The Effect of Long-Term Passage on Porcine SMCs’ Function and the Improvement of TGF-β1 on Porcine SMCs’ Secretory Function in Late Passage
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Reagent
2.2. Quantitative Real-Time PCR
2.3. Western Blot Analysis
2.4. Immunocytochemistry
2.5. Sirius Red Staining
2.6. Transcriptomics
2.7. Proteomics
2.8. Statistical Analysis
3. Results and Discussion
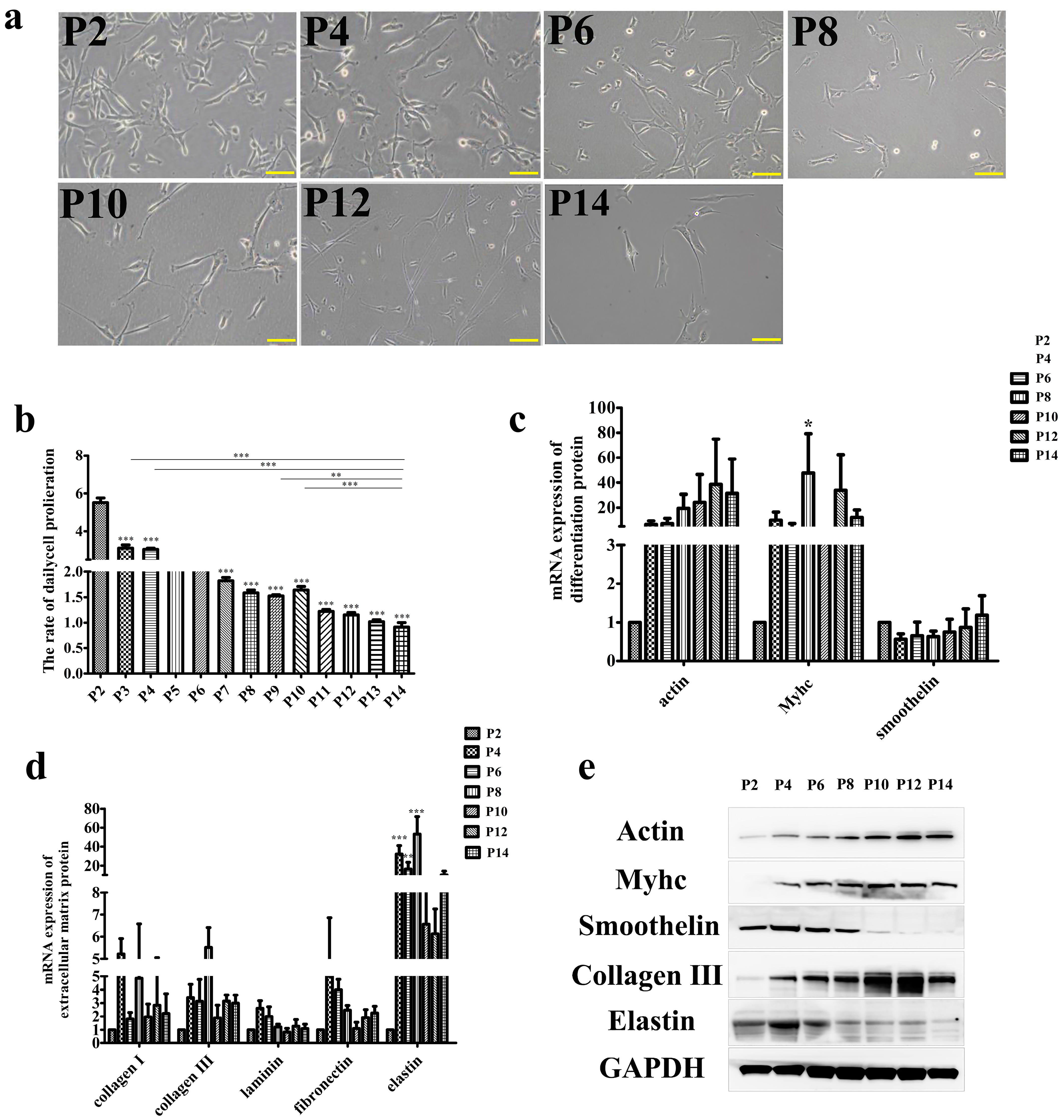
3.1. Effect of Long-Term Subculture on SMCs Function In Vitro
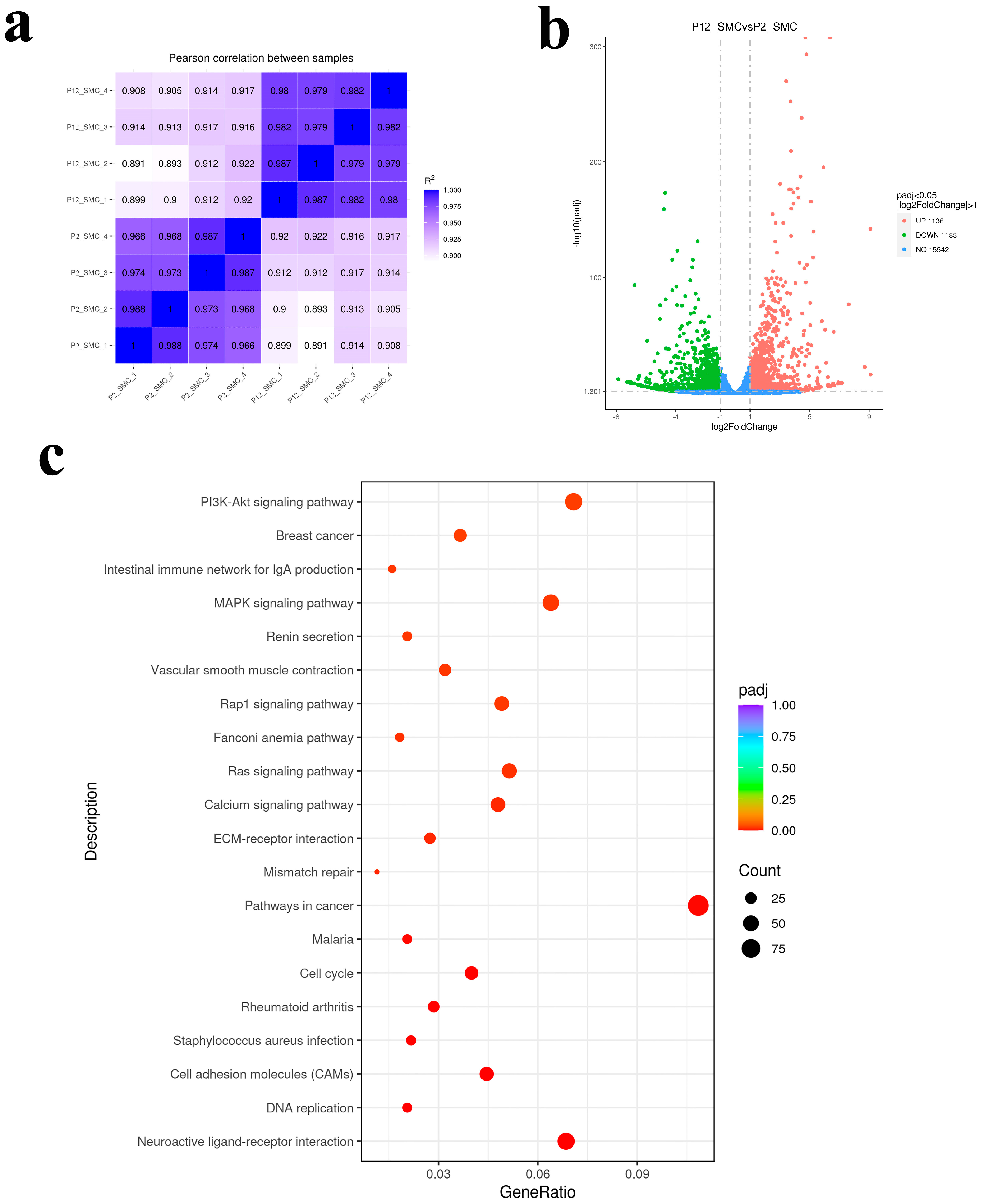
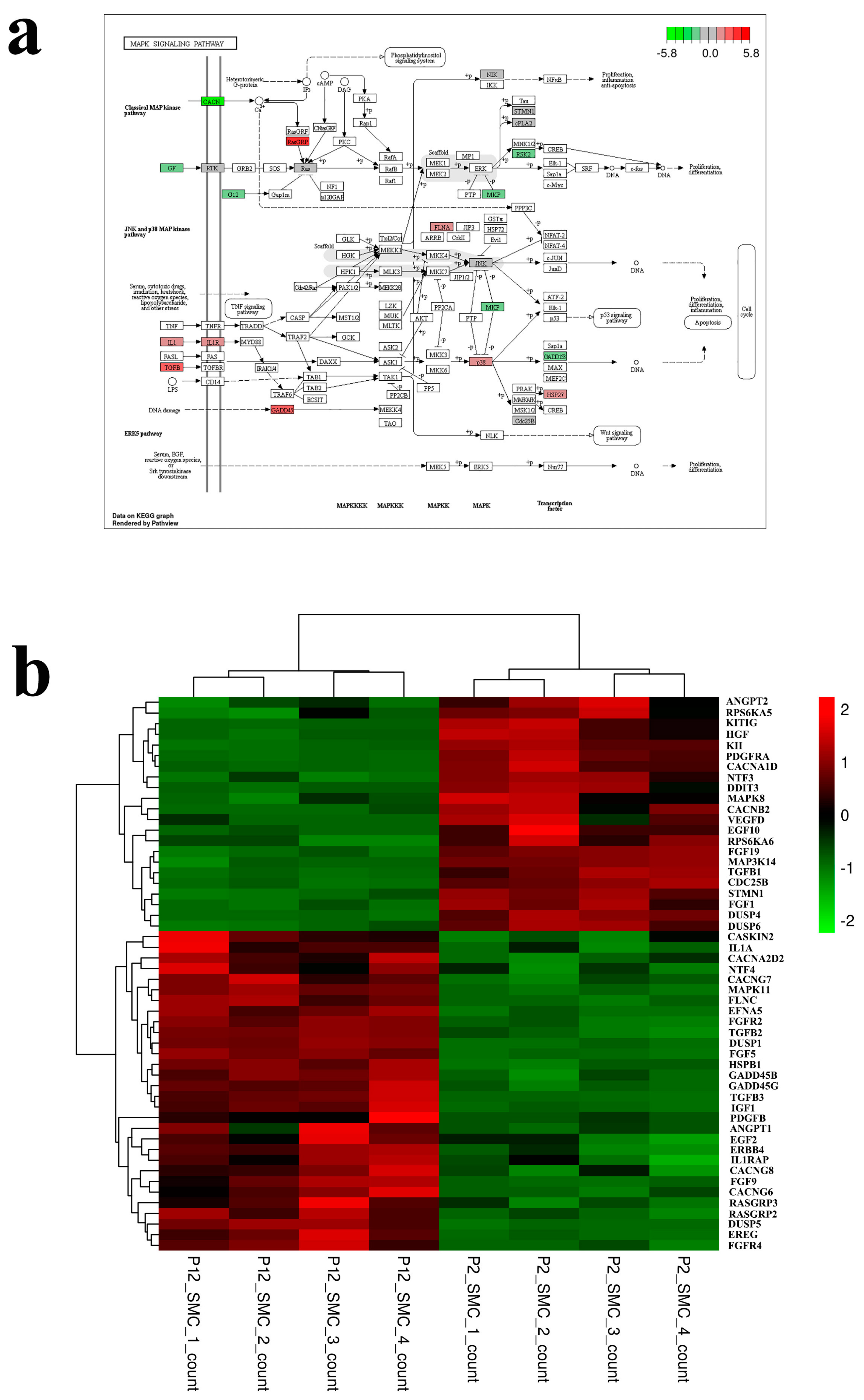
3.2. Transcriptomic Analysis of the Effect of Long Passage on SMCs
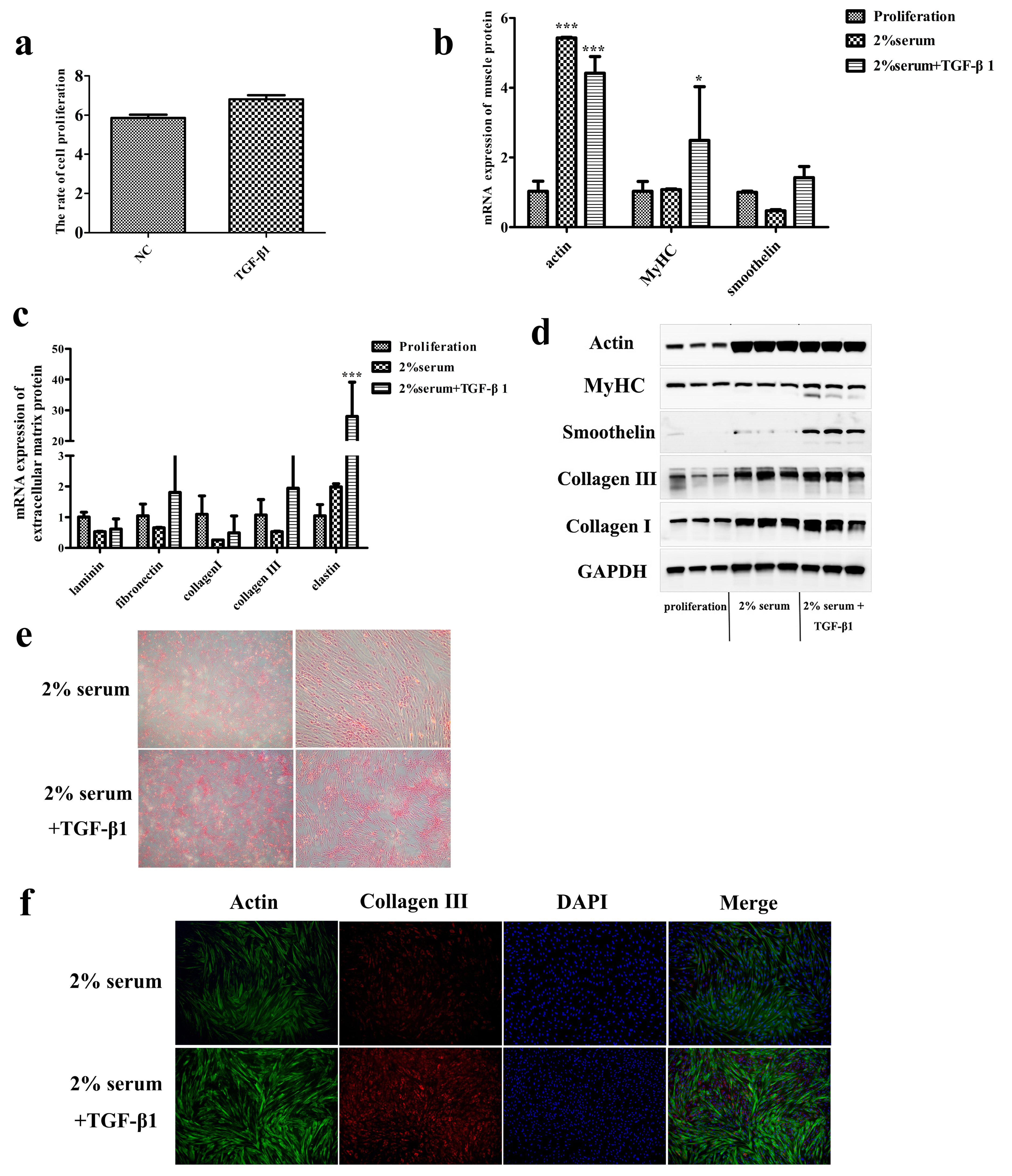
3.3. TGF-β1 Promotes the Ability of SMCs to Secrete Collagen in Late Passage
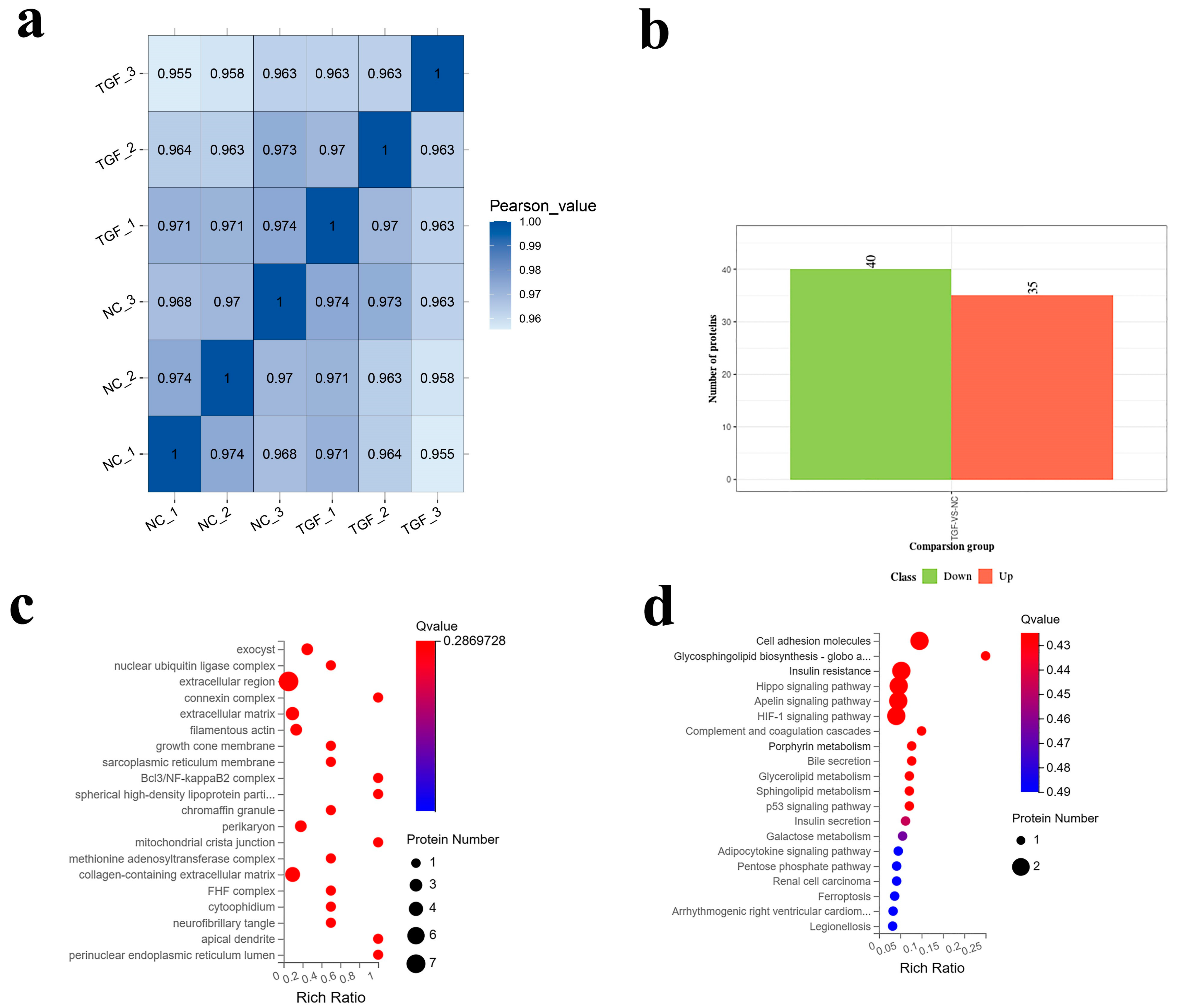
3.4. Proteomic Analysis of the Effect of TGF-β1 on SMCs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Godfray, H.C.J.; Aveyard, P.; Garnett, T.; Hall, J.W.; Key, T.J.; Lorimer, J.; Pierrehumbert, R.T.; Scarborough, P.; Springmann, M.; Jebb, S.A. Meat consumption, health, and the environment. Science 2018, 361, eaam5324. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, N. Cutting Europe’s meat and dairy consumption would benefit health and environment, says report. BMJ 2014, 348, g2949. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chaudhary, A.; Mathys, A. Dietary Change Scenarios and Implications for Environmental, Nutrition, Human Health and Economic Dimensions of Food Sustainability. Nutrients 2019, 11, 856. [Google Scholar] [CrossRef] [PubMed]
- Boukid, F.; Gagaoua, M. Meat alternatives: A proofed commodity? Adv. Food Nutr. Res. 2022, 101, 213–236. [Google Scholar]
- Eshel, G.; Stainier, P.; Shepon, A.; Swaminathan, A. Environmentally Optimal, Nutritionally Sound, Protein and Energy Conserving Plant Based Alternatives to U.S. Meat. Sci. Rep. 2019, 9, 10345. [Google Scholar] [CrossRef]
- van der Weele, C.; Feindt, P.; van der Goot, A.J.; van Mierlo, B.; van Boekel, M. Meat alternatives: An integrative comparison. Trends Food Sci. Technol. 2019, 88, 505–512. [Google Scholar] [CrossRef]
- Du, Q.; Tu, M.; Liu, J.; Ding, Y.; Zeng, X.; Pan, D. Plant-based meat analogs and fat substitutes, structuring technology and protein digestion: A review. Food Res. Int. 2023, 170, 112959. [Google Scholar] [CrossRef]
- Zhang, G.; Zhao, X.; Li, X.; Sun, X.; Zhou, J.; Du, G.; Chen, J. Application of cell culture techniques in cultured meat-a review. Sheng Wu Gong Cheng Xue Bao 2019, 35, 1374–1381. [Google Scholar]
- Guo, Y.; Ding, S.J.; Ding, X.; Liu, Z.; Wang, J.L.; Chen, Y.; Liu, P.P.; Li, H.X.; Zhou, G.H.; Tang, C.B. Effects of selected flavonoids oncellproliferation and differentiation of porcine muscle stem cells for cultured meat production. Food Res. Int. 2022, 160, 111459. [Google Scholar] [CrossRef]
- Ahmad, S.S.; Chun, H.J.; Ahmad, K.; Shaikh, S.; Lim, J.H.; Ali, S.; Han, S.S.; Hur, S.J.; Sohn, J.H.; Lee, E.J.; et al. The roles of growth factors and hormones in the regulation of muscle satellite cells for cultured meat production. J. Anim. Sci. Technol. 2023, 65, 16–31. [Google Scholar] [CrossRef]
- Zagury, Y.; Ianovici, I.; Landau, S.; Lavon, N.; Levenberg, S. Engineered marble-like bovine fat tissue for cultured meat. Commun. Biol. 2022, 5, 927. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Zhou, J.; Du, G.; Chen, J. Bioprocessing technology of muscle stem cells: Implications for cultured meat. Trends Biotechnol. 2022, 40, 721–734. [Google Scholar] [CrossRef]
- Li, C.-H.; Yang, I.-H.; Ke, C.-J.; Chi, C.-Y.; Matahum, J.; Kuan, C.-Y.; Celikkin, N.; Swieszkowski, W.; Lin, F.-H. The Production of Fat-Containing Cultured Meat by Stacking Aligned Muscle Layers and Adipose Layers Formed from Gelatin-Soymilk Scaffold. Front. Bioeng. Biotechnol. 2022, 10, 875069. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.; Seo, J.W.; Lee, H.; Jung, W.K.; Park, Y.H.; Bae, H. Efficient Myogenic/Adipogenic Transdifferentiation of Bovine Fibroblasts in a 3D Bioprinting System for Steak-Type Cultured Meat Production. Adv. Sci. 2022, 9, e2202877. [Google Scholar] [CrossRef]
- Zheng, Y.-Y.; Zhu, H.-Z.; Wu, Z.-Y.; Song, W.-J.; Tang, C.-B.; Li, C.-B.; Ding, S.-J.; Zhou, G.-H. Evaluation of the effect of smooth muscle cells on the quality of cultured meat in a model for cultured meat. Food Res. Int. 2021, 150, 110786. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhou, C.; Wang, C.; Ding, D.; Wang, J.; Li, C.; Zhou, G. Evaluating the effect of cooking temperature and time on collagen characteristics and the texture of hog maw. J. Texture Stud. 2020, 52, 207–218. [Google Scholar] [CrossRef]
- Camasão, D.B.; Pezzoli, D.; Loy, C.; Kumra, H.; Levesque, L.; Reinhardt, D.P.; Candiani, G.; Mantovani, D. Increasing Cell Seeding Density Improves Elastin Expression and Mechanical Properties in Collagen Gel-Based Scaffolds Cellularized with Smooth Muscle Cells. Biotechnol. J. 2018, 14, e1700768. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.; Gabbiani, G.; Bochaton-Piallat, M.L. Arterial smooth muscle cell heterogeneity: Implications for atherosclerosis and restenosis development. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1510–1520. [Google Scholar] [CrossRef] [PubMed]
- Rensen, S.S.M.; Doevendans, P.A.F.M.; van Eys, G.J.J.M. Regulation and characteristics of vascular smooth muscle cell phenotypic diversity. Neth. Heart J. 2007, 15, 100–108. [Google Scholar] [CrossRef]
- Zhang, Y.; Yao, F.; Qin, Z. JNJ0966 inhibits PDGF-BB-induced airway smooth muscle cell proliferation and extracellular matrix production by regulating MMP-9. Allergol. Immunopathol. 2022, 50, 57–63. [Google Scholar] [CrossRef]
- Chen, L.; Guttieres, D.; Koenigsberg, A.; Barone, P.W.; Sinskey, A.J.; Springs, S.L. Large-scale cultured meat production: Trends, challenges and promising biomanufacturing technologies. Biomaterials 2021, 280, 121274. [Google Scholar] [CrossRef] [PubMed]
- Tuomisto, H.L.; Allan, S.J.; Ellis, M.J. Prospective life cycle assessment of a bioprocess design for cultured meat production in hollow fiber bioreactors. Sci. Total Environ. 2022, 851, 158051. [Google Scholar] [CrossRef] [PubMed]
- Tyson, J.J.; Novák, B. Time-keeping and decision-making in the cell cycle. Interface Focus 2022, 12, 20210075. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.; Molkentin, J.D. Myofibroblasts: Trust your heart and let fate decide. J. Mol. Cell. Cardiol. 2013, 70, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Akhurst, R.J.; Hata, A. Targeting the TGFbeta signalling pathway in disease. Nat. Rev. Drug Discov. 2012, 11, 790–811. [Google Scholar]
- Chen, H.; Chen, H.; Liang, J.; Gu, X.; Zhou, J.; Xie, C.; Lv, X.; Wang, R.; Li, Q.; Mao, Z.; et al. TGF-beta1/IL-11/MEK/ERK signaling mediates senescence-associated pulmonary fibrosis in a stress-induced premature senescence model of Bmi-1 deficiency. Exp. Mol. Med. 2020, 52, 130–151. [Google Scholar] [CrossRef]
- Zhang, Q.; Fong, C.C.; Yu, W.K.; Chen, Y.; Wei, F.; Koon, C.M.; Lau, K.M.; Leung, P.C.; Lau, C.B.; Fung, K.P.; et al. Herbal formula Astragali Radix and Rehmanniae Radix exerted wound healing effect on human skin fibroblast cell line Hs27 via the activation of transformation growth factor (TGF-beta) pathway and promoting extracellular matrix (ECM) deposition. Phytomedicine 2012, 20, 9–16. [Google Scholar] [CrossRef]
- Milara, J.; Roger, I.; Montero, P.; Artigues, E.; Escrivá, J.; Cortijo, J. IL-11 system participates in pulmonary artery remodeling and hypertension in pulmonary fibrosis. Respir. Res. 2022, 23, 313. [Google Scholar] [CrossRef]
- Corden, B.; Adami, E.; Sweeney, M.; Schafer, S.; Cook, S.A. IL-11 in cardiac and renal fibrosis: Late to the party but a central player. Br. J. Pharmacol. 2020, 177, 1695–1708. [Google Scholar] [CrossRef]
- Zheng, Y.-Y.; Shi, Y.-F.; Zhu, H.-Z.; Ding, S.-J.; Zhou, G.-H. Quality evaluation of cultured meat with plant protein scaffold. Food Res. Int. 2022, 161, 111818. [Google Scholar] [CrossRef]
- Zheng, Y.-Y.; Chen, Y.; Zhu, H.-Z.; Li, C.-B.; Song, W.-J.; Ding, S.-J.; Zhou, G.-H. Production of cultured meat by culturing porcine smooth muscle cells in vitro with food grade peanut wire-drawing protein scaffold. Food Res. Int. 2022, 159, 111561. [Google Scholar] [CrossRef] [PubMed]
- Ben-Arye, T.; Shandalov, Y.; Ben-Shaul, S.; Landau, S.; Zagury, Y.; Ianovici, I.; Lavon, N.; Levenberg, S. Textured soy protein scaffolds enable the generation of three-dimensional bovine skeletal muscle tissue for cell-based meat. Nat. Food 2020, 1, 210–220. [Google Scholar] [CrossRef]
- Bonardi, F.; Fusetti, F.; Deelen, P.; van Gosliga, D.; Vellenga, E.; Schuringa, J.J. A Proteomics and Transcriptomics Approach to Identify Leukemic Stem Cell (LSC) Markers. Mol. Cell. Proteom. 2013, 12, 626–637. [Google Scholar] [CrossRef]
- Bodiou, V.; Moutsatsou, P.; Post, M.J. Microcarriers for Upscaling Cultured Meat Production. Front. Nutr. 2020, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Verbruggen, S.; Luining, D.; van Essen, A.; Post, M.J. Bovine myoblast cell production in a microcarriers-based system. Cytotechnology 2018, 70, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Bellani, C.F.; Ajeian, J.; Duffy, L.; Miotto, M.; Groenewegen, L.; Connon, C.J. Scale-Up Technologies for the Manufacture of Adherent Cells. Front. Nutr. 2020, 7, 575146. [Google Scholar] [CrossRef] [PubMed]
- Smetana, K.; Mikulenkova, D.; Klamova, H. The Morphology of Cell Differentiation, Terminal Differentiation and Ageing Seems to Reflect the Same Process: A Short Note. Folia Biol. 2021, 67, 70–75. [Google Scholar]
- Shen, K.; Kenche, H.; Zhao, H.; Li, J.; Stone, J. The role of extracellular matrix stiffness in regulating cytoskeletal remodeling via vinculin in synthetic smooth muscle cells. Biochem. Biophys. Res. Commun. 2019, 508, 302–307. [Google Scholar] [CrossRef]
- Ding, S.; Swennen, G.N.M.; Messmer, T.; Gagliardi, M.; Molin, D.G.M.; Li, C.; Zhou, G.; Post, M.J. Maintaining bovine satellite cells stemness through p38 pathway. Sci. Rep. 2018, 8, 10808. [Google Scholar] [CrossRef]
- Yen, F.-C.; Glusac, J.; Levi, S.; Zernov, A.; Baruch, L.; Davidovich-Pinhas, M.; Fishman, A.; Machluf, M. Cultured meat platform developed through the structuring of edible microcarrier-derived microtissues with oleogel-based fat substitute. Nat. Commun. 2023, 14, 2942. [Google Scholar] [CrossRef]
- Du, J.; Yuan, Z.; Ma, Z.; Song, J.; Xie, X.; Chen, Y. KEGG-PATH: Kyoto encyclopedia of genes and genomes-based pathway analysis using a path analysis model. Mol. Biosyst. 2014, 10, 2441–2447. [Google Scholar] [CrossRef] [PubMed]
- Tabibzadeh, S. Signaling pathways and effectors of aging. Front. Biosci. 2021, 26, 50–96. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.; Li, P.; Zhang, R.; Xu, Y.; Song, L.; Zhou, Q. Role of p38–MAPK pathway in the effects of high-magnitude compression on nucleus pulposus cell senescence in a disc perfusion culture. Biosci. Rep. 2017, 37, BSR20170718. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Wu, H.; Xiang, J.; Ruan, X.; Peng, P.; Ruan, Y.; Chen, Y.-G.; Wang, Y.; Yu, Q.; Zhang, H.; et al. Gut stem cell aging is driven by mTORC1 via a p38 MAPK-p53 pathway. Nat. Commun. 2020, 11, 37. [Google Scholar] [CrossRef]
- Tang, Q.; Markby, G.R.; MacNair, A.J.; Tang, K.; Tkacz, M.; Parys, M.; Phadwal, K.; MacRae, V.E.; Corcoran, B.M. TGF-beta-induced PI3K/AKT/mTOR pathway controls myofibroblast differentiation and secretory phenotype of valvular interstitial cells through the modulation of cellular senescence in a naturally occurring in vitro canine model of myxomatous mitral valve disease. Cell Prolif. 2023, 56, e13435. [Google Scholar]
- Yi, X.; Li, T.; Wei, X.; He, Z. Erythromycin attenuates oxidative stress-induced cellular senescence via the PI3K-mTOR signaling pathway in chronic obstructive pulmonary disease. Front. Pharmacol. 2022, 13, 1043474. [Google Scholar]
- Shin, J.-W.; Kwon, S.-H.; Choi, J.-Y.; Na, J.-I.; Huh, C.-H.; Choi, H.-R.; Park, K.-C. Molecular Mechanisms of Dermal Aging and Antiaging Approaches. Int. J. Mol. Sci. 2019, 20, 2126. [Google Scholar] [CrossRef]
- Golden, T.R.; Hinerfeld, D.A.; Melov, S. Oxidative stress and aging: Beyond correlation. Aging Cell 2002, 1, 117–123. [Google Scholar] [CrossRef]
- Kolosova, N.G.; Grishanova, A.I.; Krysanova, Z.S.; Zueva, T.V.; Sidorova, I.A.; Sinitsyna, O.I. Age-related changes in protein and lipid oxidation in the liver of prematurely aging rats OXYS. Biomed. Khim. 2004, 50, 73–78. [Google Scholar]
- Quan, T.; Fisher, G.J. Role of Age-Associated Alterations of the Dermal Extracellular Matrix Microenvironment in Human Skin Aging: A Mini-Review. Gerontology 2015, 61, 427–434. [Google Scholar] [CrossRef]
- Moses, H.L.; Roberts, A.B.; Derynck, R. The Discovery and Early Days of TGF-beta: A Historical Perspective. Cold Spring Harb. Perspect. Biol. 2016, 8, a021865. [Google Scholar] [CrossRef] [PubMed]
- van Eys, G.J.; Niessen, P.M.; Rensen, S.S. Smoothelin in Vascular Smooth Muscle Cells. Trends Cardiovasc. Med. 2007, 17, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, K.; Abarca, C.; Aguayo-Mazzucato, C. Regulation of Cellular Senescence in Type 2 Diabetes Mellitus: From Mechanisms to Clinical Applications. Diabetes Metab. J. 2023; Online ahead of print. [Google Scholar] [CrossRef]
- Roger, L.; Tomas, F.; Gire, V. Mechanisms and Regulation of Cellular Senescence. Int. J. Mol. Sci. 2021, 22, 13173. [Google Scholar] [CrossRef]
- Ohtani, N.; Hara, E. Roles and mechanisms of cellular senescence in regulation of tissue homeostasis. Cancer Sci. 2013, 104, 525–530. [Google Scholar] [CrossRef]
- Cunningham, C.; Boche, D.; Perry, V.H. Transforming growth factor beta1, the dominant cytokine in murine prion disease: Influence on inflammatory cytokine synthesis and alteration of vascular extracellular matrix. Neuropathol. Appl. Neurobiol. 2002, 28, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Kiliç, Z.M.; Ayaz, S.; Ozin, Y.; Nadir, I.; Cakal, B.; Ulker, A. Plasma transforming growth factor-beta1 level in inflammatory bowel disease. Turk. J. Gastroenterol. 2009, 20, 165–170. [Google Scholar] [CrossRef]
- Inoue, C.J.; Flauzino, T.; Gonçalves, B.P.; Paula, J.C.C.D.; Galvão, T.C.; Miyazaki, P.K.; de Alcantara, C.C.; Westmore, L.R.e.S.; Lozovoy, M.A.B.; Reiche, E.M.V.; et al. FOXP3 variants are independently associated with transforming growth factor Beta1 plasma levels in female patients with inflammatory bowel disease. Clinics 2022, 77, 100084. [Google Scholar] [CrossRef]
- Koontz, L.M.; Liu-Chittenden, Y.; Yin, F.; Zheng, Y.; Yu, J.; Huang, B.; Chen, Q.; Wu, S.; Pan, D. The Hippo Effector Yorkie Controls Normal Tissue Growth by Antagonizing Scalloped-Mediated Default Repression. Dev. Cell 2013, 25, 388–401. [Google Scholar] [CrossRef]
- Guo, T.; Lu, Y.; Li, P.; Yin, M.-X.; Lv, D.; Zhang, W.; Wang, H.; Zhou, Z.; Ji, H.; Zhao, Y.; et al. A novel partner of Scalloped regulates Hippo signaling via antagonizing Scalloped-Yorkie activity. Cell Res. 2013, 23, 1201–1214. [Google Scholar] [CrossRef]
- Ma, S.; Meng, Z.; Chen, R.; Guan, K.L. The Hippo Pathway: Biology and Pathophysiology. Annu. Rev. Biochem. 2019, 88, 577–604. [Google Scholar] [CrossRef] [PubMed]
- Bertero, T.; Cottrill, K.A.; Lu, Y.U.; Haeger, C.M.; Dieffenbach, P.; Annis, S.; Hale, A.; Bhat, B.; Kaimal, V.; Zhang, Y.-Y.; et al. Matrix Remodeling Promotes Pulmonary Hypertension through Feedback Mechanoactivation of the YAP/TAZ-miR-130/301 Circuit. Cell Rep. 2015, 13, 1016–1032. [Google Scholar] [CrossRef] [PubMed]
- Surazynski, A.; Donald, S.P.; Cooper, S.K.; Whiteside, M.A.; Salnikow, K.; Liu, Y.; Phang, J.M. Extracellular matrix and HIF-1 signaling: The role of prolidase. Int. J. Cancer 2008, 122, 1435–1440. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.-Y.; Hu, Z.-N.; Liu, Z.; Jiang, Y.-C.; Guo, R.-P.; Ding, S.-J.; Zhou, G.-H. The Effect of Long-Term Passage on Porcine SMCs’ Function and the Improvement of TGF-β1 on Porcine SMCs’ Secretory Function in Late Passage. Foods 2023, 12, 2682. https://doi.org/10.3390/foods12142682
Zheng Y-Y, Hu Z-N, Liu Z, Jiang Y-C, Guo R-P, Ding S-J, Zhou G-H. The Effect of Long-Term Passage on Porcine SMCs’ Function and the Improvement of TGF-β1 on Porcine SMCs’ Secretory Function in Late Passage. Foods. 2023; 12(14):2682. https://doi.org/10.3390/foods12142682
Chicago/Turabian StyleZheng, Yan-Yan, Ze-Nan Hu, Zheng Liu, Yi-Chen Jiang, Ren-Peng Guo, Shi-Jie Ding, and Guang-Hong Zhou. 2023. "The Effect of Long-Term Passage on Porcine SMCs’ Function and the Improvement of TGF-β1 on Porcine SMCs’ Secretory Function in Late Passage" Foods 12, no. 14: 2682. https://doi.org/10.3390/foods12142682
APA StyleZheng, Y.-Y., Hu, Z.-N., Liu, Z., Jiang, Y.-C., Guo, R.-P., Ding, S.-J., & Zhou, G.-H. (2023). The Effect of Long-Term Passage on Porcine SMCs’ Function and the Improvement of TGF-β1 on Porcine SMCs’ Secretory Function in Late Passage. Foods, 12(14), 2682. https://doi.org/10.3390/foods12142682





