Quality Improvement in Apple Ciders during Simultaneous Co-Fermentation through Triple Mixed-Cultures of Saccharomyces cerevisiae, Pichia kudriavzevii, and Lactiplantibacillus plantarum
Abstract
1. Introduction
2. Materials and Methods
2.1. Yeast and Bacterial Strains and Culture Media
2.2. Apple Cider Fermentation
2.3. Physicochemical Analysis
2.4. Determination of Antioxidant Activity
2.5. GC-TOF-MS Analysis
2.6. LC-MS/MS Analysis
2.7. Sensory Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Changes in Physicochemical Parameters
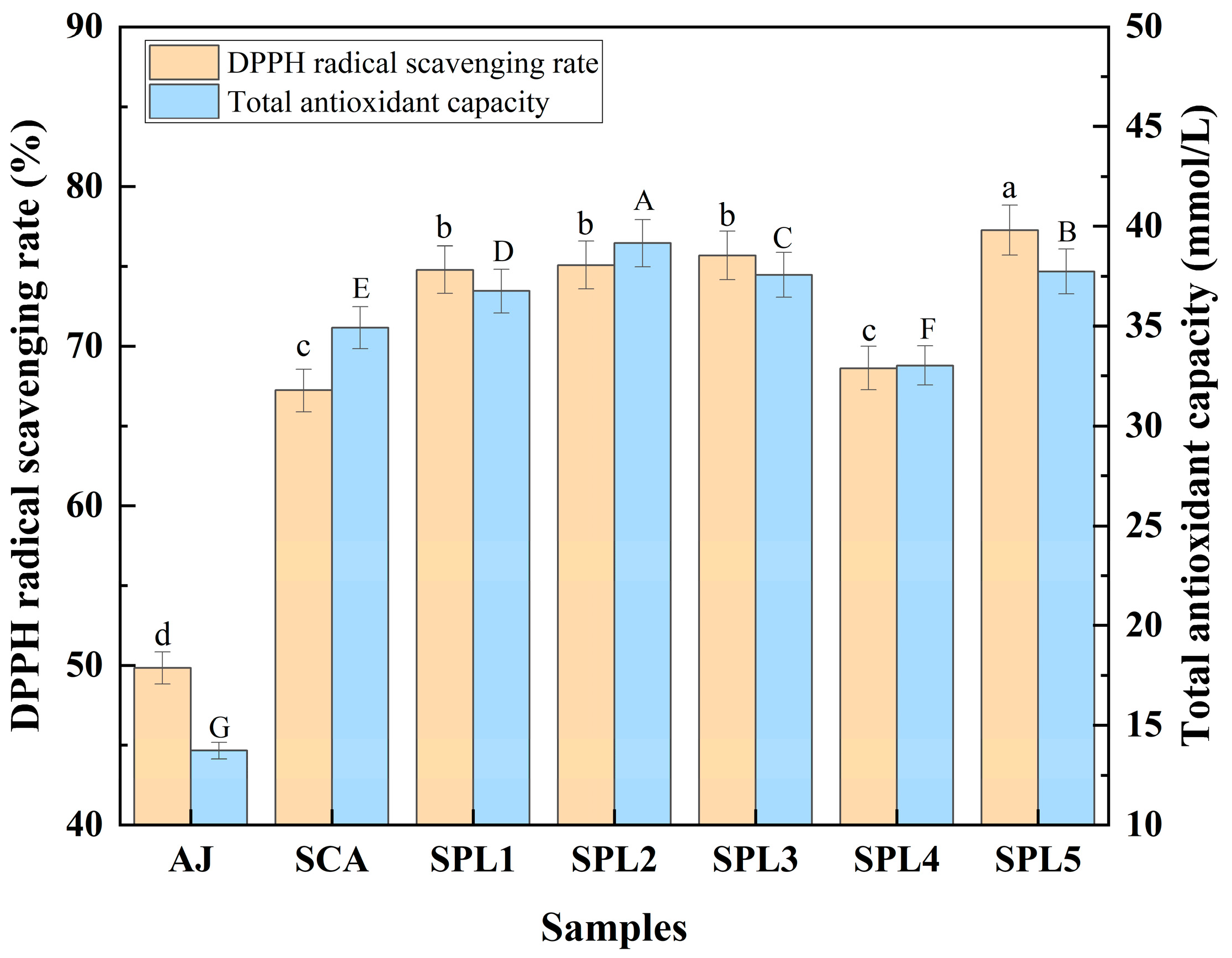
3.2. Comparative Analysis of Antioxidant Activity
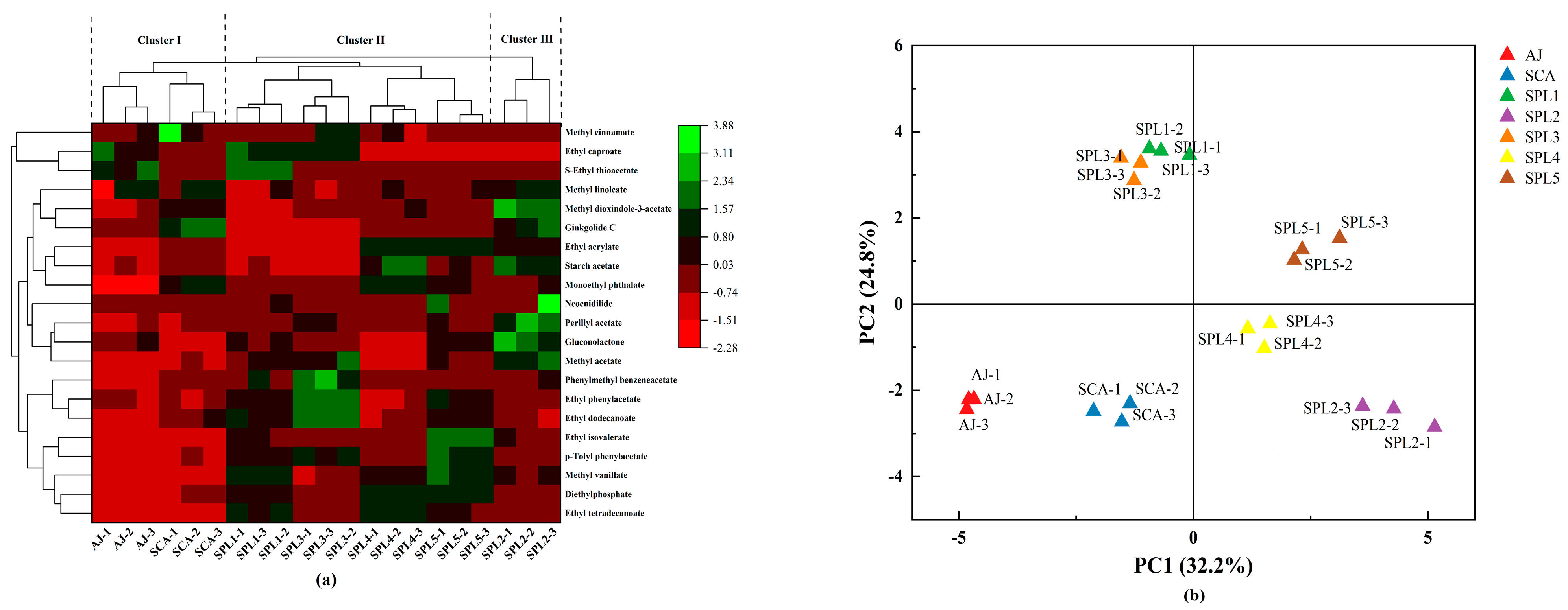
3.3. Analysis of Esters, Higher Alcohols, Aldehydes, and Ketones
3.4. Analysis of Organic Acids, Polyphenols, and Terpenoids
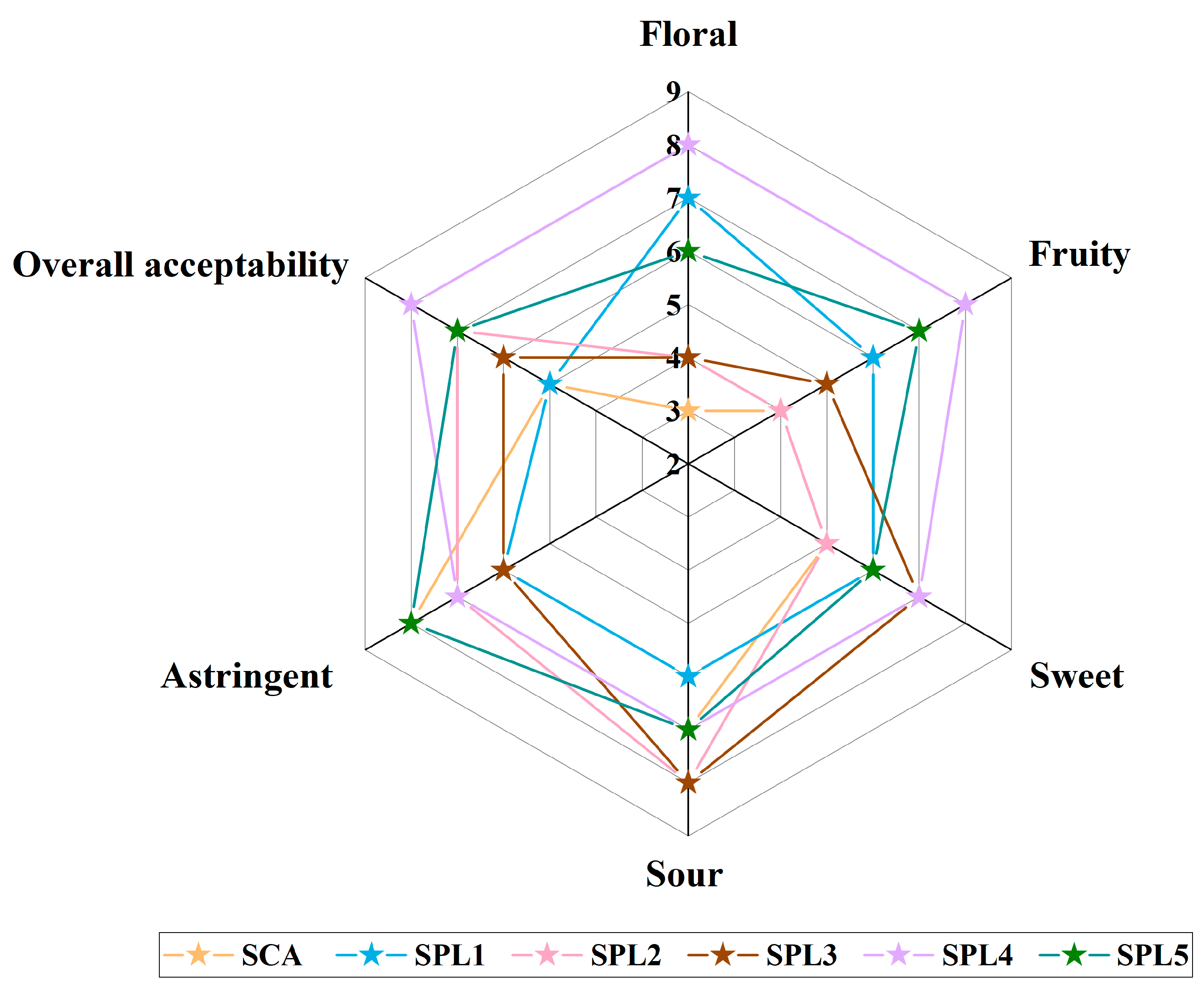
3.5. Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Francini, A.; Sebastiani, L. Phenolic compounds in apple (Malus × domestica Borkh.): Compounds characterization and stability during postharvest and after processing. Antioxidants 2013, 2, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Tsoupras, A.; Moran, D.; Pleskach, H.; Durkin, M.; Traas, C.; Zabetakis, I. Beneficial anti-platelet and anti-inflammatory properties of Irish apple juice and cider bioactives. Foods 2021, 10, 412. [Google Scholar] [PubMed]
- Feng, J.; Ren, X.L.; Tian, J.W. Comparative contents of polyphenols, soluble sugars and organic acids in Fuji apples from different growing regions. Food Sci. 2013, 34, 125–130. (In Chinese) [Google Scholar]
- Way, M.L.; Jones, J.E.; Longo, R.; Dambergs, R.G.; Swarts, N.D. A Preliminary study of yeast strain influence on chemical and sensory characteristics of apple cider. Fermentation 2022, 8, 455. [Google Scholar]
- Li, C.X.; Zhao, X.H.; Zuo, W.F.; Zhang, T.L.; Zhang, Z.Y.; Chen, X.S. The effects of simultaneous and sequential inoculation of yeast and autochthonous Oenococcus oeni on the chemical composition of red-fleshed apple cider. LWT-Food Sci. Technol. 2020, 124, 109184. [Google Scholar] [CrossRef]
- Sun, S.Y.; Che, C.Y.; Sun, T.F.; Lv, Z.Z.; He, S.X.; Gu, H.N.; Shen, W.J.; Chi, D.C.; Gao, Y. Evaluation of sequential inoculation of Saccharomyces cerevisiae and Oenococcus oeni strains on the chemical and aromatic profiles of cherry wines. Food Chem. 2013, 138, 2233–2241. [Google Scholar] [CrossRef]
- Graeme, W.; Graham, S. Saccharomyces cerevisiae in the production of fermented beverages. Beverages 2016, 2, 30. [Google Scholar]
- Li, Y.; Nguyen, T.T.H.; Jin, J.H.; Lim, J.; Lee, J.; Piao, M.Z.; Mok, I.; Kim, D. Brewing of glucuronic acid-enriched apple cider with enhanced antioxidant activities through the co-fermentation of yeast (Saccharomyces cerevisiae and Pichia kudriavzevii) and bacteria (Lactobacillus plantarum). Food Sci. Biotechnol. 2021, 30, 555–564. [Google Scholar]
- Wei, J.P.; Zhang, Y.X.; Wang, Y.W.; Ju, H.M.; Niu, C.; Song, Z.H.; Yuan, Y.H.; Yue, T.L. Assessment of chemical composition and sensorial properties of ciders fermented with different non-Saccharomyces yeasts in pure and mixed fermentations. Int. J. Food Microbiol. 2020, 318, 108471. [Google Scholar]
- Gschaedler, A.; Iniguez-Munoz, L.E.; Flores-Flores, N.Y.; Kirchmayr, M.; Arellano-Plaza, M. Use of non-Saccharomyces yeasts in cider fermentation: Importance of the nutrients addition to obtain an efficient fermentation. Int. J. Food Microbiol. 2021, 347, 109169. [Google Scholar]
- Yu, W.Y.; Zhu, Y.Y.; Zhu, R.X.; Bai, J.R.; Qiu, J.H.; Wu, Y.P.; Zhong, K.; Gao, H. Insight into the characteristics of cider fermented by single and co-culture with Saccharomyces cerevisiae and Schizosaccharomyces pombe based on metabolomic and transcriptomic approaches. LWT-Food Sci. Technol. 2022, 163, 113538. [Google Scholar]
- Ye, M.; Yue, T.; Yuan, Y. Effects of sequential mixed cultures of Wickerhamomyces anomalus and Saccharomyces cerevisiae on apple cider fermentation. FEMS Yeast Res. 2014, 14, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.P.; Zhang, Y.X.; Qiu, Y.; Guo, H.; Ju, H.M.; Wang, Y.W.; Yuan, Y.H.; Yue, T.L. Chemical composition, sensorial properties, and aroma-active compounds of ciders fermented with Hanseniaspora osmophila and Torulaspora quercuum in co- and sequential fermentations. Food Chem. 2020, 306, 125623. [Google Scholar] [CrossRef] [PubMed]
- Aung, M.T.; Lee, P.R.; Yu, B.; Liu, S.Q. Cider fermentation with three Williopsis saturnus yeast strains and volatile changes. Ann. Microbiol. 2014, 65, 921–928. [Google Scholar]
- Paradiso, V.M.; Sanarica, L.; Zara, I.; Pisarra, C.; Gambacorta, G.; Natrella, G.; Cardinale, M. Cultivar-dependent effects of non-Saccharomyces yeast starter on the oenological properties of wines produced from two autochthonous grape cultivars in southern Italy. Foods 2022, 11, 3373. [Google Scholar]
- Borren, E.; Tian, B. The important contribution of non-Saccharomyces yeasts to the aroma complexity of wine: A review. Foods 2021, 10, 13. [Google Scholar]
- Hu, K.; Zhao, H.Y.; Kang, X.T.; Ge, X.N.; Zheng, M.N.; Hu, Z.Y.; Tao, Y.S. Fruity aroma modifications in Merlot wines during simultaneous alcoholic and malolactic fermentations through mixed culture of S. cerevisiae, P. fermentans, and L. brevis. LWT-Food Sci. Technol. 2022, 154, 112711. [Google Scholar] [CrossRef]
- Al-Sahlany, S.T.G.; Khassaf, W.H.; Niamah, A.K.; Abd Al-Manhel, A.J. Date juice addition to bio-yogurt: The effects on physicochemical and microbiological properties during storage, as well as blood parameters in vivo. J. Saudi Soc. Agric. Sci. 2022; in press. [Google Scholar]
- Englezos, V.; Cachon, D.C.; Rantsiou, K.; Blanco, P.; Petrozziello, M.; Pollon, M.; Giacosa, S.; Segade, S.R.; Rolle, L.; Cocolin, L. Effect of mixed species alcoholic fermentation on growth and malolactic activity of lactic acid bacteria. Appl. Microbiol. Biotechnol. 2019, 103, 7687–7702. [Google Scholar] [CrossRef]
- Tufariello, M.; Capozzi, V.; Spano, G.; Cantele, G.; Venerito, P.; Mita, G.; Grieco, F. Effect of co-inoculation of candida zemplinina, Saccharomyces cerevisiae and Lactobacillus plantarum for the industrial production of Negroamaro wine in Apulia (southern Italy). Microorganisms 2020, 8, 726. [Google Scholar]
- Iorizzo, M.; Testa, B.; Lombardi, S.J.; García-Ruiz, A.; Muñoz-González, C.; Bartolomé, B.; Moreno-Arribas, M.V. Selection and technological potential of Lactobacillus plantarum bacteria suitable for wine malolactic fermentation and grape aroma release. LWT-Food Sci. Technol. 2016, 73, 557–566. [Google Scholar]
- Sun, S.Y.; Gong, H.S.; Liu, W.L.; Jin, C.W. Application and validation of autochthonous Lactobacillus plantarum starter cultures for controlled malolactic fermentation and its influence on the aromatic profile of cherry wines. Food Microbiol. 2016, 55, 16–24. [Google Scholar] [CrossRef]
- Grover, S.; Rashmi, H.M.; Srivastava, A.K.; Batish, V.K. Probiotics for human health—New innovations and emerging trends. Gut pathog. 2012, 4, 15. [Google Scholar] [CrossRef] [PubMed]
- Dimitrovski, D.; Velickova, E.; Langerholc, T.; Winkelhausen, E. Apple juice as a medium for fermentation by the probiotic Lactobacillus plantarum PCS 26 strain. Ann. Microbiol. 2015, 65, 2161–2170. [Google Scholar]
- Etxeberria, U.; Fernández-Quintela, A.; Milagro, F.I.; Aguirre, L.; Martínez, J.A.; Portillo, M.P. Impact of polyphenols and polyphenol-rich dietary sources on gut microbiota composition. J. Agric. Food Chem. 2013, 61, 9517–9533. [Google Scholar] [PubMed]
- Li, H.C.; Huang, J.T.; Wang, Y.Q.; Wang, X.N.; Ren, Y.C.; Yue, T.L.; Wang, Z.L.; Gao, Z.P. Study on the nutritional characteristics and antioxidant activity of dealcoholized sequentially fermented apple juice with Saccharomyces cerevisiae and Lactobacillus plantarum fermentation. Food Chem. 2021, 363, 109184. [Google Scholar] [CrossRef] [PubMed]
- He, W.J.; Liu, S.X.; Heponiemi, P.; Heinonen, M.; Marsol-Vall, A.; Ma, X.Y.; Yang, B.R.; Laaksonen, O. Effect of Saccharomyces cerevisiae and Schizosaccharomyces pombe strains on chemical composition and sensory quality of ciders made from Finnish apple cultivars. Food Chem. 2021, 345, 128833. [Google Scholar]
- Mohammed, A.A.; Niamah, A.K. Identification and antioxidant activity of hyaluronic acid extracted from local isolates of Streptococcus thermophilus. Mater. Today Proc. 2022, 60, 1523–1529. [Google Scholar]
- Zhou, B.X.; Wang, Z.H.; Yin, P.; Ma, B.S.; Ma, C.Q.; Xu, C.C.; Wang, J.C.; Wang, Z.Y.; Yin, D.F.; Xia, T. Impact of prolonged withering on phenolic compounds and antioxidant capability in white tea using LC-MS-based metabolomics and HPLC analysis: Comparison with green tea. Food Chem. 2022, 368, 130855. [Google Scholar]
- Yang, X.S.; Zhao, F.Q.; Yang, L.; Li, J.N.; Zhu, X. Enhancement of the aroma in low-alcohol apple-blended pear wine mixed fermented with Saccharomyces cerevisiae and non-Saccharomyces yeasts. LWT-Food Sci. Technol. 2022, 155, 112994. [Google Scholar] [CrossRef]
- Wu, C.Y.; Li, T.L.; Qi, J.; Jiang, T.; Xu, H.D.; Lei, H.J. Effects of lactic acid fermentation-based biotransformation on phenolic profiles, antioxidant capacity and flavor volatiles of apple juice. LWT-Food Sci. Technol. 2020, 122, 109064. [Google Scholar]
- Zhao, X.X.; Xue, Y.; Tang, F.X.; Cai, W.C.; Hao, G.F.; Shan, C.H. Quality improvement of jujube wine through mixed fermentation with Saccharomyces cerevisiae and Bacillus licheniformis. LWT-Food Sci. Technol. 2022, 164, 113444. [Google Scholar]
- Cai, W.C.; Tang, F.X.; Guo, Z.; Guo, X.; Zhang, Q.; Zhao, X.X.; Ning, M.; Shan, C.H. Effects of pretreatment methods and leaching methods on jujube wine quality detected by electronic senses and HS-SPME-GC-MS. Food Chem. 2020, 330, 127330. [Google Scholar] [CrossRef]
- Zhang, M.; Zhong, T.; Heygi, F.; Wang, Z.R.; Du, M.Y. Effects of inoculation protocols on aroma profiles and quality of plum wine in mixed culture fermentation of Metschnikowia pulcherrima with Saccharomyces cerevisiae. LWT-Food Sci. Technol. 2022, 161, 113338. [Google Scholar]
- Shi, W.K.; Wang, J.; Chen, F.S.; Zhang, X.Y. Effect of Issatchenkia terricola and Pichia kudriavzevii on wine flavor and quality through simultaneous and sequential co-fermentation with Saccharomyces cerevisiae. LWT-Food Sci. Technol. 2019, 116, 108477. [Google Scholar]
- González Álvarez, M.; González-Barreiro, C.; Cancho-Grande, B.; Simal-Gándara, J. Relationships between Godello white wine sensory properties and its aromatic fingerprinting obtained by GC-MS. Food Chem. 2011, 129, 890–898. [Google Scholar]
- Zhong, W.; Liu, S.Q.; Yang, H.; Li, E.H. Effect of selected yeast on physicochemical and oenological properties of blueberry wine fermented with citrate-degrading Pichia fermentans. LWT-Food Sci. Technol. 2021, 145, 111261. [Google Scholar]
- Loira, I.; Morata, A.; Comuzzo, P.; Callejo, M.J.; González, C.; Calderón, F.; Suárez-Lepe, J.A. Use of Schizosaccharomyces pombe and Torulaspora delbrueckii strains in mixed and sequential fermentations to improve red wine sensory quality. Food Res. Int. 2015, 76, 325–333. [Google Scholar]
- Tian, T.T.; Sun, J.Y.; Wu, D.H.; Xiao, J.B.; Lu, J. Objective measures of greengage wine quality: From taste-active compound and aroma-active compound to sensory profiles. Food Chem. 2021, 340, 128179. [Google Scholar] [CrossRef]
- Loira, I.; Morata, A.; Palomero, F.; González, C.; Suárez-Lepe, J. Schizosaccharomyces pombe: A promising biotechnology to modulate wine composition. Fermentation 2018, 4, 70. [Google Scholar]
- Laaksonen, O.; Kuldjärv, R.; Paalme, T.; Virkki, M.; Yang, B. Impact of apple cultivar, ripening stage, fermentation type and yeast strain on phenolic composition of apple ciders. Food Chem. 2017, 233, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Requena, T.; Monagas, M.; Pozo-Bayón, M.; Martín-Álvarez, P.; Bartolomé, B.; Campo, R.D.; Ávila, M.; Martínez-Cuesta, M.; Peláez, C.; Moreno-Arribas, M.V. Perspectives of the potential implications of wine polyphenols on human oral and gut microbiota. Trends Food Sci. Technol. 2010, 21, 332–344. [Google Scholar]
- Luan, Y.; Zhang, B.Q.; Duan, C.Q.; Yan, G.L. Effects of different pre-fermentation cold maceration time on aroma compounds of Saccharomyces cerevisiae co-fermentation with Hanseniaspora opuntiae or Pichia kudriavzevii. LWT-Food Sci. Technol. 2018, 92, 177–186. [Google Scholar]

| Parameters Measured | AJ (Day 0) | Treatment (n = 3) (Day 16) | |||||
|---|---|---|---|---|---|---|---|
| SCA | SPL1 | SPL2 | SPL3 | SPL4 | SPL5 | ||
| pH | 3.79 ± 0.01 b | 3.58 ± 0.00 e | 3.73 ± 0.01 c | 3.54 ± 0.01 f | 3.85 ± 0.01 a | 3.41 ± 0.01 g | 3.68 ± 0.01 d |
| Total acid (mg/mL) | 1.15 ± 0.01 e | 5.32 ± 0.12 a | 4.54 ± 0.04 b | 3.96 ± 0.04 c | 5.25 ± 0.07 a | 4.17 ± 0.04 c | 3.62 ± 0.39 d |
| SSC (°Brix) | 20.00 ± 0.00 a | 5.00 ± 0.00 e | 6.33 ± 0.29 c | 5.00 ± 0.00 e | 8.00 ± 0.00 b | 5.50 ± 0.00 d | 5.00 ± 0.00 e |
| Reducing sugar (mg/mL) | 107.56 ± 0.11 a | 7.00 ± 0.01 d | 15.89 ± 0.06 c | 5.07 ± 0.01 f | 25.44 ± 0.07 b | 4.55 ± 0.01 g | 6.07 ± 0.03 e |
| Alcohol (% v/v) | - | 11.13 ± 0.12 a | 7.97 ± 0.15 d | 9.50 ± 0.20 c | 7.30 ± 0.20 e | 10.23 ± 0.15 b | 10.17 ± 0.29 b |
| Number | Compounds | AJ | Treatment (n = 3) (μg/L) | |||||
|---|---|---|---|---|---|---|---|---|
| SCA | SPL1 | SPL2 | SPL3 | SPL4 | SPL5 | |||
| Esters | ||||||||
| 1 | Ethyl tetradecanoate | 504.85 ± 36.94 c | 681.41 ± 41.70 c | 1641.06 ± 286.23 a | 1036.07 ± 44.70 b | 1030.20 ± 61.92 b | 1659.25 ± 342.15 a | 1238.20 ± 95.31 b |
| 2 | Methyl acetate | 4.75 ± 0.34 d | 6.34 ± 0.59 d | 10.42 ± 1.11 bc | 16.09 ± 0.33 a | 13.55 ± 4.81 ab | 6.21 ± 0.29 d | 9.87 ± 1.02 c |
| 3 | Diethylphosphate | 30.85 ± 2.95 g | 64.42 ± 0.26 f | 121.39 ± 6.89 c | 83.00 ± 1.11 d | 71.69 ± 1.96 e | 189.09 ± 2.93 a | 171.63 ± 4.01 b |
| 4 | Monoethyl phthalate | - | 12.86 ± 0.31 d | 33.98 ± 2.76 b | 24.35 ± 4.78 c | 13.48 ± 1.01 d | 45.4 ± 3.22 a | 27.32 ± 3.94 c |
| 5 | Methyl vanillate | 134.79 ± 10.98 d | 131.44 ± 1.55 d | 294.64 ± 32.28 a | 233.73 ± 12.20 b | 176.62 ± 10.94 c | 242.22 ± 6.05 b | 316.94 ± 16.23 a |
| 6 | Ethyl dodecanoate | 11.01 ± 0.96 e | 37.99 ± 2.44 c | 57.94 ± 8.3 b | 29.14 ± 5.56 d | 87.73 ± 2.32 a | 29.19 ± 4.96 d | 56.26 ± 3.52 b |
| 7 | Ginkgolide C | 3.29 ± 0.68 bc | 33.29 ± 1.3 a | - | 33.18 ± 11.79 a | - | 10.13 ± 2.45 b | 11.11 ± 1.59 b |
| 8 | Gluconolactone | 122.05 ± 16.39 cde | 83.35 ± 3.42 e | 159.26 ± 9.38 bc | 296.11 ± 49.55 a | 130.9 ± 5.95 cd | 93.46 ± 3.11 de | 169.95 ± 16.58 b |
| 9 | Methyl dioxindole-3-acetate | 0.78 ± 0.05 a | - | - | 9.41 ± 0.01 a | - | - | - |
| 10 | S-ethyl thioacetate | 3.89 ± 0.74 b | - | 23.86 ± 0.05 a | - | - | - | - |
| 11 | p-tolyl phenylacetate | 1.08 ± 0.05 b | - | - | - | - | - | 8.32 ± 1 a |
| 12 | Ethyl isovalerate | 0.16 ± 0.02 c | 0.18 ± 0.01 c | 0.75 ± 0.01 c | 4.88 ± 0.27 b | 1.96 ± 0.31 bc | 9.83 ± 0.89 a | 8.26 ± 0.23 a |
| 13 | Methyl cinnamate | 0.04 ± 0.01 c | 0.02 ± 0.00 c | 0.64 ± 0.10 a | 0.35 ± 0.01 b | 0.35 ± 0.02 b | 0.25 ± 0.01 bc | 0.06 ± 0.00 c |
| 14 | Neocnidilide | 0.01 ± 0.00 d | 0.06 ± 0.01 d | 0.64 ± 0.06 c | 4.05 ± 0.03 a | 0.39 ± 0.04 cd | 0.06 ± 0.01 d | 2.06 ± 0.02 b |
| 15 | Ethyl caproate | 1.18 ± 0.02 bc | 0.34 ± 0.03 c | - | - | 2.34 ± 2.03 b | 4.27 ± 0.12 a | 2.38 ± 0.03 b |
| 16 | Methyl linoleate | 0.43 ± 0.11 b | 0.11 ± 0.01 b | 0.47 ± 0.01 b | 2.12 ± 1.84 a | 3.13 ± 0.18 a | 0.72 ± 0.06 b | 0.73 ± 0.06 b |
| 17 | Ethyl phenylacetate | 0.03 ± 0.01 c | - | 2.5 ± 0.34 b | 2.46 ± 0.46 b | 2.38 ± 0.58 b | 4.80 ± 0.44 a | 0.36 ± 0.03 c |
| 18 | Ethyl acrylate | - | 1.47 ± 0.30 a | - | - | 0.37 ± 0.05 b | - | - |
| 19 | Phenylmethyl benzeneacetate | 0.49 ± 0.18 b | - | - | 0.59 ± 1.02 b | 2.16 ± 1.91 a | - | - |
| 20 | Perillyl acetate | 0.04 ± 0.01 b | - | - | 4.93 ± 0.72 a | 1.36 ± 0.36 b | - | - |
| 21 | Starch acetate | 1.32 ± 0.27 a | - | 0.60 ± 0.04 a | - | - | 1.24 ± 0.04 a | - |
| Ʃ(Sum) | 817.75 ± 14.48 d | 1053.27 ± 67.43 d | 2348.14 ± 355.68 a | 1780.47 ± 92.68 bc | 1538.62 ± 71.77 c | 2296.11 ± 331.32 a | 2023.44 ± 134.23 ab | |
| Higher alcohols | ||||||||
| 1 | Glycerol | 3.74 ± 0.70 f | 26.39 ± 3.47 e | 46.46 ± 4.37 b | 34.49 ± 2.65 cd | 27.8 ± 0.61 de | 70.71 ± 8.16 a | 39.90 ± 0.82 bc |
| 2 | Isoeugenitol | 1.97 ± 0.84 d | 3.85 ± 0.99 d | 7.50 ± 0.37 bc | 9.72 ± 2.08 b | 10.29 ± 0.76 b | 4.68 ± 0.48 cd | 26.55 ± 4.47 a |
| 3 | 3-ethyl-1,2-benzenediol | 0.96 ± 0.31 b | 4.14 ± 0.36 a | 1.11 ± 0.15 b | 4.21 ± 0.45 a | 1.03 ± 0.56 b | 4.15 ± 0.58 a | 4.01 ± 0.95 a |
| Ʃ(Sum) | 6.67 ± 0.44 e | 34.37 ± 2.51 d | 55.08 ± 3.87 c | 48.41 ± 4.09 c | 39.12 ± 0.47 d | 79.53 ± 7.65 a | 70.46 ± 3.23 b | |
| Aldehydes | ||||||||
| 1 | Vanillin | 7.34 ± 2.44 e | 85.22 ± 4.20 bc | 55.44 ± 4.35 d | 91.69 ± 2.24 b | 45.97 ± 1.73 d | 143.06 ± 10.38 a | 77.99 ± 9.88 c |
| 2 | Pyridoxal | 5.07 ± 0.79 f | 562.62 ± 32.57 a | 183.20 ± 5.03 d | 354.90 ± 10.34 c | 113.92 ± 3.44 e | 449.05 ± 35.59 b | 591.80 ± 7.91 a |
| 3 | 4-isopropylbenzaldehyde | 16.05 ± 4.84 c | 23.11 ± 8.81 bc | 25.81 ± 6.38 bc | 45.74 ± 2.66 a | 27.13 ± 0.26 bc | 24.88 ± 4.77 bc | 33.66 ± 12.48 b |
| 4 | 2-carboxybenzaldehyde | 13.79 ± 3.52 c | 11.69 ± 4.27 c | 24.24 ± 3.96 ab | 31.28 ± 9.19 a | 17.03 ± 0.83 bc | 23.22 ± 2.37 ab | 14.38 ± 2.28 c |
| 5 | Benzaldehyde | 44.27 ± 12.09 c | 31.31 ± 4.94 d | 32.90 ± 1.87 d | 140.75 ± 4.47 a | 38.73 ± 5.01 cd | 69.69 ± 1.38 b | 48.09 ± 2.9 c |
| Ʃ(Sum) | 86.52 ± 10.88 f | 713.95 ± 31.64 b | 321.60 ± 4.63 d | 664.36 ± 16.63 c | 242.79 ± 9.37 e | 709.89 ± 38.67 b | 765.92 ± 32.97 a | |
| Ketones | ||||||||
| 1 | 1-(4-hydroxyphenyl)propan-1-one | 6.83 ± 1.10 b | 6.09 ±4.36 b | 6.81 ±0.52 b | 12.50 ± 3.68 ab | 15.45 ± 8.67 a | 7.62 ± 0.61 b | 10.75 ± 3.01 ab |
| 2 | 1,3-diphenyl-2-propen-1-one; Chalcone | 0.68 ± 1.18 c | 13.56 ± 3.16 b | 13.91 ± 0.42 b | 26.18 ± 2.55 a | 3.63 ± 2.35 c | 12.44 ± 2.31 b | 27.68 ± 3.59 a |
| Ʃ(Sum) | 7.51 ± 0.59 c | 19.65 ±7 b | 20.72 ± 0.82 b | 38.69 ± 4.74 a | 19.08 ± 7.54 b | 20.06 ± 1.82 b | 38.44 ± 5.84 a | |
| Ʃ(Sum: GC-MS) | 921.75 ± 28.57 e | 1821.25 ± 19.62 d | 2745.53 ± 327.04 bc | 2531.93 ± 115.23 c | 1839.6 ± 57.72 d | 3105.6 ± 356.62 a | 2898.26 ± 150.49 ab | |
| Number | Compounds | AJ | Treatment (n = 3) (μg/L) | |||||
|---|---|---|---|---|---|---|---|---|
| SCA | SPL1 | SPL2 | SPL3 | SPL4 | SPL5 | |||
| Organic acids | ||||||||
| 1 | Acrylic acid | 2452.89 ± 81.19 b | 594.13 ± 19.72 c | 2627.98 ± 17.83 a | 37.94 ± 12.95 d | 2726.85 ± 86.45 a | 27.8 ± 1.39 d | 52.85 ± 3.99 d |
| 2 | Glyoxylic acid | 22.56 ± 3.6 d | 35.68 ± 2.28 b | 30.80 ± 1.99 c | 34.92 ± 1.34 b | 34.36 ± 0.92 bc | 43.53 ± 2.72 a | 41.55 ± 1.32 a |
| 3 | Propionic acid | 7.52 ± 0.31 c | 8.79 ± 0.39 b | 8.80 ± 0.25 b | 8.61 ± 0.35 bc | 10.14 ± 1.19 a | 8.66 ± 0.52 bc | 9.10 ± 0.92 ab |
| 4 | Glycolic acid | 194.05 ± 2.14 b | 108.56 ± 3.86 c | 247.80 ± 7.53 a | 36.83 ± 5.27 d | 231.17 ± 19.00 a | 48.42 ± 13.94 d | 37.11 ± 7.82 d |
| 5 | Malonic acid | 107.72 ± 50.95 c | 162.42 ± 19.81 abc | 126.88 ± 15.82 bc | 194.2 ± 48.33 a | 122.76 ± 4.63 bc | 175.78 ± 29.08 ab | 214.11 ± 9.44 a |
| 6 | Palmitic acid | 289.12 ± 37.20 c | 833.94 ± 18.51 b | 1076.68 ± 148.63 a | 966.30 ± 54.73 a | 997.63 ± 54.39 a | 1061.71 ± 47.81 a | 1062 ± 57.34 a |
| 7 | Pyruvic acid | 90.77 ± 8.57 cd | 75.53 ± 2.5 d | 118.19 ± 2.37 b | 83.09 ± 6.75 d | 136.67 ± 22.85 a | 92.24 ± 8.21 cd | 103.46 ± 6.08 ab |
| 8 | Itaconic acid | 124.84 ± 34.45 f | 545.67 ± 24.07 c | 1347.57 ± 21.21 a | 480.59 ± 8.00 d | 1046.01 ± 48.14 b | 187.49 ± 20.5 e | 109.21 ± 11.97 f |
| 9 | L-malic acid | 2390.08 ± 74.17 c | 1556.56 ± 42.16 c | 1645.72 ± 24.01 c | 1245.80 ± 20.04 a | 965.81 ± 21.81 b | 998.79 ± 22.93 b | 1081.30 ± 19.02 ab |
| 10 | Maleic acid | 36.22 ± 4.19 e | 97.50 ± 7.99 d | 133.17 ± 4.33 c | 106.03 ± 4.90 d | 101.61 ± 8.44 d | 183.24 ± 6.68 b | 194.64 ± 3.67 a |
| 11 | (R)-3-hydroxybutyric acid | 2.95 ± 0.14 f | 72.88 ± 7.47 b | 97.10 ± 3.56 a | 49.55 ± 2.02 c | 22.07 ± 0.97 e | 75.14 ± 2.78 b | 32.27 ± 1.28 d |
| 12 | Quinic acid | 12,757.83 ± 74.6 d | 16,465.97 ± 52.83 b | 15,222.81 ± 81.15 c | 16,609.83 ± 74.89 b | 35,706.86 ±82.50 a | 16,225.68 ± 65.5 b | 16,861.02 ± 74.85 b |
| 13 | Maslinic acid | 3.47 ± 0.28 d | 11.09 ± 1.2 a | 5.61 ± 0.13 c | 7.86 ± 1.7 b | 2.83 ± 0.15 d | 8.31 ± 0.42 b | 10.69 ± 2.17 a |
| 14 | Malic acid | 116.65 ± 1.15 e | 153.26 ± 4.69 c | 167.31 ± 2.17 b | 134.23 ± 3.29 a | 141.98 ± 13.83 cd | 123.11 ± 16.36 de | 115.50 ± 2.73 a |
| 15 | Succinic acid | 29.45 ± 0.40 e | 9591.50 ± 20.43 b | 958.75 ± 49.66 d | 7086.79 ± 55.05 c | 878.43 ± 35.84 d | 9600.65 ± 47.45 b | 10,141.63 ± 40.74 a |
| 16 | Fumaric acid | 71.71 ± 7.32 b | 130.39 ± 9.21 ab | 87.04 ± 6.46 ab | 118.64 ± 38.84 ab | 86.82 ± 2.39 ab | 128.39 ± 46.2 ab | 136.17 ± 51.68 a |
| 17 | Phenoxyacetic acid | 2.03 ± 1.37 d | 8.33 ± 0.76 b | 11.82 ± 1.71 a | 7.73 ± 0.18 b | 10.72 ± 0.34 a | 7.27 ± 0.64 b | 4.73 ± 0.75 c |
| 18 | cis-aconitic acid | 7.25 ± 1.65 e | 904.89 ± 22.74 b | 944.47 ± 112.67 b | 592.57 ± 7.49 d | 1064 ± 3.07 a | 745.83 ± 20.46 c | 1031.97 ± 18.18 a |
| 19 | Citric acid | 1069.28 ± 109.11 f | 5039.46 ± 26.10 d | 2407.29 ± 142.65 e | 8242.77 ± 382.28 b | 6583.95 ± 333.00 c | 9564.43 ± 171.39 a | 2343.84 ± 47.19 e |
| 20 | 2-isopropylmalic acid | 35.64 ± 11.78 e | 2447.36 ± 104.28 c | 1698.46 ± 20.58 d | 3945.29 ± 4.50 b | 2797.50 ± 150.74 c | 17,209.54 ± 698.97 a | 17,272.17 ± 607.54 a |
| 21 | Succinic acid semialdehyde | 57.14 ± 4.33 e | 307.24 ± 17.9 b | 215.11 ± 2.99 c | 357.86 ± 2.84 a | 169.26 ± 11.65 d | 342.41 ± 10.21 a | 344.73 ± 13.45 a |
| 22 | Hydroxyphenyllactic acid | 96.77 ± 13.82 f | 499.86 ± 15.11 a | 184.73 ± 4.22 e | 343.44 ± 9.07 d | 179.13 ± 3.48 e | 383.38 ± 14.76 c | 424.30 ± 4.22 b |
| 23 | D-glucuronic acid | 48.60 ± 8.21 f | 6072.45 ± 21.05 b | 4604.97 ± 63.66 d | 4130.15 ± 282.25 e | 5731.96 ± 152.62 c | 4154.09 ± 184.11 e | 8264.68 ± 112.71 a |
| 24 | Phenyllactic acid | 3.37 ± 1.91 f | 6109.55 ± 116.28 a | 1937.18 ± 66.41 d | 3659.06 ± 90.28 c | 1235.05 ± 61.84 e | 5449.24 ± 253.25 b | 6149.70 ± 79.66 a |
| 25 | Trans-ferulic acid | - | 36.00 ± 1.22 b | 37.39 ± 1.68 b | 25.62 ± 3.51 c | 46.53 ± 5.11 a | 29.10 ± 5.16 c | 45.62 ± 0.29 a |
| 26 | Isocitric acid | 460.32 ± 17.58 e | 15,829.76 ± 795.11 a | 3268.20 ± 217.93 bc | 2814.06 ± 308.02 c | 3460.85 ± 183.61 b | 1364.85 ± 131.83 d | 1697.86 ± 54 d |
| 27 | D-tartaric acid | 8.60 ± 0.48 c | 7.40 ± 0.44 c | 14.50 ± 3.82 a | 9.84 ± 1.56 bc | 13.29 ± 1.35 a | 9.53 ± 0.81 bc | 11.93 ± 1.16 ab |
| 28 | Shikimic acid | 181.52 ± 41.81 b | 101.94 ± 3.63 cde | 80.10 ± 0.91 e | 235.12 ± 11.52 a | 96.21 ± 7.57 de | 131.42 ± 5.24 c | 120.91 ± 3.42 cd |
| 29 | Trans-aconitic acid | 2.33 ± 0.8 d | 81.48 ± 28.29 ab | 51.58 ± 3.36 c | 50.49 ± 7.93 c | 99.40 ± 12.31 a | 68.33 ± 5.57 bc | 75.60 ± 3.43 b |
| 30 | (R)-mandelic Acid | - | 29.87 ± 0.39 a | 23.02 ± 0.28 cd | 24.32 ± 0.21 c | 22.50 ± 1.63 cd | 21.53 ± 1.57 d | 27.07 ± 2.89 b |
| 31 | Phenylpyruvic acid | - | 274.21 ± 31.92 d | 542.66 ± 23.74 a | 162.60 ± 5.53 e | 455.96 ± 16.02 b | 354.34 ± 29.06 c | 380.55 ± 15.56 c |
| 32 | Mesaconic acid | 196.14 ± 81.4 g | 4594.37 ± 193.92 ab | 1662.10 ± 97.65 e | 4408.71 ± 76.38 bc | 1192.82 ± 55.93 f | 4728.14 ± 194.18 a | 4266.08 ± 67.07 d |
| 33 | 4-hydroxyphenylpyruvic acid | - | 1702.39 ± 105.92 b | 141.08 ± 29.12 e | 796.59 ± 36.39 d | 11.54 ± 13.27 f | 1410.06 ± 111.92 c | 2598.33 ± 39.59 a |
| 34 | L-lactic acid | 59.15 ± 6.88 e | 64.92 ± 3.82 e | 181.82 ± 0.66 cd | 192.47 ± 1.69 bc | 193.86 ± 3.85 b | 176.93 ± 3.47 d | 282.63 ± 14.26 a |
| 35 | Acetic acid | 1081.96 ± 55.07 b | 1412.84 ± 23.46 a | 1036.56 ± 22.19 b | 1436.59 ± 31.6 a | 1365.09 ± 54.61 a | 885.11 ± 17.57 c | 749.22 ± 24.4 d |
| 36 | Terephthalic acid | 7.07 ± 5.38 f | 208.48 ± 14.43 b | 153.48 ± 7.78 d | 242.22 ± 9.15 a | 81.09 ± 5.09 e | 153.39 ± 2.93 d | 191.68 ± 1.48 c |
| 37 | 3-hydroxymethylglutaric acid | 515.91 ± 88.33 d | 1073.30 ± 43.42 a | 1133.88 ± 77.81 a | 764.04 ± 81.28 bc | 1212.90 ± 69.74 a | 625.19 ± 99.14 cd | 906.52 ± 97.37 b |
| 38 | Kojic acid | 15.30 ± 1.41 c | 21.55 ± 1.07 bc | 20.38 ± 2.47 bc | 28.82 ± 0.73 a | 24.99 ± 3.58 ab | 21.14 ± 0.36 bc | 22.62 ± 0.12 bc |
| 39 | Vanillic acid | 10.82 ± 1.23 e | 81.58 ± 4.07 b | 59.13 ± 5.46 d | 73.20 ± 3.45 c | 56.11 ± 4.47 d | 53.82 ± 1.85 d | 94.62 ± 2.15 a |
| 40 | Syringic acid | 5.65 ± 1.03 d | 17.24 ± 3.57 ab | 8.37 ± 0.64 cd | 19.13 ± 1.38 a | 13.03 ± 1.5 bc | 16.75 ± 1.23 ab | 21.24 ± 3.63 a |
| 41 | Phenylacetic acid | 4.29 ± 0.85 f | 15.29 ± 1.69 c | 9.81 ± 0.22 d | 25.72 ± 0.81 a | 7.61 ± 0.43 e | 17.32 ± 1.1 b | 18.27 ± 0.96 b |
| 42 | Abscisic acid | 13.81 ± 3.75 b | 78.78 ± 4.64 a | 88.64 ± 3.46 a | 69.71 ± 5.12 a | 75.39 ± 3.29 a | 107.18 ± 58.88 a | 75.95 ± 1.35 a |
| 43 | Vanillylmandelic acid | - | 195.93 ± 8.74 a | 120.80 ± 5.14 b | 148.29 ± 5.25 b | 85.52 ± 5.96 c | 145.57 ± 23.01 b | 143.20 ± 4.21 b |
| 44 | Phthalic acid | 25.47 ± 8.38 ab | - | 38.15 ± 1.27 a | 14.86 ± 1.05 bc | 27.19 ± 7.05 ab | 5.54 ± 1.14 c | - |
| Ʃ(Sum) | 22,606.26 ± 582.12 e | 77,660.35 ± 1280.71 a | 44,577.86 ± 1331.79 d | 60,034.50 ± 1151.67 c | 69,533.42 ± 730.11 b | 77,163.20 ± 2078.21 a | 77,774.61 ± 1299.05 a | |
| Polyphenols | ||||||||
| 1 | o-cresol | 0.43 ± 0.01 e | 17.22 ± 1.73 d | 13.70 ± 2.47 d | 55.82 ± 4.78 c | 2.38 ± 0.32 e | 127.05 ± 6.37 b | 227.73 ± 11.97 a |
| 2 | p-cresol | 0.21 ± 0.07 c | 4.76 ± 0.28 b | 5.02 ± 0.12 b | 5.37 ± 0.55 b | 5.05 ± 0.68 b | 5.10 ± 0.52 b | 6.40 ± 0.53 a |
| 3 | Epicatechin | 21.25 ± 1.33 d | 76.10 ± 2.93 c | 94.51 ± 3.80 a | 77.68 ± 6.77 c | 98.16 ± 6.68 a | 71.04 ± 0.45 c | 85.67 ± 0.76 b |
| 4 | Caffeic acid | - | 821.44 ± 6.2 c | 941.15 ± 14.74 b | 1044.59 ± 21.54 a | 864.57 ± 30.87 d | 927.12 ± 12.23 b | 823.04 ± 14.61 c |
| 5 | Catechin | 169.10 ± 18.49 d | 2544.85 ± 76.94 b | 1641.13 ± 49.78 c | 4008.82 ± 69.16 a | 3112.65 ± 71.84 d | 3765.71 ± 86.35 a | 3698.22 ± 46.84 a |
| 6 | m-coumaric acid | - | 1310.58 ± 189.10 e | 3038.27 ± 25.43 a | 1430.39 ± 40.67 f | 2782.09 ± 108.46 b | 1726.14 ± 128.25 d | 2053.44 ± 53.24 c |
| 7 | Chlorogenic acid | 537.46 ± 12.59 a | 82.34 ± 15.79 d | 121.78 ± 20.42 c | 45.26 ± 4.15 e | 219.81 ± 6.04 b | 22.76 ± 2.15 f | 30.88 ± 1.09 ef |
| 8 | Genistein | 14.61 ± 2.59 d | 52.84 ± 5.9 b | 38.15 ± 0.49 c | 66.08 ± 3.58 a | 32.89 ± 2.52 c | 48.95 ± 4.34 b | 60.35 ± 4.51 a |
| 9 | 4-methylcatechol | 4.38 ± 0.71 bc | 6.44 ± 0.99 a | 3.70 ± 0.2 c | 5.76 ± 0.80 a | 5.71 ± 0.56 a | 5.17 ± 0.24 ab | 5.56 ± 0.73 ab |
| 10 | Quercetin | 1.32 ± 0.34 c | 100.68 ± 12.64 b | 299.10 ± 30.62 a | 72.34 ± 2.76 bc | 245.53 ± 18.79 a | 36.71 ± 13.16 bc | 96.16 ± 11.23 b |
| 11 | Mulberrin | 90.78 ± 2.42 d | 547.25 ± 37.55 b | 618.91 ± 9.26 a | 559.70 ± 15.14 b | 475.85 ± 25.38 c | 644.57 ± 30.57 a | 567.41 ± 19.57 b |
| 12 | Quercitrin | 18.20 ± 1.20 c | 219.87 ± 45.48 ab | 312.12 ± 43.23 a | 199.94 ± 7.11 ab | 269.43 ± 16.99 ab | 127.54 ± 28.72 bc | 150.33 ± 6.61 abc |
| 13 | Quercetin 3-galactoside | 3.64 ± 0.23 b | 28.16 ± 15.72 ab | 42.95 ± 7.88 a | 13.58 ± 2.01 b | 42.36 ± 27.15 a | 6.74 ± 2.89 b | 23.02 ± 19.03 ab |
| 14 | Procyanidin B2 | 19.95 ± 3.53 f | 56.92 ± 3.41 c | 146.48 ± 5.24 b | 33.95 ± 3.25 e | 156.10 ± 2.13 a | 30.70 ± 2.92 e | 41.17 ± 1.64 d |
| 15 | Limocitrin | 0.02 ± 0.00 e | 33.56 ± 1.05 c | 1.77 ± 0.48 e | 25.83 ± 0.83 d | 1.75 ± 0.19 e | 49.24 ± 7.98 a | 42.20 ± 4.61 b |
| 16 | Phloretin | 225.22 ± 26.54 c | 832.38 ± 66.18 b | 947.22 ± 26.44 b | 1183.27 ± 131.96 a | 1072.22 ± 34.75 a | 949.39 ± 33.71 b | 1084.25 ± 59.76 a |
| 17 | (-)-epigallocatechin | 0.97 ± 0.61 f | 17.40 ± 1.4 a | 11.68 ± 0.53 bc | 12.55 ± 0.49 b | 10.21 ± 1.34 cd | 6.95 ± 0.82 e | 9.44 ± 0.66 d |
| 18 | Phlorizin | 1092.96 ± 117 e | 4126.56 ± 43.78 d | 4579.12 ± 362.24 bc | 5568.79 ± 76.08 a | 4798.19 ± 63.58 b | 4419.42 ± 89.94 cd | 4884.58 ± 80.77 b |
| 19 | Naringenin | - | 78.52 ± 1.06 b | 154.74 ± 5.45 a | 190.69 ± 15.13 a | 121.58 ± 5.98 ab | 157.44 ± 6.43 a | 155.97 ± 4.6 a |
| 20 | Sinapic acid | 2.41 ± 0.12 d | 10.82 ± 0.58 c | 10.60 ± 0.78 c | 12.75 ± 0.31 ab | 14.24 ± 1.06 a | 12.16 ± 1.14 bc | 12.16 ± 1.71 bc |
| 21 | Myricetin | 0.52 ± 0.22 d | 11.87 ± 2.67 b | 5.68 ± 1.15 c | 10.99 ± 1.57 b | 4.75 ± 1.01 cd | 15.32 ± 2.5 ab | 18.62 ± 2.75 a |
| 22 | Bergapten | 6036.42 ± 236.66 a | 4708.60 ± 402.07 a | 4663.11 ± 195.31 a | 4923.59 ± 115.54 b | 5838.48 ± 78.85 a | 4817.93 ± 168.40 b | 4735.09 ± 199.71 b |
| 23 | Quercetin 3-arabinoside | 4.17 ± 0.20 e | 20.24 ± 1.52 b | 27.40 ± 2.41 a | 11.77 ± 0.82 cd | 25.15 ± 3.57 a | 9.27 ± 0.70 d | 13.65 ± 1.73 c |
| 24 | 3-hydroxybenzoic acid | 3.60 ± 1.08 e | 9.75 ± 0.93 b | 16.49 ± 0.83 a | 11.85 ± 1.06 cd | 7.04 ± 0.5 a | 10.29 ± 1.34 d | 11.94 ± 0.30 c |
| 25 | Daidzin | 2.27 ± 0.19 d | 11.02 ± 1.42 ab | 11.78 ± 1.03 b | 5.67 ± 0.38 c | 12.44 ± 1.23 a | 6.76 ± 0.31 c | 9.66 ± 0.83 b |
| 26 | Gallic acid | 1.24 ± 0.17 f | 156.67 ± 5.39 d | 237.25 ± 25.09 bc | 324.78 ± 17.39 a | 124.47 ± 4.19 e | 245.71 ± 15.63 b | 214.70 ± 7.38 c |
| 27 | 2-benzylbutanedioic acid | 43.93 ± 2.67 a | 18.72 ± 1.52 d | 37.32 ± 0.80 b | 17.43 ± 2.94 d | 45.60 ± 0.81 a | 15.13 ± 1.41 d | 24.29 ± 1.74 c |
| 28 | Glabranin | 1.99 ± 0.11 d | 7.51 ± 0.86 c | 10.98 ± 0.31 a | 11.89 ± 1.02 a | 9.23 ± 0.97 b | 11.85 ± 1.12 a | 12.46 ± 1.14 a |
| 29 | Isoquercitrin | 0.56 ± 0.02 d | 10.73 ± 0.69 b | 6.7 ± 0.18 c | 14.74 ± 2.85 a | 2.59 ± 0.37 d | 6.80 ± 0.97 c | 5.93 ± 0.28 c |
| 30 | Curcumin | 16.92 ± 3.07 d | 121.32 ± 5.44 a | 27.08 ± 0.44 c | 108.39 ± 2.06 b | 26.52 ± 2.53 c | 111.04 ± 6.72 b | 104.56 ± 7.72 b |
| 31 | Psoralen | 1.66 ± 0.48 d | 62.26 ± 15.37 a | 56.08 ± 3.31 a | 39.40 ± 1.00 b | 63.33 ± 2.18 a | 22.29 ± 1.67 c | 43.89 ± 2.69 b |
| 32 | Hesperidin | 2.15 ± 0.26 b | 0.91 ± 0.2 d | 1.53 ± 0.33 c | 1.17 ± 0.09 cd | 3.34 ± 0.37 a | - | - |
| 33 | Naringin | 2.16 ± 0.06 bc | 1.76 ± 0.63 cd | 2.83 ± 0.70 b | 2.51 ± 0.35 bc | 1.26 ± 0.06 de | 0.59 ± 0.04 e | 4.63 ± 0.03 a |
| 34 | 4-aminophenol | 3.72 ± 0.22 c | 5.49 ± 0.14 a | 3.90 ± 0.54 c | 5.29 ± 0.32 ab | 4.70 ± 0.48 b | 4.67 ± 0.32 b | 5.38 ± 0.33 a |
| Ʃ(Sum) | 8324.24 ± 2239.42 c | 16,115.55 ± 721.92 b | 18,130.21 ± 1630.51 ab | 20,102.62 ± 336.23 ab | 20,616.35 ± 684.21 a | 20,984.22 ± 5239.72 a | 19,296.12 ± 797.07 ab | |
| Terpenoids | ||||||||
| 1 | Geranylacetate | 25.76 ± 0.86 c | 26.88 ± 3.98 c | 396.22 ± 71.64 b | 396.71 ± 45.90 b | 51.49 ± 4.58 c | 41.09 ± 0.55 c | 694.00 ± 53.14 a |
| 2 | Bornyl acetate | 1.47 ± 0.04 d | 35.94 ± 8.98 cd | 73.45 ± 3.84 c | 15.39 ± 4.09 d | 655.98 ± 58.16 a | 14.28 ± 1.00 d | 251.55 ± 28.97 b |
| 3 | Myrcene | 1.63 ± 0.02 e | 66.25 ± 2.92 b | 74.02 ± 2.64 a | 41.59 ± 2.05 c | 25.79 ± 4.03 d | 36.91 ± 2.54 c | 74.81 ± 3.33 a |
| 4 | Nerylacetate | 21.32 ± 1.79 f | 65.19 ± 2.83 e | 266.40 ± 28.79 b | 175.01 ± 1.22 c | 751.54 ± 14.82 a | 146.69 ± 21.69 d | 187.85 ± 0.11 c |
| 5 | (+)-camphor | 27.46 ± 0.71 f | 27.63 ± 2.42 f | 361.25 ± 21.34 a | 83.12 ± 4.61 d | 60.04 ± 6.89 e | 150.39 ± 9.21 c | 168.39 ± 7.31 b |
| 6 | Terpinine-4-ol | 2.80 ± 0.88 d | 2.60 ± 0.32 d | 16.72 ± 0.60 b | 21.82 ± 2.01 b | 82.90 ± 7.74 a | 9.03 ± 0.72 c | 5.14 ± 0.81 cd |
| 7 | Citronellyl acetate | 2.69 ± 0.25 e | 5.80 ± 0.10 e | 27.48 ± 6.67 d | 227.53 ± 22.14 a | 14.22 ± 0.40 de | 113.75 ± 8.76 b | 56.50 ± 10.70 c |
| 8 | Alpha-terpineol; Patulin | 28.24 ± 5.52 a | 1.41 ± 0.08 e | 14.06 ± 1.98 c | 25.18 ± 0.96 a | 19.55 ± 4.14 b | 2.82 ± 0.41 de | 6.62 ± 0.21 d |
| Ʃ(Sum) | 111.39 ± 7.48 g | 231.69 ± 15.56 f | 1229.59 ± 57.48 c | 986.35 ± 60.28 d | 1661.52 ± 48.05 a | 514.96 ± 11.86 e | 1444.85 ± 59.73 b | |
| Ʃ (Sum: LC-MS) | 31,041.89 ± 1697.44 e | 94,007.59 ± 1229.36 ab | 63,837.66 ± 1737 d | 81,089.47 ± 729.40 c | 91,735.63 ± 510.01 b | 98,665.72 ± 6781.31 a | 98,515.58 ± 465.02 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, L.; Chen, X.; Lin, R.; Xu, T.; Xiong, D.; Li, L.; Zhao, Z. Quality Improvement in Apple Ciders during Simultaneous Co-Fermentation through Triple Mixed-Cultures of Saccharomyces cerevisiae, Pichia kudriavzevii, and Lactiplantibacillus plantarum. Foods 2023, 12, 655. https://doi.org/10.3390/foods12030655
Hu L, Chen X, Lin R, Xu T, Xiong D, Li L, Zhao Z. Quality Improvement in Apple Ciders during Simultaneous Co-Fermentation through Triple Mixed-Cultures of Saccharomyces cerevisiae, Pichia kudriavzevii, and Lactiplantibacillus plantarum. Foods. 2023; 12(3):655. https://doi.org/10.3390/foods12030655
Chicago/Turabian StyleHu, Lujun, Xiaodie Chen, Rui Lin, Teng Xu, Dake Xiong, Li Li, and Zhifeng Zhao. 2023. "Quality Improvement in Apple Ciders during Simultaneous Co-Fermentation through Triple Mixed-Cultures of Saccharomyces cerevisiae, Pichia kudriavzevii, and Lactiplantibacillus plantarum" Foods 12, no. 3: 655. https://doi.org/10.3390/foods12030655
APA StyleHu, L., Chen, X., Lin, R., Xu, T., Xiong, D., Li, L., & Zhao, Z. (2023). Quality Improvement in Apple Ciders during Simultaneous Co-Fermentation through Triple Mixed-Cultures of Saccharomyces cerevisiae, Pichia kudriavzevii, and Lactiplantibacillus plantarum. Foods, 12(3), 655. https://doi.org/10.3390/foods12030655