe) Extract Could Upregulate the Renal Expression of NRF2 and TNFα and Prevents Ethanol-Induced Toxicity in Rat Kidney. Orig. Res. Artic. 2020, 11, 134–145. [Google Scholar]
- Dugasani, S.; Pichika, M.R.; Nadarajah, V.D.; Balijepalli, M.K.; Tandra, S.; Korlakunta, J.N. Comparative Antioxidant and Anti-Inflammatory Effects of [6]-Gingerol, [8]-Gingerol, [10]-Gingerol and [6]-Shogaol. J. Ethnopharmacol. 2010, 127, 515–520. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Control Group Mean ± SEM | Intervention Mean ± SEM | p-Value 1 |
|---|---|---|---|
| Age (years) | 26.92 ± 1.64 | 30.50 ± 1.94 | 0.173 |
| Body mass index (Km/m2) | 22.74 ± 0.48 | 23.87 ± 0.50 | 0.116 |
| Weight (Kg) | 64.09 ± 2.88 | 65.46 ± 2.53 | 0.725 |
| Height (m) | 1.68 ± 0.03 | 1.64 ± 0.02 | 0.386 |
| Nutritional Parameters | Control Group Mean ± SEM | Intervention Mean ± SEM | p-Value 1 |
|---|---|---|---|
| Protein (g) | 56.33 ± 4.85 | 89.86 ± 10.63 | 0.011 |
| Carbohydrate (g) | 247.81 ± 21.91 | 306.79 ± 22.73 | 0.075 |
| Lipid (g) | 73.93 ± 6.56 | 79.71 ± 8.99 | 0.608 |
| Total energy intake (Kcal) | 1826.18 ± 110.35 | 2283.04 ± 179.09 | 0.041 |
| Time Point | Control Group Mean ± SD | Intervention Mean ± SD |
|---|---|---|
| t0 | 4.82 ± 0.12 | 4.93 ± 0.74 |
| t30 | 9.57 ± 0.43 | 7.72 ± 0.28 |
| t60 | 8.76 ± 0.66 | 6.56 ± 0.21 |
| t90 | 6.64 ± 0.36 | 5.55 ± 0.19 |
| t120 | 6.03 ± 0.28 | 5.55 ± 0.18 |
| Clinical Parameters | Control Group Mean ± SEM | Intervention Mean ± SEM | p-Value 1 |
|---|---|---|---|
| AUCi (t0–t120 min) | 334.43 ± 32.40 | 169.75 ± 17.30 | <0.001 |
| Cmax (mmol/L) | 9.57 ± 0.43 | 7.72 ± 0.28 | <0.001 |
| ∆Cmax (mmol/L | 4.75 ± 0.46 | 2.80 ± 0.26 | <0.001 |
| Compounds | Mean ± SEM |
|---|---|
| Total phenols (mg GAE/L) y = 6.431 × 103x + 1.79 × 10−2 (R2 = 0.9992) | 13.85 ± 0.15 |
| Total flavonoids (mg QCE/L) y = 2.5015 × 10−2x − 1.2673 × 10−2 (R2 = 0.99939) | 3.35 ± 0.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diakos, A.; Silva, M.L.; Brito, J.; Moncada, M.; de Mesquita, M.F.; Bernardo, M.A. The Effect of Ginger (Zingiber officinale Roscoe) Aqueous Extract on Postprandial Glycemia in Nondiabetic Adults: A Randomized Controlled Trial. Foods 2023, 12, 1037. https://doi.org/10.3390/foods12051037
Diakos A, Silva ML, Brito J, Moncada M, de Mesquita MF, Bernardo MA. The Effect of Ginger (Zingiber officinale Roscoe) Aqueous Extract on Postprandial Glycemia in Nondiabetic Adults: A Randomized Controlled Trial. Foods. 2023; 12(5):1037. https://doi.org/10.3390/foods12051037
Chicago/Turabian StyleDiakos, Alda, Maria Leonor Silva, José Brito, Margarida Moncada, Maria Fernanda de Mesquita, and Maria Alexandra Bernardo. 2023. "The Effect of Ginger (Zingiber officinale Roscoe) Aqueous Extract on Postprandial Glycemia in Nondiabetic Adults: A Randomized Controlled Trial" Foods 12, no. 5: 1037. https://doi.org/10.3390/foods12051037
APA StyleDiakos, A., Silva, M. L., Brito, J., Moncada, M., de Mesquita, M. F., & Bernardo, M. A. (2023). The Effect of Ginger (Zingiber officinale Roscoe) Aqueous Extract on Postprandial Glycemia in Nondiabetic Adults: A Randomized Controlled Trial. Foods, 12(5), 1037. https://doi.org/10.3390/foods12051037










