Physicochemical and Functional Properties of Texturized Vegetable Proteins and Cooked Patty Textures: Comprehensive Characterization and Correlation Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Proximate Composition of TVP
2.3. Protein Solubility
2.4. Water Absorption Capacity and Oil Absorption Capacity
2.5. Viscosity
2.6. Bulk Density
2.7. Rehydration Capacity
2.8. Textural Properties of Rehydrated TVP
2.9. Preparation of TVP Patties
2.10. Determination of Cooking Properties
2.11. Textural Property of TVP-Based Patty
2.12. Shear Force Measurement
2.13. Compressed Juiciness
2.14. Statistical Analysis
3. Results and Discussion
3.1. Proximate Compositions of TVP
3.2. Physicochemical Characteristics of TVP
3.3. RVA Pasting Properties of TVP
3.4. Bulk Density
3.5. Rehydration Property
3.6. Textural Properties of TVP
3.7. Cooking Properties of TVP-Based Patties
3.8. Textural Properties of TVP-Based Patties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sha, L.; Xiong, Y.L. Plant protein-based alternatives of reconstructed meat: Science, technology, and challenges. Trends Food Sci. Technol. 2020, 102, 51–61. [Google Scholar] [CrossRef]
- Bakhsh, A.; Lee, S.-J.; Lee, E.-Y.; Hwang, Y.-H.; Joo, S.-T. Evaluation of rheological and sensory characteristics of plant-based meat analog with comparison to beef and pork. Food Sci. Anim. Resour. 2021, 41, 983. [Google Scholar] [CrossRef] [PubMed]
- Tuso, P.; Stoll, S.R.; Li, W.W. A plant-based diet, atherogenesis, and coronary artery disease prevention. Perm. J. 2015, 19, 62. [Google Scholar] [CrossRef] [PubMed]
- Ball, J.J.; Wyatt, R.P.; Lambert, B.D.; Smith, H.R.; Reyes, T.M.; Sawyer, J.T. Influence of Plant-Based Proteins on the Fresh and Cooked Characteristics of Ground Beef Patties. Foods 2021, 10, 1971. [Google Scholar] [CrossRef]
- Shen, Y.; Hong, S.; Du, Z.; Chao, M.; O’Quinn, T.; Li, Y. Effect of adding modified pea protein as functional extender on the physical and sensory properties of beef patties. LWT 2022, 154, 112774. [Google Scholar] [CrossRef]
- Maningat, C.C.; Jeradechachai, T.; Buttshaw, M.R. Textured wheat and pea proteins for meat alternative applications. Cereal Chem. 2022, 99, 37–66. [Google Scholar] [CrossRef]
- Kyriakopoulou, K.; Keppler, J.K.; van der Goot, A.J. Functionality of ingredients and additives in plant-based meat analogues. Foods 2021, 10, 600. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, B.; Wei, Y. Effects of the specific mechanical energy on the physicochemical properties of texturized soy protein during high-moisture extrusion cooking. J. Food Eng. 2014, 121, 32–38. [Google Scholar] [CrossRef]
- Webb, D.; Plattner, B.J.; Donald, E.; Funk, D.; Plattner, B.S.; Alavi, S. Role of chickpea flour in texturization of extruded pea protein. J. Food Sci. 2020, 85, 4180–4187. [Google Scholar] [CrossRef]
- Asgar, M.; Fazilah, A.; Huda, N.; Bhat, R.; Karim, A. Nonmeat protein alternatives as meat extenders and meat analogs. Compr. Rev. Food Sci. Food Saf. 2010, 9, 513–529. [Google Scholar] [CrossRef]
- Samard, S.; Ryu, G.H. Physicochemical and functional characteristics of plant protein-based meat analogs. J. Food Process. Preserv. 2019, 43, e14123. [Google Scholar] [CrossRef]
- Brishti, F.H.; Chay, S.Y.; Muhammad, K.; Ismail-Fitry, M.R.; Zarei, M.; Saari, N. Texturized mung bean protein as a sustainable food source: Effects of extrusion on its physical, textural and protein quality. Innov. Food Sci. Emerg. Technol. 2021, 67, 102591. [Google Scholar] [CrossRef]
- Rehrah, D.; Ahmedna, M.; Goktepe, I.; Yu, J. Extrusion parameters and consumer acceptability of a peanut-based meat analogue. Int. J. Food Sci. Technol. 2009, 44, 2075–2084. [Google Scholar] [CrossRef]
- Hidayat, B.; Wea, A.; Andriati, N. Physicochemical, sensory attributes and protein profile by SDS-PAGE of beef sausage substituted with texturized vegetable protein. Food Res. 2018, 2, 20–31. [Google Scholar] [CrossRef]
- Bakhsh, A.; Lee, S.-J.; Lee, E.-Y.; Hwang, Y.-H.; Joo, S.-T. Characteristics of Beef Patties Substituted by Different Levels of Textured Vegetable Protein and Taste Traits Assessed by Electronic Tongue System. Foods 2021, 10, 2811. [Google Scholar] [CrossRef]
- Kassama, L.; Ngadi, M.; Raghavan, G. Structural and instrumental textural properties of meat patties containing soy protein. Int. J. Food Prop. 2003, 6, 519–529. [Google Scholar] [CrossRef]
- Vatansever, S.; Tulbek, M.C.; Riaz, M.N. Low-and high-moisture extrusion of pulse proteins as plant-based meat ingredients: A review. Cereal Foods World 2020, 65, 12–14. [Google Scholar]
- Samard, S.; Gu, B.-Y.; Kim, M.-H.; Ryu, G.-H. Influences of extrusion parameters on physicochemical properties of textured vegetable proteins and its meatless burger patty. Food Sci. Biotechnol. 2021, 30, 395–403. [Google Scholar] [CrossRef]
- Gui, Y.; Chen, G.; Tian, W.; Yang, S.; Chen, J.; Wang, F.; Li, Y. Normal rice flours perform better in gluten-free bread than glutinous rice flours. J. Food Sci. 2022, 87, 554–566. [Google Scholar] [CrossRef]
- Samard, S.; Ryu, G.H. A comparison of physicochemical characteristics, texture, and structure of meat analogue and meats. J. Sci. Food Agric. 2019, 99, 2708–2715. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.; Liu, H.; Yoon, A.; Rizvi, S.S.; Wang, Q. Changes in conformation and quality of vegetable protein during texturization process by extrusion. Crit. Rev. Food Sci. Nutr. 2019, 59, 3267–3280. [Google Scholar] [CrossRef]
- Smetana, S.; Pernutz, C.; Toepfl, S.; Heinz, V.; Van Campenhout, L. High-moisture extrusion with insect and soy protein concentrates: Cutting properties of meat analogues under insect content and barrel temperature variations. J. Insects Food Feed. 2019, 5, 29–34. [Google Scholar] [CrossRef]
- Pietsch, V.L.; Bühler, J.M.; Karbstein, H.P.; Emin, M.A. High moisture extrusion of soy protein concentrate: Influence of thermomechanical treatment on protein-protein interactions and rheological properties. J. Food Eng. 2019, 251, 11–18. [Google Scholar] [CrossRef]
- Osen, R.; Toelstede, S.; Wild, F.; Eisner, P.; Schweiggert-Weisz, U. High moisture extrusion cooking of pea protein isolates: Raw material characteristics, extruder responses, and texture properties. J. Food Eng. 2014, 127, 67–74. [Google Scholar] [CrossRef]
- Hager, D.F. Effects of extrusion upon soy concentrate solubility. J. Agric. Food Chem. 1984, 32, 293–296. [Google Scholar] [CrossRef]
- Osen, R.; Toelstede, S.; Eisner, P.; Schweiggert-Weisz, U. Effect of high moisture extrusion cooking on protein–protein interactions of pea (Pisum sativum L.) protein isolates. Int. J. Food Sci. Technol. 2015, 50, 1390–1396. [Google Scholar] [CrossRef]
- Liu, K.; Hsieh, F.-H. Protein–protein interactions during high-moisture extrusion for fibrous meat analogues and comparison of protein solubility methods using different solvent systems. J. Agric. Food Chem. 2008, 56, 2681–2687. [Google Scholar] [CrossRef]
- Chen, F.L.; Wei, Y.M.; Zhang, B. Chemical cross-linking and molecular aggregation of soybean protein during extrusion cooking at low and high moisture content. LWT-Food Sci. Technol. 2011, 44, 957–962. [Google Scholar] [CrossRef]
- Branch, S.; Maria, S. Evaluation of the functional properties of mung bean protein isolate for development of textured vegetable protein. Int. Food Res. J. 2017, 24, 1595–1605. [Google Scholar]
- Joshi, A.U.; Liu, C.; Sathe, S.K. Functional properties of select seed flours. LWT-Food Sci. Technol. 2015, 60, 325–331. [Google Scholar] [CrossRef]
- Badar, H. Functional properties of maize flour and its blends with wheat flour: Optimization of preparation conditions by response surface methodology. Pak. J. Bot 2013, 45, 2027–2035. [Google Scholar]
- Webb, D.M. Physicochemical Properties of Pea Proteins, Texturization Using Extrusion, and Application in Plant-Based Meats. Master’s Thesis, Kansas State University, Manhattan, KS, USA, 2021. [Google Scholar]
- Philipp, C.; Oey, I.; Silcock, P.; Beck, S.M.; Buckow, R. Impact of protein content on physical and microstructural properties of extruded rice starch-pea protein snacks. J. Food Eng. 2017, 212, 165–173. [Google Scholar] [CrossRef]
- Breene, W.M.; Barker, T.G. Development and application of a texture measurement procedure for textured vegetable protein. J. Texture Stud. 1975, 6, 459–472. [Google Scholar] [CrossRef]
- Wi, G.; Bae, J.; Kim, H.; Cho, Y.; Choi, M.-J. Evaluation of the physicochemical and structural properties and the sensory characteristics of meat analogues prepared with various non-animal based liquid additives. Foods 2020, 9, 461. [Google Scholar] [CrossRef]
- Lin, S.; Huff, H.; Hsieh, F. Texture and chemical characteristics of soy protein meat analog extruded at high moisture. J. Food Sci. 2000, 65, 264–269. [Google Scholar] [CrossRef]
- Shelar, G.A.; Gaikwad, S.T. Extrusion in food processing: An overview. Pharma Innov. J. 2019, 8, 562–568. [Google Scholar]
- Serdaroglu, M. The characteristics of beef patties containing different levels of fat and oat flour. Int. J. Food Sci. Technol. 2006, 41, 147–153. [Google Scholar] [CrossRef]
- Kamani, M.H.; Meera, M.S.; Bhaskar, N.; Modi, V.K. Partial and total replacement of meat by plant-based proteins in chicken sausage: Evaluation of mechanical, physico-chemical and sensory characteristics. J. Food Sci. Technol. 2019, 56, 2660–2669. [Google Scholar] [CrossRef]
- Yi, H.C.; Cho, H.; Hong, J.J.; Ryu, R.K.; Hwang, K.T.; Regenstein, J.M. Physicochemical and organoleptic characteristics of seasoned beef patties with added glutinous rice flour. Meat Sci. 2012, 92, 464–468. [Google Scholar] [CrossRef]
- Sakai, K.; Sato, Y.; Okada, M.; Yamaguchi, S. Improved functional properties of meat analogs by laccase catalyzed protein and pectin crosslinks. Sci. Rep. 2021, 11, 16631. [Google Scholar] [CrossRef]
- Kurt, Ş.; Kilincceker, O. The effects of cereal and legume flours on the quality characteristics of beef patties. Kafkas Üniversitesi Vet. Fakültesi Derg. 2012, 18, 725–730. [Google Scholar] [CrossRef][Green Version]
- Bakhsh, A.; Lee, S.-J.; Lee, E.-Y.; Sabikun, N.; Hwang, Y.-H.; Joo, S.-T. A novel approach for tuning the physicochemical, textural, and sensory characteristics of plant-based meat analogs with different levels of methylcellulose concentration. Foods 2021, 10, 560. [Google Scholar] [CrossRef]
- Sharima-Abdullah, N.; Hassan, C.; Arifin, N.; Huda-Faujan, N. Physicochemical properties and consumer preference of imitation chicken nuggets produced from chickpea flour and textured vegetable protein. Int. Food Res. J. 2018, 25, 1016–1025. [Google Scholar]
- Barbut, S.; Marangoni, A. Organogels use in meat processing–Effects of fat/oil type and heating rate. Meat Sci. 2019, 149, 9–13. [Google Scholar] [CrossRef]
- Palanisamy, M.; Töpfl, S.; Aganovic, K.; Berger, R.G. Influence of iota carrageenan addition on the properties of soya protein meat analogues. LWT 2018, 87, 546–552. [Google Scholar] [CrossRef]

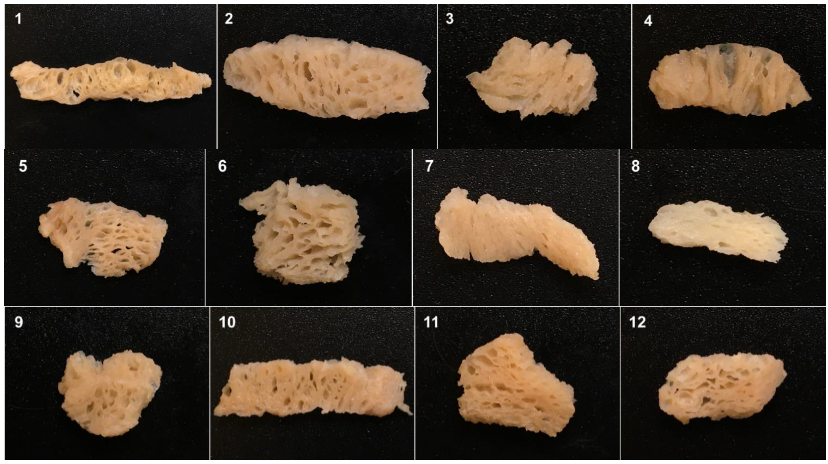
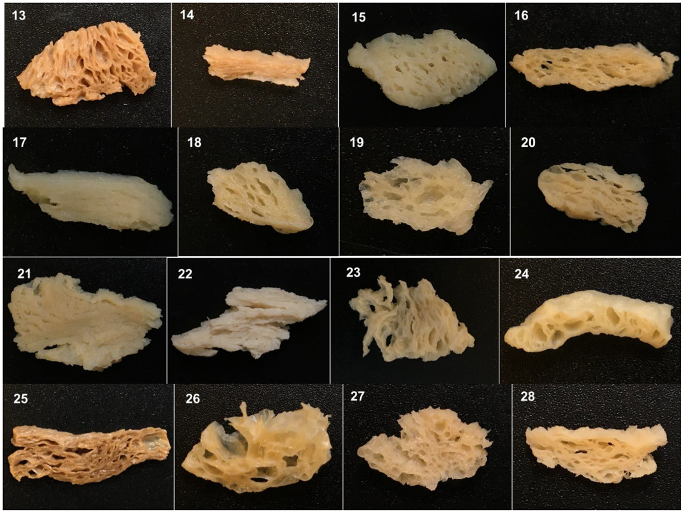
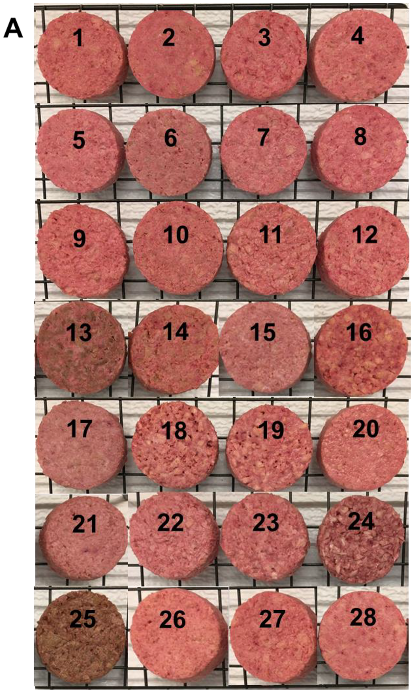

| Sample A | Protein Content (%) | Moisture Content (%) | Ash Content (%) | Fat Content (%) | Total Carbohydrate (%) |
|---|---|---|---|---|---|
| 1 | 51.1 ± 0.1 no | 7.2 ± 0.08 f | 6.1 ± 0.02 g | 2.2 ± 0.3 jkl | 33.4 |
| 2 | 51.4 ± 0.2 mn | 8.1 ± 0.03 bc | 6.0 ± 0.03 h | 2.5 ± 0.1 hi | 32.0 |
| 3 | 51.1 ± 0.0 no | 7.1 ± 0.09 g | 6.2 ± 0.01 f | 2.3 ± 0.0 ijk | 33.4 |
| 4 | 52.1 ± 0.1 l | 6.5 ± 0.07 k | 6.3 ± 0.01 d | 2.2 ± 0.0 klm | 32.9 |
| 5 | 51.3 ± 0.2 mn | 6.1 ± 0.06 m | 6.5 ± 0.04 b | 2.2 ± 0.2 ijk | 33.9 |
| 6 | 55.7 ± 0.1 k | 7.3 ± 0.07 e | 7.1 ± 0.01 a | 2.4 ± 0.1 hij | 27.4 |
| 7 | 50.5 ± 0.5 pq | 7.8 ± 0.02 d | 6.4 ± 0.00 cd | 2.1 ± 0.1 klm | 33.2 |
| 8 | 51.0 ± 0.0 no | 7.2 ± 0.02 f | 5.6 ± 0.04 i | 7.9 ± 0.2 b | 28.3 |
| 9 | 50.0 ± 0.0 q | 6.9 ± 0.03 h | 6.4 ± 0.02 c | 2.2 ± 0.0 ijk | 34.4 |
| 10 | 51.5 ± 0.0 mn | 7.8 ± 0.01 d | 6.3 ± 0.02 e | 1.9 ± 0.2 m | 32.5 |
| 11 | 51.7 ± 0.1 lm | 8.1 ± 0.02 bc | 6.3 ± 0.02 e | 2.3 ± 0.0 ijk | 31.6 |
| 12 | 50.5 ± 0.1 p | 6.7 ± 0.08 i | 6.5 ± 0.02 b | 2.1 ± 0.0 klm | 34.2 |
| 13 | 50.7 ± 0.0 op | 6.6 ± 0.01 j | 6.2 ± 0.06 f | 2.4 ± 0.1 hij | 34.1 |
| 14 | 50.5 ± 0.0 p | 5.4 ± 0.02 p | 6.2 ± 0.02 f | 2.5 ± 0.0 h | 35.4 |
| 15 | 76.6 ± 0.3 a | 5.8 ± 0.01 o | 3.9 ± 0.02 o | 4.9 ± 0.2 e | 8.8 |
| 16 | 74.0 ± 0.3 d | 6.0 ± 0.02 n | 5.6 ± 0.01 i | 5.0 ± 0.1 e | 9.4 |
| 17 | 74.1 ± 0.2 cd | 7.3 ± 0.03 e | 5.5 ± 0.03 j | 4.8 ± 0.2 e | 8.3 |
| 18 | 75.1 ± 0.4 b | 6.2 ± 0.01 l | 5.4 ± 0.01 j | 8.5 ± 0.1 a | 4.8 |
| 19 | 74.5 ± 0.1 c | 7.0 ± 0.02 g | 5.1 ± 0.00 k | 8.1 ± 0.0 b | 5.3 |
| 20 | 62.4 ± 0.2 j | 6.9 ± 0.03 h | 3.8 ± 0.03 p | 6.0 ± 0.2 d | 20.9 |
| 21 | 71.8 ± 0.1 e | 8.1 ± 0.04 bc | 4.9 ± 0.04 l | 4.9 ± 0.2 e | 10.2 |
| 22 | 66.1 ± 0.4 h | 8.2 ± 0.04 b | 2.4 ± 0.01 t | 2.8 ± 0.1 g | 20.5 |
| 23 | 70.5 ± 0.1 f | 8.1 ± 0.03 c | 2.6 ± 0.08 s | 3.1 ± 0.1 f | 15.7 |
| 24 | 72.1 ± 0.1 e | 7.3 ± 0.03 e | 3.0 ± 0.04 q | 2.9 ± 0.1 fg | 14.7 |
| 25 | 64.4 ± 0.3 i | 8.5 ± 0.05 a | 2.7 ± 0.03 r | 2.4 ± 0.0 hij | 22.0 |
| 26 | 50.4 ± 0.0 pq | 8.1 ± 0.01 bc | 4.6 ± 0.01 n | 2.0 ± 0.2 lm | 34.9 |
| 27 | 68.3 ± 0.1 g | 5.5 ± 0.03 p | 4.9 ± 0.03 l | 6.6 ± 0.1 c | 14.8 |
| 28 | 66.2 ± 0.2 h | 4.8 ± 0.01 q | 4.8 ± 0.04 m | 6.0 ± 0.1 d | 18.2 |
| Ave. soy | 51.4 ± 1.4 c | 7.9 ± 0.8 b | 6.3 ± 0.3 a | 2.7 ± 1.5 b | 32.6 ± 2.3 a |
| Ave. pea | 72.7 ± 4.7 a | 6.8 ± 0.8 b | 4.9 ± 0.8 b | 6.0 ± 1.6 a | 9.7 ± 5.4 c |
| Ave. wheat | 68.3 ± 3.6 b | 8.0 ± 0.5 a | 2.7 ± 0.2 c | 2.8 ± 0.3 b | 18.2 ± 3.6 b |
| Average B | 60.2 | 7.0 | 5.3 | 3.8 | 23.8 |
| LSD (5%) C | 0.4 | 0.1 | 0.1 | 0.2 | - |
| PC | MC | AC | FC | CC | BD | RHC | WAC | OAC | PS | PV | PT | FV | T-H | T-R | T-CO | T-S | T-CH | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PC | 1 | −0.162 | −0.611** | 0.628 ** | −0.984 ** | −0.661 ** | 0.237 | −0.073 | 0.711 ** | −0.775 ** | 0.057 | −0.652 ** | −0.274 | −0.237 | 0.386 * | 0.057 | 0.431 * | −0.182 |
| MC | 1 | −0.221 | −0.380 * | 0.164 | 0.094 | −0.226 | 0.250 | −0.394 * | −0.191 | 0.055 | −0.294 | −0.190 | 0.095 | −0.016 | −0.163 | 0.035 | 0.080 | |
| AC | 1 | −0.205 | 0.518 ** | 0.529 ** | −0.160 | 0.180 | −0.351 | 0.857 ** | −0.096 | 0.620 ** | 0.416 * | 0.340 | −0.270 | 0.014 | −0.403 * | 0.305 | ||
| FC | 1 | −0.732 ** | −0.609 ** | 0.374 * | −0.383 * | 0.852 ** | −0.195 | −0.201 | −0.321 | −0.259 | −0.170 | 0.214 | 0.418 * | 0.342 | −0.102 | |||
| CC | 1 | 0.674 ** | −0.258 | 0.099 | −0.763 ** | 0.687 ** | −0.008 | 0.633 ** | 0.277 | 0.208 | −0.375 * | −0.122 | −0.431 * | 0.149 | ||||
| BD | 1 | −0.221 | 0.393 * | −0.552 ** | 0.591 ** | 0.225 | 0.300 | 0.509 ** | 0.141 | −0.665 ** | −0.607 ** | −0.724 ** | −0.002 | |||||
| RHC | 1 | 0.032 | 0.362 | −0.019 | 0.554 ** | −0.190 | 0.404 * | −0.765 ** | −0.149 | 0.062 | 0.107 | −0.737 ** | ||||||
| WAC | 1 | −0.337 | 0.023 | 0.621 ** | −0.041 | 0.549 ** | 0.102 | −0.113 | −0.446 * | −0.082 | 0.049 | |||||||
| OAC | 1 | −0.325 | −0.071 | −0.513 ** | −0.249 | −0.234 | 0.239 | 0.312 | 0.181 | −0.188 | ||||||||
| PS | 1 | −0.133 | 0.615 ** | 0.388 * | 0.203 | −0.387 * | 0.084 | −0.414 * | 0.170 | |||||||||
| PV | 1 | −0.199 | 0.778 ** | −0.470 * | −0.190 | −0.470 * | −0.131 | 0.537 ** | ||||||||||
| PT | 1 | 0.190 | 0.271 | −0.091 | 0.166 | −0.127 | 0.273 | |||||||||||
| FV | 1 | −0.277 | −0.368 | −0.432 * | −0.336 | −0.361 | ||||||||||||
| T-H | 1 | 0.217 | 0.192 | 0.025 | 0.977 ** | |||||||||||||
| T-R | 1 | 0.646 ** | 0.786 ** | 0.343 | ||||||||||||||
| T-CO | 1 | 0.634 ** | 0.335 | |||||||||||||||
| T-S | 1 | 0.167 | ||||||||||||||||
| T-CH | 1 | |||||||||||||||||
| CL | DS | MR | FR | CJ | P-H | P-R | P-CO | P-S | P-CH | SF | ||||||||
| PC | −0.073 | −0.047 | 0.046 | −0.173 | 0.149 | −0.102 | 0.577 ** | 0.519 ** | 0.515 ** | 0.434 * | −0.094 | |||||||
| MC | −0.302 | −0.305 | 0.222 | 0.148 | −0.033 | 0.050 | 0.078 | 0.411 * | 0.372 | 0.249 | 0.097 | |||||||
| AC | 0.111 | −0.016 | −0.357 | 0.189 | −0.257 | 0.318 | −0.382 * | −0.620 ** | −0.636 ** | −0.252 | 0.273 | |||||||
| FC | 0.104 | 0.099 | −0.116 | −0.111 | 0.153 | −0.108 | 0.279 | 0.214 | 0.216 | 0.181 | 0.009 | |||||||
| CC | 0.063 | 0.056 | 0.003 | 0.150 | −0.136 | 0.074 | −0.564 ** | −0.497 ** | −0.488 ** | −0.440 ** | 0.045 | |||||||
| BD | 0.076 | 0.032 | −0.105 | 0.040 | −0.105 | 0.023 | −0.506 ** | −0.514 ** | −0.323 | −0.444 * | −0.147 | |||||||
| RHC | 0.679 ** | 0.648 ** | −0.290 | −0.415 * | 0.812 ** | −0.791 ** | −0.147 | 0.405 * | 0.387 * | −0.412 * | −0.621 ** | |||||||
| WAC | 0.341 | 0.076 | −0.508 ** | −0.361 | 0.177 | −0.068 | −0.196 | −0.023 | 0.018 | −0.242 | −0.204 | |||||||
| OAC | −0.007 | −0.005 | −0.088 | −0.071 | 0.071 | −0.104 | 0.419 * | 0.191 | 0.286 | 0.255 | −0.112 | |||||||
| PS | 0.243 | 0.137 | −0.319 | 0.123 | −0.147 | 0.108 | −0.604 ** | −0.686 ** | −0.624 ** | −0.488 ** | 0.066 | |||||||
| PV | 0.605 ** | 0.478 * | −0.391 * | −0.601 ** | 0.579 ** | −0.599 ** | −0.168 | 0.295 | 0.220 | −0.396 * | −0.587 ** | |||||||
| PT | 0.116 | 0.134 | −0.143 | 0.065 | −0.191 | 0.186 | −0.414 * | −0.528 ** | −0.663 ** | −0.360 | 0.189 | |||||||
| FV | 0.660 ** | 0.456 * | −0.530 ** | −0.552 ** | 0.368 | −0.443 * | −0.497 ** | −0.185 | −0.200 | −0.629 ** | −0.479 ** | |||||||
| T-H | −0.495 ** | −0.622 ** | 0.051 | 0.347 | −0.722 ** | 0.885 ** | 0.076 | −0.458 * | −0.536 ** | 0.365 | 0.639 ** | |||||||
| T-R | −0.074 | −0.309 | −0.155 | 0.028 | −0.408 * | 0.398 * | 0.595 ** | 0.244 | −0.013 | 0.606 ** | 0.321 | |||||||
| T-CO | −0.035 | −0.210 | −0.015 | 0.252 | −0.212 | 0.261 | 0.246 | 0.027 | −0.148 | 0.299 | 0.293 | |||||||
| T-S | 0.126 | −0.090 | −0.056 | −0.082 | −0.006 | 0.079 | 0.416 * | 0.446 * | 0.218 | 0.385 * | 0.089 | |||||||
| T-CH | −0.497 ** | −0.644 ** | 0.077 | 0.401 * | −0.719 ** | 0.889 ** | 0.128 | −0.416 * | −0.520 ** | 0.410 * | 0.673 ** | |||||||
| CL | 1 | 0.786 ** | −0.655 ** | −0.684 ** | 0.595 ** | −0.618 ** | −0.445 * | 0.012 | −0.054 | −0.634 ** | −0.528 ** | |||||||
| DS | 1 | −0.333 | −0.528 ** | 0.721 ** | −0.714 ** | −0.412 * | 0.047 | −0.050 | −0.654 ** | −0.489 ** | ||||||||
| MR | 1 | 0.560 ** | −0.074 | 0.118 | 0.257 | 0.199 | 0.230 | 0.327 | 0.157 | |||||||||
| FR | 1 | −0.437 * | 0.537 ** | 0.390 * | −0.179 | −0.079 | 0.466 * | 0.405 * | ||||||||||
| CJ | 1 | −0.883 ** | −0.312 | 0.470 * | 0.454 * | −0.540 ** | −0.653 ** | |||||||||||
| P-H | 1 | 0.387 * | −0.379 * | −0.443 * | 0.639 ** | 0.778 ** | ||||||||||||
| P-R | 1 | 0.477 * | 0.404 * | 0.888 ** | 0.246 | |||||||||||||
| P-CO | 1 | 0.819 ** | 0.388 * | −0.215 | ||||||||||||||
| P-S | 1 | 0.280 | −0.400 * | |||||||||||||||
| P-CH | 1 | 0.543 ** | ||||||||||||||||
| SF | 1 | |||||||||||||||||
| Sample A | WAC (g/g) | OAC (g/g) | Solubility (%) | Pasting Property | ||
|---|---|---|---|---|---|---|
| Peak Viscosity (cP) | Peak Time (min) | Final Viscosity (cP) | ||||
| 1 | 2.1 ± 0.01 ij | 0.69 ± 0.01 t | 81.4 ± 0.2 e | 544 ± 1 q | 7.0 ± 0.0 a | 1263 ± 17 o |
| 2 | 2.1 ± 0.00 fgh | 0.71 ± 0.01 rs | 77.1 ± 0.6 j | 651 ± 4 p | 7.0 ± 0.0 a | 1519 ± 4 n |
| 3 | 2.2 ± 0.02 efg | 0.77 ± 0.00 l | 77.6 ± 0.1 j | 2786 ± 4 g | 7.0 ± 0.0 a | 5832 ± 52 d |
| 4 | 2.1 ± 0.05 gh | 0.70 ± 0.01 rs | 82.9 ± 0.1 d | 2848 ± 9 g | 7.0 ± 0.0 a | 6049 ± 8 c |
| 5 | 2.2 ± 0.03 ef | 0.74 ± 0.02 nop | 83.6 ± 0.1 c | 2938 ± 9 f | 7.0 ± 0.0 a | 6674 ± 60 b |
| 6 | 2.3 ± 0.01 d | 0.78 ± 0.01 k | 81.5 ± 0.2 e | 1448 ± 16 m | 7.0 ± 0.0 a | 2415 ± 35 k |
| 7 | 2.1 ± 0.02 hi | 0.74 ± 0.01 op | 80.5 ± 0.2 f | 3168 ± 10 e | 7.0 ± 0.0 a | 6088 ± 16 c |
| 8 | 2.0 ± 0.02 l | 0.84 ± 0.00 g | 85.0 ± 0.1 b | 1703 ± 18 k | 6.6 ± 0.1 a | 2876 ± 31 j |
| 9 | 1.9 ± 0.02 l | 0.76 ± 0.00 lm | 90.3 ± 0.1 a | 2084 ± 16 i | 7.0 ± 0.0 a | 4615 ± 24 g |
| 10 | 2.2 ± 0.00 e | 0.69 ± 0.01 st | 74.9 ± 0.3 k | 502 ± 4 q | 7.0 ± 0.0 a | 1305 ± 3 o |
| 11 | 2.2 ± 0.04 fg | 0.76 ± 0.01 lmn | 85.3 ± 0.1 b | 2132 ± 17 i | 7.0 ± 0.0 a | 4962 ± 18 f |
| 12 | 2.1 ± 0.02 jk | 0.73 ± 0.01 pq | 85.1 ± 0.1 b | 2678 ± 29 h | 7.0 ± 0.0 a | 6901 ± 30 a |
| 13 | 1.9 ± 0.02 l | 0.72 ± 0.01 qr | 78.2 ± 0.1 i | 2152 ± 16 i | 7.0 ± 0.0 a | 4470 ± 39 h |
| 14 | 2.0 ± 0.02 k | 0.80 ± 0.00 jk | 78.8 ± 0.3 h | 2682 ± 21 h | 7.0 ± 0.0 a | 5240 ± 53 e |
| 15 | 2.9 ± 0.05 a | 0.92 ± 0.00 d | 62.8 ± 0.3 p | 4175 ± 159 b | 4.8 ± 0.6 de | 6957 ± 56 a |
| 16 | 2.0 ± 0.01 jk | 0.95 ± 0.01 c | 60.6 ± 0.1 q | 546 ± 1 q | 5.0 ± 0.1 bcd | 596 ± 3 r |
| 17 | 2.6 ± 0.02 b | 0.83 ± 0.01 gh | 60.8 ± 0.2 q | 3767 ± 1 c | 4.5 ± 0.3 ef | 4958 ± 59 f |
| 18 | 1.5 ± 0.02 q | 0.98 ± 0.01 b | 68.0 ± 0.2 n | 532 ± 1 q | 7.0 ± 0.0 a | 1024 ± 3 p |
| 19 | 1.5 ± 0.01 p | 1.04 ± 0.01 a | 68.0 ± 0.0 n | 1792 ± 4 j | 2.2 ± 0.5 g | 2969 ± 8 i |
| 20 | 1.7 ± 0.01 o | 0.97 ± 0.01 b | 73.5 ± 0.0 l | 659 ± 7 p | 5.3 ± 0.0 b | 848 ± 1 q |
| 21 | 2.5 ± 0.04 c | 0.82 ± 0.01 hi | 59.7 ± 0.4 r | 3488 ± 31 d | 4.3 ± 0.0 f | 5822 ± 30 d |
| 22 | 2.1 ± 0.01 ij | 0.86 ± 0.01 f | 47.0 ± 0.2 t | 4252 ± 52 a | 4.7 ± 0.0 de | 2424 ± 20 k |
| 23 | 1.7 ± 0.02 n | 0.76 ± 0.01 lm | 48.5 ± 0.0 s | 1061 ± 11 o | 4.9 ± 0.0 cde | 867 ± 4 q |
| 24 | 1.7 ± 0.03 o | 0.75 ± 0.01 mno | 44.7 ± 0.6 u | 1353 ± 8 n | 5.2 ± 0.0 bc | 1061 ± 10 p |
| 25 | 2.2 ± 0.01 e | 0.80 ± 0.01 ij | 43.0 ± 0.4 v | 2713 ± 1 h | 4.8 ± 0.1 cde | 1829 ± 8 l |
| 26 | 1.8 ± 0.01 m | 0.79 ± 0.00 jk | 79.5 ± 0.1 g | 1348 ± 6 n | 4.2 ± 0.1 f | 1581 ± 12 m |
| 27 | 1.6 ± 0.02 p | 0.90 ± 0.00 e | 68.6 ± 0.2 m | 1120 ± 0 o | 7.0 ± 0.0 a | 1783 ± 4 l |
| 28 | 1.5 ± 0.02 p | 0.92 ± 0.01 de | 67.1 ± 0.3 o | 1541 ± 13 l | 7.0 ± 0.0 a | 2913 ± 1 ij |
| Ave. soy | 2.1 ± 0.1 a | 0.74 ± 0.04 b | 81.6 ± 4.1 a | 2022 ± 925 a | 7.0 ± 0.1 a | 4300 ± 2037 a |
| Ave. pea | 2.1 ± 0.6 a | 0.93 ± 0.08 a | 64.8 ± 5.2 b | 2137 ± 1635 a | 4.7 ± 1.4 b | 3310 ± 2617 ab |
| Ave. wheat | 1.9 ± 0.3 a | 0.79 ± 0.05 b | 45.8 ± 2.5 c | 2345 ± 1461 a | 4.9 ± 0.2 b | 1545 ± 718 b |
| Average B | 2.0 | 0.81 | 71.2 | 2024 | 6.0 | 3423 |
| LSD (5%) C | 0.04 | 0.02 | 0.5 | 70 | 0.4 | 59 |
| Sample A | Bulk Density (g/L) | RHC (g/g) | Textural Property | ||||
|---|---|---|---|---|---|---|---|
| Hardness (g) | Resilience (%) | Cohesiveness | Springiness (%) | Chewiness (g) | |||
| 1 | 238 ± 6 kl | 2.4 ± 0.03 ij | 1061 ± 79 d | 36.2 ± 0.5 ab | 0.72 ± 0.02 a | 100.1 ± 4.6 abc | 878 ± 78 c |
| 2 | 295 ± 10 f | 1.5 ± 0.04 n | 2428 ± 118 a | 33.1 ± 1.2 efg | 0.66 ± 0.01 bc | 94.8 ± 2.1 bcdefg | 1530 ± 53 a |
| 3 | 317 ± 5 e | 2.8 ± 0.05 h | 975 ± 47 e | 27.2 ± 0.5 k | 0.65 ± 0.01 bc | 88.1 ± 1.1 ijk | 578 ± 10 e |
| 4 | 353 ± 5 c | 2.9 ± 0.01 gh | 734 ± 26 gh | 29.1 ± 1.7 ijk | 0.61 ± 0.00 fgh | 93.8 ± 3.4 defghi | 367 ± 17 hij |
| 5 | 356 ± 7 c | 3.0 ± 0.08 fg | 670 ± 15 hijk | 29.1 ± 0.9 ijk | 0.56 ± 0.01 ij | 89.6 ± 2.4 ghijk | 399 ± 14 gh |
| 6 | 343 ± 3 cd | 2.3 ± 0.04 jk | 940 ± 47 e | 32.1 ± 1.1 fg | 0.63 ± 0.02 cdef | 90.0 ± 2.1 ghijk | 561 ± 0 e |
| 7 | 330 ± 8 de | 3.0 ± 0.07 fg | 604 ± 44 klm | 23.7 ± 0.8 l | 0.59 ± 0.01 hi | 85.3 ± 1.4 k | 317 ± 12 jk |
| 8 | 259 ± 11 ik | 3.5 ± 0.09 cd | 601 ± 15 klm | 28.8 ± 0.5 ijk | 0.70 ± 0.02 a | 100.7 ± 1.5 ab | 391 ± 43 hi |
| 9 | 279 ± 5 g | 3.8 ± 0.12 b | 537 ± 38 mno | 34.3 ± 0.2 cde | 0.72 ± 0.01 a | 98.7 ± 4.2 abcde | 365 ± 8 hij |
| 10 | 330 ± 3 de | 1.8 ± 0.02 m | 1618 ± 41 b | 30.3 ± 1.0 hi | 0.65 ± 0.02 bc | 93.0 ± 7.2 efghi | 1007 ± 55 b |
| 11 | 325 ± 1 e | 3.0 ± 0.03 fg | 525 ± 37 mno | 27.7 ± 0.5 k | 0.64 ± 0.00 bcde | 92.0 ± 4.6 fghij | 343 ± 10 hijk |
| 12 | 384 ± 14 b | 3.0 ± 0.09 fg | 679 ± 44 hijk | 27.3 ± 1.2 k | 0.59 ± 0.01 hi | 85.0 ± 2.8 k | 343 ± 22 hijk |
| 13 | 354 ± 10 c | 2.5 ± 0.06 i | 842 ± 29 f | 29.64± 1.3 ij | 0.62 ± 0.02 defg | 88.1 ± 0.9 ijk | 459 ± 2 fg |
| 14 | 319 ± 14 e | 3.3 ± 0.05 e | 623 ± 19 jkl | 23.8 ± 0.4 l | 0.63 ± 0.02 cdef | 84.6 ± 2.9 k | 320 ± 20 ijk |
| 15 | 303 ± 9 f | 3.5 ± 0.05 d | 801 ± 36 fg | 33.6 ± 0.5 def | 0.62 ± 0.02 defg | 98.2 ± 5.6 abcde | 478 ± 17 f |
| 16 | 247 ± 3 jk | 2.0 ± 0.02 l | 1258 ± 49 c | 36.4 ± 0.7 ab | 0.66 ± 0.01 b | 95.9 ± 1.6 abcdef | 815 ± 1 d |
| 17 | 281 ± 6 g | 3.8 ± 0.05 b | 400 ± 16 p | 22.0 ± 0.5 m | 0.53 ± 0.02 k | 87.0 ± 4.4 jk | 208 ± 22 l |
| 18 | 202 ± 9 o | 3.1 ± 0.12 f | 829 ± 35 f | 35.7 ± 0.6 bc | 0.71 ± 0.01 a | 95.4 ± 1.5 bcdefg | 572 ± 7 e |
| 19 | 187 ± 12 p | 3.2 ± 0.02 e | 716 ± 32 hi | 35.2 ± 1.2 bcd | 0.70 ± 0.01 a | 94.7 ± 2.1 cdefgh | 470 ± 18 f |
| 20 | 230 ± 5 lm | 2.9 ± 0.07 h | 638 ± 30 ijkl | 28.4 ± 1.5 jk | 0.64 ± 0.02 bcdef | 89.0 ± 3.7 hijk | 373 ± 7 hij |
| 21 | 273 ± 2 gh | 3.5 ± 0.06 d | 510 ± 17 no | 29.5 ± 0.2 ij | 0.61 ± 0.02 efgh | 98.1 ± 2.7 abcde | 286 ± 2 k |
| 22 | 216 ± 4 mn | 3.6 ± 0.08 c | 452 ± 38 op | 37.5 ± 1.6 a | 0.66 ± 0.02 bc | 100.5 ± 1.2 abc | 281 ± 25 k |
| 23 | 222 ± 7 mn | 2.8 ± 0.05 h | 515 ± 30 no | 31.7 ± 0.7 gh | 0.64 ± 0.02 bcd | 101.7 ± 1.5 a | 340 ± 4 hijk |
| 24 | 211 ± 8 no | 2.2 ± 0.06 k | 573 ± 27 lmn | 32.6 ± 1.0 fg | 0.59 ± 0.02 gh | 97.0 ± 3.4 abcdef | 335 ± 41 hijk |
| 25 | 215 ± 9 no | 2.8 ± 0.05 h | 506 ± 34 no | 36.9 ± 2.3 ab | 0.64 ± 0.01 bcd | 95.3 ± 1.6 bcdefg | 325 ± 4 ijk |
| 26 | 453 ± 11 a | 2.9 ± 0.09 gh | 621 ± 43 jkl | 16.4 ± 0.5 n | 0.56 ± 0.02 j | 79.1 ± 3.1 l | 276 ± 11 k |
| 27 | 153 ± 1 q | 4.2 ± 0.10 a | 477 ± 22 op | 31.9 ± 0.6 gh | 0.72 ± 0.01 a | 99.0 ± 1.9 abcd | 332 ± 16 hijk |
| 28 | 264 ± 3 hi | 2.8 ± 0.03 h | 695 ± 61 hij | 28.3 ± 0.6 jk | 0.65 ± 0.01 bc | 93.6 ± 1.9 defghi | 464 ± 64 f |
| Ave. soy | 320 ± 41 a | 2.8 ± 0.6 a | 917 ± 522 a | 29.5 ± 3.6 b | 0.64 ± 0.05 a | 91.7 ± 5.5 b | 561 ± 350 a |
| Ave. pea | 246 ± 43 b | 3.1 ± 0.6 a | 736 ± 277 a | 31.5 ± 5.2 ab | 0.64 ± 0.06 a | 94.0 ± 4.4 ab | 457 ± 200 a |
| Ave. wheat | 216 ± 5 b | 2.9 ± 0.6 a | 512 ± 49.55 a | 34.7 ± 3.0 a | 0.63 ± 0.03 a | 98.6 ± 3.0 a | 320 ± 27 a |
| Average B | 284 | 2.9 | 780 | 30.3 | 0.64 | 93.1 | 479 |
| LSD (5%) C | 13 | 0.1 | 74 | 1.6 | 0.02 | 5.0 | 61 |
| Sample A | Cooking Loss (%) | Diameter Shrinkage (%) | Moisture Retention (%) | Fat Retention (%) |
|---|---|---|---|---|
| 1 | 14.7 ± 0.8 ij | 6.4 ± 0.5 jkl | 78.2 ± 0.6 bcd | 89.0 ± 0.8 b |
| 2 | 11.6 ± 0.6 m | 4.4 ± 0.5 n | 78.0 ± 1.1 bcde | 84.4 ± 0.4 cd |
| 3 | 14.8 ± 0.5 hij | 7.3 ± 0.5 fghi | 75.7 ± 0.0 ghi | 83.1 ± 1.2 de |
| 4 | 17.0 ± 0.6 bc | 8.7 ± 0.9 bc | 76.2 ± 0.7 efghi | 80.0 ± 1.0 gh |
| 5 | 16.7 ± 0.9 c | 7.7 ± 0.6 defgh | 74.8 ± 0.8 ij | 79.9 ± 1.8 gh |
| 6 | 14.9 ± 0.8 hij | 6.9 ± 0.4 hij | 73.6 ± 0.8 j | 81.0 ± 0.8 fg |
| 7 | 15.2 ± 0.5 fghi | 7.7 ± 0.6 defgh | 77.6 ± 1.3 cdefg | 79.8 ± 1.0 gh |
| 8 | 17.4 ± 0.4 b | 9.0 ± 0.7 ab | 75.5 ± 0.6 hi | 79.4 ± 1.0 gh |
| 9 | 16.4 ± 1.0 cd | 7.4 ± 0.7 efgh | 75.9 ± 1.5 fghi | 80.9 ± 0.7 fg |
| 10 | 12.3 ± 0.6 l | 4.6 ± 0.3 n | 78.1 ± 0.7 bcde | 84.9 ± 1.4 c |
| 11 | 13.7 ± 0.6 k | 6.6 ± 0.5 ijk | 77.8 ± 0.7 cdef | 85.5 ± 0.4 c |
| 12 | 15.5 ± 0.5 efg | 7.8 ± 0.9 defg | 76.3 ± 0.3 defghi | 77.4 ± 1.5 ij |
| 13 | 16.0 ± 0.6 de | 8.2 ± 0.6 bcde | 76.6 ± 1.6 defghi | 80.6 ± 0.3 fg |
| 14 | 15.9 ± 0.6 def | 8.3 ± 0.7 bcd | 78.0 ± 0.8 cde | 78.8 ± 0.5 hi |
| 15 | 18.5 ± 1.0 a | 8.2 ± 0.7 bcde | 73.2 ± 1.1 j | 70.5 ± 0.4 l |
| 16 | 11.6 ± 0.8 m | 4.6 ± 0.4 n | 77.7 ± 1.8 cdef | 92.4 ± 0.4 a |
| 17 | 15.6 ± 0.6 efg | 9.5 ± 0.7 a | 77.1 ± 0.7 defgh | 80.0 ± 0.5 gh |
| 18 | 13.7 ± 0.9 k | 6.5 ± 0.8 ijkl | 77.8 ± 1.1 cdef | 85.1 ± 1.1 c |
| 19 | 14.3 ± 0.7 jk | 6.1 ± 0.5 klm | 77.0 ± 1.1 defgh | 82.8 ± 0.7 e |
| 20 | 14.8 ± 0.7 hij | 8.0 ± 0.7 cdef | 76.0 ± 0.2 fghi | 76.0 ± 0.5 j |
| 21 | 15.9 ± 0.7 def | 5.6 ± 0.3 m | 75.4 ± 0.3 hi | 74.3 ± 0.2 k |
| 22 | 15.1 ± 0.5 ghi | 7.7 ± 0.7 defgh | 77.9 ± 1.0 cde | 81.0 ± 0.5 fg |
| 23 | 15.2 ± 0.4 ghi | 7.9 ± 0.6 cdef | 80.3 ± 0.3 a | 81.8 ± 0.4 ef |
| 24 | 12.2 ± 0.6 lm | 7.0 ± 0.5 ghij | 79.1 ± 0.3 abc | 79.5 ± 0.6 gh |
| 25 | 13.7 ± 0.7 k | 5.7 ± 0.8 lm | 77.7 ± 1.1 cdef | 80.5 ± 1.3 fg |
| 26 | 12.3 ± 0.3 l | 5.9 ± 0.5 klm | 80.5 ± 0.4 a | 91.5 ± 0.6 a |
| 27 | 15.8 ± 1.1 defg | 8.2 ± 0.7 bcd | 76.3 ± 0.9 defghi | 82.7 ± 0.6 e |
| 28 | 13.6 ± 0.5 k | 6.5 ± 0.6 ijkl | 79.8 ± 1.8 ab | 81.1 ± 0.7 fg |
| Ave. soy | 15.2 ± 1.7 a | 7.2 ± 1.4 a | 76.6 ± 1.4 b | 81.8 ± 3.2 a |
| Ave. pea | 14.9 ± 2.1 a | 6.9 ± 1.7 a | 76.3 ± 1.6 b | 80.2 ± 7.4 a |
| Ave. wheat | 14.0 ± 1.4 a | 7.1 ± 1.0 a | 78.8 ± 1.2 a | 80.7 ± 1.0 a |
| Average B | 14.8 | 7.1 | 77.1 | 81.6 |
| LSD (5%) C | 0.6 | 0.7 | 1.6 | 1.5 |
| Sample A | Hardness (g) | Resilience (%) | Cohesiveness | Springiness (%) | Chewiness (g) | Shear Force (g) | Compressed Juiciness (%) |
|---|---|---|---|---|---|---|---|
| 1 | 1554 ± 12 e | 4.7 ± 0.1 hijk | 0.20 ± 0.00 ijk | 55.4 ± 1.2 kl | 173 ± 7 h | 432 ± 47 cd | 7.1 ± 0.7 hi |
| 2 | 2768 ± 47 a | 5.3 ± 0.2 fg | 0.20 ± 0.01 jk | 50.1 ± 0.3 m | 270 ± 12 d | 527 ± 63 b | 4.4 ± 0.5 k |
| 3 | 965 ± 21 ijkl | 4.1 ± 0.3 lm | 0.20 ± 0.02 jk | 53.2 ± 3.1 lm | 95 ± 9 kl | 255 ± 13 ijkl | 8.9 ± 0.5 cde |
| 4 | 1055 ± 60 h | 4.6 ± 0.1 jk | 0.20 ± 0.01 ijk | 56.8 ± 0.9 jkl | 134 ± 14 ijk | 151 ± 23 mn | 7.7 ± 0.4 gh |
| 5 | 1249 ± 16 g | 4.7 ± 0.2 hijk | 0.20 ± 0.01 jk | 56.2 ± 2.5 kl | 136 ± 6 ijk | 325 ± 34 fg | 7.2 ± 0.5 ghi |
| 6 | 1653 ± 139 d | 5.0 ± 0.4 gh | 0.21 ± 0.00 hij | 57.6 ± 2.2 jk | 197 ± 15 fg | 441 ± 14 cd | 5.8 ± 0.5 j |
| 7 | 878 ± 6 lm | 4.3 ± 0.3 klm | 0.23 ± 0.00 fgh | 67.9 ± 1.9 efg | 141 ± 7 ij | 354 ± 43 f | 8.4 ± 0.4 ef |
| 8 | 681 ± 18 n | 5.0 ± 0.3 ghi | 0.28 ± 0.01 e | 69.4 ± 1.1 ef | 130 ± 7 jk | 318 ± 24 fgh | 10.3 ± 0.5 b |
| 9 | 1011 ± 78 hij | 5.0 ± 0.2 ghij | 0.28 ± 0.02 e | 67.4 ± 2.3 fg | 176 ± 15 h | 285 ± 45 ghi | 9.0 ± 0.4 cde |
| 10 | 2457 ± 55 c | 5.4 ± 0.4 f | 0.20 ± 0.01 ijk | 53.5 ± 2.7 lm | 260 ± 11 de | 809 ± 93 a | 4.4 ± 0.5 k |
| 11 | 929 ± 34 jkl | 4.7 ± 0.2 hijk | 0.22 ± 0.01 ghi | 60.2 ± 1.6 ij | 122 ± 5 jk | 213 ± 45 kl | 7.7 ± 0.7 gh |
| 12 | 1020 ± 32 hi | 4.6 ± 0.3 ijk | 0.20 ± 0.01 jk | 55.6 ± 2.3 kl | 113 ± 6 kl | 203 ± 36 lm | 7.1 ± 0.5 hi |
| 13 | 1388 ± 59 f | 4.4 ± 0.2 kl | 0.19 ± 0.00 k | 50.9 ± 0.6 m | 127 ± 5 jk | 369 ± 28 ef | 7.4 ± 0.3 gh |
| 14 | 803 ± 27 m | 4.5 ± 0.2 kl | 0.24 ± 0.00 fg | 62.1 ± 2.8 hi | 125 ± 5 jk | 230 ± 32 ijkl | 10.3 ± 0.4 b |
| 15 | 799 ± 21 m | 4.5 ± 0.1 kl | 0.20 ± 0.02 ijk | 62.1 ± 1.3 hi | 93 ± 5 kl | 131 ± 20 n | 8.9 ± 0.4 cde |
| 16 | 2542 ± 42 b | 11.5 ± 0.6 a | 0.22 ± 0.01 ghij | 71.3 ± 2.1 de | 391 ± 9 a | 415 ± 52 de | 4.0 ± 0.2 k |
| 17 | 559 ± 27 o | 5.2 ± 0.3 fg | 0.31 ± 0.03 cd | 73.0 ± 0.7 cd | 134 ± 12 ijk | 266 ± 11 hijk | 12.9 ± 1.0 a |
| 18 | 1598 ± 85 de | 8.2 ± 0.2 c | 0.29 ± 0.01 de | 65.0 ± 0.7 gh | 308 ± 11 b | 449 ± 10 cd | 6.6 ± 0.5 i |
| 19 | 1430 ± 67 f | 7.1 ± 0.3 d | 0.27 ± 0.02 e | 65.5 ± 1.6 gh | 294 ± 30 bc | 490 ± 32 bc | 6.7 ± 0.7 i |
| 20 | 913 ± 51 kl | 4.0 ± 0.1 m | 0.20 ± 0.01 ijk | 64.5 ± 1.7 gh | 116 ± 6 k | 235 ± 24 ijkl | 7.6 ± 0.4 gh |
| 21 | 596 ± 32 o | 5.5 ± 0.2 f | 0.38 ± 0.01 a | 90.6 ± 0.5 a | 193 ± 10 gh | 69 ± 4 o | 10.1 ± 0.5 b |
| 22 | 887 ± 23 l | 9.0 ± 0.5 b | 0.39 ± 0.02 a | 75.1 ± 1.2 c | 275 ± 3 cd | 212 ± 19 kl | 9.2 ± 0.9 cd |
| 23 | 887 ± 16 l | 6.3 ± 0.3 e | 0.32 ± 0.01 bc | 75.6 ± 1.8 c | 216 ± 4 f | 224 ± 24 jkl | 9.3 ± 1.0 c |
| 24 | 1048 ± 46 hi | 7.1 ± 0.1 d | 0.34 ± 0.01 b | 75.6 ± 1.6 c | 271 ± 29 d | 451 ± 19 cd | 7.7 ± 0.7 gh |
| 25 | 978 ± 71 hijk | 7.2 ± 0.1 d | 0.32 ± 0.01 bc | 82.6 ± 1.3 b | 245 ± 9 e | 277 ± 17 ghij | 7.9 ± 0.5 fg |
| 26 | 891 ± 49 l | 4.5 ± 0.2 kl | 0.23 ± 0.01 fgh | 79.9 ± 2.4 b | 153 ± 19 i | 151 ± 16 mn | 8.5 ± 0.3 de |
| 27 | 801 ± 11 m | 5.2 ± 0.1 fg | 0.25 ± 0.01 f | 66.9 ± 3.1 fg | 135 ± 11 ijk | 264 ± 22 hijk | 10.1 ± 0.4 b |
| 28 | 964 ± 22 ijkl | 4.5 ± 0.3 kl | 0.20 ± 0.01 ijk | 58.1 ± 2.8 jk | 117 ± 4 k | 264 ± 18 hijk | 7.8 ± 0.6 fg |
| Ave. soy | 1315 ± 618 a | 4.7 ± 0.4 b | 0.22 ± 0.03 b | 58.3 ± 6.3 b | 157 ± 53 b | 351 ± 168 a | 7.5 ± 1.8 a |
| Ave. pea | 1205 ± 711 a | 6.6 ± 2.6 a | 0.27 ± 0.06 b | 70.3 ± 9.8 a | 218 ± 114 ab | 294 ± 163 a | 8.1 ± 2.8 a |
| Ave. wheat | 950 ± 78 a | 7.4 ± 1.1 a | 0.34 ± 0.03 a | 77.2 ± 3.6 a | 252 ± 28 a | 291 ± 11 a | 8.5 ± 0.9 a |
| Average B | 1189 | 5.6 | 0.25 | 65.1 | 184 | 315 | 8.0 |
| LSD (5%) C | 75 | 0.4 | 0.02 | 3.2 | 20 | 52 | 0.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, S.; Shen, Y.; Li, Y. Physicochemical and Functional Properties of Texturized Vegetable Proteins and Cooked Patty Textures: Comprehensive Characterization and Correlation Analysis. Foods 2022, 11, 2619. https://doi.org/10.3390/foods11172619
Hong S, Shen Y, Li Y. Physicochemical and Functional Properties of Texturized Vegetable Proteins and Cooked Patty Textures: Comprehensive Characterization and Correlation Analysis. Foods. 2022; 11(17):2619. https://doi.org/10.3390/foods11172619
Chicago/Turabian StyleHong, Shan, Yanting Shen, and Yonghui Li. 2022. "Physicochemical and Functional Properties of Texturized Vegetable Proteins and Cooked Patty Textures: Comprehensive Characterization and Correlation Analysis" Foods 11, no. 17: 2619. https://doi.org/10.3390/foods11172619
APA StyleHong, S., Shen, Y., & Li, Y. (2022). Physicochemical and Functional Properties of Texturized Vegetable Proteins and Cooked Patty Textures: Comprehensive Characterization and Correlation Analysis. Foods, 11(17), 2619. https://doi.org/10.3390/foods11172619








