Valorization of Rice Husk (Oryza sativa L.) as a Source of In Vitro Antiglycative and Antioxidant Agents
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Plant Material
2.3. Microwave-Assisted Extraction Process
2.4. Experimental Design
2.4.1. Screening Study of Process Variables
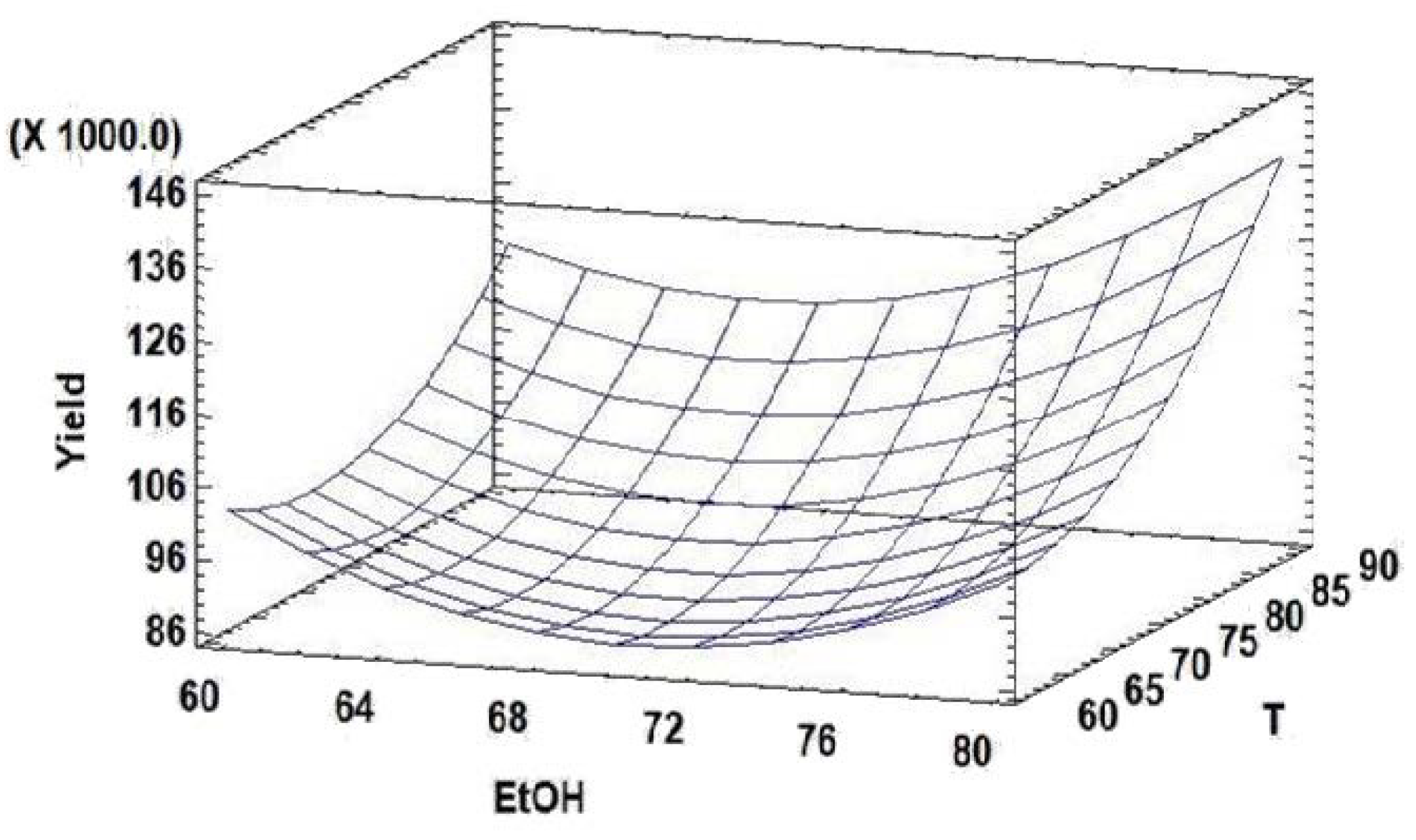
2.4.2. Response Surface Methodology
2.5. RP-HPLC-DAD Analyses
2.6. RP-HPLC-DAD-ESI/MSn Analyses
2.7. Evaluation of Amadori Products Inhibition
2.8. Evaluation of Antiglycative Capacities
2.9. Evaluation of Methylglyoxal and Glyoxal Trapping Capacities
2.10. Determination of Free Amino Groups
2.11. DPPH Assay
2.12. ABTS Assay
2.13. Statistial Analysis
3. Results and Discussion
3.1. Microwave-Assisted Extraction (MAE) Optimization
3.1.1. Screening Phase
3.1.2. Optimization Phase
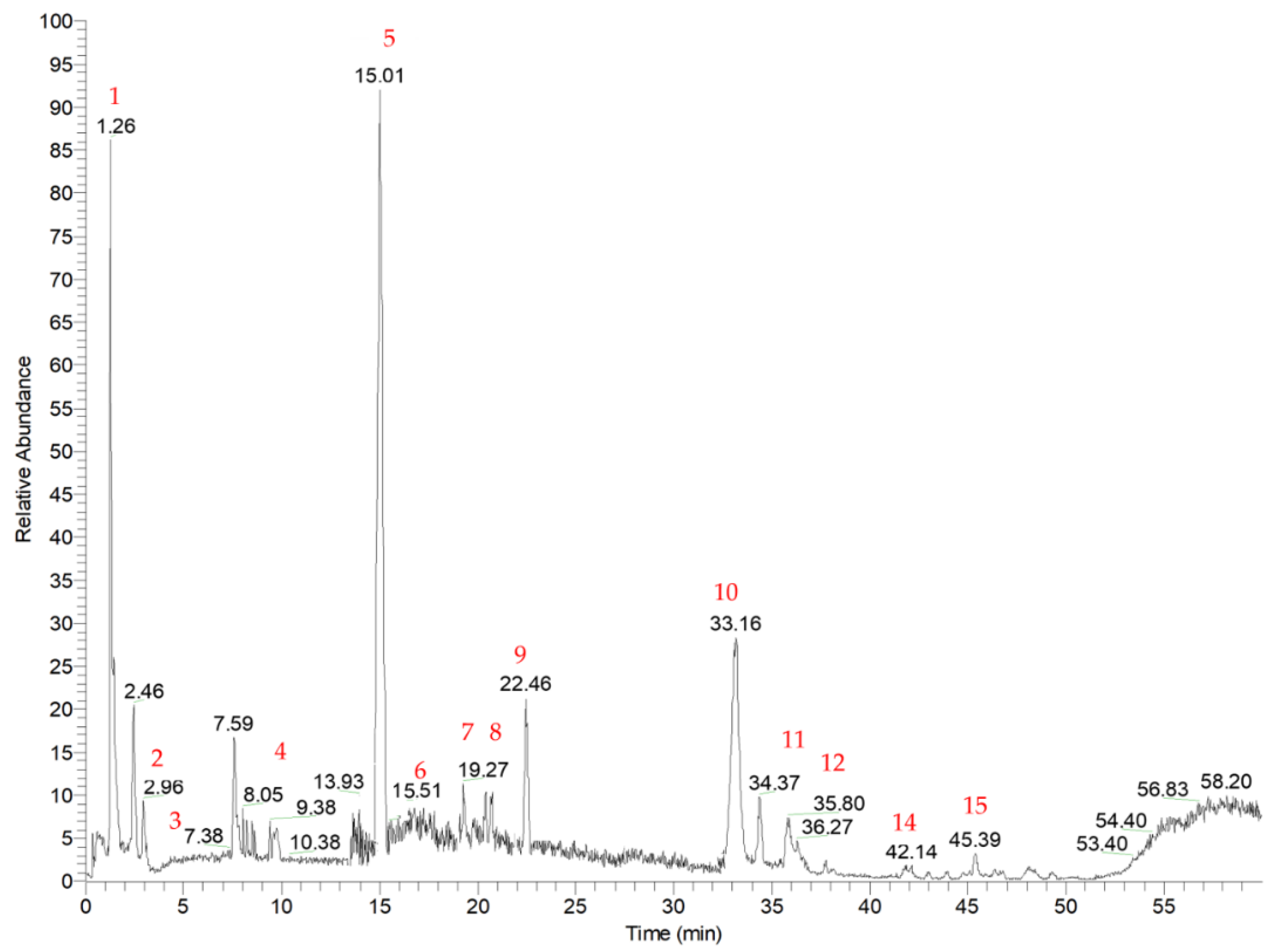
3.2. Identification of Phytochemicals
| Compound | Rt (min) | Precursor Ion (m/z) | HPLC-ESI-MSn m/z (% of Base Peak) | Compound Identity | Refs |
|---|---|---|---|---|---|
| 1 | 1.26 | 387 | MS2 [387]: 341(100) MS3 [341]: 179 (100), 119 (70), 113 (50), 161 (45), 143 (40), 131 (20) | 1-Caffeoyl-β-D-glucose | [30] |
| 2 | 2.96 | 133 | MS2 [133]: 115 (100), 71 (10) | Levulinic acid | [44] |
| 3 | 3.24 | 191 | MS2 [191]: 173 (100), 111 (50), 155 (10) | Quinic acid | [32] |
| 4 | 10.38 | 151 | MS2 [151]: 136 (100), 123 (5), 107 (5), 88 (5) | Vanillin | [31] |
| 5 | 15.01 | 163 | MS2 [163]: 11 9(100) | p-Coumaric acid | [31] |
| 6 | 17.20 | 563 | MS2 [563]: 443 (100), 473 (70), 353 (40), 383 (30) | Apigenin 8-C-arabinoside-6-C-glucoside | [33,34,35] |
| 7 | 17.27 | 563 | MS2 [563]: 473 (100), 443 (70), 353 (65), 383 (45) | Apigenin 6-C-arabinoside-8-C-glucoside | [33,34,35] |
| 8 | 20.70 | 187 | MS2 [187]: 125 (100), 169 (5), 97 (5) | Hydroxy gallic acid | [42,43] |
| 9 | 22.46 | 355 | MS2 [355]: 337 (100), 219(60), 325 (20), 204 (10), 176 (5) | Unidentified | - |
| 10 | 33.16 | 329 | MS2 [329]: 314 (100), 299 (5) | Tricin | [36] |
| 11 | 35.80 | 329 | MS2 [329]: 229 (100), 211 (90) 314 (70), 311 (20), 295 (30), 171 (10), 155 (40) | tri-OH oleic acid derivative | [46] |
| 12 | 36.59 | 671 | MS2 [671]: 329 (100), 314 (30) | Tricin derivative | [38,39] |
| 13 | 38.32 | 315* | MS2 [315]: 271 (100), 269 (10), 199 | 2′,7 dihydroxy-4′,5′-dimethoxyisoflavone | [41] |
| 14 | 42.14 | 311 | MS2 [311]: 293 (100), 171 (50), 201 (40), 211 (30), 197 (35) | Unidentified | - |
| 15 | 45.39 | 295 | MS2 [295]: 171 (100), 277 (80), 179 (35), 195 (30) | Hydroxy-octadienoic acid | [45] |
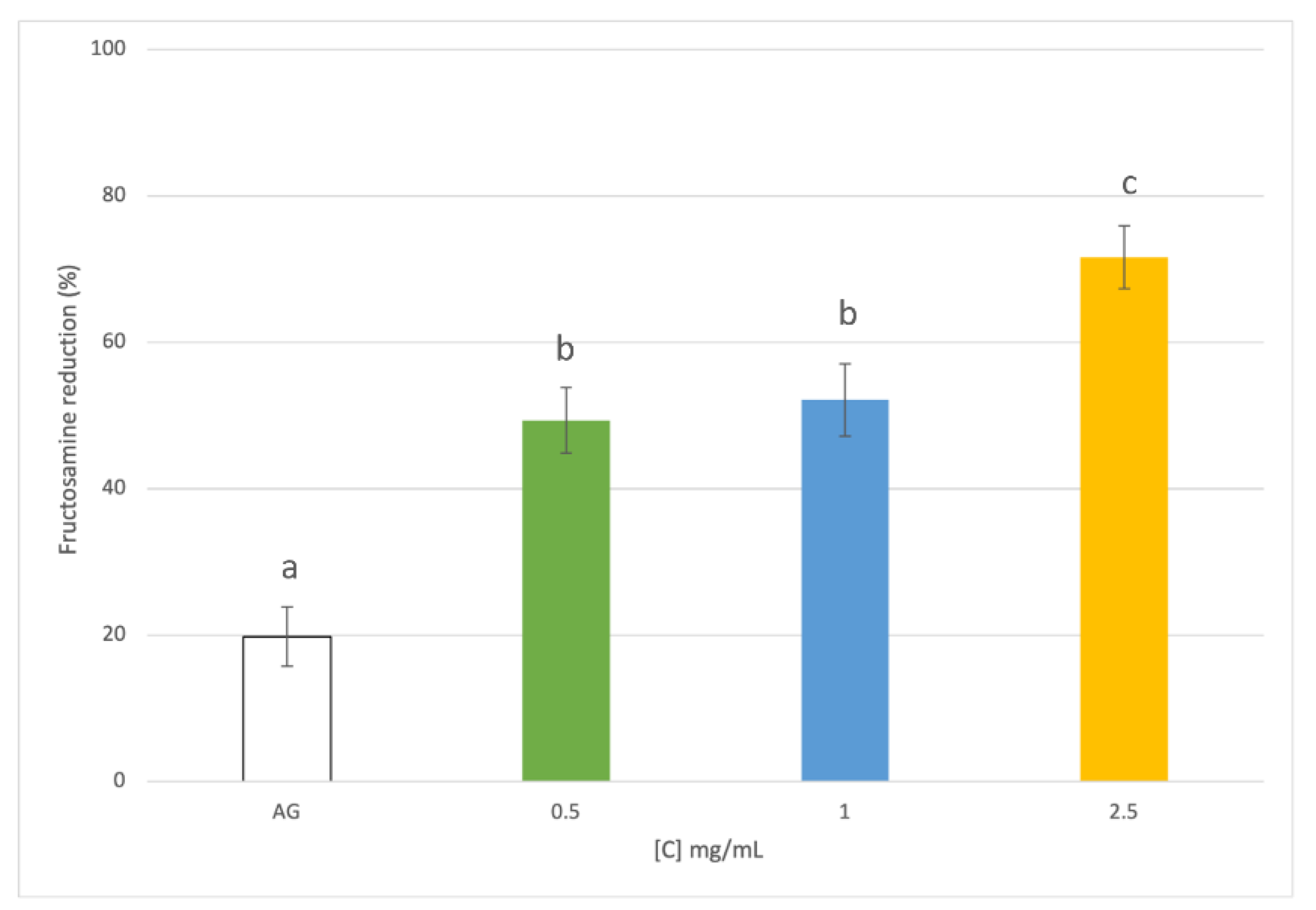
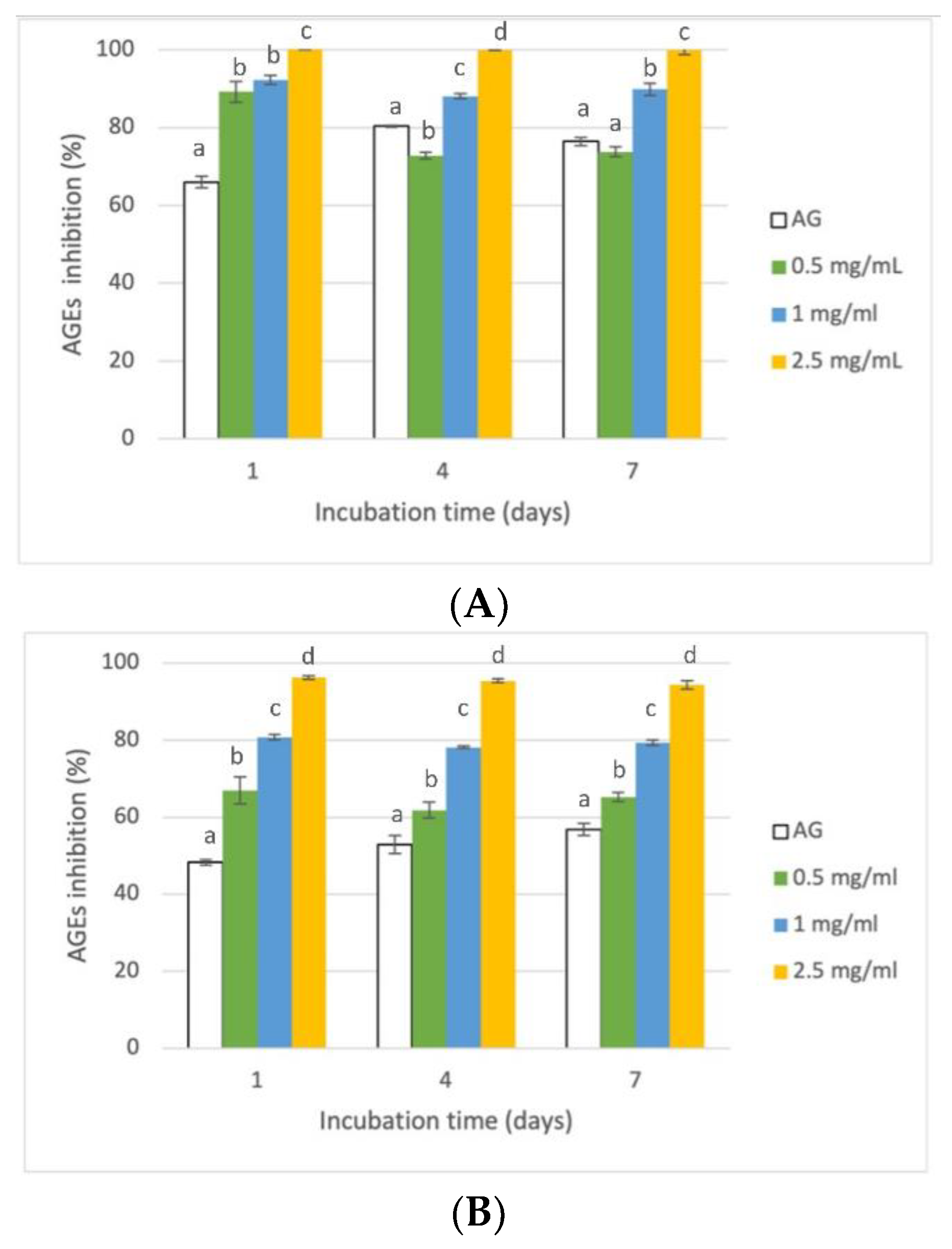
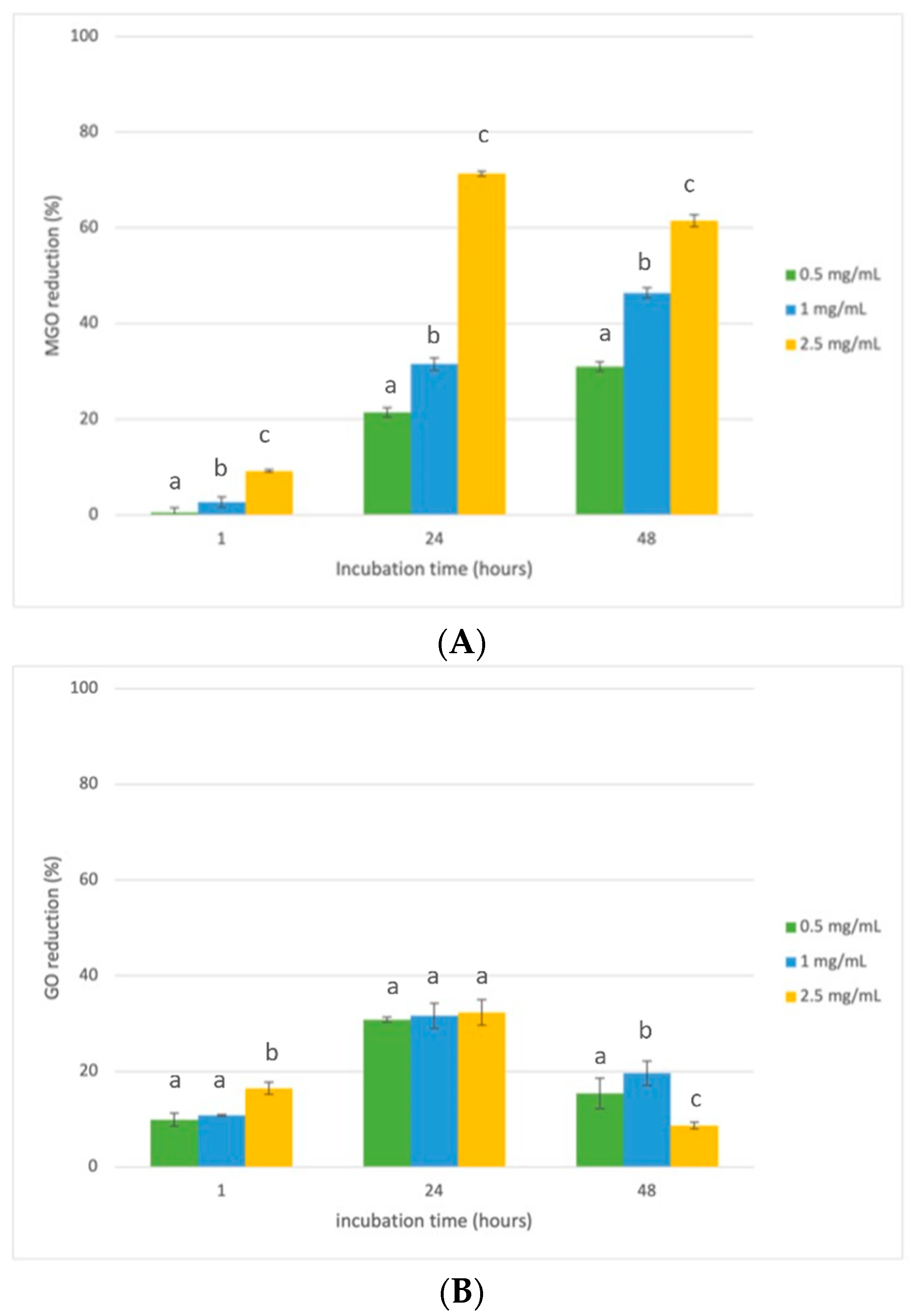
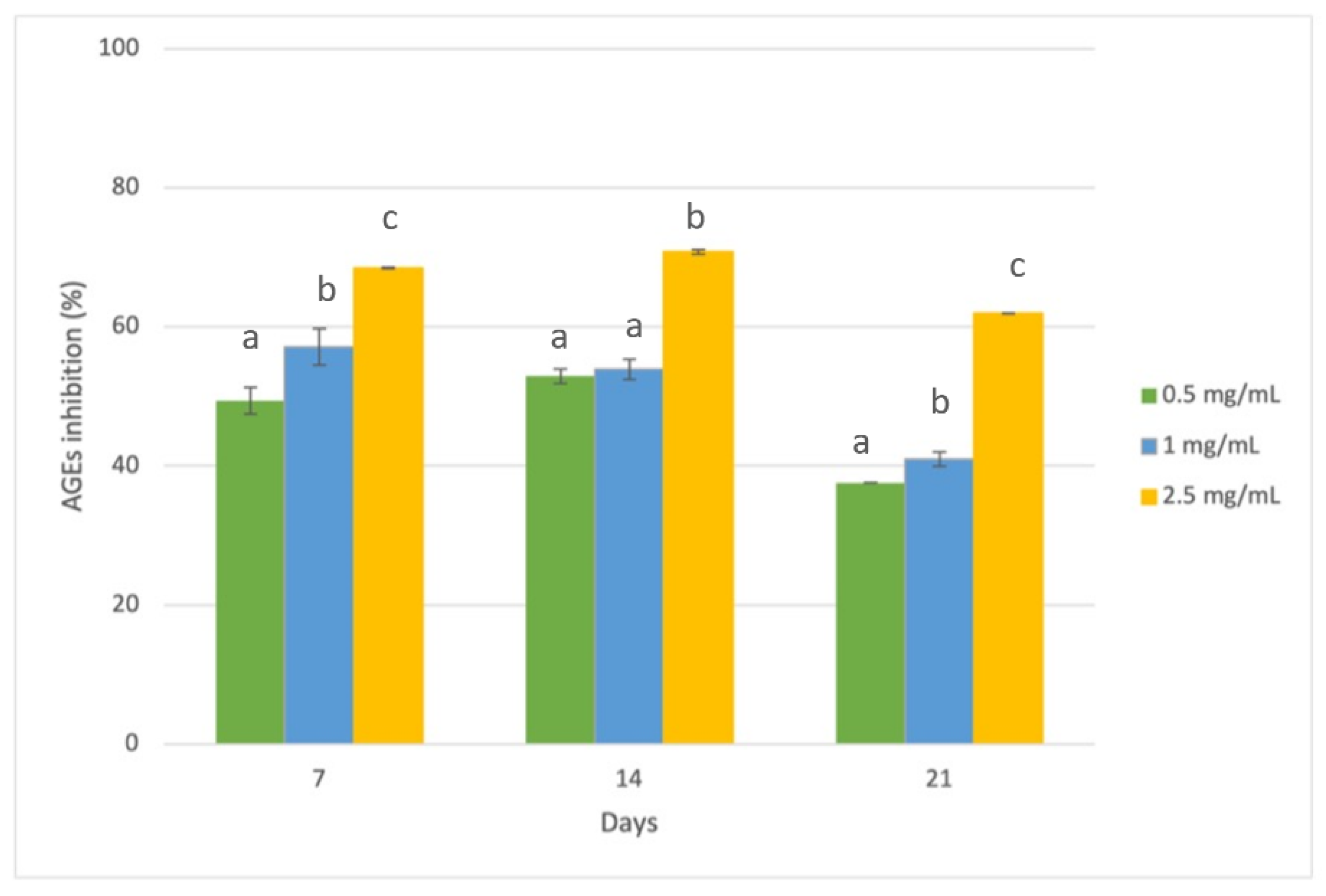
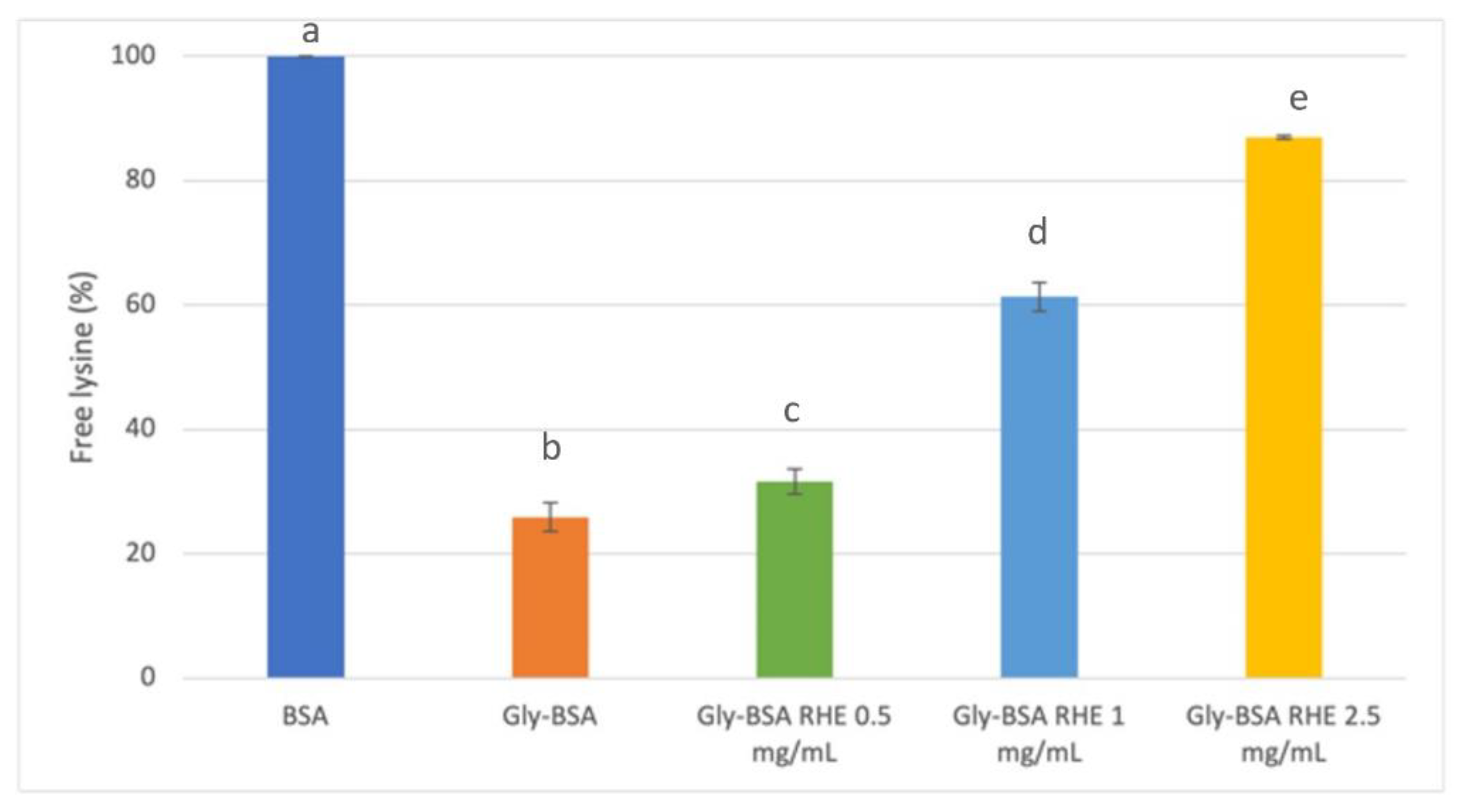
3.3. Evaluation of Antiglycative Capacities of Rice Husk Extract
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Atlas Big. World Rice Production by Country. Available online: https://www.atlasbig.com/en-au/countries-by-rice-production (accessed on 3 August 2022).
- Esa, N.M.; Ling, T.B.; Peng, L.S. By-products of rice processing: An overview of health benefits and applications. Rice Res. 2013, 1, 1–11. [Google Scholar]
- Peanparkdee, M.; Iwamoto, S. Bioactive compounds from by-products of rice cultivation and rice processing: Extraction and application in the food and pharmaceutical industries. Trends Food Sci. Technol. 2019, 86, 109–117. [Google Scholar] [CrossRef]
- Butsat, S.; Siriamornpun, S. Antioxidant capacities and phenolic compounds of the husk, bran and endosperm of Thai rice. Food Chem. 2010, 119, 606–613. [Google Scholar] [CrossRef]
- Wanyo, P.; Kaewseejan, N.; Meeso, N.; Siriamornpun, S. Bioactive compounds and antioxidant properties of different solvent extracts derived from Thai rice by-products. Appl. Biol. Chem. 2016, 59, 1–12. [Google Scholar] [CrossRef]
- Lourith, N.; Kanlayavattanakul, M. Appraisal of Thai glutinous rice husk for health promotion products. J. Cereal Sci. 2013, 57, 343–347. [Google Scholar] [CrossRef]
- Wanyo, P.; Meeso, N.; Siriamornpun, S. Effects of different treatments on the antioxidant properties and phenolic compounds of rice bran and rice husk. Food Chem. 2014, 157, 457–463. [Google Scholar] [CrossRef]
- Irakli, M.; Kleisiaris, F.; Kadoglidou, K.; Katsantionis, D. Optimizing extraction conditions of free and bound phenolic compounds from rice by-products and their antioxidant effects. Foods 2018, 7, 93. [Google Scholar] [CrossRef]
- Gao, Y.; Guo, X.; Liu, Y.; Fang, Z.; Zhang, M.; Zhang, R.; You, L.; Li, T.; Liu, R.H. A full utilization of rice husk to evaluate phytochemical bioactivities and prepare cellulose nanocrystals. Sci. Rep. 2018, 8, 10482. [Google Scholar] [CrossRef]
- Naqvi, S.A.Z.; Irfan, A.; Zaheer, S.; Sultan, A.; Shajahan, S.; Rubab, S.L.; Ain, Q.; Acevedo, R. Proximate composition of orange peel, pea peel and rice husk wastes and their potential use as antimicrobial agents and antioxidants. Vegetos 2021, 34, 470–476. [Google Scholar] [CrossRef]
- Nilnumkhum, A.; Punvittayagul, C.; Chariayakornkul, A.; Wongpoomchai, R. Effects of hydrophilic compounds in purple rice husk on AFB1-induced mutagenesis. Mol. Cell. Toxicol. 2017, 13, 171–178. [Google Scholar] [CrossRef]
- Rahman, S.; Jahan, R.; Rahmatullah, M. Effect of paddy husk extracts on glucose tolerance in glucose-induced hyperglycemic mice. World J. Pharm. Pharm. Sci. 2014, 8, 111–120. [Google Scholar]
- Roberts, R.O.; Knopman, D.S.; Przybelski, S.A.; Mielke, M.M.; Kantarci, K.; Preboske, G.M.; Senjem, M.L.; Pankratz, V.S.; Geda, Y.E.; Boeve, B.F.; et al. Association of type 2 diabetes with brain atrophy and cognitive impairment. Neurology 2014, 82, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Velinchova, S.; Foubert, K.; Pieters, L. Natural products as a source of inspiration for novel inhibitors on advanced glycation endproducts (AGEs) formation. Planta Med. 2021, 87, 780–802. [Google Scholar]
- Khan, M.S.; Alokail, M.S.; Alenad, A.M.H.; Altwaijry, N.; Alafaleq, N.O.; Alamri, A.M.; Zawba, M.A. Binding studies of caffeic and p-coumaric acid with α-amylase: Multispectroscopic and computational approaches deciphering the effect on advanced glycation end products (AGEs). Molecules 2022, 27, 3992. [Google Scholar] [CrossRef]
- Yeh, W.-J.; Hsia, S.-M.; Lee, W.-H.; Wu, C.-H. Polyphenols with antiglycation activity and mechanism of action: A review of recent finding. J. Food Drug Anal. 2017, 25, 84–92. [Google Scholar] [CrossRef]
- Zhang, L.-S.; Wang, X.; Dong, L.-L. Antioxidant and antiglycation of polysaccharides from Misgurnus anguillicaudatus. Food Chem. 2011, 124, 183–187. [Google Scholar] [CrossRef]
- Maietta, M.; Colombo, R.; Lavecchia, R.; Sorrenti, M.; Zuorro, A.; Papetti, A. Artichoke (Cynara cardunculus L. var. scolymus) waste as a natural source of carbonyl trapping and antiglycative agents. Food Res. Int. 2017, 100, 780–790. [Google Scholar] [CrossRef] [PubMed]
- Maietta, M.; Colombo, R.; Corana, F.; Papetti, A. Cretan tea (Origanum dictamnus L.) as a functional beverage: An investigation on antiglycative and carbonyl trapping activities. Food Funct. 2018, 9, 1545–1556. [Google Scholar] [CrossRef]
- Mesías, M.; Navarro, M.; Gökmen, V.; Morales, F.J. Antiglycative effect of fruit and vegetable seed extracts: Inhibition of AGE formation and carbonyl-trapping abilities. J. Sci. Food Agric. 2013, 93, 2037–2044. [Google Scholar] [CrossRef]
- Fayle, S.E.; Healy, J.P.; Brown, P.A.; Reid, E.A.; Gerrard, J.A.; Ames, J.M. Novel approaches to the analysis of Maillard reaction proteins. Electrophoresis 2011, 22, 1518–1525. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evance, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Das, A.B.; Goud, V.V.; Das, C. Extraction and characterization of phenolic content from purple and black rice (Oryza sativa L.) bran and its antioxidant activity. Food Meas. 2018, 12, 332–345. [Google Scholar] [CrossRef]
- Rodríguez Amado, I.; Franco, D.; Sánchez, M.; Zapata, C.; Vázquez, J.A. Optimization of antioxidant extraction from Solanum tuberosum potato peel waste by surface response methodology. Food Chem. 2014, 165, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Liazid, A.; Palma, M.; Brigui, J.; Barroso, C.G. Investigation on phenolic compounds stability during microwave-assisted extraction. J. Chromatrogr. A 2014, 1140, 29–34. [Google Scholar] [CrossRef]
- Galan, A.M.; Calinescu, I.; Trifan, A.; Winkworth-Smith, C.; Calvo-Carrascal, M.; Dodds, C.; Binner, E. New insights into the role of selective and volumetric heating during microwave extraction: Investigation of the extraction of polyphenolic compounds from sea buckthorn leaves using microwave-assisted extraction and conventional solvent extraction. Chem. Eng. Process. 2017, 116, 29–39. [Google Scholar] [CrossRef]
- Frosi, I.; Montagna, I.; Colombo, R.; Milanese, C.; Papetti, A. Recovery of chlorogenic acids from agri-food wastes: Updates on green extraction techniques. Molecules 2021, 26, 4515. [Google Scholar] [CrossRef]
- Mena-García, A.; Rodríguez-Sánchez, S.; Ruiz-Matute, A.I.; Sanz, M.L. Exploitation of artichoke byproducts to obtain bioactive extracts enriched in inositols and caffeoylquinic acids by Microwave Assisted Extraction. J. Chromatrogr. A. 2020, 1613, 460703. [Google Scholar] [CrossRef]
- Tranfić Bakić, M.; Pedisić, S.; Zorić, Z.; Dragovic-Uzelac, V.; Nincević Grassino, A. Effect of microwave-assisted extraction on polyphenols recovery from tomato peel waste. Acta Chim. Slov. 2019, 66, 367–377. [Google Scholar] [CrossRef]
- Goufo, P.; Singh, R.K.; Cortez, I. A reference list of phenolic compounds (including stilbenes) in grapevine (Vitis vinifera L.) roots, woods, canes, stems and leaves. Antioxidants 2020, 9, 398. [Google Scholar] [CrossRef]
- Lopez-Fernández, O.; Domínguez, R.; Pateiro, M.; Munekata, P.E.S.; Rocchetti, G.; Lorenzo, J.M. Determination of polyphenols using liquid chromatography-tandem mass spectrometry technique (LC-MS/MS): A review. Antioxidants 2022, 9, 479. [Google Scholar] [CrossRef]
- Carazzone, C.; Mascherpa, D.; Gazzani, G.; Papetti, A. Identification of phenolic constituents in red chicory salads (Chichorium intybus) by high-performance liquid chromatography with diode array detection and electrospray ionization tandem mass spectrometry. Food Chem. 2013, 138, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, R.M.; El-Halawany, A.M.; Saleh, D.O.; El Naggar, E.M.B.; El-Shabrawy, A.E.R.O.; El-Hawary, S.S. HPLC-DAD-MS/MS profiling of phenolics from Securigera securidaca flowers and its anti-hyperglycemic and anti-hyperlipidemic activities. Rev. Bras. Farmacogn. 2015, 25, 134–141. [Google Scholar] [CrossRef]
- Benjad, Z.; Gómez-Cordovés, C.; Es-Safi, N.E. Characterization of flavonoid glycosides from fenugreek (Trigonella foenum-graecum) crude seed by HPLC-DAD-ESI/MS analysis. Int. J. Mol. Sci. 2014, 15, 20668–20685. [Google Scholar]
- Righi, A.A.; Negri, G.; Salatino, A. Comparative chemistry of propolis from eight brazilian localities. Evid. Complement. Alternat. Med. 2013, 2013, 267878. [Google Scholar] [CrossRef] [PubMed]
- Taamalli, A.; Arráez-Román, D.; Abaza, L.; Iswaldi, I.; Fernández-Gutiérrez, A.; Zarrouk, M.; Segura-Sarretero, A. LC-MS-based metabolite profiling of methanolic extracts from medicinal and aromatic species Mentha pulegium and Origanum majorana. Phytochem. Anal. 2015, 26, 320–330. [Google Scholar] [CrossRef]
- Poulev, A.; Chen, M.-H.; Cherravuru, S.; Raskin, I.; Belanger, F.C. Variation in levels in the flavone tricin in bran from rice genotypes varying in pericarp color. J. Cereal Sci. 2018, 79, 226–232. [Google Scholar] [CrossRef]
- Li, M.; Pu, Y.; Yoo, C.G.; Ragauskas, A.J. The occurrence of tricin and its derivatives in plants. Green Chem. 2016, 18, 1439–1454. [Google Scholar] [CrossRef]
- Duarte-Almeida, J.M.; Negri, G.; Salatino, A.; de Carvalho, J.E.; Lajolo, F.M. Antiproliferative and antioxidant activities of a tricin acylated glycoside from sugarcane (Saccharum officinarum) juice. Phytochemistry 2007, 68, 1165–1171. [Google Scholar] [CrossRef]
- Stochmal, A.; Simonet, A.M.; Marcias, F.A.; Oleszek, W. Alfalfa (Medicago sativa L.) flavonoids. 2. Tricin and chrysoeriol glycosides from aerial parts. J. Agric. Food Chem. 2001, 49, 5310–5314. [Google Scholar] [CrossRef]
- Tang, J.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF/MS characterization of phenolic compounds from medicinal plants (hops and juniper berries) and their antioxidant activity. Foods 2020, 9, 7. [Google Scholar] [CrossRef]
- Fathoni, A.; Saepudin, E.; Cahyana, A.H.; Rahayu, D.U.C.; Haib, J. Identification of Nonvolatile Compounds in Clove (Syzygium aromaticum) from Manado. In Proceedings of the AIP Conference, Pizzo Calabro, Italy, 19–25 September 2016; Volume 1862, p. 030079. [Google Scholar]
- Thepthanee, C.; Liu, C.-C.; Yu, H.-S.; Huang, H.-S.; Yen, C.-H.; Li, Y.-H.; Lee, M.-R.; Liaw, E.-T. Evaluation of phytochemical contents and in vitro antioxidant, anti-inflammatory, and anticancer activities of black rice leaf (Oryza sativa L.) extract and its fractions. Foods 2021, 10, 2987. [Google Scholar] [CrossRef] [PubMed]
- Bevilaqua, D.B.; Rambo, M.K.D.; Rizzetti, T.M.; Cardoso, A.L.; Martins, A.F. Cleaner production: Levulinic acid from rice husks. J. Clean. Prod. 2013, 47, 96–101. [Google Scholar] [CrossRef]
- Nastić, N.; Borrás-Linares, I.; Lozano-Sánchez, J.; Švarc-Gajić, J.; Segura-Carratero, A. Comparative assesment of phytochemical profiles of comfrey (Symphytum officinale L.) roots extracts obtained by different extraction techniques. Molecules 2008, 25, 837. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Ganf, N.; Li, Q.; Zhang, J.; Luo, H.; Roins, M.; Badger, T.M. In vitro actions on human cancer cells and the liquid chromatography-mass specrtrometry/mass spectrometry fingerprint of phytochemicals in rice protein isolate. J. Agric. Food Chem. 2006, 54, 4482–4492. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.; Khan, S.; Almatroudi, A.; Khan, A.A.; Alsahli, M.A.; Almatroodi, S.A.; Rahmani, A.H. A review on mechanism of inhibition of advanced glycation end products formation by plants derived polyphenolic compounds. Mol. Biol. Rep. 2021, 48, 787–805. [Google Scholar] [CrossRef]
- Marchetti, L.; Truzzi, E.; Frosi, I.; Papetti, A.; Cappellozza, S.; Saviane, A.; Pellati, F.; Bertelli, D. In vitro bioactivity evaluation of mulberry leaf extracts as nutraceutucals for the management of diabetes mellitus. Food Funct. 2022, 13, 4344–4359. [Google Scholar] [CrossRef]
- Li, X.; Zheng, T.; Sang, S.; Lv, L. Quercetin inhibits advanced glycation end product formation by trapping methylglyoxal and glyoxal. J. Agric. Food Chem. 2014, 62, 12152–12158. [Google Scholar] [CrossRef]
- Sadowska-Bartosz, I.; Galiniak, S.; Bartosz, G. Kinetics of glycoxidation of bovine serum albumin by glucose, fructose and ribose and its prevention by food components. Molecules 2014, 19, 18828–18849. [Google Scholar] [CrossRef]
- Singh, R.; Barden, A.; Mori, T.; Beilin, L. Advanced glycation end-products: A review. Diabetologia 2001, 44, 129–146. [Google Scholar] [CrossRef]
- Wang, R.; Khalifa, I.; Du, X.; Li, K.; Xu, Y.; Li, C. Effects of anthocyanins on β-lactoglobulin glycoxidation: A study of mechanisms and structure-activity relationship. Food Funct. 2021, 12, 10550–10562. [Google Scholar] [CrossRef]
- Aalim, H.; Belwal, T.; Jiang, L.; Huang, H.; Meng, X.; Luo, Z. Extraction optimization, antidiabetic and antiglycation potentials of aqueous glycerol extract from rice (Oryza sativa L.) bran. Lebensm. Wiss. Technol. 2018, 103, 147–154. [Google Scholar] [CrossRef]
- Premakumara, G.A.S.; Abeysekera, W.K.S.M.; Ratnasooriya, W.D.; Chandrasekharan, N.V.; Benota, A.P. Antioxidant, anti-amylase and anti-glycation potential of brans of some Sri Lankan traditional and improved rice (Oryza sativa L.) varieties. J. Cereal Sci. 2018, 58, 451–456. [Google Scholar] [CrossRef]
- Ferron, L.; Colombo, R.; Mannucci, B.; Papetti, A. A new Italian purple corn variety (Moradyn) byproduct extract: Antiglycative and hypogycemic in vitro activities and preliminary bioaccessibility studies. Molecules 2020, 25, 1958. [Google Scholar] [CrossRef] [PubMed]
- Senevirathne, I.G.N.H.; Abeysekera, W.K.S.M.; Abeysekera, W.P.K.M.; Jayanath, N.Y.; Premakumara Galbada Arachchige, S.; Wijewardana, D.C.M.S.I. Antiamylase, antiglucosidase, and antiglycation properties of millets and sorghum from Sri Lanka. Evid. Based Complemt. Alternat. Med. 2021, 2021, 5834915. [Google Scholar] [CrossRef]
- Chen, G.; Madl, R.L.; Smith, J.S. Cereal bran extracts inhibit the formation of advanced glycation end products in bovine serum albumin/glucose model. Cereal Chem. 2018, 95, 625–633. [Google Scholar] [CrossRef]
- Haldipur, A.C.; Srividya, N. Multi-mechanistic in vitro evaluation of antihyperglycemic, antioxidant and antiglycation activities of three phenolic-rich Indian red rice genotypes and in silico evaluation of their phenolic metabolites. Foods 2018, 10, 2818. [Google Scholar] [CrossRef]
- Butstat, S.; Siriamornpun, S. Phenolic acids and antioxidant activities in husk of different Thai rice varieties. Food Sci. Technol. Int. 2010, 16, 329–335. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, X. Structures required of polyphenols for inhibiting advanced glycation end products formation. Curr. Drug Metab. 2013, 14, 414–431. [Google Scholar] [CrossRef]
- Anandan, S.; Kotebagilu, N.P.; Shivanna, L.M.; Urooj, A. Inhibitory potency of C-glycosyl flavonoids from morus sp. on advanced glycation end products. J. Biol. Act. Prod. Nat. 2017, 5, 391–400. [Google Scholar] [CrossRef]

| Sample | Ethanol (%) | Temperature (°C) | Time (min) | Solvent to Solid Ratio (mL/g) | Yield (mAU*s) |
|---|---|---|---|---|---|
| 1 | 30 | 40 | 5 | 15 | 44,026.1 |
| 2 | 30 | 40 | 5 | 35 | 53,250.2 |
| 3 | 30 | 80 | 5 | 15 | 73,121.4 |
| 4 | 30 | 80 | 35 | 35 | 67,167.3 |
| 5 | 30 | 40 | 35 | 15 | 46,771.2 |
| 6 | 30 | 40 | 35 | 35 | 60,918.1 |
| 7 | 30 | 80 | 35 | 15 | 10,712.0 |
| 8 | 30 | 80 | 5 | 35 | 69,380.9 |
| 9 | 70 | 40 | 5 | 15 | 62,104.9 |
| 10 | 70 | 40 | 5 | 35 | 79,735.8 |
| 11 | 70 | 80 | 5 | 15 | 93,851.4 |
| 12 | 70 | 80 | 5 | 35 | 108,931.0 |
| 13 | 70 | 40 | 35 | 15 | 71,067.7 |
| 14 | 70 | 40 | 35 | 35 | 84,906.3 |
| 15 | 70 | 80 | 35 | 15 | 111,471.3 |
| 16 | 70 | 80 | 35 | 35 | 128,500.3 |
| 17 | 50 | 60 | 20 | 25 | 94,467.9 |
| 18 | 50 | 60 | 20 | 25 | 97,625.2 |
| 19 | 50 | 60 | 20 | 25 | 98,631.8 |
| Sample | Ethanol (%) | Temperature (°C) | Actual Yield (mAU*s) | Predicted Yield (mAU*s) |
|---|---|---|---|---|
| 1 | 60 | 60 | 102564.9 | 102,381.0 |
| 2 | 80 | 60 | 96,972.2 | 99,914.4 |
| 3 | 60 | 90 | 120,588.4 | 118,411.0 |
| 4 | 80 | 90 | 136,819.6 | 137,771.0 |
| 5 | 60 | 75 | 98,178.2 | 100,539.0 |
| 6 | 80 | 75 | 112,884.1 | 108,987.0 |
| 7 | 70 | 60 | 90,576.6 | 87,815.2 |
| 8 | 70 | 90 | 113,531.8 | 114,757.0 |
| 9 | 70 | 75 | 89,423.5 | 91,429.1 |
| 10 | 70 | 75 | 91,898.6 | 91,429.1 |
| Source | Sum of Squares | Df | Mean Square | F Ratio | p Value |
|---|---|---|---|---|---|
| A:EtOH | 1.07056 × 108 | 1 | 1.07056 × 108 | 8.83 | 0.0411 |
| B:T | 1.08881 × 109 | 1 | 1.08881 × 109 | 89.83 | 0.0007 |
| AA | 4.14862 × 108 | 1 | 4.14862 × 108 | 34.23 | 0.0043 |
| AB | 1.19071 × 108 | 1 | 1.19071 × 108 | 9.82 | 0.0350 |
| BB | 2.26714 × 108 | 1 | 2.26714 × 108 | 18.70 | 0.0124 |
| Total error | 4.84828 × 107 | 4 | 1.21208 × 107 | ||
| PRESS | 4.92509 × 108 | ||||
| R2 | 0.9772 | ||||
| R2adj | 0.9487 |
| RHE Concentration | ABTS•+ Inhibition (%) | DPPH Inhibition (%) |
|---|---|---|
| 0.5 mg/mL | 41.55 ± 0.30 | 20.89 ± 0.28 |
| 1 mg/mL | 65.57 ± 2.28 | 31.59 ± 0.21 |
| 2.5 mg/mL | 95.12 ± 2.06 | 47.49 ± 0.06 |
| Assay | DPPH (R2) | ABTS•+ (R2) |
|---|---|---|
| NBT | 0.9566 | 0.9399 |
| MGO | 0.9705 | 0.9818 |
| GLU | 0.9604 | 0.9443 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frosi, I.; Vallelonga, D.; Colombo, R.; Milanese, C.; Papetti, A. Valorization of Rice Husk (Oryza sativa L.) as a Source of In Vitro Antiglycative and Antioxidant Agents. Foods 2023, 12, 529. https://doi.org/10.3390/foods12030529
Frosi I, Vallelonga D, Colombo R, Milanese C, Papetti A. Valorization of Rice Husk (Oryza sativa L.) as a Source of In Vitro Antiglycative and Antioxidant Agents. Foods. 2023; 12(3):529. https://doi.org/10.3390/foods12030529
Chicago/Turabian StyleFrosi, Ilaria, Daniela Vallelonga, Raffaella Colombo, Chiara Milanese, and Adele Papetti. 2023. "Valorization of Rice Husk (Oryza sativa L.) as a Source of In Vitro Antiglycative and Antioxidant Agents" Foods 12, no. 3: 529. https://doi.org/10.3390/foods12030529
APA StyleFrosi, I., Vallelonga, D., Colombo, R., Milanese, C., & Papetti, A. (2023). Valorization of Rice Husk (Oryza sativa L.) as a Source of In Vitro Antiglycative and Antioxidant Agents. Foods, 12(3), 529. https://doi.org/10.3390/foods12030529








