Optimization of Ultrasonic-Assisted Extraction Conditions for Bioactive Components and Antioxidant Activity of Poria cocos (Schw.) Wolf by an RSM-ANN-GA Hybrid Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design of Response Surface Methodology(RSM) for Extraction
2.3. Determination of Contents and Activity
2.3.1. Triterpene Acids Content
2.3.2. Total Polysaccharide Content (TPs)
2.3.3. Total Phenolic Content (TPc)
2.3.4. Antioxidant Activity
2.4. Statistical Analysis
2.4.1. RSM Modeling
2.4.2. Artificial Neural Network-Genetic Algorithm (ANN-GA) Modeling
3. Result and Discussion
3.1. Principle Component Analysis (PCA)
3.2. RSM Modeling
3.3. RSM-ANN-GA Modeling
3.4. Optimum Ultrasonic Extraction Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lee, S.; Lee, D.; Lee, S.O.; Ryu, J.-Y.; Choi, S.-Z.; Kang, K.S.; Kim, K.H. Anti-inflammatory activity of the sclerotia of edible fungus, Poria cocos Wolf and their active lanostane triterpenoids. J. Funct. Foods 2017, 32, 27–36. [Google Scholar] [CrossRef]
- Cheng, Y.; Xie, Y.; Ge, J.C.; Wang, L.; Peng, D.Y.; Yu, N.J.; Zhang, Y.; Jiang, Y.H.; Luo, J.P.; Chen, W.D. Structural characterization and hepatoprotective activity of a galactoglucan from Poria cocos. Carbohydr. Polym. 2021, 263, 117979. [Google Scholar] [CrossRef]
- Li, X.; He, Y.; Zeng, P.; Liu, Y.; Zhang, M.; Hao, C.; Wang, H.; Lv, Z.; Zhang, L. Molecular basis for Poria cocos mushroom polysaccharide used as an antitumour drug in China. J. Cell. Mol. Med. 2019, 23, 4–20. [Google Scholar] [CrossRef]
- Guo, Y.; Li, Y.; Li, Z.; Yan, W.; Chen, P.; Yao, S. Extraction assisted by far infrared radiation and hot air circulation with deep eutectic solvent for bioactive polysaccharides from Poria cocos (Schw.) wolf. Green Chem. 2021, 23, 7170–7192. [Google Scholar] [CrossRef]
- Khomsi, M.E.; Imtara, H.; Kara, M.; Hmamou, A.; Assouguem, A.; Bourkhiss, B.; Tarayrah, M.; AlZain, M.N.; Alzamel, N.M.; Noman, O. Antimicrobial and Antioxidant Properties of Total Polyphenols of Anchusa italica Retz. Molecules 2022, 27, 416. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, L.; Li, P.; Zhao, J.; Duan, J. Antidepressant and immunosuppressive activities of two polysaccharides from Poria cocos (Schw.) Wolf. Int. J. Biol. Macromol. 2018, 120, 1696–1704. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Wang, H.; Xie, W.; Wang, Q.; Wang, X.; Wang, C.; Du, Y.; Huo, C.; Wang, Q. Comparison of triterpene compounds of four botanical parts from Poria cocos (Schw.) wolf using simultaneous qualitative and quantitative method and metabolomics approach. Food Res. Int. 2019, 121, 666–677. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, C.; Pan, Z.; Zheng, Z. Vacuum and infrared-assisted hot air impingement drying for improving the processing performance and quality of Poria cocos (Schw.) wolf cubes. Foods 2021, 10, 992. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.; Mohamed, A.; Sahena, F.; Jahurul, M.; Ghafoor, K.; Norulaini, N.; Omar, A. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Mai, X.; Liu, Y.; Tang, X.; Wang, L.; Lin, Y.; Zeng, H.; Luo, L.; Fan, H.; Li, P. Sequential extraction and enrichment of flavonoids from Euonymus alatus by ultrasonic-assisted polyethylene glycol-based extraction coupled to temperature-induced cloud point extraction. Ultrason. Sonochem. 2020, 66, 105073. [Google Scholar] [CrossRef]
- Wang, Q.-Y.; Dong, X.; Yang, J.; Hu, Y.-H.; Peng, L.-Q.; Zheng, H.; Cao, J. Vesicle based ultrasonic-assisted extraction of saponins in Panax notoginseng. Food Chem. 2020, 303, 125394. [Google Scholar] [CrossRef] [PubMed]
- Teh, S.-S.; Birch, E.J. Effect of ultrasonic treatment on the polyphenol content and antioxidant capacity of extract from defatted hemp, flax and canola seed cakes. Ultrason. Sonochem. 2014, 21, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Izadiyan, P.; Hemmateenejad, B. Multi-response optimization of factors affecting ultrasonic assisted extraction from Iranian basil using central composite design. Food Chem. 2016, 190, 864–870. [Google Scholar] [CrossRef]
- Song, R.; Lin, Y.; Li, Z. Ultrasonic-assisted preparation of eucalyptus oil nanoemulsion: Process optimization, in vitro digestive stability, and anti-Escherichia coli activity. Ultrason. Sonochem. 2022, 82, 105904. [Google Scholar] [CrossRef] [PubMed]
- Tasfiyati, A.N.; Antika, L.D.; Dewi, R.T.; Septama, A.W.; Sabarudin, A.; Ernawati, T. An experimental design approach for the optimization of scopoletin extraction from Morinda citrifolia L. using accelerated solvent extraction. Talanta 2022, 238, 123010. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Chen, J.; Tian, S.; Gu, H.; Li, N.; Sun, Y.; Ru, J.; Wang, J. Extraction optimization, preliminary characterization and immunological activities in vitro of polysaccharides from Elaeagnus angustifolia L. pulp. Carbohydr. Polym. 2016, 151, 348–357. [Google Scholar] [CrossRef]
- Riciputi, Y.; Diaz-de-Cerio, E.; Akyol, H.; Capanoglu, E.; Cerretani, L.; Caboni, M.F.; Verardo, V. Establishment of ultrasound-assisted extraction of phenolic compounds from industrial potato by-products using response surface methodology. Food Chem. 2018, 269, 258–263. [Google Scholar] [CrossRef]
- Xu, W.-J.; Zhai, J.-W.; Cui, Q.; Liu, J.-Z.; Luo, M.; Fu, Y.-J.; Zu, Y.-G. Ultra-turrax based ultrasound-assisted extraction of five organic acids from honeysuckle (Lonicera japonica Thunb.) and optimization of extraction process. Sep. Purif. Technol. 2016, 166, 73–82. [Google Scholar] [CrossRef]
- Midya, R.; Wang, Z.; Asapu, S.; Joshi, S.; Li, Y.; Zhuo, Y.; Song, W.; Jiang, H.; Upadhay, N.; Rao, M. Artificial neural network (ANN) to spiking neural network (SNN) converters based on diffusive memristors. Adv. Electron. Mater. 2019, 5, 1900060. [Google Scholar] [CrossRef]
- Aung, T.; Kim, S.-J.; Eun, J.-B. A hybrid RSM-ANN-GA approach on optimisation of extraction conditions for bioactive component-rich laver (Porphyra dentata) extract. Food Chem. 2022, 366, 130689. [Google Scholar] [CrossRef]
- Choi, H.-J.; Naznin, M.; Alam, M.B.; Javed, A.; Alshammari, F.H.; Kim, S.; Lee, S.-H. Optimization of the Extraction Conditions of Nypa fruticans Wurmb. Using Response Surface Methodology and Artificial Neural Network. Food Chem. 2022, 381, 132086. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Cui, P.; Ge, S.; Cai, X.; Li, Q.; Xue, H. Ultrasound assisted aqueous two-phase extraction of polysaccharides from Cornus officinalis fruit: Modeling, optimization, purification, and characterization. Ultrason. Sonochem. 2022, 84, 105966. [Google Scholar] [CrossRef]
- Hamdia, K.M.; Zhuang, X.; Rabczuk, T. An efficient optimization approach for designing machine learning models based on genetic algorithm. Neural Comput. Appl. 2021, 33, 1923–1933. [Google Scholar] [CrossRef]
- Bakht, M.A.; Geesi, M.H.; Riadi, Y.; Imran, M.; Ali, M.I.; Ahsan, M.J.; Ajmal, N. Ultrasound-assisted extraction of some branded tea: Optimization based on polyphenol content, antioxidant potential and thermodynamic study. Saudi J. Biol. Sci. 2019, 26, 1043–1052. [Google Scholar] [CrossRef]
- Feng, C.-H. Optimizing procedures of ultrasound-assisted extraction of waste orange peels by response surface methodology. Molecules 2022, 27, 2268. [Google Scholar] [CrossRef] [PubMed]
- Meregalli, M.M.; Puton, B.M.S.; Camera, F.D.M.; Amaral, A.U.; Zeni, J.; Cansian, R.L.; Mignoni, M.L.; Backes, G.T. Conventional and ultrasound-assisted methods for extraction of bioactive compounds from red araçá peel (Psidium cattleianum Sabine). Arab. J. Chem. 2020, 13, 5800–5809. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, X.; Song, L.; Liu, S.; Li, P. The mechanism by which Enteromorpha Linza polysaccharide promotes Bacillus subtilis growth and nitrate removal. Int. J. Biol. Macromol. 2022, 209, 840–849. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Song, Q.; Li, X.; Chen, Y.; Liu, C.; Zhu, X.; Liu, J.; Granato, D.; Wang, Y.; Huang, J. Effects of different dietary polyphenols on conformational changes and functional properties of protein–polyphenol covalent complexes. Food Chem. 2021, 361, 130071. [Google Scholar] [CrossRef]
- Islam, S.; Alam, M.B.; Ahmed, A.; Lee, S.; Lee, S.-H.; Kim, S. Identification of secondary metabolites in Averrhoa carambola L. bark by high-resolution mass spectrometry and evaluation for α-glucosidase, tyrosinase, elastase, and antioxidant potential. Food Chem. 2020, 332, 127377. [Google Scholar] [CrossRef]
- Yahya, H.S.M.; Abbas, T.; Amin, N.A.S. Optimization of hydrogen production via toluene steam reforming over Ni–Co supported modified-activated carbon using ANN coupled GA and RSM. Int. J. Hydrogen Energy 2021, 46, 24632–24651. [Google Scholar] [CrossRef]
- Li, Y.; Jia, M.; Han, X.; Bai, X.-S. Towards a comprehensive optimization of engine efficiency and emissions by coupling artificial neural network (ANN) with genetic algorithm (GA). Energy 2021, 225, 120331. [Google Scholar] [CrossRef]
- Liu, L.; Moayedi, H.; Rashid, A.S.A.; Rahman, S.S.A.; Nguyen, H. Optimizing an ANN model with genetic algorithm (GA) predicting load-settlement behaviours of eco-friendly raft-pile foundation (ERP) system. Eng. Comput. 2020, 36, 421–433. [Google Scholar] [CrossRef]
- Tao, Y.; Wu, D.; Zhang, Q.-A.; Sun, D.-W. Ultrasound-assisted extraction of phenolics from wine lees: Modeling, optimization and stability of extracts during storage. Ultrason. Sonochem. 2014, 21, 706–715. [Google Scholar] [CrossRef]
- Ringnér, M. What is principal component analysis? Nat. Biotechnol. 2008, 26, 303–304. [Google Scholar] [CrossRef]
- Granato, D.; Santos, J.S.; Escher, G.B.; Ferreira, B.L.; Maggio, R.M. Use of principal component analysis (PCA) and hierarchical cluster analysis (HCA) for multivariate association between bioactive compounds and functional properties in foods: A critical perspective. Trends Food Sci. Technol. 2018, 72, 83–90. [Google Scholar] [CrossRef]
- Dao, T.A.T.; Webb, H.K.; Malherbe, F. Optimization of pectin extraction from fruit peels by response surface method: Conventional versus microwave-assisted heating. Food Hydrocoll. 2021, 113, 106475. [Google Scholar] [CrossRef]
- Taofiq, O.; Silva, A.R.; Costa, C.; Ferreira, I.; Nunes, J.; Prieto, M.A.; Simal-Gandara, J.; Barros, L.; Ferreira, I.C. Optimization of ergosterol extraction from Pleurotus mushrooms using response surface methodology. Food Funct. 2020, 11, 5887–5897. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wei, S.; Liao, M. Optimization of ultrasonic extraction of phenolic compounds from Euryale ferox seed shells using response surface methodology. Ind. Crops Prod. 2013, 49, 837–843. [Google Scholar] [CrossRef]
- Ciric, A.; Krajnc, B.; Heath, D.; Ogrinc, N. Response surface methodology and artificial neural network approach for the optimization of ultrasound-assisted extraction of polyphenols from garlic. Food Chem. Toxicol. 2020, 135, 110976. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, R.; Mohammadifar, M.A.; Mortazavian, A.M.; Rouhi, M.; Ghasemi, J.B.; Delshadian, Z. Extraction optimization of pepsin-soluble collagen from eggshell membrane by response surface methodology (RSM). Food Chem. 2016, 190, 186–193. [Google Scholar] [CrossRef]
- Chakraborty, S.; Uppaluri, R.; Das, C. Optimization of ultrasound-assisted extraction (UAE) process for the recovery of bioactive compounds from bitter gourd using response surface methodology (RSM). Food Bioprod. Process. 2020, 120, 114–122. [Google Scholar] [CrossRef]
- Chen, C.; Shao, Y.; Tao, Y.; Wen, H. Optimization of dynamic microwave-assisted extraction of Armillaria polysaccharides using RSM, and their biological activity. LWT-Food Sci. Technol. 2015, 64, 1263–1269. [Google Scholar] [CrossRef]
- Cui, R.; Zhu, F. Ultrasound modified polysaccharides: A review of structure, physicochemical properties, biological activities and food applications. Trends Food Sci. Technol. 2021, 107, 491–508. [Google Scholar] [CrossRef]
- Wang, P.; Cheng, C.; Ma, Y.; Jia, M. Degradation behavior of polyphenols in model aqueous extraction system based on mechanical and sonochemical effects induced by ultrasound. Sep. Purif. Technol. 2020, 247, 116967. [Google Scholar] [CrossRef]
- Gómez-Cruz, I.; Contreras, M.d.M.; Romero, I.; Castro, E. Sequential Extraction of Hydroxytyrosol, Mannitol and Triterpenic Acids Using a Green Optimized Procedure Based on Ultrasound. Antioxidants 2021, 10, 1781. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Tang, Q.; Chen, Y.; Wang, W.; Li, S. Simultaneous extraction of polysaccharides from Poria cocos by ultrasonic technique and its inhibitory activities against oxidative injury in rats with cervical cancer. Carbohydr. Polym. 2010, 79, 409–413. [Google Scholar] [CrossRef]
- Jia, X.; Ma, L.; Li, P.; Chen, M.; He, C. Prospects of Poria cocos polysaccharides: Isolation process, structural features and bioactivities. Trends Food Sci. Technol. 2016, 54, 52–62. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, Y.; Gapter, L.A.; Ling, H.; Agarwal, R.; Ng, K.-Y. Cytotoxic and anti-oxidant activities of lanostane-type triterpenes isolated from Poria cocos. Chem. Pharm. Bull. 2008, 56, 1459–1462. [Google Scholar] [CrossRef]
- Wu, Y.; Li, D.; Wang, H.; Wan, X. Protective Effect of Poria Cocos Polysaccharides on Fecal Peritonitis-Induced Sepsis in Mice Through Inhibition of Oxidative Stress, Inflammation, Apoptosis, and Reduction of Treg Cells. Front. Microbiol. 2022, 13, 887949. [Google Scholar] [CrossRef]
- Taghavifar, H.; Hu, C.; Taghavifar, L.; Qin, Y.; Na, J.; Wei, C. Optimal robust control of vehicle lateral stability using damped least-square backpropagation training of neural networks. Neurocomputing 2020, 384, 256–267. [Google Scholar] [CrossRef]
- Cheung, C.-C.; Lui, A.K.; Xu, S.S. Solving the local minimum and flat-spot problem by modifying wrong outputs for feed-forward neural networks. In Proceedings of the The 2013 International Joint Conference on Neural Networks (IJCNN), Dallas, TX, USA, 4–9 August 2013; pp. 1–7. [Google Scholar]
- López, G.; Arboleya, P. Short-term wind speed forecasting over complex terrain using linear regression models and multivariable LSTM and NARX networks in the Andes Mountains, Ecuador. Renew. Energy 2022, 183, 351–368. [Google Scholar] [CrossRef]
- Lahiri, S.; Ghanta, K.C. Artificial neural network model with parameter tuning assisted by genetic algorithm technique: Study of critical velocity of slurry flow in pipeline. Asia-Pac. J. Chem. Eng. 2010, 5, 763–777. [Google Scholar] [CrossRef]
- Bülbül, M.A.; Harirchian, E.; Işık, M.F.; Aghakouchaki Hosseini, S.E.; Işık, E. A Hybrid ANN-GA Model for an Automated Rapid Vulnerability Assessment of Existing RC Buildings. Appl. Sci. 2022, 12, 5138. [Google Scholar] [CrossRef]
- Yang, W.Y.; Cao, W.; Kim, J.; Park, K.W.; Park, H.-H.; Joung, J.; Ro, J.-S.; Lee, H.L.; Hong, C.-H.; Im, T. Applied Numerical Methods Using MATLAB; John Wiley & Sons: Hoboken, NJ, USA, 2020. [Google Scholar]
- Al-Saadi, S.N.; Al-Jabri, K.S. Optimization of envelope design for housing in hot climates using a genetic algorithm (GA) computational approach. J. Build. Eng. 2020, 32, 101712. [Google Scholar] [CrossRef]
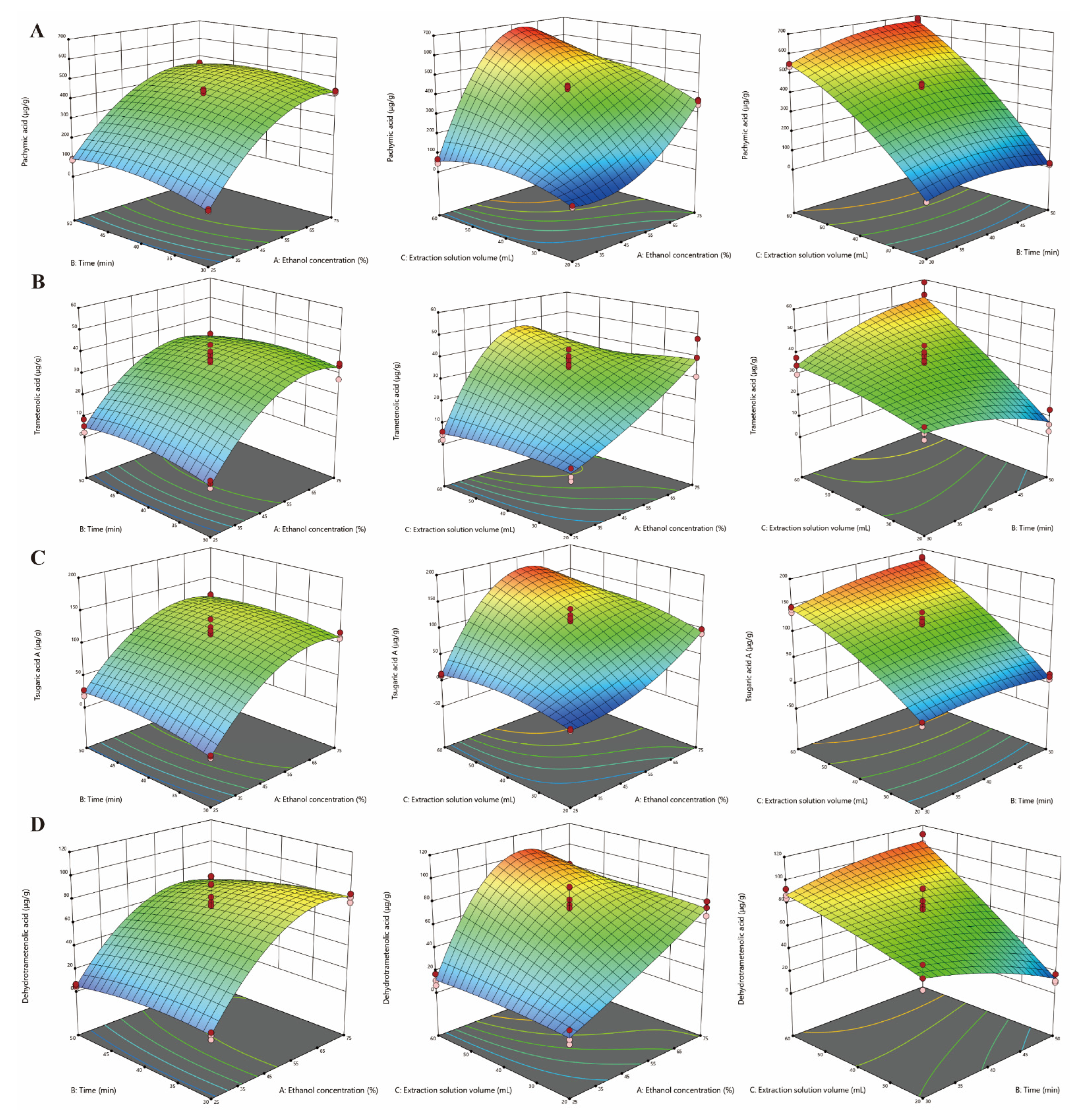
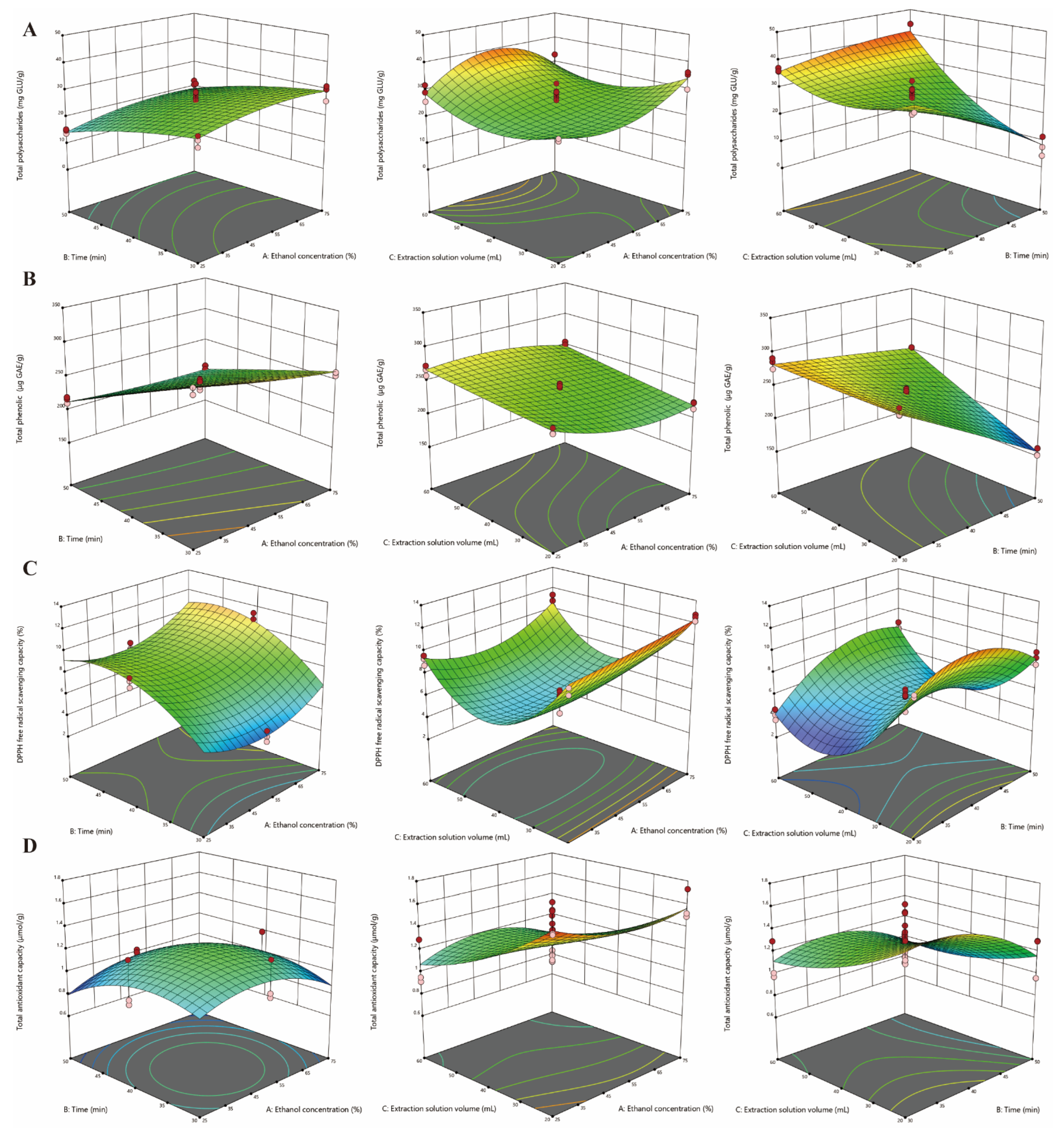
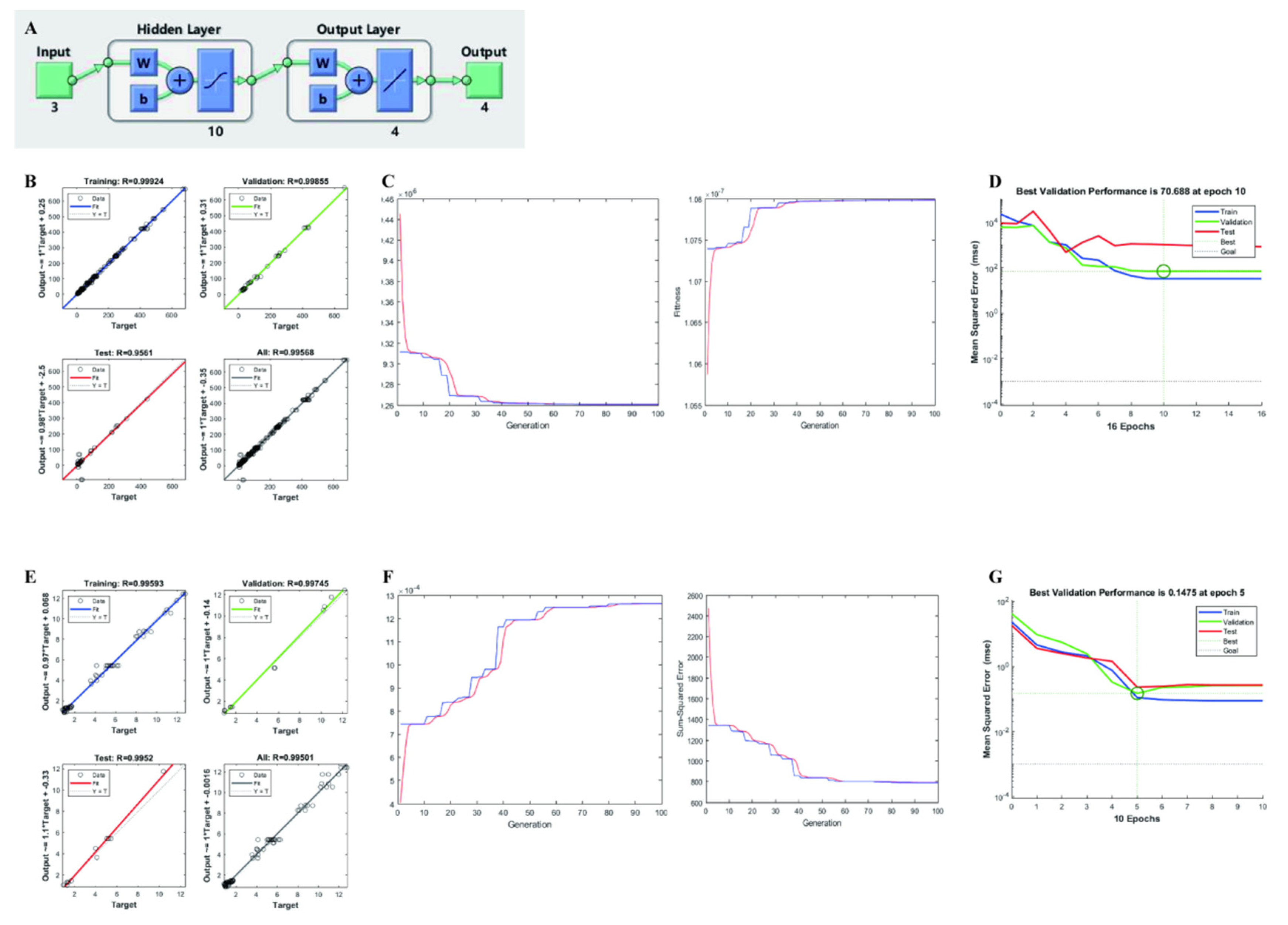
| Run | Variables | Responses | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A (%) | B (min) | C (mL) | PA (μg/g) | TA (μg/g) | TAA (μg/g) | DA (μg/g) | |||||||||
| Exp | Pred | Exp | Pred | Exp | Pred | Exp | Pred | ||||||||
| RSM | ANN | RSM | ANN | RSM | ANN | RSM | ANN | ||||||||
| 1 | 50 | 40 | 40 | 434.57 ± 10.16 | 422.08 | 419.80 | 35.78 ± 4.01 | 34.78 | 34.56 | 109.94 ± 6.95 | 113.24 | 113.37 | 68.75 ± 5.12 | 71.17 | 72.89 |
| 2 | 50 | 50 | 20 | 17.54 ± 3.84 | 13.77 | 10.88 | 7.36 ± 4.36 | 7.30 | 13.21 | 13.43 ± 4.39 | 12.22 | 11.79 | 12.89 ± 3.26 | 11.39 | 13.69 |
| 3 | 75 | 40 | 20 | 355.47 ± 10.52 | 355.32 | 359.32 | 39.44 ± 7.00 | 38.61 | 43.74 | 94.36 ± 3.82 | 95.00 | 97.10 | 74.90 ± 5.06 | 69.26 | 75.55 |
| 4 | 75 | 40 | 60 | 487.26 ± 5.02 | 487.11 | 495.19 | 29.06 ± 5.25 | 28.23 | 25.86 | 119.07 ± 5.62 | 119.70 | 119.36 | 75.48 ± 5.57 | 78.51 | 79.15 |
| 5 | 50 | 30 | 20 | 38.00 ± 2.64 | 41.78 | 37.87 | 24.81 ± 2.30 | 24.86 | 24.77 | 20.54 ± 3.54 | 21.75 | 24.32 | 59.08 ± 8.18 | 59.58 | 59.28 |
| 6 | 25 | 50 | 40 | 89.34 ± 2.62 | 92.97 | 91.50 | 5.36 ± 2.60 | 4.59 | 5.39 | 22.10 ± 4.00 | 23.94 | 19.38 | 5.14 ± 1.12 | 3.66 | 5.19 |
| 7 | 50 | 50 | 60 | 672.10 ± 9.77 | 668.33 | 660.04 | 51.25 ± 6.53 | 51.20 | 42.75 | 179.60 ± 3.88 | 178.39 | 178.38 | 104.45 ± 5.52 | 107.96 | 105.11 |
| 8 | 50 | 40 | 40 | 421.83 ± 13.04 | 422.08 | 419.80 | 37.65 ± 4.45 | 34.78 | 34.56 | 117.05 ± 6.67 | 113.24 | 113.37 | 81.98 ± 9.02 | 71.17 | 72.89 |
| 9 | 50 | 40 | 40 | 426.13 ± 12.66 | 422.08 | 419.80 | 33.03 ± 4.57 | 34.78 | 34.56 | 106.05 ± 2.83 | 113.24 | 113.37 | 67.32 ± 3.80 | 71.17 | 72.89 |
| 10 | 25 | 30 | 40 | 33.57 ± 5.03 | 29.65 | 34.59 | 3.42 ± 1.40 | 2.54 | 3.31 | 7.80 ± 1.95 | 7.23 | 6.08 | 11.51 ± 2.46 | 13.71 | 11.51 |
| 11 | 50 | 30 | 60 | 542.10 ± 6.40 | 545.87 | 548.24 | 33.99 ± 3.22 | 34.05 | 32.08 | 142.41 ± 4.29 | 143.62 | 146.57 | 88.08 ± 3.44 | 89.57 | 92.62 |
| 12 | 25 | 40 | 20 | 26.51 ± 4.86 | 26.66 | 28.90 | 5.12 ± 2.22 | 5.94 | 2.71 | 6.25 ± 1.63 | 5.62 | 6.17 | 10.39 ± 4.81 | 9.03 | 10.39 |
| 13 | 50 | 40 | 40 | 412.60 ± 6.02 | 422.08 | 419.80 | 30.97 ± 3.40 | 34.78 | 34.56 | 111.01 ± 6.80 | 113.24 | 113.37 | 68.27 ± 5.37 | 71.17 | 72.89 |
| 14 | 75 | 50 | 40 | 452.91 ± 10.50 | 456.83 | 449.44 | 29.45 ± 1.84 | 30.32 | 31.92 | 118.06 ± 3.66 | 118.64 | 119.62 | 63.65 ± 3.92 | 63.11 | 61.84 |
| 15 | 50 | 40 | 40 | 415.27 ± 5.11 | 422.08 | 419.80 | 36.49 ± 2.06 | 34.78 | 34.56 | 122.14 ± 12.26 | 113.24 | 113.37 | 79.18 ± 11.13 | 71.17 | 72.89 |
| 16 | 25 | 40 | 60 | 55.72 ± 9.37 | 55.87 | 68.54 | 4.10 ± 1.59 | 4.93 | 4.10 | 12.12 ± 1.63 | 11.49 | 8.09 | 11.49 ± 4.24 | 10.13 | 6.62 |
| 17 | 75 | 30 | 40 | 429.31 ± 4.72 | 425.69 | 433.54 | 32.00 ± 3.25 | 32.77 | 32.00 | 111.96 ± 4.14 | 110.11 | 111.88 | 81.72 ± 2.99 | 82.87 | 81.47 |
| Run | Variables | Responses | |||||||||||||
| A (%) | B (min) | C (mL) | TPs (mg GLU/g) | TPc (μg GAE/g) | DPPH-SC (%) | T-AOC (μmol/g) | |||||||||
| Exp | Pred | Exp | Pred | Exp | Pred | Exp | Pred | ||||||||
| RSM | ANN | RSM | ANN | RSM | ANN | RSM | ANN | ||||||||
| 1 | 50 | 40 | 40 | 29.24 ± 2.54 | 25.61 | 25.61 | 241.14 ± 6.11 | 242.71 | 241.76 | 5.55 ± 0.25 | 5.45 | 5.44 | 1.27 ± 0.11 | 1.28 | 1.32 |
| 2 | 50 | 50 | 20 | 7.71 ± 2.93 | 8.36 | 7.79 | 154.71 ± 4.89 | 156.76 | 150.76 | 8.89 ± 0.47 | 8.82 | 8.29 | 1.18 ± 0.16 | 1.16 | 1.15 |
| 3 | 75 | 40 | 20 | 33.83 ± 2.80 | 34.02 | 29.89 | 220.61 ± 4.53 | 219.60 | 218.77 | 12.50 ± 0.24 | 12.34 | 12.12 | 1.65 ± 0.20 | 1.62 | 1.56 |
| 4 | 75 | 40 | 60 | 28.99 ± 2.92 | 29.18 | 28.98 | 249.83 ± 4.45 | 248.82 | 252.23 | 10.78 ± 0.47 | 10.62 | 10.77 | 1.03 ± 0.12 | 1.00 | 1.01 |
| 5 | 50 | 30 | 20 | 36.60 ± 2.20 | 35.95 | 37.00 | 281.69 ± 4.55 | 279.63 | 280.08 | 10.46 ± 0.37 | 10.52 | 10.76 | 1.53 ± 0.08 | 1.55 | 1.49 |
| 6 | 25 | 50 | 40 | 14.66 ± 0.69 | 14.19 | 14.23 | 215.27 ± 3.93 | 212.21 | 219.85 | 4.23 ± 0.30 | 4.14 | 4.36 | 0.95 ± 0.06 | 0.95 | 0.93 |
| 7 | 50 | 50 | 60 | 40.73 ± 2.52 | 41.38 | 41.30 | 250.96 ± 4.55 | 253.02 | 249.22 | 8.27 ± 0.31 | 8.21 | 11.28 | 0.99 ± 0.03 | 0.97 | 1.10 |
| 8 | 50 | 40 | 40 | 22.75 ± 3.10 | 25.61 | 25.61 | 243.32 ± 2.03 | 242.71 | 241.76 | 5.80 ± 0.35 | 5.45 | 5.44 | 1.25 ± 0.14 | 1.28 | 1.32 |
| 9 | 50 | 40 | 40 | 26.31 ± 2.34 | 25.61 | 25.61 | 243.49 ± 4.66 | 242.71 | 241.76 | 5.29 ± 0.22 | 5.45 | 5.44 | 1.27 ± 0.20 | 1.28 | 1.32 |
| 10 | 25 | 30 | 40 | 24.90 ± 1.59 | 25.74 | 23.98 | 301.98 ± 6.95 | 303.03 | 301.85 | 3.57 ± 0.45 | 3.35 | 3.60 | 1.35 ± 0.01 | 1.30 | 1.36 |
| 11 | 50 | 30 | 60 | 36.32 ± 0.58 | 35.67 | 36.33 | 284.16 ± 6.67 | 282.11 | 283.83 | 4.13 ± 0.41 | 4.19 | 3.87 | 1.09 ± 0.14 | 1.11 | 0.94 |
| 12 | 25 | 40 | 20 | 26.24 ± 1.41 | 26.05 | 25.25 | 250.68 ± 3.52 | 251.68 | 250.01 | 11.10 ± 0.65 | 11.26 | 11.33 | 1.69 ± 0.02 | 1.71 | 1.70 |
| 13 | 50 | 40 | 40 | 23.08 ± 3.45 | 25.61 | 25.61 | 240.52 ± 2.97 | 242.71 | 241.76 | 5.27 ± 0.84 | 5.45 | 5.44 | 1.29 ± 0.23 | 1.28 | 1.32 |
| 14 | 75 | 50 | 40 | 19.89 ± 2.30 | 19.05 | 20.72 | 203.07 ± 3.83 | 202.02 | 202.05 | 5.44 ± 0.27 | 5.66 | 5.34 | 0.90 ± 0.06 | 0.94 | 0.98 |
| 15 | 50 | 40 | 40 | 26.67 ± 3.11 | 25.61 | 25.61 | 245.10 ± 2.79 | 242.71 | 241.76 | 5.32 ± 0.21 | 5.45 | 5.44 | 1.33 ± 0.16 | 1.28 | 1.32 |
| 16 | 25 | 40 | 60 | 28.85 ± 2.50 | 28.67 | 27.43 | 265.78 ± 6.10 | 266.79 | 266.57 | 9.23 ± 0.36 | 9.39 | 9.22 | 1.05 ± 0.17 | 1.08 | 1.05 |
| 17 | 75 | 30 | 40 | 28.91 ± 2.39 | 29.37 | 28.84 | 260.09 ± 2.26 | 263.16 | 270.32 | 4.03 ± 0.36 | 4.13 | 4.03 | 1.14 ± 0.06 | 1.14 | 1.16 |
| Source | PA | TA | TAA | DA | TPs | TPc | DPPH-SC | T-AOC |
|---|---|---|---|---|---|---|---|---|
| Sum of Squares (model) | 2.24 × 106 | 1.06 × 104 | 1.50 × 105 | 5.03 × 104 | 3.03 × 103 | 5.47 × 103 | 402.52 | 2.36 |
| df | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Mean Squares (model) | 2.24 × 105 | 1.06 × 103 | 1.50 × 104 | 5.03 × 103 | 3.03 × 102 | 5.47 × 102 | 40.25 | 0.24 |
| F-value (model) | 2002.85 | 48.25 | 311.73 | 94.24 | 29.98 | 174.22 | 159.14 | 11.16 |
| p-value (model) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| F-value (Lack of Fit) | 1.58 | 0.36 | 0.45 | 0.09 | 0.54 | 2.12 | 1.45 | 0.43 |
| p-value (Lack of Fit) | 0.22 | 0.70 | 0.64 | 0.91 | 0.59 | 0.13 | 0.25 | 0.67 |
| Std. Dev | 10.57 | 4.68 | 6.93 | 7.31 | 3.18 | 5.60 | 0.50 | 0.15 |
| R2 | 0.99 | 0.92 | 0.98 | 0.96 | 0.88 | 0.98 | 0.98 | 0.74 |
| Adeq precision | 133.39 | 22.40 | 53.66 | 29.65 | 22.36 | 56.19 | 38.52 | 11.32 |
| Variables | Group 1 | Group 2 | |||
|---|---|---|---|---|---|
| RSM | RSM-ANN-GA | RSM | RSM-ANN-GA | ||
| Input (process parameters) | Ethanol concentration (v/v, %) | 55.97 | 53.53 | 25.00 | 40.49 |
| Time (min) | 49.30 | 48.64 | 30.00 | 30.25 | |
| Extraction solution volume (mL) | 60.00 | 60.00 | 20.00 | 20.00 | |
| Output (responses) | PA (μg/g) | 697.92 | 674.09 | ||
| TA (μg/g) | 51.93 | 43.10 | |||
| TAA (μg/g) | 184.87 | 184.02 | |||
| DA (μg/g) | 108.86 | 107.44 | |||
| TPs (mg GLU/g) | 38.82 | 35.33 | |||
| TPc (μg GAE/g) | 319.78 | 283.73 | |||
| DPPH-SC (%) | 10.24 | 10.58 | |||
| T-AOC (μmol/g) | 1.77 | 1.61 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Zhang, H.; Yang, L.; Zhang, S.; Jiang, H. Optimization of Ultrasonic-Assisted Extraction Conditions for Bioactive Components and Antioxidant Activity of Poria cocos (Schw.) Wolf by an RSM-ANN-GA Hybrid Approach. Foods 2023, 12, 619. https://doi.org/10.3390/foods12030619
Chen S, Zhang H, Yang L, Zhang S, Jiang H. Optimization of Ultrasonic-Assisted Extraction Conditions for Bioactive Components and Antioxidant Activity of Poria cocos (Schw.) Wolf by an RSM-ANN-GA Hybrid Approach. Foods. 2023; 12(3):619. https://doi.org/10.3390/foods12030619
Chicago/Turabian StyleChen, Shiqi, Huixia Zhang, Liu Yang, Shuai Zhang, and Haiyang Jiang. 2023. "Optimization of Ultrasonic-Assisted Extraction Conditions for Bioactive Components and Antioxidant Activity of Poria cocos (Schw.) Wolf by an RSM-ANN-GA Hybrid Approach" Foods 12, no. 3: 619. https://doi.org/10.3390/foods12030619
APA StyleChen, S., Zhang, H., Yang, L., Zhang, S., & Jiang, H. (2023). Optimization of Ultrasonic-Assisted Extraction Conditions for Bioactive Components and Antioxidant Activity of Poria cocos (Schw.) Wolf by an RSM-ANN-GA Hybrid Approach. Foods, 12(3), 619. https://doi.org/10.3390/foods12030619







