Protein Nanofibrils from Fava Bean and Its Major Storage Proteins: Formation and Ability to Generate and Stabilise Foams
Abstract
1. Introduction
2. Materials and Methods
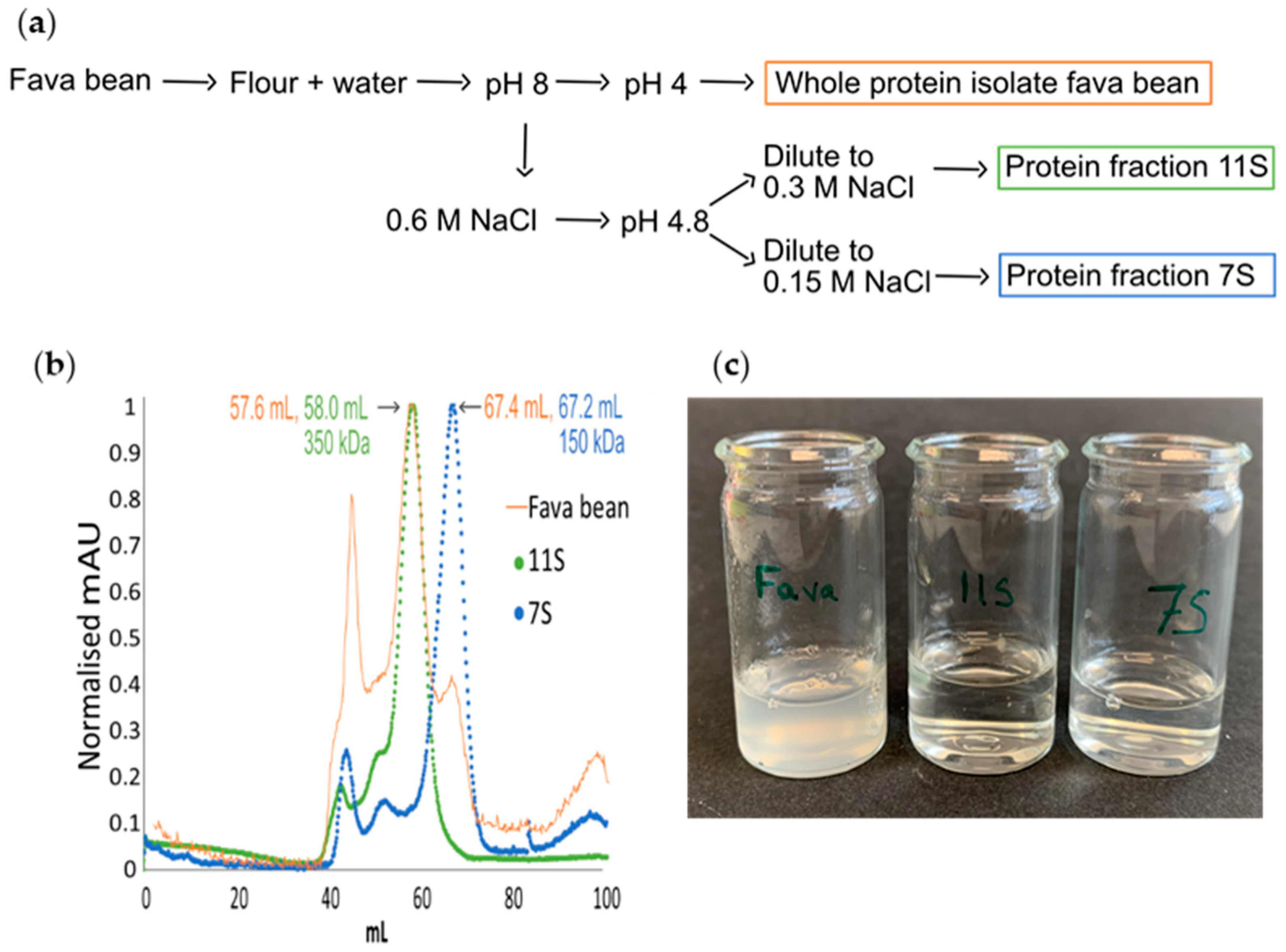
2.1. Extraction of Protein from Fava Bean, 11S, and 7S
2.1.1. Fava Bean Protein Isolate
2.1.2. 11S and 7S
- (i)
- The supernatant was diluted to double the volume with deionised water and centrifuged at 1000× g for 15 min. The supernatant was saved overnight at 4 °C, followed by another round of centrifugation at 1000× g (15 min). The obtained supernatant was diluted with cold distilled water to double the volume and centrifuged again at 1000× g for 15 min, and the pellet was collected and freeze-dried (7S).
- (ii)
- The pellet was dissolved in 1000 mL of 0.6 M NaCl solution and stirred to complete dissolution, followed by centrifugation at 5000× g (30 min). The supernatant was diluted with distilled water to double the volume. The solution was then left at room temperature, and the sediment was collected and freeze-dried (11S).
2.1.3. Protein Content
2.2. Size Exclusion Chromatography
2.3. Preparation of Protein Nanofibrils from Fava Bean, 11S, and 7S Protein Isolate
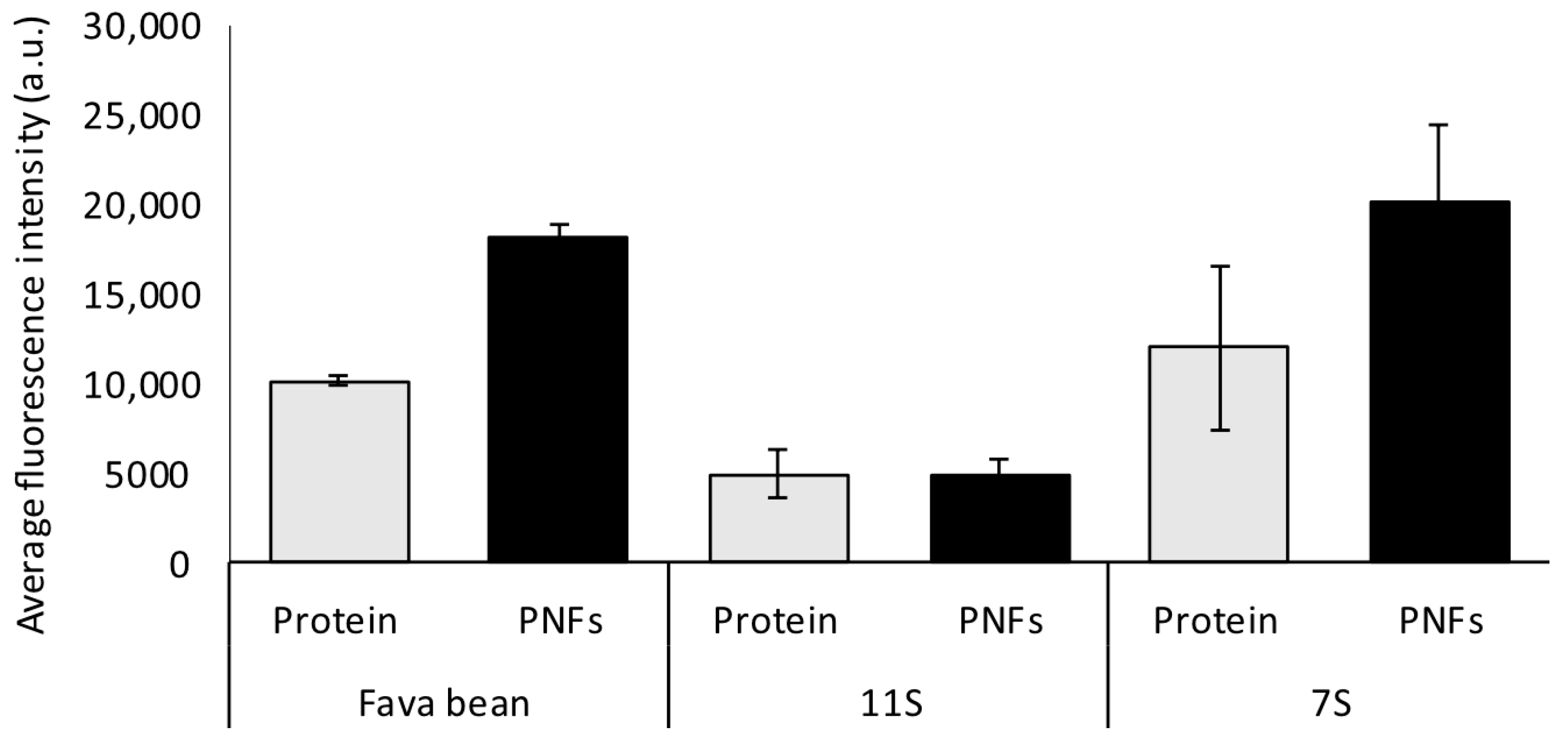
2.4. Thioflavin T Fluorescence
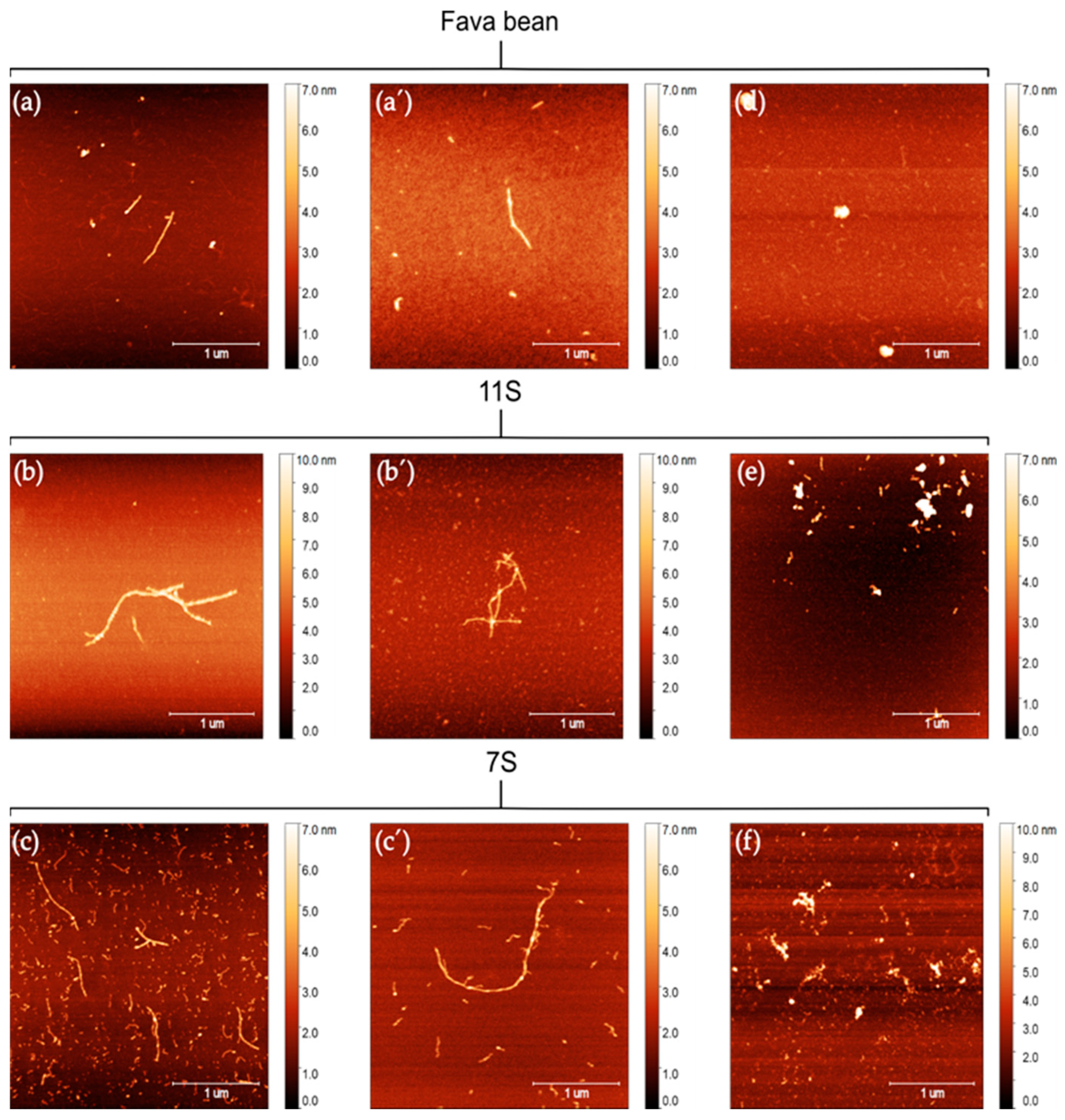
2.5. Atomic Force Microscopy
2.6. Rheological Measurements
2.7. Zeta Potential
2.8. Foaming Properties
2.9. Confocal Microscopy
3. Results and Discussion
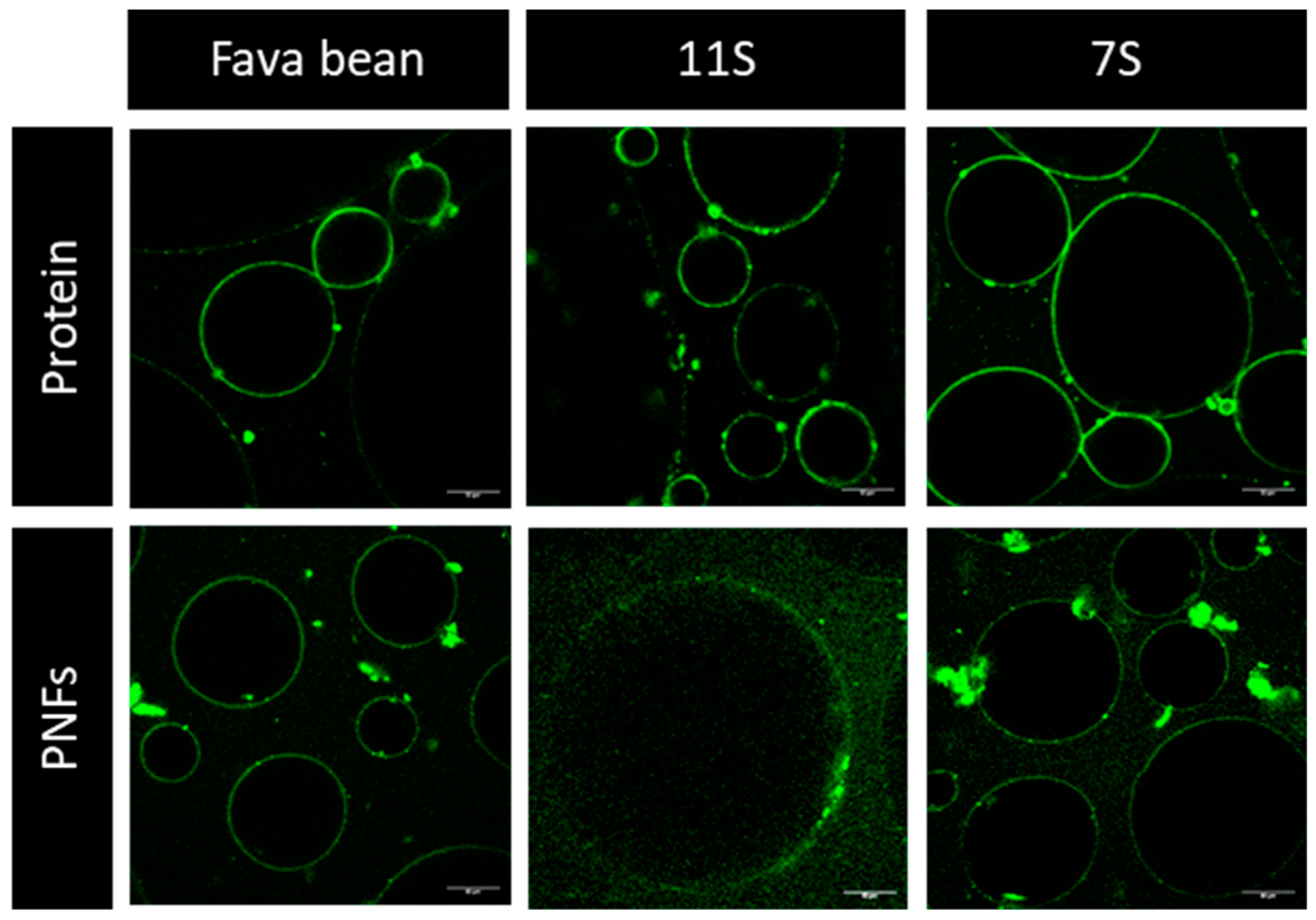
3.1. Characterisation of Protein and Nanofibrils
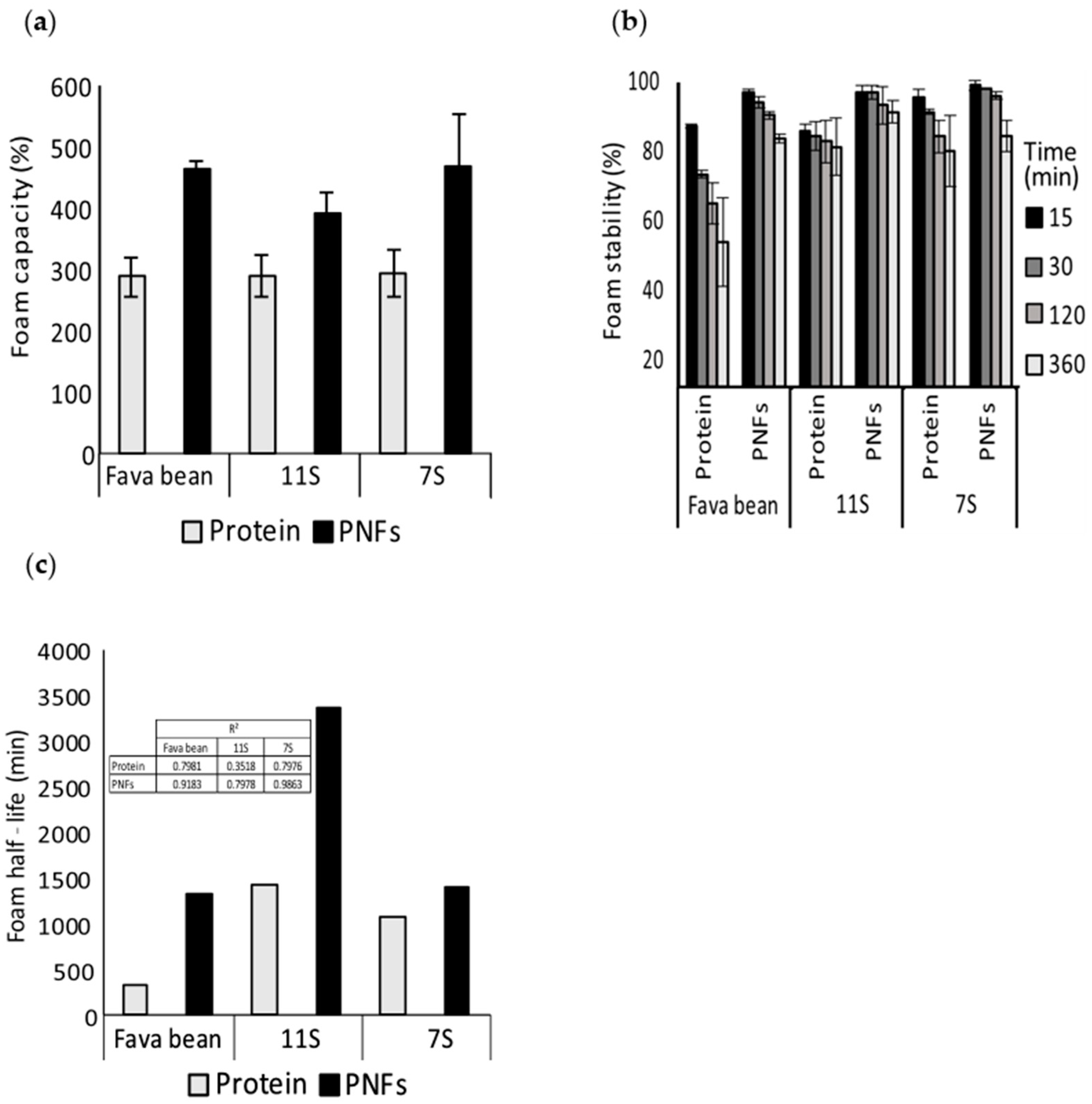
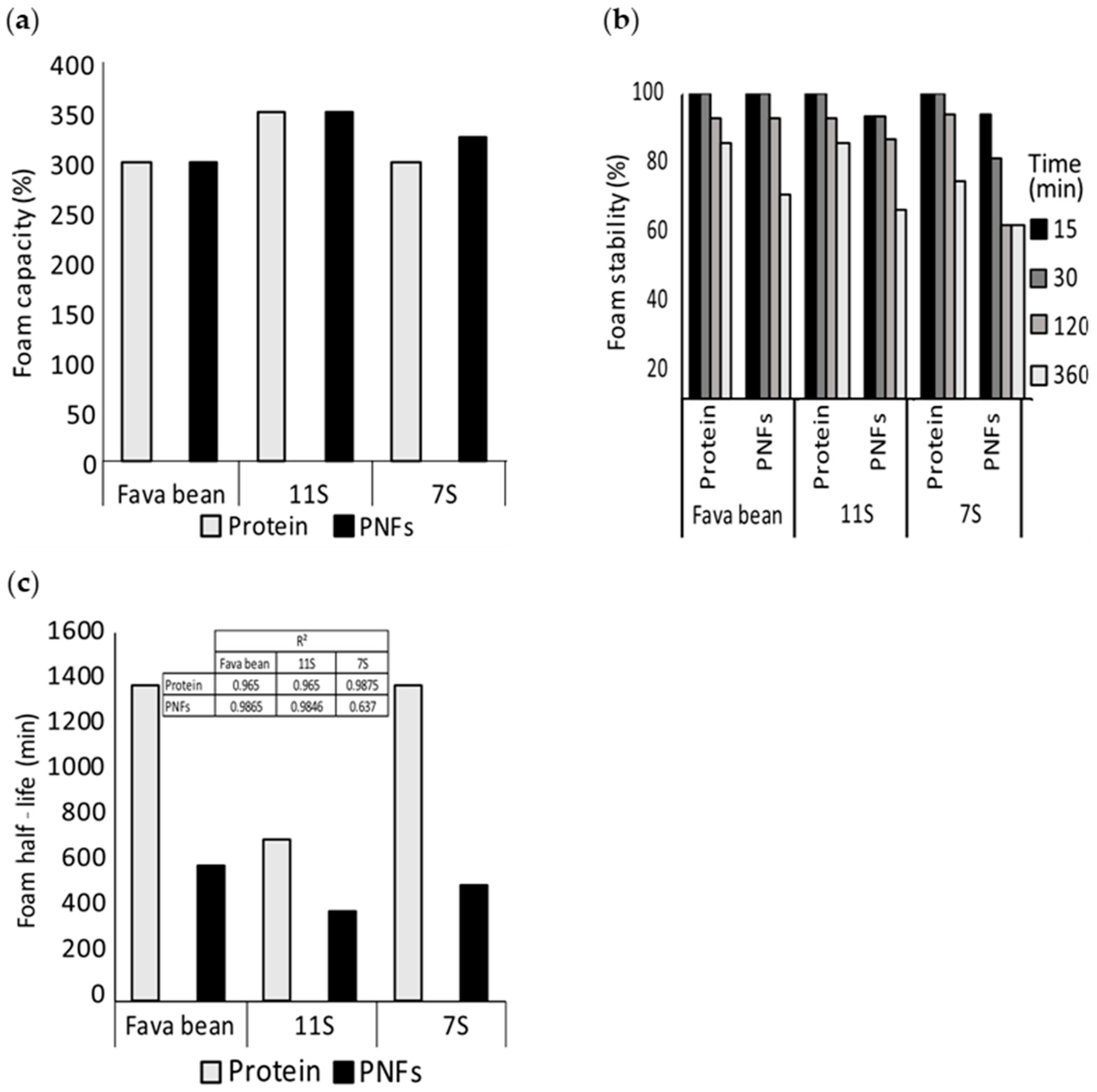
3.2. Foaming Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT-Lancet Commission on Healthy Diets from Sustainable Food Systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef] [PubMed]
- Crépon, K.; Marget, P.; Peyronnet, C.; Carrouée, B.; Arese, P.; Duc, G. Nutritional Value of Faba Bean (Vicia Faba L.) Seeds for Feed and Food. F. Crop. Res. 2010, 115, 329–339. [Google Scholar] [CrossRef]
- Warsame, A.O.; O’Sullivan, D.M.; Tosi, P. Seed Storage Proteins of Faba Bean (Vicia Faba L.): Current Status and Prospects for Genetic Improvement. J. Agric. Food Chem. 2018, 66, 12617–12626. [Google Scholar] [CrossRef] [PubMed]
- Lonkila, A.; Kaljonen, M. Promises of Meat and Milk Alternatives: An Integrative Literature Review on Emergent Research Themes. Agric. Human Values 2021, 38, 625–639. [Google Scholar] [CrossRef]
- Campbell, G.M.; Mougeot, E. Creation and Characterisation of Aerated Food Products. Trends Food Sci. Technol. 1999, 10, 283–296. [Google Scholar] [CrossRef]
- Narsimhan, G.; Xiang, N. Role of Proteins on Formation, Drainage, and Stability of Liquid Food Foams. Annu. Rev. Food Sci. Technol. 2018, 9, 45–63. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Lendel, C.; Langton, M.; Olsson, R.T.; Hedenqvist, M.S. Protein Nanofibrils: Preparation, Properties, and Possible Applications in Industrial Nanomaterials; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128157497. [Google Scholar]
- Cao, Y.; Mezzenga, R. Food Protein Amyloid Fibrils: Origin, Structure, Formation, Characterization, Applications and Health Implications. Adv. Colloid Interface Sci. 2019, 269, 334–356. [Google Scholar] [CrossRef]
- Hill, S.E.; Miti, T.; Richmond, T.; Muschol, M. Spatial Extent of Charge Repulsion Regulates Assembly Pathways for Lysozyme Amyloid Fibrils. PLoS ONE 2011, 6, e18171. [Google Scholar] [CrossRef]
- Herneke, A.; Lendel, C.; Johansson, D.; Newson, W.; Hedenqvist, M.; Karkehabadi, S.; Jonsson, D.; Langton, M. Protein Nanofibrils for Sustainable Food–Characterization and Comparison of Fibrils from a Broad Range of Plant Protein Isolates. ACS Food Sci. Technol. 2021, 1, 854–864. [Google Scholar] [CrossRef]
- Pereira, I.; Gomes, A.M.P.; Pintado, M.E.; Madureira, A.R.; Malcata, F.X. Bovine Whey Proteins—Overview on Their Main Biological Properties. Food Res. Int. 2007, 40, 1197–1211. [Google Scholar] [CrossRef]
- Langton, M.; Ehsanzamir, S.; Karkehabadi, S.; Feng, X.; Johansson, M.; Johansson, D.P. Gelation of Faba Bean Proteins—Effect of Extraction Method, PH and NaCl. Food Hydrocoll. 2020, 103, 105622. [Google Scholar] [CrossRef]
- Duranti, M.; Consonni, A.; Magni, C.; Sessa, F.; Scarafoni, A. The Major Proteins of Lupin Seed: Characterisation and Molecular Properties for Use as Functional and Nutraceutical Ingredients. Trends Food Sci. Technol. 2008, 19, 624–633. [Google Scholar] [CrossRef]
- Mäkinen, O.E.; Sozer, N.; Ercili-Cura, D.; Poutanen, K. Protein From Oat: Structure, Processes, Functionality, and Nutrition; Academic Press: Cambridge, MA, USA, 2016; ISBN 9780128027769. [Google Scholar]
- Wanasundara, J.P.D.; Abeysekara, S.J.; McIntosh, T.C.; Falk, K.C. Solubility Differences of Major Storage Proteins of Brassicaceae Oilseeds. JAOCS, J. Am. Oil Chem. Soc. 2012, 89, 869–881. [Google Scholar] [CrossRef]
- Barac, M.; Stanojevic, S.; Jovanovic, S.; Pesic, M. Soy Protein Modification: A Review. Acta Period. Technol. 2004, 280, 3–16. [Google Scholar] [CrossRef]
- Oboroceanu, D.; Wang, L.; Magner, E.; Auty, M.A.E. Fibrillization of Whey Proteins Improves Foaming Capacity and Foam Stability at Low Protein Concentrations. J. Food Eng. 2014, 121, 102–111. [Google Scholar] [CrossRef]
- Peng, D.; Yang, J.; Li, J.; Tang, C.; Li, B. Foams Stabilized by β-Lactoglobulin Amyloid Fibrils: Effect of PH. J. Agric. Food Chem. 2017, 65, 10658–10665. [Google Scholar] [CrossRef]
- Wan, Z.; Yang, X.; Sagis, L.M.C. Contribution of Long Fibrils and Peptides to Surface and Foaming Behavior of Soy Protein Fibril System. Langmuir 2016, 32, 8092–8101. [Google Scholar] [CrossRef]
- Herneke, A.; Karkehabadi, S.; Lu, J.; Lendel, C.; Langton, M. Protein Nanofibrils from Mung Bean: The Effect of PH on Morphology and the Ability to Form and Stabilise Foams. Food Hydrocoll. 2023, 136, 108315. [Google Scholar] [CrossRef]
- Biancalana, M.; Koide, S. Molecular Mechanism of Thioflavin-T Binding to Amyloid Fibrils. Biochim. Biophys. Acta—Proteins Proteom. 2010, 1804, 1405–1412. [Google Scholar] [CrossRef]
- Suchkov, V.V.; Popello, I.A.; Grinberg, V.Y.; Tolstogusov, V.B. Isolation and Purification of 7S and 11S Globulins from Broad Beans and Peas. J. Agric. Food Chem. 1990, 38, 92–95. [Google Scholar] [CrossRef]
- Mossé, J. Nitrogen to Protein Conversion Factor for Ten Cereals and Six Legumes or Oilseeds. A Reappraisal of Its Definition and Determination. Variation According to Species and to Seed Protein Content. J. Agric. Food Chem. 1990, 38, 18–24. [Google Scholar] [CrossRef]
- Johansson, M.; Johansson, D.; Ström, A.; Rydén, J.; Nilsson, K.; Karlsson, J.; Moriana, R.; Langton, M. Effect of Starch and Fibre on Faba Bean Protein Gel Characteristics. Food Hydrocoll. 2022, 131, 107741. [Google Scholar] [CrossRef]
- Tang, C.H.; Wang, C.S. Formation and Characterization of Amyloid-like Fibrils from Soy β-Conglycinin and Glycinin. J. Agric. Food Chem. 2010, 58, 11058–11066. [Google Scholar] [CrossRef]
- Kimura, A.; Takako, F.; Meili, Z.; Shiori, M.; Maruyama, N.; Utsumi, S. Comparison of Physicochemical Properties of 7S and 11S Globulins from Pea, Fava Bean, Cowpea, and French Bean with Those of Soybean-French Bean 7S Globulin Exhibits Excellent Properties. J. Agric. Food Chem. 2008, 56, 10273–10279. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.; Campanella, O.G.; Jones, O. Electrostatic Stabilization of β-Lactoglobulin Fibrils at Increased PH with Cationic Polymers. Biomacromolecules 2014, 15, 3119–3127. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Guo, S. The Formation and Disaggregation of Soy Protein Isolate Fibril: Effects of PH. Food Biophys. 2019, 14, 164–172. [Google Scholar] [CrossRef]
- Loveday, S.M.; Su, J.; Rao, M.A.; Anema, S.G.; Singh, H. Whey Protein Nanofibrils: The Environment-Morphology-Functionality Relationship in Lyophilization, Rehydration, and Seeding. J. Agric. Food Chem. 2012, 60, 5229–5236. [Google Scholar] [CrossRef]
- Kamada, A.; Herneke, A.; Lopez-Sanchez, P.; Harder, C.; Ornithopoulou, E.; Wu, Q.; Wei, X.; Schwartzkopf, M.; Müller-Buschbaum, P.; Roth, S.V.; et al. Hierarchical Propagation of Structural Features in Protein Nanomaterials. Nanoscale 2022, 14, 2502–2510. [Google Scholar] [CrossRef]
- Lendel, C.; Solin, N. Protein Nanofibrils and Their Use as Building Blocks of Sustainable Materials. RSC Adv. 2021, 11, 39188–39215. [Google Scholar] [CrossRef]
- Vogelsang-o’Dwyer, M.; Petersen, I.L.; Joehnke, M.S.; Sørensen, J.C.; Bez, J.; Detzel, A.; Busch, M.; Krueger, M.; Mahony, J.A.O.; Arendt, E.K.; et al. Comparison of Faba Bean Protein Ingredients Environmental Performance. Foods 2020, 9, 322. [Google Scholar]
- Jiang, J.; Wang, Q.; Xiong, Y.L. A PH Shift Approach to the Improvement of Interfacial Properties of Plant Seed Proteins. Curr. Opin. Food Sci. 2018, 19, 50–56. [Google Scholar] [CrossRef]

| Sample | pH | K (Pa.s) | n |
|---|---|---|---|
| Fava bean | 2 | 0.217 ± 0.016 | −0.770 ± 0.021 |
| 7 | 0.009 ± 0.001 | −0.293 ± 0.081 | |
| 11S | 2 | 0.087 ± 0.063 | −0.727 ± 0.005 |
| 7 | 0.011 ± 0.047 | −0.360 ± 0.129 | |
| 7S | 2 | 0.076 ± 0.012 | −0.720 ± 0.029 |
| 7 | 0.013 ± 0.007 | −0.424 ± 0.014 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herneke, A.; Lendel, C.; Karkehabadi, S.; Lu, J.; Langton, M. Protein Nanofibrils from Fava Bean and Its Major Storage Proteins: Formation and Ability to Generate and Stabilise Foams. Foods 2023, 12, 521. https://doi.org/10.3390/foods12030521
Herneke A, Lendel C, Karkehabadi S, Lu J, Langton M. Protein Nanofibrils from Fava Bean and Its Major Storage Proteins: Formation and Ability to Generate and Stabilise Foams. Foods. 2023; 12(3):521. https://doi.org/10.3390/foods12030521
Chicago/Turabian StyleHerneke, Anja, Christofer Lendel, Saeid Karkehabadi, Jing Lu, and Maud Langton. 2023. "Protein Nanofibrils from Fava Bean and Its Major Storage Proteins: Formation and Ability to Generate and Stabilise Foams" Foods 12, no. 3: 521. https://doi.org/10.3390/foods12030521
APA StyleHerneke, A., Lendel, C., Karkehabadi, S., Lu, J., & Langton, M. (2023). Protein Nanofibrils from Fava Bean and Its Major Storage Proteins: Formation and Ability to Generate and Stabilise Foams. Foods, 12(3), 521. https://doi.org/10.3390/foods12030521