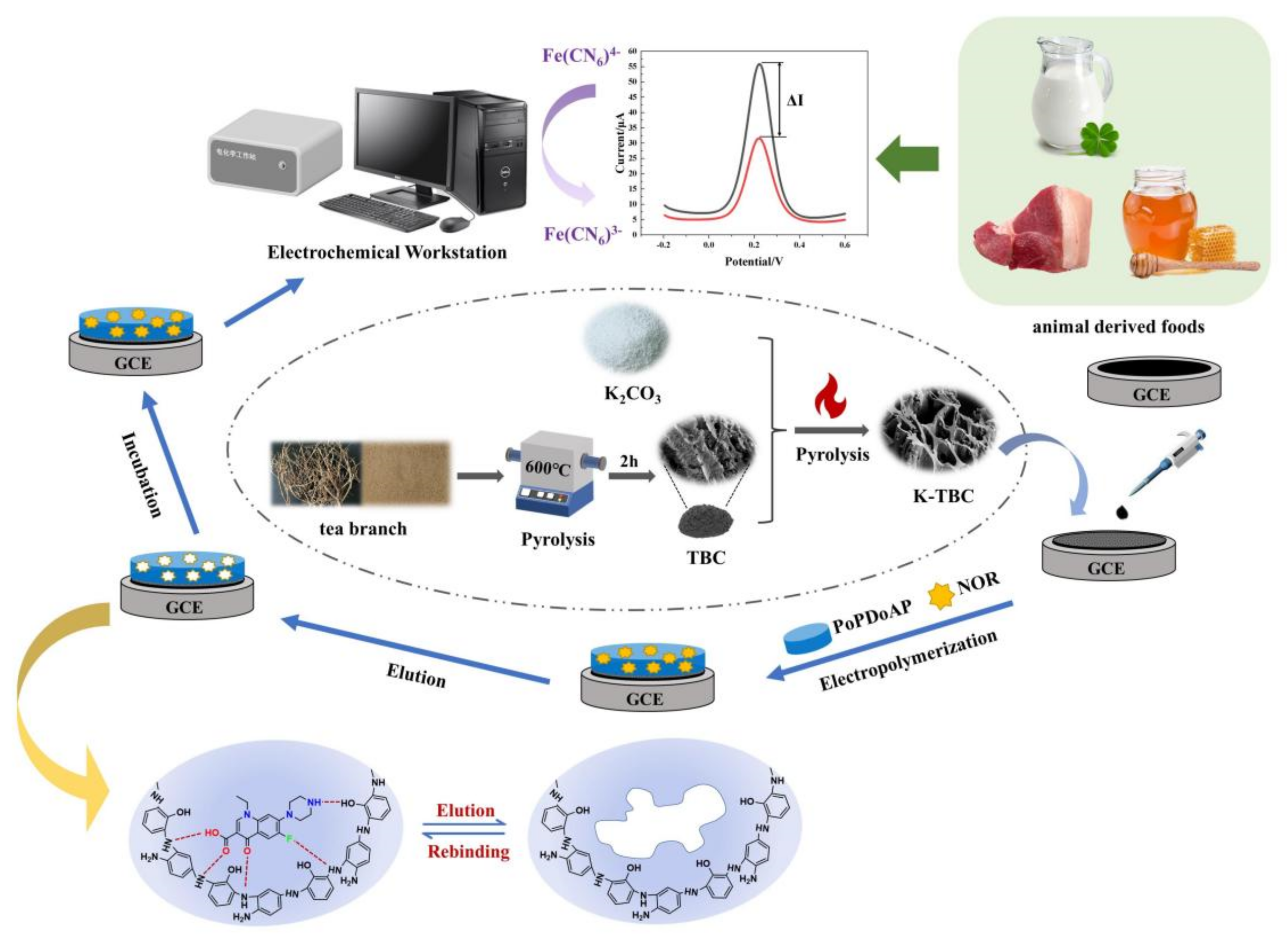
Construction of a Molecularly Imprinted Sensor Modified with Tea Branch Biochar and Its Rapid Detection of Norfloxacin Residues in Animal-Derived Foods
Abstract
1. Introduction
2. Materials and Methods
2.1. Apparatus and Reagents
2.2. Preparation of Tea Tree Branch Biochar
2.3. Preparation of the Biochar-Modified GCE
2.4. Preparation of the MIPs Electrochemical Sensor
2.5. Electrochemical Measurement and NOR Detection
2.6. Sample Preparation
2.7. Statistical Analysis
3. Results and Discussion
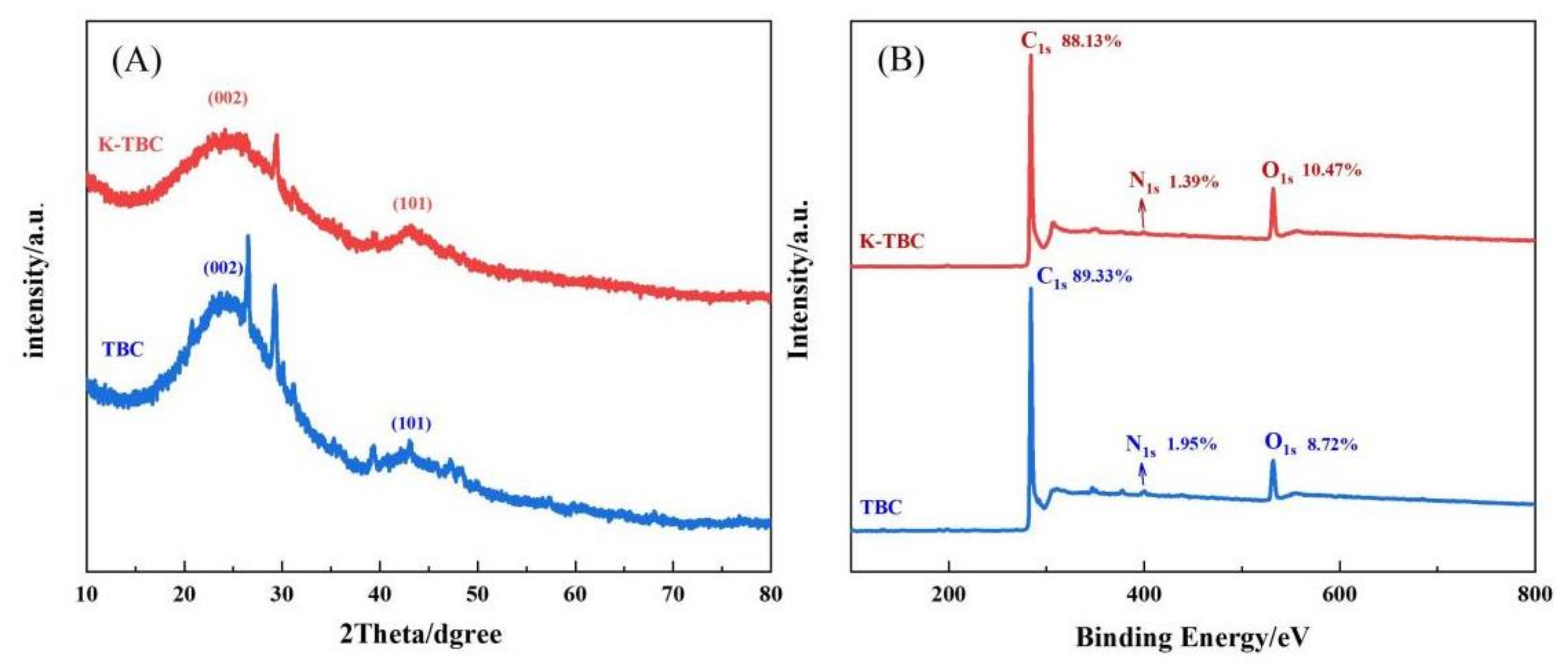
3.1. Characterization of Materials and Polymers
3.2. Characterization of Electrochemical Properties
3.3. Optimization of Experimental Conditions
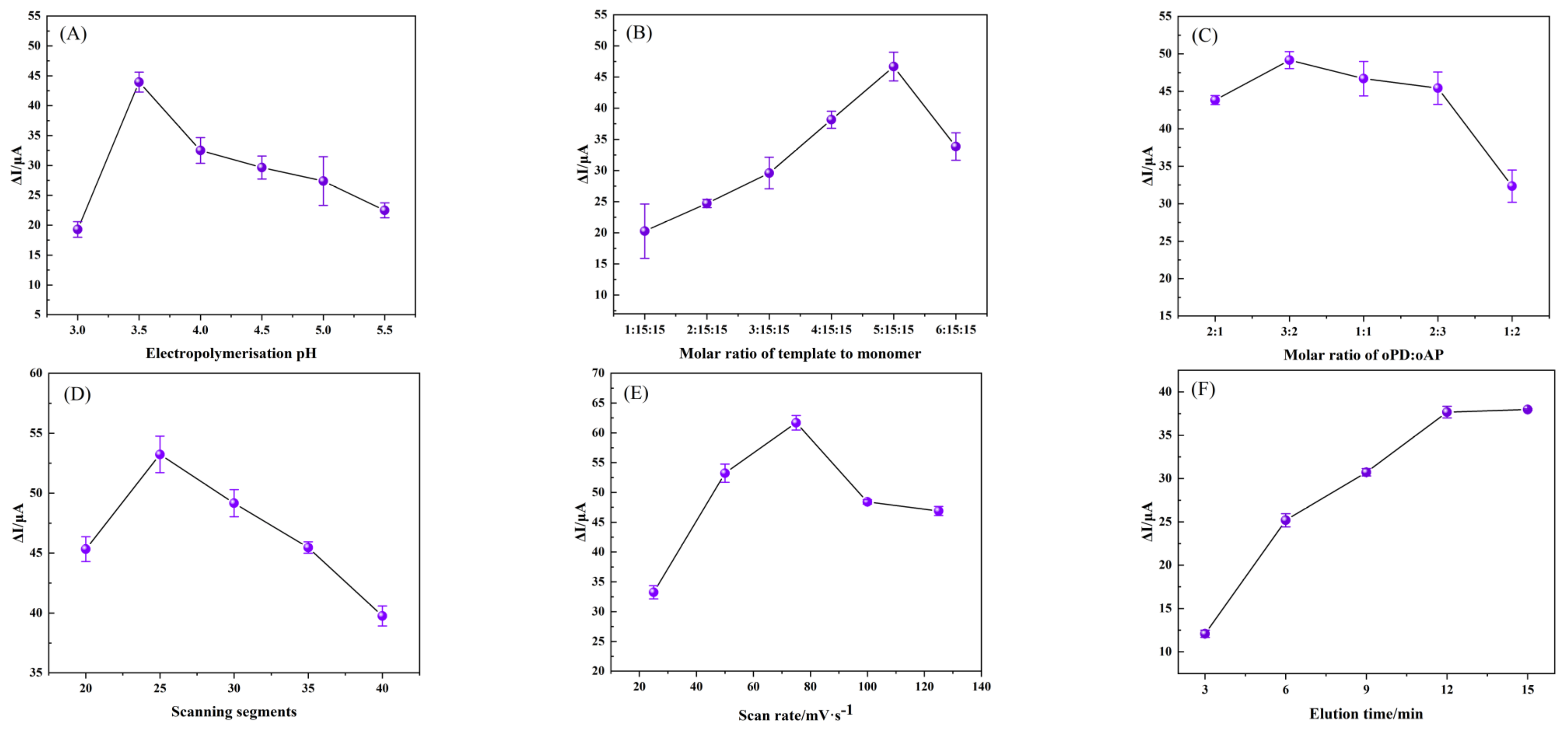
3.3.1. Optimization of Conditions for the Fabrication of the TBC/GCE
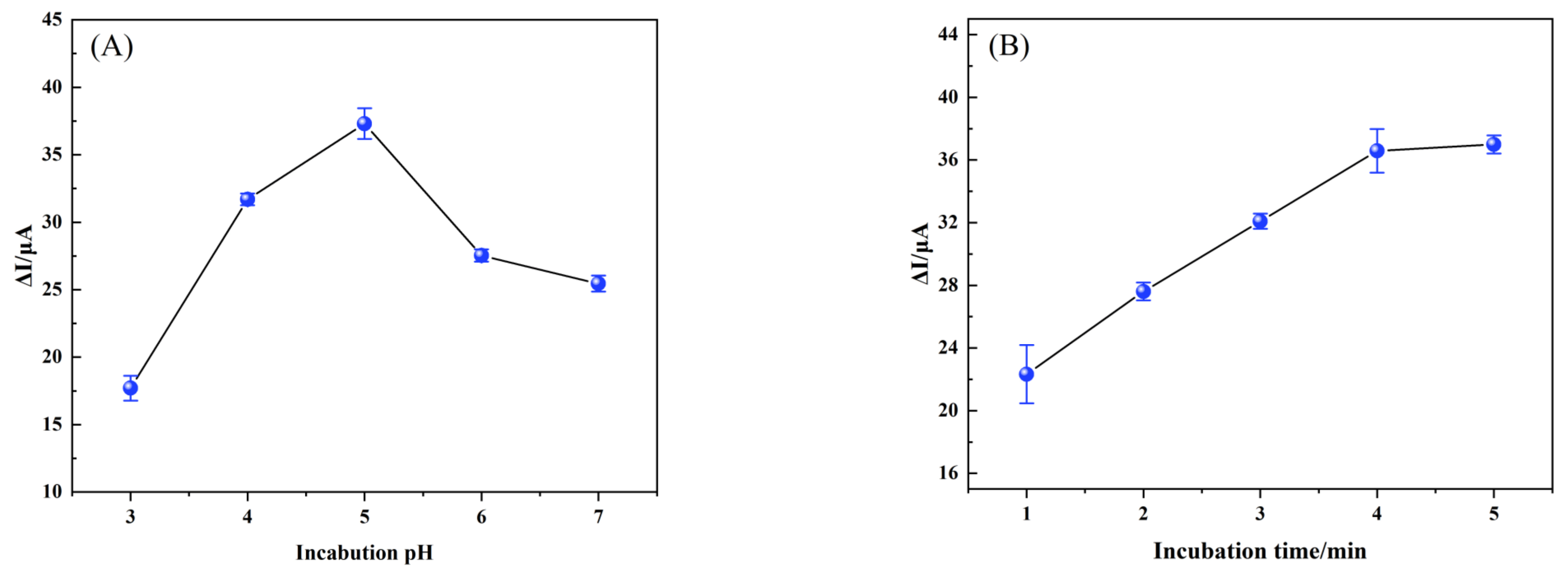
3.3.2. Optimization of Conditions for the Fabrication of the MIP/TBC/GCE
3.3.3. Optimization of Conditions for the Fabrication of the MIP/TBC/GCE
3.4. Study on the Properties of the MIP/TBC/GCE Sensor
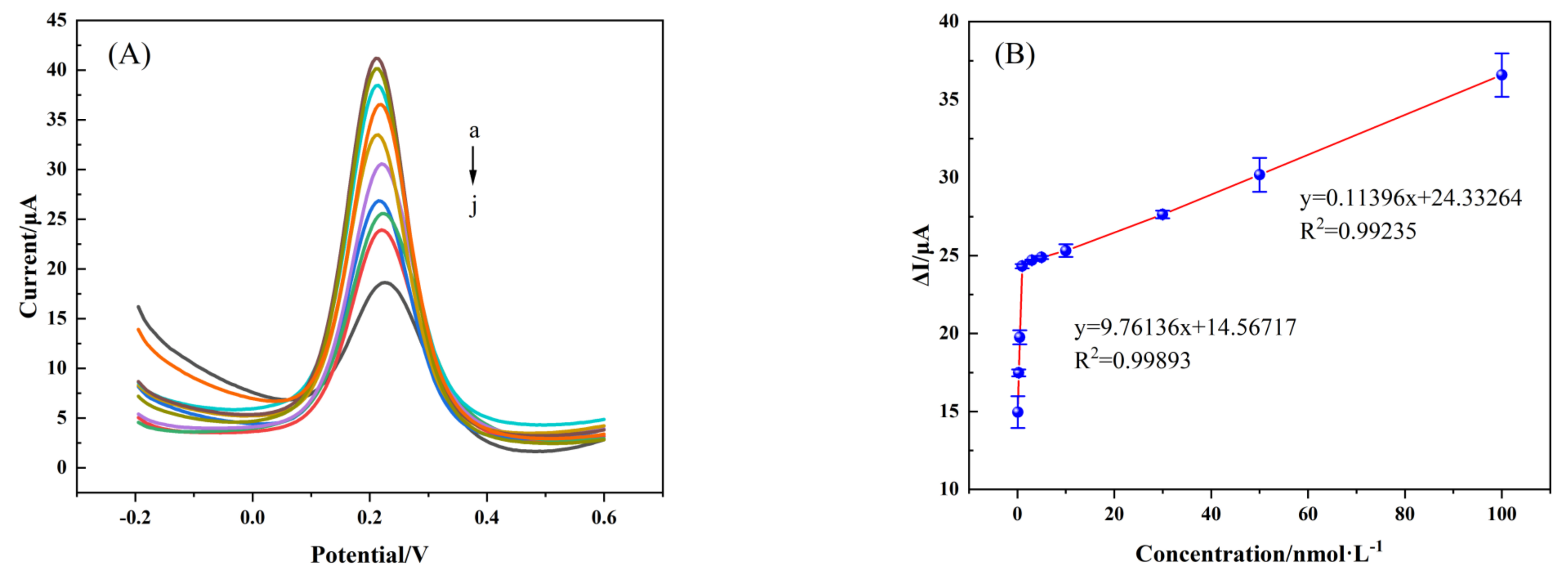
3.4.1. Linear Range and Limit of Detection
3.4.2. Selectivity, Reproducibility and Stability
3.4.3. Application of Real Sample Detection
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Norrby, S.R.; Jonsson, M. Antibacterial activity of norfloxacin. Antimicrob. Agents Chemother. 1983, 23, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Lucas, C.; Hérida, R.N.S. Review of Properties and Analytical Methods for the Determination of Norfloxacin. Crit. Rev. Anal. Chem. 2015, 46, 22–39. [Google Scholar] [CrossRef]
- Califf, R.M. Oral Fluoroquinolones. J. Am. Coll. Cardiol. 2019, 74, 1451–1453. [Google Scholar] [CrossRef]
- Ribeiro, N.V.; Melo, R.G.; Guerra, N.C.; Guerra, N.C.; Nobre, A.; Fernandes, R.M.; Pedro, L.M.; Costa, J.; Costa, F.J.; Caldeira, D. Fluoroquinolones are associated with increased risk of aortic aneurysm or dissection: Systematic review and meta-analysis. Semin. Thorac. Cardiovasc. Surg. 2021, 33, 907–918. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.L.; Mo, Y.M.; Wu, Z.Q.; Rad, S.; Song, X.H.; Zeng, H.H.; Bashir, S.; Kang, B.; Chen, Z.B. Occurrence, distribution, and health risk assessmentof quinolone antibiotics in watcr, sediment, and fish species of Qingshitan reservoir, South China. Sci. Rep. 2020, 10, 15777. [Google Scholar] [CrossRef]
- The Ministry of Agriculture of the People’s Republic of China. Bulletin of the Ministry of Agriculture of the People’s Republic of China; No. 2292; Ministry of Agriculture: Beijing, China, 2015. Available online: http://www.moa.gov.cn/govpublic/SYJ/201509/t20150907_4819267.htm (accessed on 26 October 2022).
- The Ministry of Agriculture of the People’s Republic of China. Circular on the Monitoring Results of Veterinary Drug Residues in Animals and Animal Products in 2020; No. 8; Ministry of Agriculture: Beijing, China, 2021. Available online: http://www.moa.gov.cn/govpublic/xmsyj/202101/t20210126_6360537.htm (accessed on 30 October 2022).
- Weng, R.; Sun, L.S.; Jiang, L.P.; Li, N.; Ruan, G.H.; Li, J.P.; Du, F.Y. Electrospun Graphene Oxide–Doped Nanofiber-Based Solid Phase Extraction Followed by High-Performance Liquid Chromatography for the Determination of Tetracycline Antibiotic Residues in Food Samples. Food Anal. Methods 2019, 12, 1594–1603. [Google Scholar] [CrossRef]
- Zou, W.; Wen, X.K.; Xia, C.H.; Nie, L.; Zhou, Q.; Chen, X.C.; Fang, C.Y.; Wang, Y.C.; Zhang, L. LC-Q-TOF-MS based plasma metabolomic profile of subclinical pelvic inflammatory disease: A pilot study. Clin. Chim. Acta 2018, 483, 164–169. [Google Scholar] [CrossRef]
- Kanda, M.; Kusano, T.; Kanai, S.; Hayashi, H.; Matushima, Y.; Nakajima, T.; Takeba, K.; Sasamoto, T.; Nagayma, T. Rapid Determination of Fluoroquinolone Residues in Honey by a Microbiological Screening Method and Liquid Chromatography. J. AOAC Int. 2010, 93, 1331–1339. [Google Scholar] [CrossRef]
- Moonsun, J.; Insook, R.P. Quantitative detection of tetracycline residues in honey by a simple sensitive immunoassay. Anal. Chim. Acta 2008, 626, 180–185. [Google Scholar] [CrossRef]
- Patrícia, B.D.; Romeu, C.R.; Orlando, F. A new and simple method for the simultaneous determination of amoxicillin and nimesulide using carbon black within a dihexadecylphosphate film as electrochemical sensor. Talanta 2018, 179, 115–123. [Google Scholar] [CrossRef]
- Manel, R.; Mabrouk, B.B.; Youssef, S. Electrochemical determination of levofloxacin antibiotic in biological samples using boron doped diamond electrode. J. Electroanal. Chem. 2017, 794, 175–181. [Google Scholar] [CrossRef]
- Ahmed, W.; Xu, T.W.; Mahmood, M.; Nunez-Delgado, A.; Ali, S.; Shakoor, A.; Qaswar, M.; Zhao, H.W.; Liu, W.J.; Li, W.D.; et al. Nano-hydroxyapatite modified biochar: Insights into the dynamic adsorption and performance of lead (II) removal from aqueous solution. Environ. Res. 2022, 214, 113827. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xu, L.; Zhang, Y.G.; Zhou, X.; Zhang, L.T.; Yasin, A.; Wang, L.L.; Zhi, K.K. Facile synthesis of bio-based nitrogen- and oxygen-doped porous carbon derived from cotton for supercapacitors. RSC Adv. 2018, 8, 3869–3877. [Google Scholar] [CrossRef]
- Kumar, A.; Upadhyay, S.N.; Mishra, P.K.; Mondal, M.K. Multivariable modeling, optimization and experimental study of Cr (VI) removal from aqueous solution using peanut shell biochar. Environ. Res. 2022, 215, 114287. [Google Scholar] [CrossRef]
- Zhou, J.Z.; Luo, A.R.; Zhao, Y.C. Preparation and characterisation of activated carbon from waste tea by physical activation using steam. J. Air Waste Manag. 2018, 68, 1269–1277. [Google Scholar] [CrossRef]
- Asadi-Sangachini, Z.; Galangash, M.M.; Younesi, H.; Nowrouzi, M. The feasibility of cost-effective manufacturing activated carbon derived from walnut shells for large-scale CO2 capture. Environ. Sci. Pollut. R. 2019, 26, 26542–26552. [Google Scholar] [CrossRef]
- Liang, Q.L.; Liu, Y.C.; Chen, M.Y.; Ma, L.L.; Yang, B.; Li, L.L.; Liu, Q. Optimized preparation of activated carbon from coconut shell and civil for municipal sludge. Mater. Chem. Phys. 2020, 241, 122327. [Google Scholar] [CrossRef]
- Han, C.; Wang, M.; Ren, Y.; Zhang, L.; Ji, Y.; Zhu, W.; Song, Y.; He, J. Characterization of pruned tea branch biochar and the mechanisms underlying its adsorption for cadmium in aqueous solution. RSC Adv. 2021, 11, 26832–26843. [Google Scholar] [CrossRef] [PubMed]
- Akgül, G.; Iglesias, D.; Ocon, P.; Jiménez, E.M. Valorization of Tea-Waste Biochar for Energy Storage. Bioenerg. Res. 2019, 12, 1012–1020. [Google Scholar] [CrossRef]
- Gui, R.; Hui, J.; Guo, H.; Wang, Z. Recent advances and future prospects in molecularly imprinted polymers-based electrochemical biosensors. Biosens. Bioelectron. 2018, 100, 56–70. [Google Scholar] [CrossRef]
- Singh, M.; Singh, S.; Singh, S.P.; Patel, S.S. Recent advancement of carbon nanomaterials engrained molecular imprinted polymer for environmental matrix. Trends Environ. Anal. Chem. 2020, 27, e00092. [Google Scholar] [CrossRef]
- Kong, Y.; Li, X.Y.; Ni, J.H.; Yao, C.; Chen, Z.D. Enantioselective recognition of glutamic acid enantiomers based on poly (aniline-co-m-aminophenol) electrode column. Electrochem. Commun. 2012, 14, 17–20. [Google Scholar] [CrossRef]
- Kong, Y.; Shan, X.; Ma, J.; Chen, M.; Chen, Z. A novel voltammetric sensor for ascorbic acid based on molecularly imprinted poly(o-phenylenediamine-co-o-aminophenol). Anal. Chim. Acta 2014, 809, 54–60. [Google Scholar] [CrossRef]
- Khumngern, S.; Thavarungkul, P.; Kanatharana, P.; Bejrananda, T.; Numnuam, A. Molecularly imprinted electrochemical sensor based on poly(o-phenylenediamine-co-o-aminophenol) incorporated with poly(styrenesulfonate) doped poly(3,4-ethylenedioxythiophene) ferrocene composite modified screen-printed carbon electrode for highly sensitive and selective detection of prostate cancer biomarker. Microchem. J. 2022, 177, 107311. [Google Scholar] [CrossRef]
- Pang, P.; Yan, F.; Chen, M.; Li, H.Y.; Zhang, Y.L.; Wang, H.B.; Wu, Z.; Yang, W.R. Promising biomass-derived activated carbon and gold nanoparticle nanocomposites as a novel electrode material for electrochemical detection of rutin. RSC Adv. 2016, 6, 90446–90454. [Google Scholar] [CrossRef]
- Ye, Y.; Jian, J.; Pi, F.; Yang, H.C.; Liu, J.; Zhang, Y.Z. A novel electrochemical biosensor for antioxidant evaluation of phloretin based on cell-alginate/-cysteine/gold nanoparticle-modified glassy carbon electrode. Biosens. Bioelectron. 2018, 119, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhao, N.; Tong, L.H.; Lv, Y.Z. Structural and adsorption characteristics of potassium carbonate activated biochar. RSC Adv. 2018, 8, 21012–21019. [Google Scholar] [CrossRef]
- Madhu, R.; Veeramani, V.; Chen, S.M. Heteroatom-enriched and renewable banana-stem-derived porous carbon for the electrochemical determination of nitrite in various water samples. Sci. Rep. 2014, 4, 4679. [Google Scholar] [CrossRef]
- Goswami, R.; Shim, J.; Deka, S.; Kumari, D.; Kataki, R.; Kumar, M. Characterization of cadmium removal from aqueous solution by biochar produced from Ipomoea fistulosa at different pyrolytic temperatures. Ecol. Eng. 2016, 97, 444–451. [Google Scholar] [CrossRef]
- Yang, F.; Sun, L.L.; Xie, W.L.; Jiang, Q.; Gao, Y.; Zhang, W.; Zhang, Y. Nitrogen-functionalization biochars derived from wheat straws via molten salt synthesis: An efficient adsorbent for atrazine removal. Sci. Total Environ. 2017, 607–608, 1391–1399. [Google Scholar] [CrossRef]
- Chen, L.F.; Zhang, X.D.; Liang, H.W.; Kong, M.G.; Guan, Q.F.; Chen, P.; Wu, Z.Y.; Yu, S.H. Synthesis of Nitrogen-Doped Porous Carbon Nanofibers as an Efficient Electrode Material for Supercapacitors. ACS Nano 2012, 6, 7092–7102. [Google Scholar] [CrossRef] [PubMed]
- Li, X.X.; Li, H.Y.; Liu, T.T.; Hei, Y.S.; Hassan, M.; Zhang, S.Y.; Lin, J.X.; Wang, T.S.; Bo, X.J.; Wang, H.L.; et al. The biomass of ground cherry husks derived carbon nanoplates for electrochemical sensing. Sens. Actuators B Chem. 2018, 255, 3248–3256. [Google Scholar] [CrossRef]
- Kong, J.J.; Zheng, Y.J.; Xiao, L.J.; Dai, B.L.; Meng, Y.J.; Ma, Z.Y.; Wang, J.H.; Huang, X.C. Synthesis and comparison studies of activated carbons based folium cycas for ciprofloxacin adsorption. Colloids Surf. A 2020, 606, 125519. [Google Scholar] [CrossRef]
- Zhao, C.; Li, Y.; He, Z.; Jiang, Y.; Li, L.; Jiang, F. KHCO3 activated carbon microsphere as excellent electrocatalyst for VO~(2+)/VO2~+ redox couple for vanadium redox flow battery. J. Energy Chem. 2019, 29, 103–110. [Google Scholar] [CrossRef]
- Chen, C.; Qian, S.; Yao, T.H.; Guo, J.H.; Wang, H.K. Plate-like carbon-supported Fe3C nanoparticles with superior electrochemical performance. Rare Metals 2021, 40, 1402–1411. [Google Scholar] [CrossRef]
- Wang, L.L.; Wang, J.; Ng, D.; Li, S.; Zou, B.B.; Cui, Y.X.; Liu, X.H.; El-Khodary, S.A.; Qiu, J.X.; Lian, J.B. Operando mechanistic and dynamic studies of N/P co-doped hard carbon nanofibers for efficient sodium storage. Chem. Commun. 2021, 57, 9610–9613. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liu, J.W.; Ling, P.; Zhang, X.; Xu, K.; He, L.M.; Wang, Y.; Su, S.; Hu, S.; Xiang, J. Raman spectroscopy of biochar from the pyrolysis of three typical Chinese biomasses: A novel method for rapidly evaluating the biochar property. Energy 2020, 202, 117644. [Google Scholar] [CrossRef]
- Anthonysamy, S.I.; Lahijani, P.; Mohammadi, M.; Mohamed, A.R. Alkali-modified biochar as a sustainable adsorbent for the low-temperature uptake of nitric oxide. Int. J. Environ. Sci. Technol. 2022, 19, 7127–7140. [Google Scholar] [CrossRef]
- Tang, C.G.; Liu, Y.J.; Yang, D.G.; Yang, M.; Li, H.M. Oxygen and nitrogen co-doped porous carbons with finely-layered schistose structure for high-rate-performance supercapacitors. Carbon 2017, 122, 538–546. [Google Scholar] [CrossRef]
- Li, X.P.; Zhang, J.G.; Liu, B. A critical review on the application and recent developments of post-modifiedbiochar in supercapacitors. J. Clean. Prod. 2021, 310, 127428. [Google Scholar] [CrossRef]
- Rao, H.B.; Chen, M.; Ge, H.W.; Lu, Z.W.; Liu, X.; Zou, P.; Wang, X.X.; He, H.; Zeng, X.Y.; Wang, Y.Y. A novel electrochemical sensor based on Au@PANI composites film modified glassy carbon electrode binding molecular imprinting technique for the determination of melamine. Biosens. Bioelectron. 2017, 87, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Celiktas, M.S.; Alptekin, F.M. Conversion of model biomass to carbon-based material with high conductivity by using carbonization. Energy 2019, 188, 116089. [Google Scholar] [CrossRef]
- An, J.; Li, L.; Ding, Y. A novel molecularly imprinted electrochemical sensor based on Prussian blue analogue generated by iron metal organic frameworks for highly sensitive detection of melamine. Electrochim. Acta 2019, 326, 134946. [Google Scholar] [CrossRef]
- Dong, X.X.; Li, M.Y.; Feng, N.N. A nanoporous MgO based nonenzymatic electrochemical sensor for rapid screening of hydrogen peroxide in milk. RSC Adv. 2015, 5, 86485–86489. [Google Scholar] [CrossRef]
- Liu, Z.; Jin, M.; Cao, J. High-sensitive Electrochemical Sensor for Determination of Norfloxacin and Its Metabolism Using MWCNT-CPE/pRGO-ANSA/Au. Sens. Actuators B Chem. 2017, 257, 1065–1075. [Google Scholar] [CrossRef]
- Liu, Z.; Jin, M.; Lu, H. Molecularly imprinted polymer decorated 3D-framework of functionalized multi-walled carbon nanotubes for ultrasensitive electrochemical sensing of Norfloxacin in pharmaceutical formulations and rat plasma. Sens. Actuators B Chem. 2019, 288, 363–372. [Google Scholar] [CrossRef]
- Ye, C.; Chen, X.; Zhang, D. Study on the properties and reaction mechanism of polypyrrole@norfloxacin molecularly imprinted electrochemical sensor based on three-dimensional CoFe-MOFs/AuNPs. Electrochim. Acta 2021, 379, 138174. [Google Scholar] [CrossRef]
- Wang, Q.T.; Cheng, S.N.; Ren, S.F. Construction of Molecularly Imprinted Voltammetric Sensor Based on Cu-N-C Polyhedron Porous Carbon from Cu Doping ZIF-8 for the Selective Determination of Norfloxacin. Microchem. J. 2022, 183, 107963. [Google Scholar] [CrossRef]






| Interfering Substances | Concentration Multiple | Relative Error (%) | Interfering Substances | Concentration Multiple | Relative Error (%) |
|---|---|---|---|---|---|
| Na+ | 500 | −1.21 | Fe2+ | 200 | −3.21 |
| Cl− | 500 | −1.21 | glucose | 80 | −3.72 |
| K+ | 500 | +3.26 | Phenylalanine (Phe) | 50 | −0.96 |
| NO3− | 500 | +3.26 | Cysteine (Cys) | 50 | −4.08 |
| Ca2+ | 200 | −2.00 | Lysine (Lys) | 50 | −2.76 |
| ΔI1/(μA) | ΔI2/(μA) | ΔI3/(μA) | ΔI4/(μA) | ΔI5/(μA) | RSD (%) | |
|---|---|---|---|---|---|---|
| Different electrodes | 34.9 | 35.43 | 35.31 | 35.36 | 35.92 | 0.92 |
| Same electrode | 35.48 | 34.97 | 34.4 | 34.31 | 34.27 | 1.36 |
| Samples | Added (nM) | ΔI0 (μA) | ΔI1 (μA) | Found (nM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|---|---|
| Milk-1 | 0 | 4.84 | - | nd a | - | - |
| Milk-2 | 6 | - | 29.87 | 6.12 | 102.0 ± 1.21 | 4.05 |
| Milk-3 | 30 | - | 32.65 | 30.51 | 101.7 ± 0.10 | 0.31 |
| Milk-4 | 60 | - | 35.68 | 57.10 | 95.2 ± 0.39 | 1.08 |
| Honey-1 | 0 | 7.80 | - | nd a | ||
| Honey-2 | 6 | - | 32.83 | 6.12 | 102.0 ± 0.07 | 0.22 |
| Honey-3 | 30 | - | 35.56 | 30.08 | 100.3 ± 0.14 | 0.40 |
| Honey-4 | 60 | - | 38.74 | 57.97 | 96.6 ± 0.05 | 0.12 |
| Pork-1 | 0 | 8.80 | - | nd a | - | - |
| Pork-2 | 6 | - | 33.72 | 5.15 | 85.9 ± 0.04 | 0.11 |
| Pork-3 | 30 | - | 36.61 | 30.51 | 101.7 ± 0.3 | 0.83 |
| Pork-4 | 60 | - | 39.80 | 58.5 | 97.5 ± 0.60 | 1.50 |
| Modified Electrodes | Methods | Samples | Linear Range (mol L−1) | LOD (mol L−1) | Incubation Time (min) | Ref. |
|---|---|---|---|---|---|---|
| MWCNT-CPE/pRGO-ANSA/Au | DPV | drug tablet | 3 × 10−8–5 × 10−5 | 1.6 × 10−8 | 3.5 | [47] |
| fMWCNTs-MIP/GCE | DPV | drug tablet and rat plasma | 3 × 10−9–3.13 × 10−6 | 1.58 × 10−9 | 9.0 | [48] |
| MIP/CoFe-MOFs/AuNPs/GCE | DPV | drug capsules and milk | 5 × 10−12–6 × 10−9 | 1.31 × 10−13 | 3 | [49] |
| MIP/Cu-N-C/GCE | DPV | drug capsules | 1 × 10−8–5 × 10−7, 5 × 10−7–1 × 10−4 | 3.33 × 10−9 | 1.5 | [50] |
| Sensor in this work | DPV | milk, honey and pork | 1 × 10−10–5 × 10−10, 5 × 10−10–1 × 10−7 | 2.8 × 10−11 | 4 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Zhu, Y.; Han, J.; Zhang, T.; Chou, R.; Liu, A.; Liu, S.; Yang, Y.; Hu, K.; Zou, L. Construction of a Molecularly Imprinted Sensor Modified with Tea Branch Biochar and Its Rapid Detection of Norfloxacin Residues in Animal-Derived Foods. Foods 2023, 12, 544. https://doi.org/10.3390/foods12030544
Chen S, Zhu Y, Han J, Zhang T, Chou R, Liu A, Liu S, Yang Y, Hu K, Zou L. Construction of a Molecularly Imprinted Sensor Modified with Tea Branch Biochar and Its Rapid Detection of Norfloxacin Residues in Animal-Derived Foods. Foods. 2023; 12(3):544. https://doi.org/10.3390/foods12030544
Chicago/Turabian StyleChen, Shujuan, Yiting Zhu, Jing Han, Tianyi Zhang, Runwen Chou, Aiping Liu, Shuliang Liu, Yong Yang, Kaidi Hu, and Likou Zou. 2023. "Construction of a Molecularly Imprinted Sensor Modified with Tea Branch Biochar and Its Rapid Detection of Norfloxacin Residues in Animal-Derived Foods" Foods 12, no. 3: 544. https://doi.org/10.3390/foods12030544
APA StyleChen, S., Zhu, Y., Han, J., Zhang, T., Chou, R., Liu, A., Liu, S., Yang, Y., Hu, K., & Zou, L. (2023). Construction of a Molecularly Imprinted Sensor Modified with Tea Branch Biochar and Its Rapid Detection of Norfloxacin Residues in Animal-Derived Foods. Foods, 12(3), 544. https://doi.org/10.3390/foods12030544






