Valorization of Fruit Waste for Bioactive Compounds and Their Applications in the Food Industry
Abstract
1. Introduction
2. Materials and Methods
3. Fruit Loss and Processing Waste
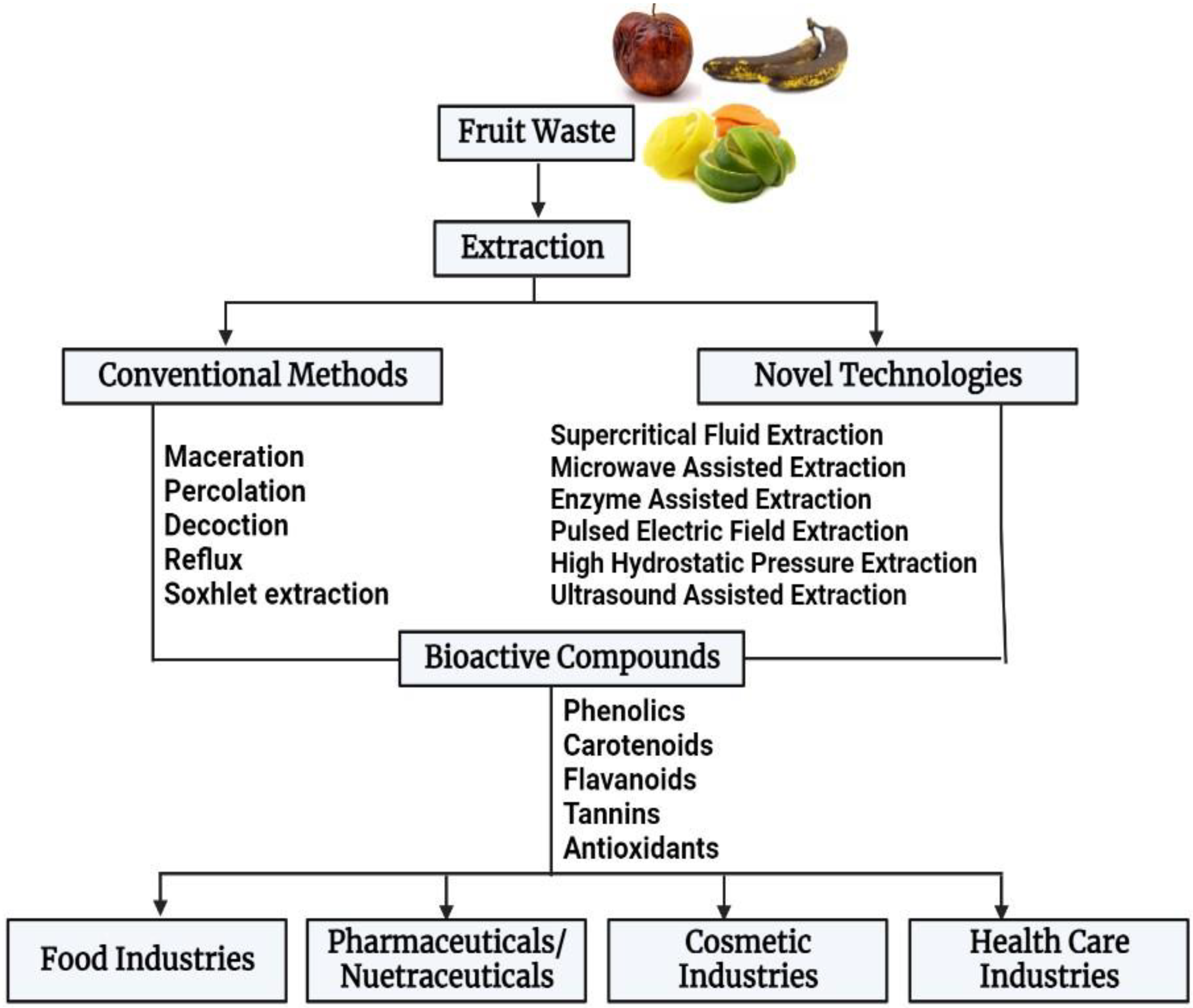
4. Bioactive Compounds from Fruit Waste
5. Extraction Techniques
5.1. Conventional Methods
5.2. Novel Emerging Methods
5.2.1. Supercritical Fluid Extraction (SFE)
5.2.2. Microwave-Assisted Extraction (MAE)
5.2.3. Enzyme-Assisted Extraction (EAE)
5.2.4. Pulsed Electric Field Extraction (PEFE)
5.2.5. High-Pressure Extraction
5.2.6. Ultrasound-Assisted Extraction (UAE)
6. Bioactivities of Active Compounds Extracted from Fruit Waste
6.1. Antioxidant Activity
6.2. Antimicrobial Activity
6.3. Other Properties
7. Application of Bioactive Compounds in the Food Industry
7.1. Food Fortification
7.2. Food Preservation
8. Challenges and Future Direction
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baysal, S.S.; Ülkü, M.A. Food loss and waste: A sustainable supply chain perspective. In Disruptive Technologies and Eco-Innovation for Sustainable Development; Ulas, A., Ed.; IGI global: Hershey, PA, USA, 2022; pp. 90–108. [Google Scholar]
- FAO. Food Wastage Foot Print Impacts on Natural Resources. Available online: https://www.fao.org/news/story/en/item/196402/icode/ (accessed on 20 November 2022).
- Trigo, J.P.; Alexandre, E.M.; Saraiva, J.A.; Pintado, M.E. High value-added compounds from fruit and vegetable by-products–Characterization, bioactivities, and application in the development of novel food products. Crit. Rev. Food Sci. Nutr. 2022, 60, 1388–1416. [Google Scholar] [CrossRef] [PubMed]
- Galanakis, C.M. Recovery of high added-value components from food wastes: Conventional, emerging technologies and commercialized applications. Trends Food Sci. Technol. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- Rifna, E.J.; Misra, N.N.; Dwivedi, M. Recent advances in extraction technologies for recovery of bioactive compounds derived from fruit and vegetable waste peels: A review. Crit. Rev. Food Sci. Nutr. 2021, 1–34. [Google Scholar] [CrossRef]
- Sindhu, R.; Gnansounou, E.; Rebello, S.; Binod, P.; Varjani, S.; Thakur, I.S.; Nair, R.B.; Pandey, A. Conversion of food and kitchen waste to value-added products. J. Environ. Manag. 2019, 241, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Esparza, I.; Jiménez-Moreno, N.; Bimbela, F.; Ancín-Azpilicueta, C.; Gandía, L.M. Fruit and vegetable waste management: Conventional and emerging approaches. J. Environ. Manag. 2020, 265, 110510. [Google Scholar] [CrossRef]
- UN. International Day of Awareness on Food Loss and Waste Reduction. Available online: https://www.un.org/en/observances/end-food-waste-day#:~:text=Globally%2C%20around%2014%20percent%20of,and%202%20percent%20in%20retail (accessed on 20 November 2022).
- WWF. Driven to Waste: The Global Impact of Food Loss and Waste on Farms. Available online: https://www.worldwildlife.org/publications/driven-to-waste-the-global-impact-of-food-loss-and-waste-on-farms (accessed on 20 November 2022).
- De Laurentiis, V.; Corrado, S.; Sala, S. Quantifying household waste of fresh fruit and vegetables in the EU. Waste Manag. 2018, 77, 238–251. [Google Scholar] [CrossRef]
- GERVASI, A. This Is How Much Fruit You’re Throwing Away Each Year. Available online: https://www.mashed.com/230012/this-is-how-much-fruit-youre-throwing-away-each-year/ (accessed on 20 November 2022).
- USFDA. Food Loss and Waste. Available online: https://www.fda.gov/food/consumers/food-loss-and-waste#:~:text=In%20the%20United%20States%2C%20food,worth%20of%20food%20in%202010 (accessed on 20 November 2022).
- The World Counts. Available online: https://www.theworldcounts.com/challenges/people-and-poverty/hunger-and-obesity/food-waste-statistics (accessed on 20 November 2022).
- Mattsson, L.; Williams, H.; Berghel, J. Waste of fresh fruit and vegetables at retailers in Sweden—Measuring and calculation of mass, economic cost and climate impact. Resour. Conserv. Recycl. 2018, 130, 118–126. [Google Scholar] [CrossRef]
- Zhang, Y.; Liao, J.; Qi, J. Functional and structural properties of dietary fiber from citrus peel affected by the alkali combined with high-speed homogenization treatment. Lwt 2020, 128, 109397. [Google Scholar] [CrossRef]
- Leong, Y.K.; Chang, J.S. Valorization of fruit wastes for circular bioeconomy: Current advances, challenges, and opportunities. Bioresour. Technol. 2022, 359, 127459. [Google Scholar] [CrossRef]
- de Medeiros, V.P.B.; Pimentel, T.C.; Varandas, R.C.R.; Dos Santos, S.A.; de Souza Pedrosa, G.T.; da Costa Sassi, C.F.; da Conceicao, M.M.; Magnani, M. Exploiting the use of agro-industrial residues from fruit and vegetables as alternative microalgae culture medium. Food Res. Int. 2020, 137, 109722. [Google Scholar] [CrossRef] [PubMed]
- Caipang, C.M.A.; Mabuhay-Omar, J.; Gonzales-Plasus, M.M. Plant and fruit waste products as phytogenic feed additives in aquaculture. Aquac. Aquar. Conserv. Legis. 2019, 12, 261–268. [Google Scholar]
- Chew, K.W.; Yap, J.Y.; Show, P.L.; Suan, N.H.; Juan, J.C.; Ling, T.C.; Lee, D.J.; Chang, J.S. Microalgae biorefinery: High value products perspectives. Bioresour. Technol. 2017, 229, 53–62. [Google Scholar] [CrossRef]
- Sharma, M.; Usmani, Z.; Gupta, V.K.; Bhat, R. Valorization of fruits and vegetable wastes and by-products to produce natural pigments. Crit. Rev. Biotechnol. 2021, 41, 535–563. [Google Scholar] [CrossRef] [PubMed]
- Ran, X.L.; Zhang, M.; Wang, Y.; Adhikari, B. Novel technologies applied for recovery and value addition of high value compounds from plant byproducts: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 450–461. [Google Scholar] [CrossRef]
- Rudra, S.G.; Nishad, J.; Jakhar, N.; Kaur, C. Food industry waste: Mine of nutraceuticals. Int. J. Sci. Environ. Technol 2015, 4, 205–229. [Google Scholar]
- Shabir, I.; Pandey, V.K.; Dar, A.H.; Pandiselvam, R.; Manzoor, S.; Mir, S.A.; Shams, R.; Dash, K.K.; Fayaz, U.; Khan, S.A.; et al. Nutritional Profile, Phytochemical Compounds, Biological Activities, and Utilisation of Onion Peel for Food Applications: A Review. Sustainability 2022, 14, 11958. [Google Scholar] [CrossRef]
- Kumar, M.; Barbhai, M.D.; Hasan, M.; Punia, S.; Dhumal, S.; Rais, N.; Chandran, D.; Pandiselvam, R.; Kothakota, A.; Tomar, M. Onion (Allium cepa L.) peels: A review on bioactive compounds and biomedical activities. Biomed. Pharmacother. 2022, 146, 112498. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and Vegetable Waste: Bioactive Compounds, Their Extraction, and Possible Utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef]
- Wolfe, K.; Wu, X.; Liu, R.H. Antioxidant activity of apple peels. J. Agric. Food Chem. 2003, 51, 609–614. [Google Scholar] [CrossRef]
- Zadernowski, R.; Czaplicki, S.; Naczk, M. Phenolic acid profiles of mangosteen fruits (Garcinia mangostana). Food Chem. 2009, 112, 685–689. [Google Scholar] [CrossRef]
- Wittenauer, J.; Falk, S.; Schweiggert-Weisz, U.; Carle, R. Characterisation and quantification of xanthones from the aril and pericarp of mangosteens (Garcinia mangostana L.) and a mangosteen containing functional beverage by HPLC–DAD–MSn. Food Chem. 2012, 134, 445–452. [Google Scholar] [CrossRef]
- Daud, N.H.; Aung, C.S.; Hewavitharana, A.K.; Wilkinson, A.S.; Pierson, J.T.; Roberts-Thomson, S.J.; Shaw, P.N.; Monteith, G.R.; Gidley, M.J.; Parat, M.O. Mango extracts and the mango component mangiferin promote endothelial cell migration. J. Agric. Food Chem. 2010, 58, 5181–5186. [Google Scholar] [CrossRef]
- Dorta, E.; Lobo, M.G.; González, M. Using drying treatments to stabilise mango peel and seed: Effect on antioxidant activity. LWT Food Sci. Technol. 2012, 45, 261–268. [Google Scholar] [CrossRef]
- Ng, L.; Ang, Y.; Khoo, H.; Yim, H. Influence of different extraction parameters on antioxidant properties of Carica papaya peel and seed. Res. J. Phytochem. 2012, 6, 61–74. [Google Scholar]
- Parni, B.; Verma, Y. Biochemical properties in peel, pulp and seeds of Carica papaya. Plant Arch. 2014, 14, 565–568. [Google Scholar]
- Santos, C.M.D.; Abreu, C.M.P.D.; Freire, J.M.; Queiroz, E.D.R.; Mendonça, M.M. Chemical characterization of the flour of peel and seed from two papaya cultivars. Food Sci. Technol. 2014, 34, 353–357. [Google Scholar] [CrossRef]
- López-Vargas, J.H.; Fernández-López, J.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Chemical, physico-chemical, technological, antibacterial and antioxidant properties of dietary fiber powder obtained from yellow passion fruit (Passiflora edulis var. flavicarpa) co-products. Food Res. Int. 2013, 51, 756–763. [Google Scholar] [CrossRef]
- Liaotrakoon, W.; Van Buggenhout, S.; Christiaens, S.; Houben, K.; De Clercq, N.; Dewettinck, K.; Hendrickx, M.E. An explorative study on the cell wall polysaccharides in the pulp and peel of dragon fruits (Hylocereus spp.). Eur. Food Res. Technol. 2013, 237, 341–351. [Google Scholar] [CrossRef]
- Kumar, M.; Tomar, M.; Bhuyan, D.J.; Punia, S.; Grasso, S.; Sa, A.G.A.; Carciofi, B.A.M.; Arrutia, F.; Changan, S.; Radha; et al. Tomato (Solanum lycopersicum L.) seed: A review on bioactives and biomedical activities. Biomed Pharm. 2021, 142, 112018. [Google Scholar] [CrossRef]
- Kumar, M.; Kapoor, S.; Dhumal, S.; Tkaczewska, J.; Changan, S.; Saurabh, V.; Mekhemar, M.; Radha; Rais, N.; Satankar, V.; et al. Guava (Psidium guajava L.) seed: A low-volume, high-value byproduct for human health and the food industry. Food Chem. 2022, 386, 132694. [Google Scholar] [CrossRef]
- Kumar, M.; Hasan, M.; Lorenzo, J.M.; Dhumal, S.; Nishad, J.; Rais, N.; Verma, A.; Changan, S.; Barbhai, M.D.; Chandran, D. Jamun (Syzygium cumini (L.) Skeels) seed bioactives and its biological activities: A review. Food Biosci. 2022, 50, 102109. [Google Scholar] [CrossRef]
- Freitas, A.; Moldao-Martins, M.; Costa, H.S.; Albuquerque, T.G.; Valente, A.; Sanches-Silva, A. Effect of UV-C radiation on bioactive compounds of pineapple (Ananas comosus L. Merr.) by-products. J. Sci. Food Agric. 2015, 95, 44–52. [Google Scholar] [CrossRef]
- Johnson, J.; Abam, K.; Ujong, U.; Odey, M.; Inekwe, V.; Dasofunjo, K.; Inah, G.J.I.J.o.S. Vitamins composition of pulp, seed and rind of fresh and dry Rambutan Nephelium lappaceum and squash Cucurbita pepo L. Int. J. Sci. Technol. 2013, 2, 71–76. [Google Scholar]
- Sogi, D.S.; Siddiq, M.; Greiby, I.; Dolan, K.D. Total phenolics, antioxidant activity, and functional properties of ‘Tommy Atkins’ mango peel and kernel as affected by drying methods. Food Chem. 2013, 141, 2649–2655. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Keum, Y.S. Carotenoid extraction methods: A review of recent developments. Food Chem. 2018, 240, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Rammuni, M.N.; Ariyadasa, T.U.; Nimarshana, P.H.V.; Attalage, R.A. Comparative assessment on the extraction of carotenoids from microalgal sources: Astaxanthin from H. pluvialis and beta-carotene from D. salina. Food Chem. 2019, 277, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Szabo, K.; Catoi, A.F.; Vodnar, D.C. Bioactive Compounds Extracted from Tomato Processing by-Products as a Source of Valuable Nutrients. Plant Foods Hum. Nutr. 2018, 73, 268–277. [Google Scholar] [CrossRef]
- Imran, M.; Ghorat, F.; Ul-Haq, I.; Ur-Rehman, H.; Aslam, F.; Heydari, M.; Shariati, M.A.; Okuskhanova, E.; Yessimbekov, Z.; Thiruvengadam, M.; et al. Lycopene as a Natural Antioxidant Used to Prevent Human Health Disorders. Antioxidants 2020, 9, 706. [Google Scholar] [CrossRef]
- Cha, J.H.; Kim, W.K.; Ha, A.W.; Kim, M.H.; Chang, M.J. Anti-inflammatory effect of lycopene in SW480 human colorectal cancer cells. Nutr. Res. Pract. 2017, 11, 90–96. [Google Scholar] [CrossRef]
- Ochoa Becerra, M.; Mojica Contreras, L.; Hsieh Lo, M.; Mateos Díaz, J.; Castillo Herrera, G. Lutein as a functional food ingredient: Stability and bioavailability. J. Funct. Foods 2020, 66, 103771. [Google Scholar] [CrossRef]
- Sun, Y.; Li, L. Cyanidin-3-glucoside inhibits inflammatory activities in human fibroblast-like synoviocytes and in mice with collagen-induced arthritis. Clin. Exp. Pharmacol. Physiol. 2018, 45, 1038–1045. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Han, X.; Guo, J.; Guo, Y.; Yin, M.; Liu, G.; Huang, W.; Zhan, J. Cyanidin-3-glucoside attenuates high-fat and high-fructose diet-induced obesity by promoting the thermogenic capacity of brown adipose tissue. J. Funct. Foods 2018, 41, 62–71. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F. Flavonols (kaempeferol, quercetin, myricetin) contents of selected fruits, vegetables and medicinal plants. Food Chem. 2008, 108, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Vijayalakshmi, S.; Nadanasabapathi, S. Health Benefits of Quercetin. Def. Life Sci. J. 2017, 2, 142–151. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Sudha, M.L.; Baskaran, V.; Leelavathi, K. Apple pomace as a source of dietary fiber and polyphenols and its effect on the rheological characteristics and cake making. Food Chem. 2007, 104, 686–692. [Google Scholar] [CrossRef]
- Kumar, M.; Barbhai, M.D.; Esatbeyoglu, T.; Zhang, B.; Sheri, V.; Dhumal, S.; Rais, N.; Al Masry, E.M.S.; Chandran, D.; Pandiselvam, R. Apple (Malus domestica Borkh.) seed: A review on health promoting bioactivities and its application as functional food ingredient. Food Biosci. 2022, 50, 102155. [Google Scholar] [CrossRef]
- Reissner, A.M.; Al-Hamimi, S.; Quiles, A.; Schmidt, C.; Struck, S.; Hernando, I.; Turner, C.; Rohm, H. Composition and physicochemical properties of dried berry pomace. J. Sci. Food Agric. 2019, 99, 1284–1293. [Google Scholar] [CrossRef]
- Valiente, C.; Arrigoni, E.; Esteban, R.; Amado, R. Grape pomace as a potential food fiber. J. Food Sci. 1995, 60, 818–820. [Google Scholar] [CrossRef]
- Ajila, C.M.; Prasada Rao, U.J.S. Mango peel dietary fibre: Composition and associated bound phenolics. J. Funct. Foods 2013, 5, 444–450. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Cujic, N.; Savikin, K.; Jankovic, T.; Pljevljakusic, D.; Zdunic, G.; Ibric, S. Optimization of polyphenols extraction from dried chokeberry using maceration as traditional technique. Food Chem. 2016, 194, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Masi, E.; Taiti, C.; Heimler, D.; Vignolini, P.; Romani, A.; Mancuso, S. PTR-TOF-MS and HPLC analysis in the characterization of saffron (Crocus sativus L.) from Italy and Iran. Food Chem. 2016, 192, 75–81. [Google Scholar] [CrossRef]
- Naviglio, D.; Formato, A.; Vitulano, M.; Cozzolino, I.; Ferrara, L.; Zanoelo, E.F.; Gallo, M. Comparison Between the Kinetics of Conventional Maceration and A Cyclic Pressurization Extraction Process for the Production of Lemon Liqueur Using A Numerical Model. J. Food Process Eng. 2017, 40, e12350. [Google Scholar] [CrossRef]
- Rathore, S.K.; Bhatt, S.; Dhyani, S.; Jain, A. Preliminary phytochemical screening of medicinal plant Ziziphus mauritiana Lam. fruits. Int. J. Curr. Pharm. Res. 2012, 4, 160–162. [Google Scholar]
- Leela, C.; Shashank, B.; Suresh, D. Phytochemical screening of secondary metabolites of euphorbia Neriifolia linn. Glob. J. Res. Med. Plants Indig. Med. 2013, 2, 292. [Google Scholar]
- Tandon, S.; Rane, S. Decoction and Hot Continuous Extraction Techniques. In Extraction Technologies for Medicinal and Aromatic Plants; Handa, S.S., Khanuja, S.P.S., Longo, G., Rakesh, D.D., Eds.; ICS-UNIDO: Trieste, Italy, 2008; Volume 93. [Google Scholar]
- Kaneria, M.; Kanani, B.; Chanda, S. Assessment of effect of hydroalcoholic and decoction methods on extraction of antioxidants from selected Indian medicinal plants. Asian Pac. J. Trop. Biomed. 2012, 2, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Rijo, P.; Falé, P.L.; Serralheiro, M.L.; Simões, M.F.; Gomes, A.; Reis, C. Optimization of medicinal plant extraction methods and their encapsulation through extrusion technology. Measurement 2014, 58, 249–255. [Google Scholar] [CrossRef]
- Garcia-Salas, P.; Morales-Soto, A.; Segura-Carretero, A.; Fernandez-Gutierrez, A. Phenolic-compound-extraction systems for fruit and vegetable samples. Molecules 2010, 15, 8813–8826. [Google Scholar] [CrossRef]
- Aliboudhar, H.; Tigrine-Kordjani, N. Effect of extraction technique on the content and antioxidant activity of crude extract of Anacyclus clavatus flowers and their essential oil composition. Nat. Prod. Res. 2014, 28, 2140–2149. [Google Scholar] [CrossRef]
- Luque de Castro, M.D.; Priego-Capote, F. Soxhlet extraction: Past and present panacea. J. Chromatogr. A 2010, 1217, 2383–2389. [Google Scholar] [CrossRef] [PubMed]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C. Soxhlet extraction of phenolic compounds from Vernonia cinerea leaves and its antioxidant activity. J. Appl. Res. Med. Aromat. Plants 2018, 11, 12–17. [Google Scholar] [CrossRef]
- Kumar, P. Effect of temperature and time intervals on the solvent extraction of essential oil from azadirachta indica (neem) leaf powder by using soxhlet extraction method. World J. Pharm. Res. 2018, 8, 939–946. [Google Scholar]
- Feng, C.H.; García-Martín, J.F.; Broncano Lavado, M.; López-Barrera, M.d.C.; Álvarez-Mateos, P. Evaluation of different solvents on flavonoids extraction efficiency from sweet oranges and ripe and immature Seville oranges. Int. J. Food Sci. Technol. 2020, 55, 3123–3134. [Google Scholar] [CrossRef]
- Da Silva, R.P.; Rocha-Santos, T.A.; Duarte, A.C. Supercritical fluid extraction of bioactive compounds. Trends Anal. Chem. 2016, 76, 40–51. [Google Scholar] [CrossRef]
- Zhang, H.-F.; Yang, X.-H.; Wang, Y. Microwave assisted extraction of secondary metabolites from plants: Current status and future directions. Trends Food Sci. Technol. 2011, 22, 672–688. [Google Scholar] [CrossRef]
- Sridhar, A.; Ponnuchamy, M.; Kumar, P.S.; Kapoor, A.; Vo, D.N.; Prabhakar, S. Techniques and modeling of polyphenol extraction from food: A review. Environ. Chem. Lett. 2021, 19, 3409–3443. [Google Scholar] [CrossRef]
- Gligor, O.; Mocan, A.; Moldovan, C.; Locatelli, M.; Crișan, G.; Ferreira, I.C.F.R. Enzyme-assisted extractions of polyphenols—A comprehensive review. Trends Food Sci. Technol. 2019, 88, 302–315. [Google Scholar] [CrossRef]
- Nadar, S.S.; Rao, P.; Rathod, V.K. Enzyme assisted extraction of biomolecules as an approach to novel extraction technology: A review. Food Res. Int. 2018, 108, 309–330. [Google Scholar] [CrossRef]
- Yan, L.G.; He, L.; Xi, J. High intensity pulsed electric field as an innovative technique for extraction of bioactive compounds—A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 2877–2888. [Google Scholar] [CrossRef]
- Redondo, D.; Venturini, M.E.; Luengo, E.; Raso, J.; Arias, E. Pulsed electric fields as a green technology for the extraction of bioactive compounds from thinned peach by-products. Innov. Food Sci. Emerg. Technol. 2018, 45, 335–343. [Google Scholar] [CrossRef]
- El Kantar, S.; Boussetta, N.; Lebovka, N.; Foucart, F.; Rajha, H.N.; Maroun, R.G.; Louka, N.; Vorobiev, E. Pulsed electric field treatment of citrus fruits: Improvement of juice and polyphenols extraction. Innov. Food Sci. Emerg. Technol. 2018, 46, 153–161. [Google Scholar] [CrossRef]
- Rajha, H.N.; Boussetta, N.; Louka, N.; Maroun, R.G.; Vorobiev, E. A comparative study of physical pretreatments for the extraction of polyphenols and proteins from vine shoots. Food Res. Int. 2014, 65, 462–468. [Google Scholar] [CrossRef]
- Medina-Meza, I.G.; Barbosa-Cánovas, G.V. Assisted extraction of bioactive compounds from plum and grape peels by ultrasonics and pulsed electric fields. J. Food Eng. 2015, 166, 268–275. [Google Scholar] [CrossRef]
- Khan, S.A.; Aslam, R.; Makroo, H.A. High pressure extraction and its application in the extraction of bio-active compounds: A review. J. Food Process Eng. 2019, 42, e12896. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Zhang, H.; Dzah, C.S.; Zandile, M.; Duan, Y.; Ma, H.; Luo, X. Advances in ultrasound assisted extraction of bioactive compounds from cash crops–A review. Ultrason. Sonochem. 2018, 48, 538–549. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef]
- Vilkhu, K.; Mawson, R.; Simons, L.; Bates, D. Applications and opportunities for ultrasound assisted extraction in the food industry—A review. Innov. Food Sci. Emerg. Technol. 2008, 9, 161–169. [Google Scholar] [CrossRef]
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 1–26. [Google Scholar] [CrossRef]
- Azwanida. A Review on the Extraction Methods Use in Medicinal Plants, Principle, Strength and Limitation. Med. & Arom. Plants 2015, 4, 196. [Google Scholar] [CrossRef]
- Mena-García, A.; Ruiz-Matute, A.I.; Soria, A.C.; Sanz, M.L. Green techniques for extraction of bioactive carbohydrates. TrAC, Trends Anal. Chem. 2019, 119, 115612. [Google Scholar] [CrossRef]
- Maza, M.; Álvarez, I.; Raso, J. Thermal and Non-Thermal Physical Methods for Improving Polyphenol Extraction in Red Winemaking. Beverages 2019, 5, 47. [Google Scholar] [CrossRef]
- Uwineza, P.A.; Waskiewicz, A. Recent Advances in Supercritical Fluid Extraction of Natural Bioactive Compounds from Natural Plant Materials. Molecules 2020, 25, 3847. [Google Scholar] [CrossRef]
- Carrara, V.; Garcia, V.; Faiões, V.; Cunha-Júnior, E.; Torres-Santos, E.; Cortez, D. Supercritical fluid extraction of pyrrolidine alkaloid from leaves of Piper amalago L. Evid. -Based Complement. Altern. Med. 2017, 2017, 7401748. [Google Scholar] [CrossRef]
- İçen, H.; Gürü, M. Effect of ethanol content on supercritical carbon dioxide extraction of caffeine from tea stalk and fiber wastes. J. Supercrit. Fluids 2010, 55, 156–160. [Google Scholar] [CrossRef]
- Ellington, E.; Bastida, J.; Viladomat, F.; Codina, C. Supercritical carbon dioxide extraction of colchicine and related alkaloids from seeds of Colchicum autumnale L. Phytochem. Anal. Int. J. Plant Chem. Biochem. Tech. 2003, 14, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Falcão, M.A.; Scopel, R.; Almeida, R.N.; do Espirito Santo, A.T.; Franceschini, G.; Garcez, J.J.; Vargas, R.M.; Cassel, E. Supercritical fluid extraction of vinblastine from Catharanthus roseus. J. Supercrit. Fluids 2017, 129, 9–15. [Google Scholar] [CrossRef]
- Conde-Hernández, L.A.; Espinosa-Victoria, J.R.; Guerrero-Beltrán, J.Á. Supercritical extraction of essential oils of Piper auritum and Porophyllum ruderale. J. Supercrit. Fluids 2017, 127, 97–102. [Google Scholar] [CrossRef]
- Favareto, R.; Teixeira, M.B.; Soares, F.A.L.; Belisário, C.M.; Corazza, M.L.; Cardozo-Filho, L. Study of the supercritical extraction of Pterodon fruits (Fabaceae). J. Supercrit. Fluids 2017, 128, 159–165. [Google Scholar] [CrossRef]
- Ouédraogo, J.C.W.; Dicko, C.; Kini, F.B.; Bonzi-Coulibaly, Y.L.; Dey, E.S. Enhanced extraction of flavonoids from Odontonema strictum leaves with antioxidant activity using supercritical carbon dioxide fluid combined with ethanol. J. Supercrit. Fluids 2018, 131, 66–71. [Google Scholar] [CrossRef]
- Pimentel-Moral, S.; Borrás-Linares, I.; Lozano-Sánchez, J.; Arráez-Román, D.; Martínez-Férez, A.; Segura-Carretero, A. Supercritical CO2 extraction of bioactive compounds from Hibiscus sabdariffa. J. Supercrit. Fluids 2019, 147, 213–221. [Google Scholar] [CrossRef]
- Vasudev, H.; Singh, G.; Bansal, A.; Vardhan, S.; Thakur, L. Microwave heating and its applications in surface engineering: A review. Mater. Res. Express 2019, 6, 102001. [Google Scholar] [CrossRef]
- Sun, J.; Wang, W.; Yue, Q. Review on Microwave-Matter Interaction Fundamentals and Efficient Microwave-Associated Heating Strategies. Materials 2016, 9, 231. [Google Scholar] [CrossRef] [PubMed]
- Ekezie, F.-G.C.; Sun, D.-W.; Cheng, J.-H. Acceleration of microwave-assisted extraction processes of food components by integrating technologies and applying emerging solvents: A review of latest developments. Trends Food Sci. Technol. 2017, 67, 160–172. [Google Scholar] [CrossRef]
- Baydar, N.G.; Özkan, G.; Yaşar, S. Evaluation of the antiradical and antioxidant potential of grape extracts. Food Control 2007, 18, 1131–1136. [Google Scholar] [CrossRef]
- Rostagno, M.A.; Palma, M.; Barroso, C.G. Microwave assisted extraction of soy isoflavones. Anal. Chim. Acta 2007, 588, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xie, M.-Y.; Gong, X.-F. Microwave-assisted extraction used for the isolation of total triterpenoid saponins from Ganoderma atrum. J. Food Eng. 2007, 81, 162–170. [Google Scholar] [CrossRef]
- Raman, G.; Gaikar, V.G. Microwave-assisted extraction of piperine from Piper nigrum. Ind. Eng. Chem. Res. 2002, 41, 2521–2528. [Google Scholar] [CrossRef]
- Kiss, G.A.C.; Forgacs, E.; Cserháti, T.; Mota, T.; Morais, H.; Ramos, A. Optimisation of the microwave-assisted extraction of pigments from paprika (Capsicum annuum L.) powders. J. Chromatogr. A 2000, 889, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Ávila, N.; Priego-Capote, F.; Ruiz-Jiménez, J.; de Castro, M.L. Fast and selective determination of triterpenic compounds in olive leaves by liquid chromatography–tandem mass spectrometry with multiple reaction monitoring after microwave-assisted extraction. Talanta 2009, 78, 40–48. [Google Scholar] [CrossRef]
- Srinivas, Y.; Mathew, S.M.; Kothakota, A.; Sagarika, N.; Pandiselvam, R. Microwave assisted fluidized bed drying of nutmeg mace for essential oil enriched extracts: An assessment of drying kinetics, process optimization and quality. Innov. Food Sci. Emerg. Technol. 2020, 66, 102541. [Google Scholar] [CrossRef]
- Sagarika, N.; Prince, M.; Kothakota, A.; Pandiselvam, R.; Sreeja, R.; Mathew, S.M. Characterization and optimization of microwave assisted process for extraction of nutmeg (Myristica fragrans Houtt.) mace essential oil. J. Essent. Oil Bear. Plants 2018, 21, 895–904. [Google Scholar] [CrossRef]
- Lal, A.N.; Prince, M.; Kothakota, A.; Pandiselvam, R.; Thirumdas, R.; Mahanti, N.K.; Sreeja, R. Pulsed electric field combined with microwave-assisted extraction of pectin polysaccharide from jackfruit waste. Innov. Food Sci. Emerg. Technol. 2021, 74, 102844. [Google Scholar] [CrossRef]
- Danalache, F.; Mata, P.; Alves, V.D.; Moldão-Martins, M. Enzyme-assisted extraction of fruit juices. In Fruit Juices; Elsevier: Amsterdam, The Netherlands, 2018; pp. 183–200. [Google Scholar]
- Stambuk, P.; Tomaskovic, D.; Tomaz, I.; Maslov, L.; Stupic, D.; Karoglan Kontic, J. Application of pectinases for recovery of grape seeds phenolics. 3 Biotech 2016, 6, 224. [Google Scholar] [CrossRef] [PubMed]
- Huynh, N.T.; Smagghe, G.; Gonzales, G.B.; Van Camp, J.; Raes, K. Enzyme-assisted extraction enhancing the phenolic release from cauliflower (Brassica oleracea L. var. botrytis) outer leaves. J. Agric. Food Chem. 2014, 62, 7468–7476. [Google Scholar] [CrossRef]
- Paolucci-Jeanjean, D.; Belleville, M.-P.; Zakhia, N.; Rios, G.M. Kinetics of cassava starch hydrolysis with Termamyl® enzyme. Biotechnol. Bioeng. 2000, 68, 71–77. [Google Scholar] [CrossRef]
- Dal Magro, L.; Dalagnol, L.M.G.; Manfroi, V.; Hertz, P.F.; Klein, M.P.; Rodrigues, R.C. Synergistic effects of Pectinex Ultra Clear and Lallzyme Beta on yield and bioactive compounds extraction of Concord grape juice. LWT Food Sci. Technol. 2016, 72, 157–165. [Google Scholar] [CrossRef]
- Swer, T.L.; Chauhan, K.; Paul, P.K.; Mukhim, C. Evaluation of enzyme treatment conditions on extraction of anthocyanins from Prunus nepalensis L. Int. J. Biol. Macromol. 2016, 92, 867–871. [Google Scholar] [CrossRef]
- Pinelo, M.; Zornoza, B.; Meyer, A.S. Selective release of phenols from apple skin: Mass transfer kinetics during solvent and enzyme-assisted extraction. Sep. Purif. Technol. 2008, 63, 620–627. [Google Scholar] [CrossRef]
- Manasa, D.; Srinivas, P.; Sowbhagya, H. Enzyme-assisted extraction of bioactive compounds from ginger (Zingiber officinale Roscoe). Food Chem. 2013, 139, 509–514. [Google Scholar]
- Benucci, I.; Segade, S.R.; Cerreti, M.; Giacosa, S.; Paissoni, M.A.; Liburdi, K.; Bautista-Ortín, A.B.; Gómez-Plaza, E.; Gerbi, V.; Esti, M. Application of enzyme preparations for extraction of berry skin phenolics in withered winegrapes. Food Chem. 2017, 237, 756–765. [Google Scholar] [CrossRef]
- Hardouin, K.; Bedoux, G.; Burlot, A.-S.; Donnay-Moreno, C.; Bergé, J.-P.; Nyvall-Collén, P.; Bourgougnon, N. Enzyme-assisted extraction (EAE) for the production of antiviral and antioxidant extracts from the green seaweed Ulva armoricana (Ulvales, Ulvophyceae). Algal Res. 2016, 16, 233–239. [Google Scholar] [CrossRef]
- Teixeira, C.B.; Macedo, G.A.; Macedo, J.A.; da Silva, L.H.M.; Rodrigues, A.M.D.C. Simultaneous extraction of oil and antioxidant compounds from oil palm fruit (Elaeis guineensis) by an aqueous enzymatic process. Bioresour. Technol. 2013, 129, 575–581. [Google Scholar] [CrossRef]
- Niu, D.; Zeng, X.A.; Ren, E.F.; Xu, F.Y.; Li, J.; Wang, M.S.; Wang, R. Review of the application of pulsed electric fields (PEF) technology for food processing in China. Food Res. Int. 2020, 137, 109715. [Google Scholar] [CrossRef]
- Huang, H.-W.; Hsu, C.-P.; Yang, B.B.; Wang, C.-Y. Advances in the extraction of natural ingredients by high pressure extraction technology. Trends Food Sci. Technol. 2013, 33, 54–62. [Google Scholar] [CrossRef]
- del Castillo, M.D.; Iriondo-DeHond, A.; Martinez-Saez, N.; Fernandez-Gomez, B.; Iriondo-DeHond, M.; Zhou, J.-R. Applications of recovered compounds in food products. In Handbook of Coffee Processing By-Products; Elsevier: Amsterdam, The Netherlands, 2017; pp. 171–194. [Google Scholar]
- Guil-Guerrero, J.L.; Ramos, L.; Moreno, C.; Zúñiga-Paredes, J.; Carlosama-Yepez, M.; Ruales, P. Plant Foods By-Products as Sources of Health-Promoting Agents for Animal Production: A Review Focusing on the Tropics. Agron. J. 2016, 108, 1759–1774. [Google Scholar] [CrossRef]
- Zeb, A. Concept, mechanism, and applications of phenolic antioxidants in foods. J. Food Biochem. 2020, 44, e13394. [Google Scholar] [CrossRef] [PubMed]
- Duda-Chodak, A.; Tarko, T. Antioxidant properties of different fruit seeds and peels. Acta Sci. Pol. Technol. Aliment. 2007, 6, 29–36. [Google Scholar]
- Panwar, D.; Panesar, P.S.; Chopra, H.K. Recent trends on the valorization strategies for the management of citrus by-products. Food Rev. Int. 2021, 37, 91–120. [Google Scholar] [CrossRef]
- Antonic, B.; Jancikova, S.; Dordevic, D.; Tremlova, B. Apple pomace as food fortification ingredient: A systematic review and meta-analysis. J. Food Sci. 2020, 85, 2977–2985. [Google Scholar] [CrossRef]
- Peschel, W.; Sánchez-Rabaneda, F.; Diekmann, W.; Plescher, A.; Gartzía, I.; Jiménez, D.; Lamuela-Raventos, R.; Buxaderas, S.; Codina, C. An industrial approach in the search of natural antioxidants from vegetable and fruit wastes. Food Chem. 2006, 97, 137–150. [Google Scholar] [CrossRef]
- Sharma, K.; Mahato, N.; Cho, M.H.; Lee, Y.R. Converting citrus wastes into value-added products: Economic and environmently friendly approaches. Nutrition 2017, 34, 29–46. [Google Scholar] [CrossRef]
- Benavente-Garcia, O.; Castillo, J. Update on uses and properties of citrus flavonoids: New findings in anticancer, cardiovascular, and anti-inflammatory activity. J. Agric. Food Chem. 2008, 56, 6185–6205. [Google Scholar] [CrossRef]
- Chen, X.-M.; Tait, A.R.; Kitts, D.D. Flavonoid composition of orange peel and its association with antioxidant and anti-inflammatory activities. Food Chem. 2017, 218, 15–21. [Google Scholar] [CrossRef]
- Xi, W.; Lu, J.; Qun, J.; Jiao, B. Characterization of phenolic profile and antioxidant capacity of different fruit part from lemon (Citrus limon Burm.) cultivars. J. Food Sci. Technol. 2017, 54, 1108–1118. [Google Scholar] [CrossRef] [PubMed]
- Jamal, P.; Akbar, I.; Jaswir, I.; Zuhanis, Y. Quantification of Total Phenolic compounds in Papaya fruit peel. Pertanika J. Trop. Agric. Sci. 2017, 40. [Google Scholar]
- Sultana, B.; Hussain, Z.; Asif, M.; Munir, A. Investigation on the antioxidant activity of leaves, peels, stems bark, and kernel of mango (Mangifera indica L.). J. Food Sci. 2012, 77, C849–C852. [Google Scholar] [CrossRef] [PubMed]
- Okino Delgado, C.H.; Fleuri, L.F. Orange and mango by-products: Agro-industrial waste as source of bioactive compounds and botanical versus commercial description—A review. Food Rev. Int. 2016, 32, 1–14. [Google Scholar] [CrossRef]
- Li, Y.; Guo, C.; Yang, J.; Wei, J.; Xu, J.; Cheng, S. Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Food Chem. 2006, 96, 254–260. [Google Scholar] [CrossRef]
- Palanisamy, U.; Cheng, H.M.; Masilamani, T.; Subramaniam, T.; Ling, L.T.; Radhakrishnan, A.K. Rind of the rambutan, Nephelium lappaceum, a potential source of natural antioxidants. Food Chem. 2008, 109, 54–63. [Google Scholar] [CrossRef]
- Lee, F.Y.; Vo, G.T.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A. Mango rejects and mango waste: Characterization and quantification of phenolic compounds and their antioxidant potential. J. Food Process. Preserv. 2021, 45, e15618. [Google Scholar] [CrossRef]
- Adnan, L.; Osman, A.; Abdul Hamid, A. Antioxidant activity of different extracts of red pitaya (Hylocereus polyrhizus) seed. Int. J. Food Prop. 2011, 14, 1171–1181. [Google Scholar] [CrossRef]
- Kim, H.; Moon, J.Y.; Kim, H.; Lee, D.-S.; Cho, M.; Choi, H.-K.; Kim, Y.S.; Mosaddik, A.; Cho, S.K. Antioxidant and antiproliferative activities of mango (Mangifera indica L.) flesh and peel. Food Chem. 2010, 121, 429–436. [Google Scholar] [CrossRef]
- Marina, Z.; Noriham, A. Quantification of total phenolic compound and in vitro antioxidant potential of fruit peel extracts. Int. Food Res. J. 2014, 21, 1925–1929. [Google Scholar]
- Fakhlaei, R.; Khoo, H.E.; Chiaw Mei Sia, W.; Yim, H.S. Extraction And Evaluation of Antioxidant Potential in Rambutan Rind as Food Waste. Carpathian J. Food Sci. Technol. 2015, 7, 35–44. [Google Scholar]
- Jeon, G.; Choi, Y.; LEE, S.M.; Kim, Y.; Oh, M.; JEONG, H.S.; Lee, J. Antioxidant and antiproliferative properties of hot pepper (Capsicum annuum L.) seeds. J. Food Biochem. 2012, 36, 595–603. [Google Scholar] [CrossRef]
- Coman, M.M.; Oancea, A.M.; Verdenelli, M.C.; Cecchini, C.; Bahrim, G.E.; Orpianesi, C.; Cresci, A.; Silvi, S. Polyphenol content and in vitro evaluation of antioxidant, antimicrobial and prebiotic properties of red fruit extracts. Eur. Food Res. Technol. 2018, 244, 735–745. [Google Scholar] [CrossRef]
- Jiménez-Moreno, N.; Esparza, I.; Bimbela, F.; Gandía, L.M.; Ancín-Azpilicueta, C. Valorization of selected fruit and vegetable wastes as bioactive compounds: Opportunities and challenges. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2061–2108. [Google Scholar] [CrossRef]
- Chen, Z.-T.; Chu, H.-L.; Chyau, C.-C.; Chu, C.-C.; Duh, P.-D. Protective effects of sweet orange (Citrus sinensis) peel and their bioactive compounds on oxidative stress. Food Chem. 2012, 135, 2119–2127. [Google Scholar] [CrossRef]
- Sanz-Puig, M.; Pina-Pérez, M.C.; Saenz, J.; Marañón, I.; Rodrigo, D.; Martínez-López, A. Effect of polyphenol content on the antimicrobial activity of natural extracts from agro-industrial by-products. J. Food Saf. Food Qual. Arch. 2015, 66, 24. [Google Scholar]
- Gunwantrao, B.B.; Bhausaheb, S.K.; Ramrao, B.S.; Subhash, K.S. Antimicrobial activity and phytochemical analysis of orange (Citrus aurantium L.) and pineapple (Ananas comosus (L.) Merr.) peel extract. Ann. Phytomedicine 2016, 5, 156–160. [Google Scholar] [CrossRef]
- Xu, C.; Yagiz, Y.; Hsu, W.-Y.; Simonne, A.; Lu, J.; Marshall, M.R. Antioxidant, antibacterial, and antibiofilm properties of polyphenols from muscadine grape (Vitis rotundifolia Michx.) pomace against selected foodborne pathogens. J. Agric. Food Chem. 2014, 62, 6640–6649. [Google Scholar] [CrossRef]
- Mordi, R.; Fadiaro, A.; Owoeye, T.; Olanrewaju, I.; Uzoamaka, G.; Olorunshola, S. Identification by GC-MS of the components of oils of banana peels extract, phytochemical and antimicrobial analyses. Res. J. Phytochem. 2016, 10, 39–44. [Google Scholar] [CrossRef]
- Mutua, J.K.; Imathiu, S.; Owino, W. Evaluation of the proximate composition, antioxidant potential, and antimicrobial activity of mango seed kernel extracts. Food Sci. Nutr. 2017, 5, 349–357. [Google Scholar] [CrossRef]
- Rauha, J.-P.; Remes, S.; Heinonen, M.; Hopia, A.; Kähkönen, M.; Kujala, T.; Pihlaja, K.; Vuorela, H.; Vuorela, P. Antimicrobial effects of Finnish plant extracts containing flavonoids and other phenolic compounds. Int. J. Food Microbiol. 2000, 56, 3–12. [Google Scholar] [CrossRef]
- Asif, A.; Farooq, U.; Akram, K.; Hayat, Z.; Shafi, A.; Sarfraz, F.; Sidhu, M.A.I.; Rehman, H.-U.; Aftab, S. Therapeutic potentials of bioactive compounds from mango fruit wastes. Trends Food Sci. Technol. 2016, 53, 102–112. [Google Scholar] [CrossRef]
- Aboul-Enein, A.M.; Salama, Z.A.; Gaafar, A.A.; Aly, H.F.; Abou-Elella, F.; Ahmed, H. Identification of phenolic compounds from banana peel (Musa paradaisica L.) as antioxidant and antimicrobial agents. J. Chem. Pharm. Res. 2016, 8, 46–55. [Google Scholar]
- Kanatt, S.R.; Chander, R.; Sharma, A. Antioxidant and antimicrobial activity of pomegranate peel extract improves the shelf life of chicken products. Int. J. Food Sci. Technol. 2010, 45, 216–222. [Google Scholar] [CrossRef]
- Trošt, K.; Klančnik, A.; Mozetič Vodopivec, B.; Sternad Lemut, M.; Jug Novšak, K.; Raspor, P.; Smole Možina, S. Polyphenol, antioxidant and antimicrobial potential of six different white and red wine grape processing leftovers. J. Sci. Food Agric. 2016, 96, 4809–4820. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Saeed, M.T. Potential application of waste fruit peels (orange, yellow lemon and banana) as wide range natural antimicrobial agent. J. King Saud Univ. -Sci. 2020, 32, 805–810. [Google Scholar] [CrossRef]
- Fattouch, S.; Caboni, P.; Coroneo, V.; Tuberoso, C.; Angioni, A.; Dessi, S.; Marzouki, N.; Cabras, P. Comparative analysis of polyphenolic profiles and antioxidant and antimicrobial activities of Tunisian pome fruit pulp and peel aqueous acetone extracts. J. Agric. Food Chem. 2008, 56, 1084–1090. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Tian, L. Diverse phytochemicals and bioactivities in the ancient fruit and modern functional food pomegranate (Punica granatum). Molecules 2017, 22, 1606. [Google Scholar] [CrossRef] [PubMed]
- Palazzolo, E.; Laudicina, V.A.; Germanà, M.A. Current and potential use of citrus essential oils. Curr. Org. Chem. 2013, 17, 3042–3049. [Google Scholar] [CrossRef]
- Calvano, C.D.; Tamborrino, A. Valorization of Olive By-Products: Innovative Strategies for Their Production, Treatment and Characterization. Foods 2022, 11, 768. [Google Scholar] [CrossRef]
- Gomez-Mejia, E.; Rosales-Conrado, N.; Leon-Gonzalez, M.E.; Madrid, Y. Citrus peels waste as a source of value-added compounds: Extraction and quantification of bioactive polyphenols. Food Chem. 2019, 295, 289–299. [Google Scholar] [CrossRef]
- Mahato, N.; Sharma, K.; Sinha, M.; Cho, M.H. Citrus waste derived nutra-/pharmaceuticals for health benefits: Current trends and future perspectives. J. Funct. Foods 2018, 40, 307–316. [Google Scholar] [CrossRef]
- Multari, S.; Licciardello, C.; Caruso, M.; Martens, S. Monitoring the changes in phenolic compounds and carotenoids occurring during fruit development in the tissues of four citrus fruits. Food Res. Int. 2020, 134, 109228. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic composition, antioxidant potential and health benefits of citrus peel. Food Res. Int. 2020, 132, 109114. [Google Scholar] [CrossRef] [PubMed]
- Kuo, P.C.; Liao, Y.R.; Hung, H.Y.; Chuang, C.W.; Hwang, T.L.; Huang, S.C.; Shiao, Y.J.; Kuo, D.H.; Wu, T.S. Anti-Inflammatory and Neuroprotective Constituents from the Peels of Citrus grandis. Molecules 2017, 226, 967. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Silva, A.M.; Nunes, F.M. Citrus reticulata Blanco peels as a source of antioxidant and anti-proliferative phenolic compounds. Ind. Crops Prod. 2018, 111, 141–148. [Google Scholar] [CrossRef]
- Ruviaro, A.R.; Barbosa, P.d.P.M.; Martins, I.M.; de Ávila, A.R.A.; Nakajima, V.M.; Dos Prazeres, A.R.; Macedo, J.A.; Macedo, G.A. Flavanones biotransformation of citrus by-products improves antioxidant and ACE inhibitory activities in vitro. Food Biosci. 2020, 38, 100787. [Google Scholar] [CrossRef]
- Iqbal, A.; Schulz, P.; Rizvi, S.S.H. Valorization of bioactive compounds in fruit pomace from agro-fruit industries: Present Insights and future challenges. Food Biosci. 2021, 44, 101384. [Google Scholar] [CrossRef]
- Reis, S.F.; Rai, D.K.; Abu-Ghannam, N. Apple pomace as a potential ingredient for the development of new functional foods. Int. J. Food Sci. Technol. 2014, 49, 1743–1750. [Google Scholar] [CrossRef]
- Bandyopadhyay, K.; Chakraborty, C.; Bhattacharyya, S. Fortification of mango peel and kernel powder in cookies formulation. J. Acad. Ind. Res. 2014, 2, 661–664. [Google Scholar]
- Younis, K.; Ahmad, S. Quality evaluation of buffalo meat patties incorporated with apple pomace powder. Buffalo Bull. 2018, 37, 389–401. [Google Scholar]
- Younis, K.; Ahmad, S.; Ahmad, S. Waste utilization of apple pomace as a source of functional ingredient in buffalo meat sausage. Cogent Food Agric. 2015, 1, 1119397. [Google Scholar] [CrossRef]
- Wang, X.; Kristo, E.; LaPointe, G. The effect of apple pomace on the texture, rheology and microstructure of set type yogurt. Food Hydrocoll. 2019, 91, 83–91. [Google Scholar] [CrossRef]
- Wang, J.; Mukhtar, H.; Ma, L.; Pang, Q.; Wang, X. VHH Antibodies: Reagents for Mycotoxin Detection in Food Products. Sensor 2018, 18, 485. [Google Scholar] [CrossRef]
- Soncu, E.D.; Kolsarici, N.; Cicek, N.; Ozturk, G.S.; Akoglu, I.T.; Arici, Y.K. The Comparative Effect of Carrot and Lemon Fiber as a Fat Replacer on Physico-chemical, Textural, and Organoleptic Quality of Low-fat Beef Hamburger. Korean J. Food Sci. Anim. Resour. 2015, 35, 370–381. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Pan, T.; Wu, J.; Ren, F. The improvement effect and mechanism of citrus fiber on the water-binding ability of low-fat frankfurters. J. Food Sci. Technol. 2016, 53, 4197–4204. [Google Scholar] [CrossRef]
- Bhat, T.A.; Rather, A.H.; Hussain, S.Z.; Naseer, B.; Qadri, T.; Nazir, N. Efficacy of ascorbic acid, citric acid, ethylenediaminetetraacetic acid, and 4-hexylresorcinol as inhibitors of enzymatic browning in osmo-dehydrated fresh cut kiwis. J. Food Meas. Charact. 2021, 15, 4354–4370. [Google Scholar] [CrossRef]
- Grozdanovic, M.M.; Burazer, L.; Gavrovic-Jankulovic, M. Kiwifruit (Actinidia deliciosa) extract shows potential as a low-cost and efficient milk-clotting agent. Int. Dairy J. 2013, 32, 46–52. [Google Scholar] [CrossRef]
- Kim, D.H.; Jeong, D.; Kim, H.; Seo, K.H. Modern perspectives on the health benefits of kefir in next generation sequencing era: Improvement of the host gut microbiota. Crit. Rev. Food Sci. Nutr. 2019, 59, 1782–1793. [Google Scholar] [CrossRef]
- da Costa, M.R.; de Alencar, E.R.; Dos Santos Leandro, E.; Mendonca, M.A.; de Souza Ferreira, W.F. Characterization of the kefir beverage produced from yam (Colocasia esculenta L.), sesame seed (Sesamum indicum L.) and bean (Phaseolus vulgaris L.) extracts. J. Food Sci. Technol. 2018, 55, 4851–4858. [Google Scholar] [CrossRef]
- Selim, A.; Ismaael, O.H.; Abdel Bary, M. Influence of incorporation of orange juice by-product on the quality properties of sponge cake and low-fat beef burger. Food Sci. Technol 2019, 4, 860–887. [Google Scholar] [CrossRef]
- Parkar, S.G.; Simmons, L.; Herath, T.D.; Phipps, J.E.; Trower, T.M.; Hedderley, D.I.; McGhie, T.K.; Blatchford, P.; Ansell, J.; Sutton, K.H. Evaluation of the prebiotic potential of five kiwifruit cultivars after simulated gastrointestinal digestion and fermentation with human faecal bacteria. Int. J. Food Sci. Technol. 2018, 53, 1203–1210. [Google Scholar] [CrossRef]
- Martin, H.; Cordiner, S.B.; McGhie, T. Kiwifruit actinidin digests salivary amylase but not gastric lipase. Food Funct. 2017, 8, 3339–3345. [Google Scholar] [CrossRef]
- Qu, L.; Liu, Q.; Zhang, Q.; Liu, D.; Zhang, C.; Fan, D.; Deng, J.; Yang, H. Kiwifruit seed oil ameliorates inflammation and hepatic fat metabolism in high-fat diet-induced obese mice. J. Funct. Foods 2019, 52, 715–723. [Google Scholar] [CrossRef]
- Leontowicz, H.; Leontowicz, M.; Latocha, P.; Jesion, I.; Park, Y.-S.; Katrich, E.; Barasch, D.; Nemirovski, A.; Gorinstein, S. Bioactivity and nutritional properties of hardy kiwi fruit Actinidia arguta in comparison with Actinidia deliciosa ‘Hayward’ and Actinidia eriantha ‘Bidan’. Food Chem. 2016, 196, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Spanos, G.A.; Wrolstad, R.E. Phenolics of apple, pear, and white grape juices and their changes with processing and storage. A review. J. Agric. Food Chem. 1992, 40, 1478–1487. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Atanasova-Penichon, V.; Barreau, C.; Richard-Forget, F. Antioxidant secondary metabolites in cereals: Potential involvement in resistance to Fusarium and mycotoxin accumulation. Front. Microbiol. 2016, 7, 566. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- de Camargo, A.C.; Schwember, A.R.; Parada, R.; Garcia, S.; Marostica, M.R.J.; Franchin, M.; Regitano-d’Arce, M.A.B.; Shahidi, F. Opinion on the Hurdles and Potential Health Benefits in Value-Added Use of Plant Food Processing By-Products as Sources of Phenolic Compounds. Int. J. Mol. Sci. 2018, 19, 3498. [Google Scholar] [CrossRef]
- Middleton, E., Jr.; Kandaswami. Potential health-promoting properties of citrus flavonoids. J. Food Technol. 1994, 48, 115–119. [Google Scholar]
- Hollman, P.; Katan, M. Absorption, metabolism and health effects of dietary flavonoids in man. Biomed. Pharmacother. 1997, 51, 305–310. [Google Scholar] [CrossRef]
- He, J.; Giusti, M.M. Anthocyanins: Natural colorants with health-promoting properties. Annu. Rev. Food Sci. Technol 2010, 1, 163–187. [Google Scholar] [CrossRef] [PubMed]
- Weber, H.A.; Hodges, A.E.; Guthrie, J.R.; O’Brien, B.M.; Robaugh, D.; Clark, A.P.; Harris, R.K.; Algaier, J.W.; Smith, C.S. Comparison of proanthocyanidins in commercial antioxidants: Grape seed and pine bark extracts. J. Agric. Food Chem. 2007, 55, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Mielink, M.; Olsen, E.; Vogt, G.; Adeline, D.; Skrede, G. Grape seed extract as antioxidant in cook, cold store turkey meat. LWT 2006, 39, 191–198. [Google Scholar] [CrossRef]
- Rababah, T.M.; Hettiarachchy, N.S.; Horax, R. Total phenolics and antioxidant activities of fenugreek, green tea, black tea, grape seed, ginger, rosemary, gotu kola, and ginkgo extracts, vitamin E, and tert-butylhydroquinone. J. Agric. Food Chem. 2004, 52, 5183–5186. [Google Scholar] [CrossRef]
- Shirahigue, L.D.; Plata-Oviedo, M.; De Alencar, S.M.; D’Arce, M.A.B.R.; De Souza Vieira, T.M.F.; Oldoni, T.L.C.; Contreras-Castillo, C.J. Wine industry residue as antioxidant in cooked chicken meat. Int. J. Food Sci. Technol. 2010, 45, 863–870. [Google Scholar] [CrossRef]
- Brown, J.C.; Huang, G.; Haley-Zitlin, V.; Jiang, X. Antibacterial effects of grape extracts on Helicobacter pylori. Appl. Environ. Microbiol. 2009, 75, 848–852. [Google Scholar] [CrossRef] [PubMed]
- Baydar, N.G.; Özkan, G.; Sağdiç, O. Total phenolic contents and antibacterial activities of grape (Vitis vinifera L.) extracts. Food Control 2004, 15, 335–339. [Google Scholar] [CrossRef]
- Jayaprakash, G.; Selvi, T.; Sakariah, K. Antibacterial and antioxidant activities of grape (Vitis vinifera) seed extract. Food Res. Int. 2003, 36, 117–122. [Google Scholar] [CrossRef]
- Pastorkova, E.; Zakova, T.; Landa, P.; Novakova, J.; Vadlejch, J.; Kokoska. Growth inhibitory effect of grape phenolics against wine spoilage yeasts and acetic acid bacteria. Int. J. Food Microbiol. 2013, 161, 209–213. [Google Scholar] [CrossRef]
- Perumalla, A.; Hettiarachchy, N.S. Green tea and grape seed extracts—Potential applications in food safety and quality. Food Res. Int. 2011, 44, 827–839. [Google Scholar] [CrossRef]
- Katalinić, V.; Možina, S.S.; Skroza, D.; Generalić, I.; Abramovič, H.; Miloš, M.; Ljubenkov, I.; Piskernik, S.; Pezo, I.; Terpinc, P. Polyphenolic profile, antioxidant properties and antimicrobial activity of grape skin extracts of 14 Vitis vinifera varieties grown in Dalmatia (Croatia). Food Chem. 2010, 119, 715–723. [Google Scholar] [CrossRef]
- Filocamo, A.; Bisignano, C.; Mandalari, G.; Navarra, M. In vitro antimicrobial activity and effect on biofilm production of a white grape juice (Vitis vinifera) extract. Evid. -Based Complement. Altern. Med. 2015, 2015, 856243. [Google Scholar] [CrossRef]
- Silván, J.M.; Mingo, E.; Hidalgo, M.; de Pascual-Teresa, S.; Carrascosa, A.V.; Martinez-Rodriguez, A.J. Antibacterial activity of a grape seed extract and its fractions against Campylobacter spp. Food Control 2013, 29, 25–31. [Google Scholar] [CrossRef]
- Shan, B.; Cai, Y.-Z.; Brooks, J.D.; Corke, H. Potential application of spice and herb extracts as natural preservatives in cheese. J. Med. Food 2011, 14, 284–290. [Google Scholar] [CrossRef]
- Ahmad, M.M.; Iqbal, Z.; Anjum, F.M.; Sultan, J.I. Genetic variability to essential oil composition in four citrus fruit species. Pak. J. Bot. 2006, 38, 319. [Google Scholar]
- de Souza Pedrosa, G.T.; de Carvalho, R.J.; Berdejo, D.; de Souza, E.L.; Pagán, R.; Magnani, M. Control of autochthonous spoilage lactic acid bacteria in apple and orange juices by sensorially accepted doses of Citrus spp. essential oils combined with mild heat treatments. J. Food Sci. 2019, 84, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Jahurul, M.; Zaidul, I.; Ghafoor, K.; Al-Juhaimi, F.Y.; Nyam, K.-L.; Norulaini, N.; Sahena, F.; Omar, A.M. Mango (Mangifera indica L.) by-products and their valuable components: A review. Food Chem. 2015, 183, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Torres-León, C.; Rojas, R.; Contreras-Esquivel, J.C.; Serna-Cock, L.; Belmares-Cerda, R.E.; Aguilar, C.N. Mango seed: Functional and nutritional properties. Trends Food Sci. Technol. 2016, 55, 109–117. [Google Scholar] [CrossRef]
- Kabuki, T.; Nakajima, H.; Arai, M.; Ueda, S.; Kuwabara, Y.; Dosako, S.i. Characterization of novel antimicrobial compounds from mango (Mangifera indica L.) kernel seeds. Food Chem. 2000, 71, 61–66. [Google Scholar] [CrossRef]
- Kanatt, S.R.; Chawla, S. Shelf life extension of chicken packed in active film developed with mango peel extract. J. Food Saf. 2018, 38, e12385. [Google Scholar] [CrossRef]
- Hurtado-Fernandez, E.; Carrasco-Pancorbo, A.; Fernandez-Gutierrez, A.; Chemistry, F. Profiling LC-DAD-ESI-TOF MS method for the determination of phenolic metabolites from avocado (Persea americana). J. Agric. FoodChem. 2011, 59, 2255–2267. [Google Scholar] [CrossRef]
- Figueroa, J.G.; Borrás-Linares, I.; Lozano-Sánchez, J.; Segura-Carretero, A. Comprehensive identification of bioactive compounds of avocado peel by liquid chromatography coupled to ultra-high-definition accurate-mass Q-TOF. Food Chem. 2018, 245, 707–716. [Google Scholar] [CrossRef]
- Figueroa, J.G.; Borrás-Linares, I.; Lozano-Sánchez, J.; Segura-Carretero, A. Comprehensive characterization of phenolic and other polar compounds in the seed and seed coat of avocado by HPLC-DAD-ESI-QTOF-MS. Food Res. Int. 2018, 105, 752–763. [Google Scholar] [CrossRef]
- Raymond Chia, T.W.; Dykes, G.A. Antimicrobial activity of crude epicarp and seed extracts from mature avocado fruit (Persea americana) of three cultivars. Pharm. Biol. 2010, 48, 753–756. [Google Scholar] [CrossRef]
- Anna, B.; Vizma, N.; Dmitry, B. Anti-Helicobacter activity of certain food plant extracts and juices and their composition in vitro. Food Nutr. Sci. 2011, 2011, 868–877. [Google Scholar]
- Almeida, D.; Pinto, D.; Santos, J.; Vinha, A.F.; Palmeira, J.; Ferreira, H.N.; Rodrigues, F.; Oliveira, M.B.P. Hardy kiwifruit leaves (Actinidia arguta): An extraordinary source of value-added compounds for food industry. Food Chem. 2018, 259, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Obied, H.K.; Karuso, P.; Prenzler, P.D.; Robards, K. Novel secoiridoids with antioxidant activity from Australian olive mill waste. J. Agric. Food Chem. 2007, 55, 2848–2853. [Google Scholar] [CrossRef] [PubMed]
- Szabo, K.; Dulf, F.V.; Diaconeasa, Z.; Vodnar, D.C. Antimicrobial and antioxidant properties of tomato processing byproducts and their correlation with the biochemical composition. Lwt 2019, 116, 108558. [Google Scholar] [CrossRef]
- John, S.; Priyadarshini, S.; Monica, S.J.; Arumugam, P. Phytochemical profile and thin layer chromatographic studies of Daucus carota peel extracts. Int. J. Food Sci. Nutr. 2017, 2, 23–26. [Google Scholar]
- Hacke, A.C.M.; Granato, D.; Maciel, L.G.; Weinert, P.L.; Prado-Silva, L.d.; Alvarenga, V.O.; de Souza Sant’Ana, A.; Bataglion, G.A.; Eberlin, M.N.; Rosso, N.D. Jabuticaba (Myrciaria cauliflora) seeds: Chemical characterization and extraction of antioxidant and antimicrobial compounds. J. Food Sci. 2016, 81, C2206–C2217. [Google Scholar] [CrossRef] [PubMed]
- Terefe, N.S.; Matthies, K.; Simons, L.; Versteeg, C. Combined high pressure-mild temperature processing for optimal retention of physical and nutritional quality of strawberries (Fragaria× ananassa). Innov. Food Sci. Emerg. Technol. 2009, 10, 297–307. [Google Scholar] [CrossRef]
- Albuquerque, B.R.; Heleno, S.A.; Oliveira, M.B.P.; Barros, L.; Ferreira, I. Phenolic compounds: Current industrial applications, limitations and future challenges. Food Funct. 2021, 12, 14–29. [Google Scholar] [CrossRef]
| Technique | Advantages | Disadvantages | Bioactive Component | References |
|---|---|---|---|---|
| Maceration |
|
| Polyphenols, anthocyanins, flavonoids, and essential oils | [59,60,61] |
| Percolation |
|
| Alkaloids, Sterols, flavonoids, glycosides, saponins, phenols, lignins, sterols, and tannins | [62,63] |
| Decoction |
|
| Antioxidants and polyphenol | [64,65,66] |
| Reflux or solid–liquid extraction |
|
| Essential oils, flavonoids, and polyphenols | [25,67,68] |
| Soxhlet extraction |
|
| Phenolics, antioxidants, essential oils, and flavonoids | [69,70,71,72] |
| Supercritical fluid extraction (SFE) |
|
| Flavonoids, antioxidants, carotenoids, fatty acids, essential oils, terpenes, and polyphenols | [73] |
| Microwave-assisted extraction (MAE) |
|
| Phenolic compounds, glycosides, flavonoids, terpenoids, essential oils, alkaloids, and saponins | [74,75] |
| Enzyme-assisted extraction (EAE) |
|
| Anthocyanins, polyphenols, carotene, terpenes, and flavonoids | [76,77] |
| Pulsed electric field extraction (PEFE) |
|
| Phenols, flavonoids, proteins, anthocyanins, and carbohydrates | [78,79,80,81,82] |
| High-hydrostatic-pressure extraction |
|
| Phenolic compounds, carotenoids, flavonoids, pectin, lutein, lycopene, and catechin | [83] |
| Ultrasound-assistedextraction (UAE) |
|
| Phenolic compounds, flavonoids, oils, and anthocyanins | [58,84,85,86] |
| Sl No | Fruit Waste | Bioactive Compounds | Antioxidant Activity/Results | Reference |
|---|---|---|---|---|
| 1 | Mango waste | Catechin, epicatechin, andkaempferol. | A significant amount of these phenolic compounds contributes to the potential activity | [141] |
| 2 | Red pitaya seeds | Flavonoids and phenolic acids | The total phenolic content of the sample was found to be 13.56 ± 2.04 mg GAE/g dry weight | [142] |
| 3 | Pomegranate peels | Flavonoids and phenolic acids | Higher antioxidant activity in the peel than in the edible portions | [139] |
| 4 | Mango by-products | Phenolic acids, sterols, carotenoids, and tocopherols | A safer alternative to the synthetic antioxidants in biscuits, vegetable oils, and other different food formulations | [143] |
| 5 | Apple peel and seeds | Polyphenols and tannins | Superiority of bioactivity was observed in the case of peels compared with the seed portions | [130] |
| 6 | Citrus by-products | Flavonoids and phenolic acids | Depended on the species, cultivar, type of by-product, and harvesting conditions | [129] |
| 7 | Mango, papaya, and guava peels | Polyphenols | Antioxidant activities from the four assays indicated that mango peel extract possessed higher antioxidant properties. | [144] |
| 8 | Rambutan by-products | Phenolic acids and ellagitannins | Constituents contributed to the antioxidant potential of rinds | [145] |
| 9 | Pepper seed extracts | Capsaicin and di-hydrocapsaicin | Total polyphenolic content was 10.9 mg gallic acid equivalents/g residue | [146] |
| 10 | Plum, grapes, and elderberry fruit by-products | Anthocyanins | The highest values of 90.19 and 89.86% were attributed to elderberry fruit and Italian red grape extracts respectively | [147] |
| 11 | Pomegranate by-products | Flavonoids and condensed and hydrolyzabletannins | Bioactive compounds found in by-products have antioxidant properties that help protect cells from various stimuli-induced oxidative stresses and cell death | [148] |
| 12 | Orange by-products | Ascorbic acid, flavonoids, and phenylpropanoids | Flavonoids are an important subgroup exhibiting high antioxidant activity | [149] |
| 13 | Grape seeds | Phenolic acids and flavonoids | Higher polyphenol concentration and antioxidant potential of the sample when compared with bagasse extract | [103] |
| Sl No | Fruit Waste | Observation | Reference |
|---|---|---|---|
| 1 | Citrus essential oil | Antimicrobial activity against species such as Trichoderma viride, Cladosporium herbarum, and Aspergillus flavus | [138] |
| 2 | Plum, grapes, and elderberry fruit by-products | Constituted sizeable contents of anthocyanins and significantly inhibited the growth of B. cereus | [147] |
| 3 | Grape by-products | Antimicrobial activities of winemaking by-products were verified against foodborne pathogens, with the lowest MICs for Gram-positive bacteria and medium influences on the MICs of Gram-negative bacteria | [159] |
| 4 | Muscadine grapes | Muscadine polyphenols at 4 × minimum inhibitory concentration caused nearly a 5 log10 CFU/mL decrease in cell viability for S. aureus in 6 h with lysis | [152] |
| 5 | Banana peels | The antimicrobial potential was due to the presence of tannins and phenolics | [157] |
| 6 | Mango kernel extracts | Greater inhibition against S. aureas at various concentrations than against E. coli | [154] |
| 7 | Orange and pineapple peels | The pineapple sample showed the largest zone of inhibition against Klebsiella and the smallest against Bacillus subtilis | [151] |
| 8 | Pomegranate by-products | Peel extract displayed excellent antioxidant activity, while the seed extract did not have any substantial activity | [158] |
| 9 | Mandarin, broccoli, and orange by-products | All samples showed inhibitory effects against Salmonella spp., Escherichia coli, Bacillus cereus, and Listeria monocytogenes. | [150] |
| 10 | Orange, banana, and lemon peels | Effectiveness was found to be higher in yellow lemon, followed by orange and banana peels. Klebsiella spp. showed the highest sensitivity to the extract of yellow lemon peel and showed the largest zone of inhibition | [160] |
| 11 | Quince fruit peel | Effective against bacteria growth owing to flavonoid proportions in the peel in conjunction with chlorogenic acid | [161] |
| Food Waste/Bioactive Compound | Food Preservation Effect | Reference |
|---|---|---|
| Apple pomace | Inhibitory effect against pathogens Helicobacter pylori | [221] |
| Kiwi leaves (alcoholic and hydroalcoholic extracts) | Antimicrobial effect against S. aureus | [222] |
| Olive mill wastewater (phenols) | Antimicrobial action against E. coli, P. aeruginosa, S. aureus, and B. subtilis strains | [223] |
| Tomato wastes | Antimicrobial activity of tomato waste extracts against S. aureus correlated moderately with isochlorogenic acid content | [224] |
| Acetone and methanol carrot peel extracts | Growth inhibition of Shigella flexneri, E.coli, S. aureus, and Klebsiella pneumoniae | [225] |
| Jabuticaba seeds | Ellagitannins and ellagic acid in the extracts contained antimicrobial and antioxidant properties. | [226] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nirmal, N.P.; Khanashyam, A.C.; Mundanat, A.S.; Shah, K.; Babu, K.S.; Thorakkattu, P.; Al-Asmari, F.; Pandiselvam, R. Valorization of Fruit Waste for Bioactive Compounds and Their Applications in the Food Industry. Foods 2023, 12, 556. https://doi.org/10.3390/foods12030556
Nirmal NP, Khanashyam AC, Mundanat AS, Shah K, Babu KS, Thorakkattu P, Al-Asmari F, Pandiselvam R. Valorization of Fruit Waste for Bioactive Compounds and Their Applications in the Food Industry. Foods. 2023; 12(3):556. https://doi.org/10.3390/foods12030556
Chicago/Turabian StyleNirmal, Nilesh Prakash, Anandu Chandra Khanashyam, Anjaly Shanker Mundanat, Kartik Shah, Karthik Sajith Babu, Priyamvada Thorakkattu, Fahad Al-Asmari, and Ravi Pandiselvam. 2023. "Valorization of Fruit Waste for Bioactive Compounds and Their Applications in the Food Industry" Foods 12, no. 3: 556. https://doi.org/10.3390/foods12030556
APA StyleNirmal, N. P., Khanashyam, A. C., Mundanat, A. S., Shah, K., Babu, K. S., Thorakkattu, P., Al-Asmari, F., & Pandiselvam, R. (2023). Valorization of Fruit Waste for Bioactive Compounds and Their Applications in the Food Industry. Foods, 12(3), 556. https://doi.org/10.3390/foods12030556









