Influences of Illumination Pretreatment on Soybean Oil Activated Clay Bleaching Effects and Soybean Oil Quality Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Visible Light Exposure Pretreatment for the Soybean Oils
2.3. Soybean Oil Activated Clay Bleaching
2.4. Bleaching Process Designed for Exploring the Possibiltiy of a Decrease in Processing Temperature
2.5. Color Evaluation of the Bleached Soybean Oils
2.6. Fatty Acid Composition Analysis by Gas Chromatography (GC)
2.7. Determination of the Soybean Oil Oxidation Stability Parameters
2.8. Sterols Composition and Tocopherols Content Analysis
2.8.1. Phytosterol Quantification by Gas Chromatography–Mass Spectrometry (GC–MS)
2.8.2. Tocopherols Quantification by High-Performance Liquid Chromatography (HPLC)
2.9. Statistical Analysis
3. Results and Discussion
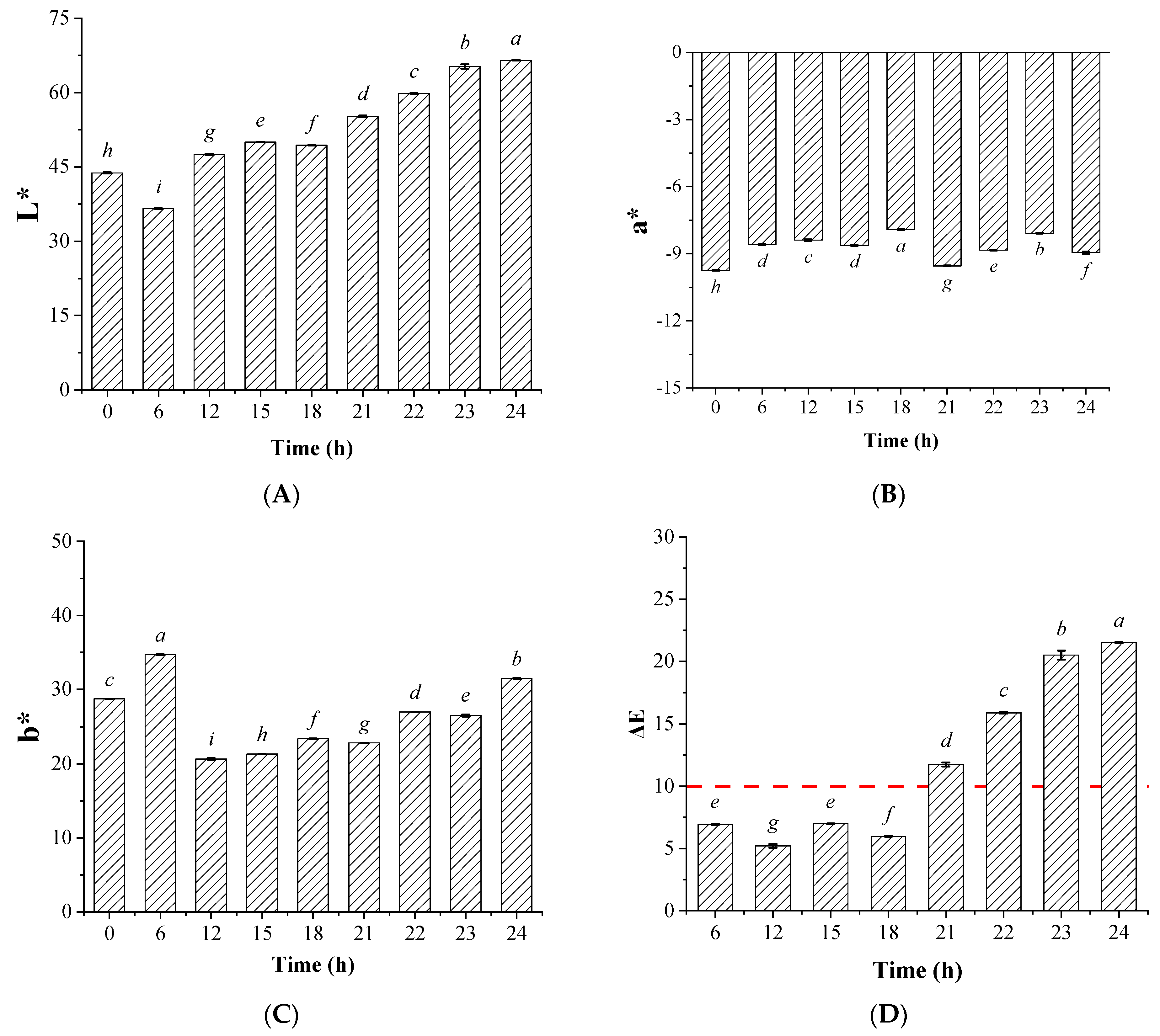
3.1. Influences of the Illumination Pretreatment on the Soybean Oil Activated Clay Decoloring Effects
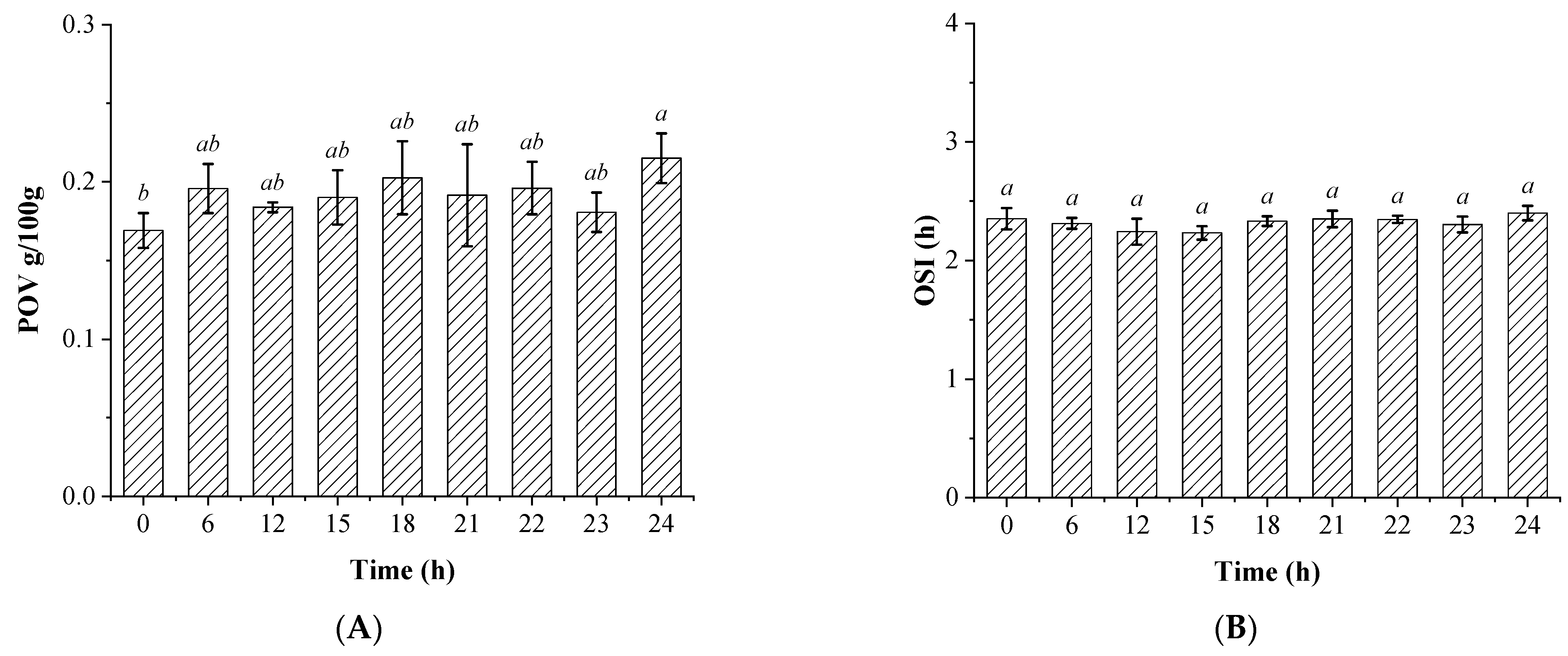
3.2. The Changes in the Fatty Acids Compositions and Oil Oxidation Stability of the Soybean Oils
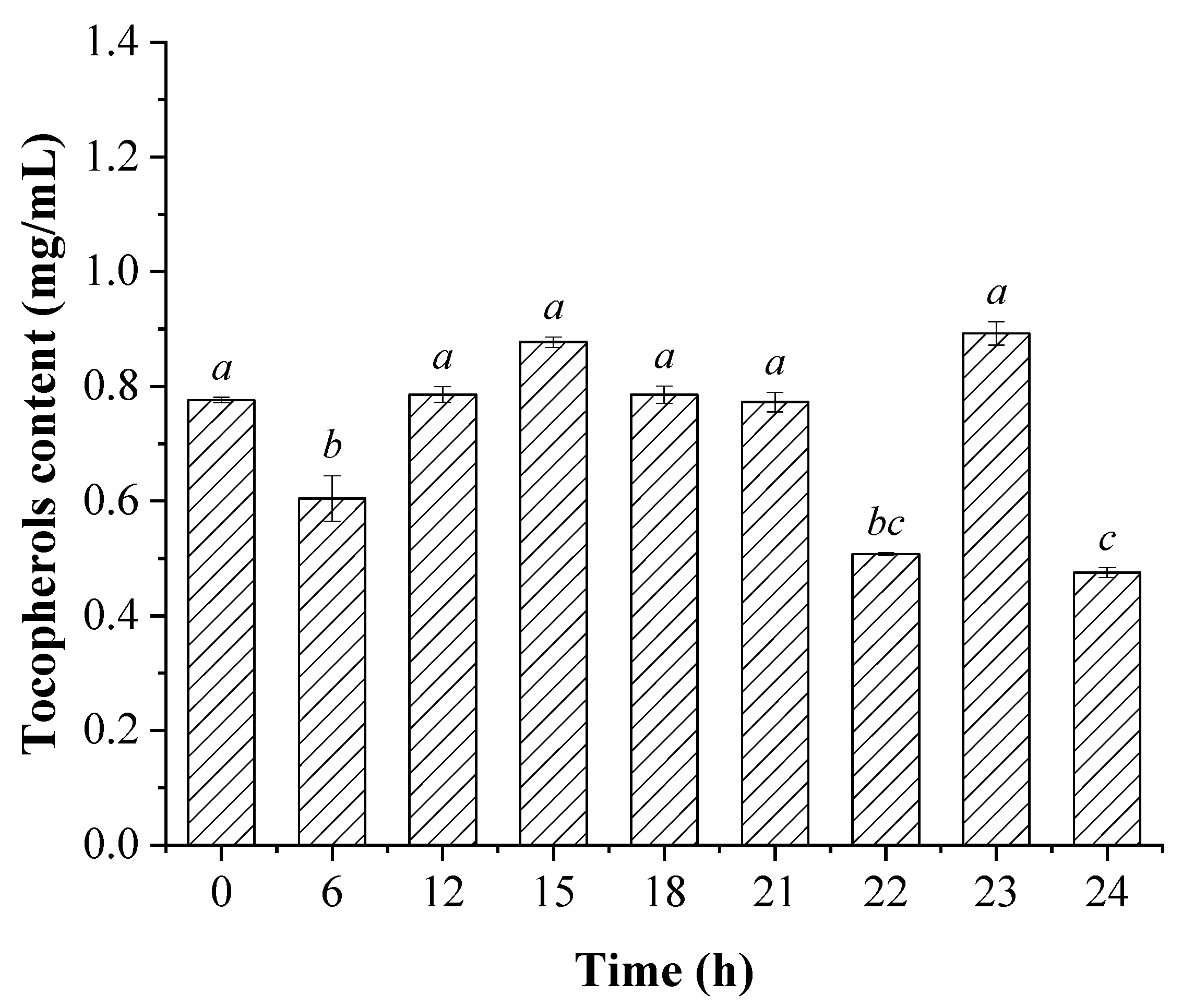
3.3. The Changes in the Contents and Compositions of Sterols and Tocopherols
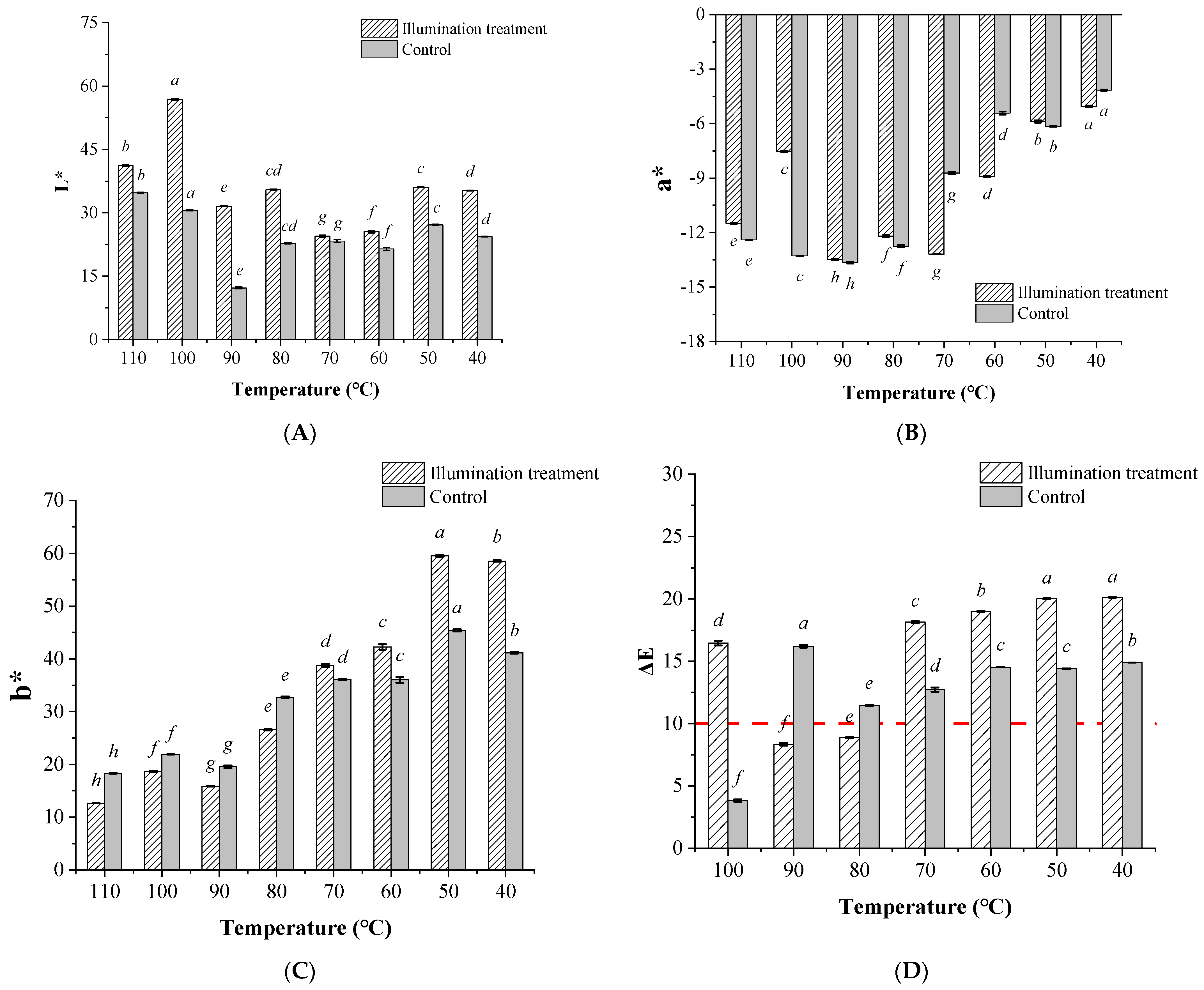
3.4. Effects of Illumination Pretreatment on the Soybean Oil Bleaching Temperature
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| GC | gas chromatography |
| GC–MS | gas chromatography–mass spectrometry |
| HPLC | High Performance Liquid Chromatography |
| MUFA | monounsaturated fatty acids |
| OSI | oxidative stability indexes |
| POV | peroxide values |
| PUFA | polyunsaturated fatty acids |
| SFA | Saturated fatty acids |
References
- Chew, S.C.; Nyam, K.L. Chapter 6 Refining of edible oils. In Lipids and Edible Oils: Properties, Processing, and Applications; Galanakis, C.M., Ed.; Academic Press: Washington, DC, USA, 2020; pp. 213–241. [Google Scholar]
- Ye, Z.; Qiao, X.; Luo, Z.; Hu, C.; Liu, L.; He, D. Optimization and comparison of water degumming and phospholipase C degumming for rapeseed oil. CYTA–J. Food 2016, 14, 604–612. [Google Scholar] [CrossRef]
- Durmaz, G.; Gokmen, V. Effect of refining on bioactive composition and oxidative stability of hazelnut oil. Food Res. Int. 2019, 116, 586–591. [Google Scholar] [CrossRef]
- Chew, S.C.; Ali, M.A. Recent advances in ultrasound technology applications of vegetable oil refining. Trends Food Sci. Technol. 2021, 116, 468–479. [Google Scholar] [CrossRef]
- Elena, A.; Maryam, G.; Mehrdad, G. Investigation of using egg shell powder for bleaching of soybean oil. Lwt-Food Sci. Technol. 2021, 140, 110859. [Google Scholar]
- Nidzam, M.S.; Hossain, M.S.; Ismail, N.; Abdul Latip, R.; Mohammad Ilias, M.K.; Mobin Siddique, M.B.; Zulkifli, M. Influence of the Degumming Process Parameters on the Formation of Glyceryl Esters and 3-MCPDE in Refined Palm Oil: Optimization and Palm Oil Quality Analyses. Foods 2022, 11, 124. [Google Scholar] [CrossRef] [PubMed]
- Seçilmiş, Ş.S.; Yanık, D.K.; Fadiloğlu, S.; Göğüş, F. A comparative study on performance of industrial and microwave techniques for sunflower oil bleaching process. Food Chem. 2021, 365, 130488. [Google Scholar] [CrossRef] [PubMed]
- Bera, D.; Lahiri, D.; Nag, A. Kinetic studies on bleaching of edible oil using charred sawdust as a new adsorbent. J. Food Eng. 2004, 65, 33–36. [Google Scholar] [CrossRef]
- Farihahusnah, H.; Kheireddine, A.M.; Wan, D.W.M.A. Textural characteristics, surface chemistry and activation of bleaching earth: A review. Chem. Eng. J. 2011, 170, 90–106. [Google Scholar]
- Brooks, D.D.; Berbesi, R.; Hodgson, A.S. Optimization of Bleaching Process. Available online: https://lipidlibrary.aocs.org/edible-oil-processing/optimization-of-bleaching-process (accessed on 1 May 2022).
- Taylor, D. Chapter 3 Adsorbents. In Bleaching and Purifying Fats and Oils: Theory and Practice, 2nd ed.; List, G.R., Ed.; AOCS Press: Urbana, IL, USA, 2009; pp. 69–95. [Google Scholar]
- Rossi, M.; Gianazza, M.; Alamprese, C.; Stanga, F. The role of bleaching clays and synthetic silica in palm oil physical refining. Food Chem. 2003, 82, 291–296. [Google Scholar] [CrossRef]
- Łaska-Zieja, B.; Marcinkowski, D.; Golimowski, W.; Niedbała, G.; Wojciechowska, E. Low-Cost Investment with High Quality Performance. Bleaching Earths for Phosphorus Reduction in the Low-Temperature Bleaching Process of Rapeseed Oil. Foods 2020, 9, 603. [Google Scholar] [CrossRef]
- Dan, S.; Tianyu, X.; Dongyan, G.; Yong, C.; Yuan, J.; Weinong, Z.; Tao, W. Ultrasonic bleaching of rapeseed oil: Effects of bleaching conditions and underlying mechanisms. J. Food Eng. 2013, 117, 8–13. [Google Scholar]
- Tahereh, D.; Ali, S.M.; Mohsen, B. Bleaching of Olive Oil by Membrane Filtration. Eur. J. Lipid Sci. Tech. 2020, 122, 1900151. [Google Scholar]
- Nemiwal, M.; Zhang, T.C.; Kumar, D. Recent progress in g-C3N4, TiO2 and ZnO based photocatalysts for dye degradation: Strategies to improve photocatalytic activity. Sci. Total Environ. 2021, 767, 144896. [Google Scholar] [CrossRef]
- Guo, Q.; Zhou, C.; Ma, Z.; Yang, X. Fundamentals of TiO2 Photocatalysis: Concepts, Mechanisms, and Challenges. Adv. Mater. 2019, 31, 1901997. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Zhang, K.; Liu, X.; Shi, Y. High efficiency inactivation of microalgae in ballast water by a new proposed dual-wave UV-photocatalysis system (UVA/UVC-TiO2). Environ. Sci. Pollut. Res. 2019, 26, 7785–7792. [Google Scholar] [CrossRef] [PubMed]
- Bevilacqua, A.C.; Köhler, M.H.; Iglesias, B.A.; Piquini, P.C. Photophysical and photocatalytic properties of corrophyll and chlorophyll. Comp. Mater. Sci. 2019, 158, 228–234. [Google Scholar] [CrossRef]
- Luo, M.R.; Cui, G.; Rigg, B. The development of the CIE 2000 colour-difference formula: CIEDE2000. Color Res. Appl. 2001, 26, 340–350. [Google Scholar] [CrossRef]
- AOCS. AOCS Official Method Ce 2-66. In Official Methods and Recommended Practices of the American Oil Chemists’ Society, 6th ed.; AOCS: Champaign, IL, USA, 2009. [Google Scholar]
- Ye, Z.; Xu, Y.J.; Liu, Y. Different typical dietary lipid consumption affects the bile acid metabolism and the gut microbiota structure: An animal trial using Sprague-Dawley rats. J. Sci. Food Agr. 2022, 102, 3179–3192. [Google Scholar] [CrossRef]
- AOCS. AOCS Official Methods Cd 8-53. In Official Methods and Recommended Practices of the American Oil Chemists’ Society; AOCS: Champaign, IL, USA, 2003. [Google Scholar]
- Ting, L.; Yan, S.; Han, W.J.; Kai, X.H.; Fu, W.Y.; Qi, Z.; Yong, Z.D.; Fereidoon, S. Improving oxidative stability of flaxseed oil with a mixture of antioxidants. J. Food Process Pres. 2019, 44, e14355. [Google Scholar]
- Jun, J.; Pembe, W.; Hongyan, M.; Youfeng, Z.; Liang, J.; Jiahui, M.; Dan, X.; Jianhua, H.; Qingzhe, J.; Xingguo, W. Characteristics of Mango Kernel Fats Extracted from 11 China-Specific Varieties and Their Typically Fractionated Fractions. J. Am. Oil Chem. Soc. 2016, 93, 1115–1125. [Google Scholar] [CrossRef]
- Wang, W.; Yang, X.; Ye, Z.; Li, Y.; Liu, Y.; Cao, P. Extraction Technology Can Impose Influences on Peanut Oil Functional Quality: A Study to Investigate the Lipid Metabolism by Sprague-Dawley Rat Model. J. Food Sci. 2019, 84, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Polo, C.; Muñoz, M.P.; Lorenzo Luengo, M.C.; Vicente, P.; Galindo, P.; Martín Casado, A.M. Comparison of the CIELab and CIEDE2000 color difference formulas. J. Prosthet. Dent. 2016, 115, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yaguchi, H.; Shioiri, S. Testing CIELAB-based color-difference formulae using large color differences. Opt. Rev. 2001, 8, 478–494. [Google Scholar] [CrossRef]
- Esposto, S.; Taticchi, A.; Urbani, S.; Selvaggini, R.; Veneziani, G.; Di Maio, I.; Sordini, B.; Servili, M. Effect of light exposure on the quality of extra virgin olive oils according to their chemical composition. Food Chem. 2017, 229, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Cihat, I.N.; Zeki, D.M. Ultrasound-assisted bleaching of canola oil: Improve the bleaching process by central composite design. Lwt-Food Sci. Technol. 2018, 97, 640–647. [Google Scholar]
- Francesco, C.; Teresa, B.M.; Antonella, P.; Ewa, S.; Tommaso, G. Influence of the exposure to light on extra virgin olive oil quality during storage. Eur. Food Res. Technol. 2005, 221, 92–98. [Google Scholar]
- Edwald, L.; Haechun, A.; Eunok, C. Effects of light and lipids on chlorophyll degradation. Food Sci. Biotechnol. 2014, 23, 1061–1065. [Google Scholar]
- Yasuda, M.; Oda, K.; Ueda, T.; Tabata, M. Physico-chemical chlorophyll-a species in aqueous alcohol solutions determine the rate of its discoloration under UV light. Food Chem. 2019, 277, 463–470. [Google Scholar] [CrossRef]
- Verleyen, T.; Sosinska, U.; Ioannidou, S.; Verhe, R.; Dewettinck, K.; Huyghebaert, A.; De Greyt, W. Influence of the vegetable oil refining process on free and esterified sterols. J. Am. Oil Chem. Soc. 2002, 79, 947–953. [Google Scholar] [CrossRef]
- Ge, B.; Chuanguo, M.; Xiaowei, C. Phytosterols in edible oil: Distribution, analysis and variation during processing. Grain & Oil Sci. Technol. 2021, 4, 33–44. [Google Scholar]
- Dijkstra, A.J. Soybean Oil. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Washington, DC, USA, 2016; pp. 58–63. [Google Scholar]
- Sabliov, C.M.; Fronczek, C.; Astete, C.E.; Khachaturyan, M.; Khachatryan, L.; Leonardi, C. Effects of Temperature and UV Light on Degradation of α-Tocopherol in Free and Dissolved Form. J. Am. Oil Chem. Soc. 2009, 86, 895–902. [Google Scholar] [CrossRef]
- De Vaugelade, S.; Nicol, E.; Vujovic, S.; Bourcier, S.; Pirnay, S.; Bouchonnet, S. UV–vis degradation of α-tocopherol in a model system and in a cosmetic emulsion—Structural elucidation of photoproducts and toxicological consequences. J. Chromatogr. A 2017, 1517, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yousef, Y.A.; Li, H.; Melø, T.B.; Naqvi, K.R. Comparison of the Photochemical Behaviors of α-Tocopherol and its Acetate in Organic and Aqueous Micellar Solutions. J. Phys. Chem. A 2011, 115, 8242–8247. [Google Scholar] [CrossRef] [PubMed]
- Eunok, C. Interaction of Light and Temperature on Tocopherols During Oxidation of Sunflower Oil. J. Am. Oil Chem. Soc. 2013, 90, 1851–1857. [Google Scholar]
- Verleyen, T.; Verhe, R.; Huyghebaert, A.; Dewettinck, K.; Greyt, W.D. Identification of alpha-tocopherol oxidation products in triolein at elevated temperatures. J. Agr. Food Chem. 2001, 49, 1508–1511. [Google Scholar] [CrossRef]
- Olkkonen, V.M.; Gylling, H.; Ikonen, E. Plant sterols, cholesterol precursors and oxysterols: Minute concentrations-Major physiological effects. J. Steroid Biochem. 2017, 169, 4–9. [Google Scholar] [CrossRef]
- Shahidi, F.; Pinaffi-Langley, A.C.; Fuentes, J.; Speisky, H.; de Camargo, A.C. Vitamin E as an essential micronutrient for human health: Common, novel, and unexplored dietary sources. Free Radical Bio. Med. 2021, 176, 312–321. [Google Scholar] [CrossRef]
- Buzzetti, L.; Crisenza, G.E.; Melchiorre, P. Mechanistic Studies in Photocatalysis. Angew. Chem. Int. Ed. Engl. 2019, 58, 3730–3747. [Google Scholar] [CrossRef]
| Fatty Acid | Time of Illumination Treatment (h) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 6 | 12 | 15 | 18 | 21 | 22 | 23 | 24 | |
| C16:0 | 12.62 ± 0.00 a | 12.39 ± 0.00 a | 14.58 ± 0.00 a | 12.86 ± 0.00 a | 12.47 ± 0.01 a | 14.41 ± 0.03 a | 12.48 ± 0.00 a | 12.71 ± 0.00 a | 12.55 ± 0.00 a |
| C16:1 | 0.42 ± 0.00 a | 0.37 ± 0.00 a | 0.36 ± 0.00 a | 0.49 ± 0.00 a | 0.40 ± 0.00 a | 0.38 ± 0.00 a | 0.37 ± 0.00 a | 0.44 ± 0.00 a | 0.53 ± 0.00 a |
| C17:0 | 0.42 ± 0.00 a | 0.43 ± 0.00 a | 0.40 ± 0.00 a | 0.59 ± 0.00 a | 0.42 ± 0.00 a | 0.37 ± 0.00 a | 0.46 ± 0.00 a | 0.50 ± 0.00 a | 0.68 ± 0.00 a |
| C17:1 | 0.28 ± 0.00 a | 0.29 ± 0.00 a | 0.30 ± 0.00 a | 0.32 ± 0.00 a | 0.41 ± 0.00 a | 0.26 ± 0.00 a | 0.36 ± 0.00 a | 0.28 ± 0.00 a | 0.43 ± 0.00 a |
| C18:0 | 4.61 ± 0.00 a | 4.67 ± 0.00 a | 4.79 ± 0.00 a | 4.83 ± 0.00 a | 4.75 ± 0.00 a | 4.90 ± 0.00 a | 4.67 ± 0.00 a | 4.76 ± 0.00 a | 4.71 ± 0.00 a |
| C18:1 | 21.87 ± 0.00 a | 21.63 ± 0.00 a | 20.91 ± 0.01 a | 21.56 ± 0.00 a | 21.62 ± 0.00 a | 21.05 ± 0.01 a | 21.74 ± 0.00 a | 21.67 ± 0.00 a | 21.34 ± 0.00 a |
| C18:2ω6 | 52.12 ± 0.00 a | 52.76 ± 0.01 a | 51.28 ± 0.01 a | 51.76 ± 0.00 a | 52.27 ± 0.01 a | 51.06 ± 0.01 a | 52.26 ± 0.01 a | 51.83 ± 0.00 a | 51.93 ± 0.01 a |
| C18:3ω6 | 5.90 ± 0.11 a | 5.96 ± 0.17 a | 5.54 ± 0.14 a | 5.82 ± 0.07 a | 5.81 ± 0.19 a | 5.50 ± 0.35 a | 5.87 ± 0.23 a | 5.82 ± 0.14 a | 5.97 ± 0.28 a |
| C18:3ω3 | 0.45 ± 0.01 a | 0.52 ± 0.13 a | 0.66 ± 0.07 a | 0.50 ± 0.03 a | 0.60 ± 0.26 a | 0.77 ± 0.10 a | 0.52 ± 0.12 a | 0.51 ± 0.10 a | 0.53 ± 0.10 a |
| C20:0 | 0.55 ± 0.00 a | 0.26 ± 0.00 a | 0.51 ± 0.00 a | 0.55 ± 0.00 a | 0.50 ± 0.00 a | 0.60 ± 0.00 a | 0.58 ± 0.00 a | 0.78 ± 0.00 a | 0.59 ± 0.00 a |
| C22:1 | 0.78 ± 0.00 a | 0.72 ± 0.00 a | 0.67 ± 0.00 a | 0.73 ± 0.00 a | 0.75 ± 0.00 a | 0.71 ± 0.00 a | 0.69 ± 0.00 a | 0.71 ± 0.00 a | 0.73 ± 0.00 a |
| ∑SFA | 18.20 ± 0.26 a | 17.75 ± 0.97 a | 20.29 ± 2.33 a | 18.83 ± 0.42 a | 18.15 ± 0.80 a | 20.27 ± 2.69 a | 18.19 ± 0.96 a | 18.75 ± 0.24 a | 18.54 ± 0.67 a |
| ∑MUFA | 23.34 ± 0.26 a | 23.01 ± 0.13 a | 2.24 ± 1.41 a | 23.10 ± 0.18 a | 23.18 ± 0.26 a | 22.40 ± 1.25 a | 23.16 ± 0.19 a | 23.10 ± 0.26 a | 23.03 ± 0.25 a |
| ∑PUFA | 58.47 ± 0.42 a | 59.24 ± 1.01 a | 57.48 ± 0.94 a | 58.08 ± 0.36 a | 58.68 ± 1.05 a | 57.33 ± 1.44 a | 58.65 ± 0.97 a | 58.15 ± 0.39 a | 58.43 ± 0.92 a |
| Time (h) | Composition (mg/g) | ||||
|---|---|---|---|---|---|
| Total Sterols | Campesterol | Stigmasterol | β-Sitosterol | β-Sitosterenol | |
| 0 | 3.61 ± 0.05 a | 0.66 ± 0.01 a | 0.78 ± 0.01 a | 2.03 ± 0.07 a | 0.14 ± 0.02 a |
| 6 | 3.65 ± 0.55 a | 0.65 ± 0.09 a | 0.75 ± 0.06 a | 2.09 ± 0.46 a | 0.15 ± 0.02 a |
| 12 | 3.40 ± 0.48 a | 0.60 ± 0.05 a | 0.88 ± 0.04 a | 1.78 ± 0.13 a | 0.14 ± 0.06 a |
| 15 | 4.28 ± 0.15 a | 0.85 ± 0.18 a | 0.90 ± 0.07 a | 2.38 ± 0.17 a | 0.16 ± 0.03 a |
| 18 | 4.23 ± 0.90 a | 0.75 ± 0.18 a | 0.95 ± 0.22 a | 2.34 ± 0.47 a | 0.19 ± 0.06 a |
| 21 | 3.77 ± 0.09 a | 0.69 ± 0.05 a | 0.85 ± 0.01 a | 2.08 ± 0.06 a | 0.17 ± 0.03 a |
| 22 | 3.73 ± 0.01 a | 0.72 ± 0.01 a | 0.79 ± 0.01 a | 2.10 ± 0.01 a | 0.12 ± 0.00 b |
| 23 | 3.51 ± 0.18 a | 0.67 ± 0.09 a | 0.78 ± 0.05 a | 1.90 ± 0.09 a | 0.16 ± 0.03 a |
| 24 | 3.84 ± 0.31 a | 0.67 ± 0.08 a | 0.81 ± 0.12 a | 2.18 ± 0.14 a | 0.18 ± 0.01 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, Z.; Luo, S.; Lv, Y.; Liu, Y. Influences of Illumination Pretreatment on Soybean Oil Activated Clay Bleaching Effects and Soybean Oil Quality Evaluation. Foods 2023, 12, 1038. https://doi.org/10.3390/foods12051038
Ye Z, Luo S, Lv Y, Liu Y. Influences of Illumination Pretreatment on Soybean Oil Activated Clay Bleaching Effects and Soybean Oil Quality Evaluation. Foods. 2023; 12(5):1038. https://doi.org/10.3390/foods12051038
Chicago/Turabian StyleYe, Zhan, Shufan Luo, Yaping Lv, and Yuanfa Liu. 2023. "Influences of Illumination Pretreatment on Soybean Oil Activated Clay Bleaching Effects and Soybean Oil Quality Evaluation" Foods 12, no. 5: 1038. https://doi.org/10.3390/foods12051038
APA StyleYe, Z., Luo, S., Lv, Y., & Liu, Y. (2023). Influences of Illumination Pretreatment on Soybean Oil Activated Clay Bleaching Effects and Soybean Oil Quality Evaluation. Foods, 12(5), 1038. https://doi.org/10.3390/foods12051038





