Small Contaminations on Broiler Carcasses Are More a Quality Matter than a Food Safety Issue
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Used for Input in the Calculations
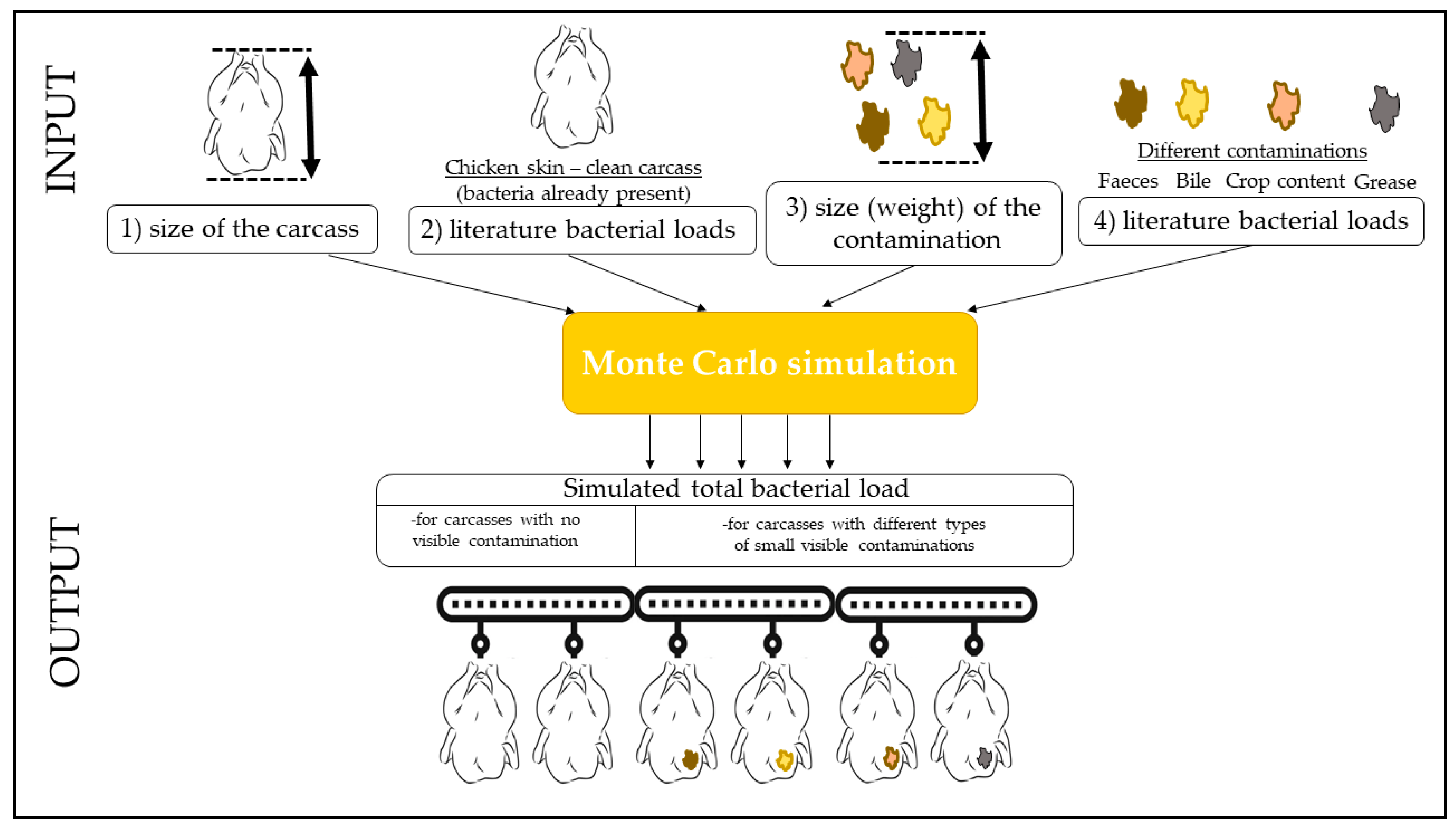
2.2. Monte Carlo Simulation
3. Results
4. Discussion
4.1. Bacterial Counts Used for Calculations
4.2. Monte Carlo Simulation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klaharn, K.; Pichpol, D.; Meeyam, T.; Harintharanon, T.; Lohaanukul, P.; Punyapornwithaya, V. Bacterial Contamination of Chicken Meat in Slaughterhouses and the Associated Risk Factors: A Nationwide Study in Thailand. PLoS ONE 2022, 17, e0269416. [Google Scholar] [CrossRef] [PubMed]
- Emanowicz, M.; Meade, J.; Bolton, D.; Golden, O.; Gutierrez, M.; Byrne, W.; Egan, J.; Lynch, H.; O’Connor, L.; Coffey, A.; et al. The Impact of Key Processing Stages and Flock Variables on the Prevalence and Levels of Campylobacter on Broiler Carcasses. Food Microbiol. 2021, 95, 103688. [Google Scholar] [CrossRef] [PubMed]
- Berends, B.R.; Snijders, J.M.A. De Hazard Analysis Critical Control Point benadering bij de productie van vlees. Tijdschr Diergeneeskd 1994, 119. [Google Scholar]
- Rasekh, J.; Thaler, A.M.; Engeljohn, D.L.; Pihkala, N.H. Food Safety and Inspection Service Policy for Control of Poultry Contaminated by Digestive Tract Contents: A Review. J. Appl. Poult. Res. 2005, 14, 603–611. [Google Scholar] [CrossRef]
- Rouger, A.; Tresse, O.; Zagorec, M. Bacterial Contaminants of Poultry Meat: Sources, Species, and Dynamics. Microorganisms 2017, 5, 50. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union One Health 2020 Zoonoses Report. EFSA J. 2021, 19, 20. [Google Scholar] [CrossRef]
- European Food Safety Authority. EFSA Panel on Biological Hazards (BIOHAZ); Scientific Opinion on Campylobacter in broiler meat production: Control options and performance objectives and/or targets at different stages of the food chain. EFSA J. 2011, 9, 8. Available online: http://www.efsa.europa.eu/en/efsajournal/pub/2105 (accessed on 4 November 2022).
- Pacholewicz, E.; Swart, A.; Wagenaar, J.A.; Lipman, L.J.A.; Havelaar, A.H. Explanatory variables associated with Campylobacter and Escherichia coli concentrations on broiler chicken carcasses during processing in two slaughterhouses. J. Food Protect. 2016, 79, 2038–2047. [Google Scholar] [CrossRef]
- Tang, Y.; Jiang, Q.; Tang, H.; Wang, Z.; Yin, Y.; Ren, F.; Kong, L.; Jiao, X.; Huang, J. Characterization and Prevalence of Campylobacter spp. from Broiler Chicken Rearing Period to the Slaughtering Process in Eastern China. Front. Vet. Sci. 2020, 7, 227. [Google Scholar] [CrossRef]
- Pacholewicz, E.; Swart, A.; Schipper, M.; Gortemaker, B.G.M.; Wagenaar, J.A.; Havelaar, A.H.; Lipman, L.J.A. A comparison of fluctuations of Campylobacter and Escherichia coli concentrations on broiler chicken carcasses during processing in two slaughterhouses. Int. J. Food Microbiol. 2015, 205, 119–127. [Google Scholar] [CrossRef]
- Nauta, M.J.; Jacobs-Reitsma, W.F.; Havelaar, A.H. A Risk assessment Model for Campylobacter in Broiler Meat. Risk Anal. 2007, 27, 845–861. [Google Scholar] [CrossRef] [PubMed]
- Nauta, M.; Bolton, D.; Crotta, M.; Ellis-Iversen, J.; Alter, T.; Hempen, M.; Messens, W.; Chemaly, M. An Updated Assessment of the Effect of Control Options to Reduce Campylobacter Concentrations in Broiler Caeca on Human Health Risk in the European Union. Microb. Risk Anal. 2022, 21, 100197. [Google Scholar] [CrossRef]
- Russell, S.M.; Walker, J.M. The Effect of Evisceration on Visible Contamination and the Microbiological Profile of Fresh Broiler Chicken Carcasses Using the Nu-Tech Evisceration System or the Conventional Streamlined Inspection System. Poult. Sci. 1997, 76, 780–784. [Google Scholar] [CrossRef] [PubMed]
- Russell, S.M. The effects of airsacculitis on bird weights, uniformity, fecal contamination, processing errors and populations of Campylobacter spp. and Escherichia coli. Poult. Sci. 2003, 82, 1326–1331. [Google Scholar] [CrossRef] [PubMed]
- Brizio, A.P.; Marin, G.; Schittler, L.; Prentice, C. Visible Contamination in Broiler Carcasses and its Relation to The Stages of Evisceration in Poultry Slaughter. Int. Food Res. J. 2015, 22, 59–63. [Google Scholar]
- Fletcher, D.L.; Craig, E.W.; Arnold, J.W. An evaluation of on-line “reprocessing” on visual contamination and microbiological quality of broilers. J Appl. Poultry Res. 1997, 6, 436–442. [Google Scholar] [CrossRef]
- Pacholewicz, E.; Sura Barus, S.A.; Swart, A.; Havelaar, A.H.; Lipman, L.J.A.; Luning, P.A. Influence of Food Handlers’ Compliance with Procedures of Poultry Carcasses Contamination: A Case Study Concerning Evisceration in Broiler Slaughterhouses. Food Control 2016, 68, 367–378. [Google Scholar] [CrossRef]
- Cibin, V.; Mancin, M.; Pedersen, K.; Barrucci, F.; Belluco, S.; Roccato, A.; Cocola, F.; Ferrarini, S.; Sandri, A.; Lau Baggesen, D.; et al. Usefulness of Escherichia coli and Enterobacteriaceae as Process Hygiene Criteria in poultry: Experimental study. EFSA J. 2014, 121, 25. Available online: https://www.efsa.europa.eu/en/supporting/pub/en-635 (accessed on 4 November 2022). [CrossRef]
- Jiménez, S.M.; Salsi, M.S.; Tiburzi, M.C.; Pirovani, M.E. A comparison between broiler chicken carcasses with and without visible fecal contamination during the slaughtering process on hazard identification of Salmonella spp. J. Appl. Microbiol. 2002, 93, 593–598. [Google Scholar] [CrossRef]
- Jiménez, S.M.; Tiburzi, M.C.; Salsi, M.S.; Pirovani, M.E.; Moguilevsky, M.A. The role of visible fecal material as a vehicle for generic Escherichia coli, coliform, and other enterobacteria contaminating poultry carcasses during slaughtering. J. Appl. Microbiol. 2003, 95, 451–456. [Google Scholar] [CrossRef]
- Giombelli, A.; Gloria, M.B.A. Prevalence of Salmonella and Campylobacter on Broiler Chickens from Farm to Slaughter and Efficiency of Methods to Remove Visible Fecal Contamination. J. Food Prot. 2014, 77, 1851–1859. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Joossens, M.; Houf, K. Analyses of the Bacterial Contamination on Belgian Broiler Carcasses at Retail Level. Front. Microbiol. 2020, 11, 539540. [Google Scholar] [CrossRef] [PubMed]
- Vose, D. Risk Analysis: A Guide to Monte Carlo Simulation Modeling; John Wiley and Sons: Chichester, NY, USA, 1996. [Google Scholar]
- European Food Safety Authority. EFSA Panel on Biological Hazards (BIOHAZ); Scientific Opinion on Quantitative Assessment of The Residual BSE Risk in Bovine-Derived Products. EFSA J. 2005, 307, 34–35. [Google Scholar] [CrossRef]
- Ellis-Iversen, J.; Gantzhorn, M.R.; Borck Høg, B.; Foddai, A.; Nauta, M. The Ability to Detect Campylobacter Presence and Concentration Using Different Chicken Carcass Samples. Food Control 2020, 115, 107294. [Google Scholar] [CrossRef]
- Nauta, M.J.; Jacobs-Reitsma, W.F.; Evers, E.G.; van Pelt, W.; Havelaar, A.H. Risk Assessment of Campylobacter in the Netherlands via Broiler Meat and Other Routes; Report 250911006/2005; National Institute for Public Health and the Environment (RIVM): Bilthoven, The Netherlands, 2005.
- Elfadil, A.A.; Vaillantcourt, J.P.; Duncan, I.J.H. Comparative study of body characteristics of different strains of broiler chickens. J. Appl. Poult. Sci. 1998, 7, 268–272. [Google Scholar] [CrossRef]
- Cussler, E.L.; Fennell Evans, D.; DePalma, R. A Model for Gallbladder Function and Cholesterol Gallstone Formation. Proc. Natl. Ac. Sci. USA 1970, 67, 400–407. [Google Scholar] [CrossRef]
- Van Der Meer, J. Het verschijnsel van de drijvende feces. Ned. Tijdschr. Voor Geneeskd. 1972, 116, 1662. [Google Scholar]
- Seol, K.-H.; Han, G.-S.; Kim, H.W.; Chang, O.-K.; Oh, M.-H.; Park, B.-Y.; Ham, J.-S. Prevalence and Microbial Flora of Chicken Slaughtering and Processing Procedure. J. Food. Sci. Anim. Resour. 2012, 32, 763–768. [Google Scholar] [CrossRef]
- Goksoy, E.O.; Kirkan, S.; Kok, F. Microbiological Quality of Broiler Carcasses during Processing in Two Slaughterhouses in Turkey. Poult. Sci. 2004, 83, 1427–1432. [Google Scholar] [CrossRef]
- Altekruse, S.F.; Berrang, M.E.; Marks, H.; Patel, B.; Shaw, W.K.; Saini, P.; Bennett, P.A.; Bailey, J.S. Enumeration of Escherichia Coli Cells on Chicken Carcasses as a Potential Measure of Microbial Process Control in a Random Selection of Slaughter Establishments in the United States. Appl. Environ. Microbiol. 2009, 75, 3522–3527. [Google Scholar] [CrossRef]
- Danek-Majewska, A.; Kwiecień, M.; Samolińska, W.; Kowalczyk-Pecka, D.; Nowakowicz-Dębek, B.; Winiarska-Mieczan, A. Effect of Raw Chickpea in the Broiler Chicken Diet on Intestinal Histomorphology and Intestinal Microbial Populations. Animals 2022, 12, 1767. [Google Scholar] [CrossRef] [PubMed]
- Śliżewska, K.; Markowiak-Kopeć, P.; Żbikowski, A.; Szeleszczuk, P. The Effect of Synbiotic Preparations on the Intestinal Microbiota and Her Metabolism in Broiler Chickens. Sci. Rep. 2020, 10, 4281. [Google Scholar] [CrossRef] [PubMed]
- Berrang, M.E.; Cox, N.A.; Meinersmann, R.J.; Bowker, B.C.; Zhuang, H.; Huff, H.C. Mild Heat and Freezing to Lessen Bacterial Numbers on Chicken Liver. J. Appl. Poult. Res. 2020, 29, 251–257. [Google Scholar] [CrossRef]
- Dourou, D.; Grounta, A.; Argyri, A.A.; Froutis, G.; Tsakanikas, P.; Nychas, G.-J.E.; Doulgeraki, A.I.; Chorianopoulos, N.G.; Tassou, C.C. Rapid Microbial Quality Assessment of Chicken Liver Inoculated or Not with Salmonella Using FTIR Spectroscopy and Machine Learning. Front. Microbiol. 2021, 11, 623788. [Google Scholar] [CrossRef]
- Stromberg, Z.R.; Johnson, J.R.; Fairbrother, J.M.; Kilbourne, J.; Van Goor, A.; Curtiss, R.; Mellata, M. Evaluation of Escherichia Coli Isolates from Healthy Chickens to Determine Their Potential Risk to Poultry and Human Health. PLoS ONE 2017, 12, e0180599. [Google Scholar] [CrossRef]
- Whyte, R.; Hudson, J.A.; Graham, C. Campylobacter in Chicken Livers and Their Destruction by Pan Frying. Lett. Appl. Microbiol. 2006, 43, 591–595. [Google Scholar] [CrossRef]
- Smith, D.P.; Berrang, M.E. Prevalence and Numbers of Bacteria in Broiler Crop and Gizzard Contents. Poult. Sci. 2006, 85, 144–147. [Google Scholar] [CrossRef]
- Musgrove, M.T.; Berrang, M.E.; Byrd, J.A.; Stern, N.J.; Cox, N.A. Detection of Campylobacter Spp. in Ceca and Crops with and Without Enrichment. Poult. Sci. 2001, 80, 825–828. [Google Scholar] [CrossRef]
- Food and Drug Administration Office of Regulatory Affairs. Pharmaceutical Microbiology Manual, ORA.007; Food and Drug Administration: Silver Spring, MD, USA, 2020.
- Smith, D.P.; Northcutt, J.K.; Musgrove, M.T. Microbiology of Contaminated or Visibly Clean Broiler Carcasses Processed with an Inside-Outside Bird Washer. Int. J. Poutry Sci. 2005, 4, 955–958. [Google Scholar] [CrossRef]
- Cason, J.A.; Berrang, M.E.; Buhr, R.J.; Cox, N.A. Effect of Prechill Fecal Contamination on Numbers of Bacteria Recovered from Broiler Chicken Carcasses Before and After Immersion Chilling. J. Food Prot. 2004, 67, 1829–1833. [Google Scholar] [CrossRef]
- Burfoot, D.; Hall, J.; Nicholson, K.; Holmes, K.; Hanson, C.; Handley, S.; Mulvey, E. Effect of Rapid Surface Cooling on Campylobacter Numbers on Poultry Carcasses. Food Control 2016, 70, 293–301. [Google Scholar] [CrossRef]
- Musavian, H.S.; Krebs, N.H.; Nonboe, U.; Corry, J.E.L.; Purnell, G. Combined Steam and Ultrasound Treatment of Broilers at Slaughter: A Promising Intervention to Significantly Reduce Numbers of Naturally Occurring Campylobacters on Carcasses. Int. J. Food Microbiol. 2014, 176, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Stefani, L.M.; Backes, R.G.; Faria, G.A.; Biffi, C.P.; de Almeida, J.M.; da Silva, H.K.; das Neves, G.B.; Langaro, A. Trimming and Washing Poultry Carcass to Reduce Microbial Contamination: A Comparative Study. Poult. Sci. 2014, 93, 3119–3122. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Regulation (EC) No 2073/2005 of 15 November 2005 on Microbiological Criteria for Foodstuffs (Consolidated Version, Text with EAA Relevance). 2005. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02005R2073-20200308 (accessed on 4 November 2022).
- Swart, A.N.; Mangen, M.J.J.; Havelaar, A.H. Microbiological Criteria as a Decision Tool for Controlling Campylobacter in the Broiler Meat Chain; RIVM Letter Report 330331008/2013; National Institute for Public Health and the Environment (RIVM): Bilthoven, The Netherlands, 2013.


| Type of Contamination | Minimal Amount (g) | Maximal Amount (g) |
|---|---|---|
| Feces | 0.001 | 0.01 |
| Bile | 0.0375 | 0.15 |
| Crop content | 0.05 | 0.2 |
| Grease | 0.01 | 0.04 |
| Total Aerobic Count | Enterobacteriaceae | E. coli | Campylobacter spp. | |
|---|---|---|---|---|
| Skin | ||||
| mean ± sd (log10/g) | 4.15 ± 0.46 | 3.77 ± 0.13 | 3.3 ± 0.6 | 2.99 ± 0.7 |
| reference | [30] | [31] | [32] | [26] |
| Feces | ||||
| mean ± sd (log10/g) | 3.36 ± 1.37 | 8.62 ± 0.58 | 8.44 ± 0.35 | 6.0 ± 1.52 |
| reference | [33] | [34] | [34] | [26] |
| Bile | ||||
| mean ± sd (log10/g) | 6.0 ± 0.7 | 3.1 ± 0.5 | 1.9 ± 1.1 | 2.795 ± 1.641 |
| reference | [35] | [36] | [37] | [38] |
| Crop content | ||||
| mean ± sd (log10/g) | 5.6 ± 0.1 | 4.2 ± 0.2 | 3.9 ± 0.2 | 3.63 ± 1.12 |
| reference | [39] | [39] | [39] | [40] |
| Grease | ||||
| mean ± sd (log10/g) | 3.40 ± 0.16 | 1.86 ± 0.41 | 0.86 ± 1.19 | 0.83 ± 0.67 |
| reference | own data not published | own data not published | own data not published | own data not published |
| Bacterial Species | Type of Contamination | |||||||
|---|---|---|---|---|---|---|---|---|
| Feces | Bile | Crop Content | Grease | |||||
| No Contam. | With Contam. | No Contam. | With Contam. | No Contam. | With Contam. | No Contam. | With Contam. | |
| Total aerobic count | ||||||||
| mean | 7.3535 | 7.3536 | 7.3535 | 7.3625 | 7.3535 | 7.3552 | 7.3535 | 7.3535 |
| sd | 0.4604 | 0.4604 | 0.4606 | 0.4528 | 0.4603 | 0.4585 | 0.4606 | 0.4605 |
| minimum | 5.3536 | 5.3536 | 5.3394 | 5.5386 | 5.4267 | 5.4681 | 5.4143 | 5.4144 |
| maximum | 9.3717 | 9.3717 | 9.4324 | 9.4324 | 9.4114 | 9.4114 | 9.2766 | 9.2766 |
| Enterobacteriaceae | ||||||||
| mean | 6.9735 | 7.1181 | 6.9735 | 6.9735 | 6.9735 | 6.9736 | 6.9735 | 6.9735 |
| sd | 0.1316 | 0.1907 | 0.1318 | 0.1318 | 0.1319 | 0.1319 | 0.1320 | 0.1320 |
| minimum | 6.4232 | 6.4665 | 6.3800 | 6.3800 | 6.4164 | 6.4171 | 6.3775 | 6.3775 |
| maximum | 7.5920 | 8.0262 | 7.5122 | 7.5122 | 7.5480 | 7.5480 | 7.5612 | 7.5612 |
| E. coli | ||||||||
| mean | 6.5035 | 6.7640 | 6.5035 | 6.5036 | 6.5035 | 6.5039 | 6.5035 | 6.5035 |
| sd | 0.6003 | 0.4368 | 0.6003 | 0.6002 | 0.6005 | 0.6000 | 0.6004 | 0.6004 |
| minimum | 3.5649 | 5.1184 | 3.7284 | 3.7287 | 3.9146 | 4.0222 | 3.9726 | 3.9726 |
| maximum | 9.1898 | 9.1915 | 9.15620 | 9.1562 | 9.2393 | 9.2393 | 9.0849 | 9.0849 |
| Campylobacter spp. | ||||||||
| mean | 6.1936 | 6.2610 | 6.1936 | 6.2011 | 6.1936 | 6.2005 | 6.1936 | 6.1936 |
| sd | 0.7001 | 0.6784 | 0.7002 | 0.6946 | 0.7002 | 0.6931 | 0.7003 | 0.7003 |
| minimum | 3.3425 | 3.5869 | 3.3131 | 3.3245 | 3.3419 | 3.60291 | 3.2279 | 3.2287 |
| maximum | 9.2117 | 9.2117 | 9.26841 | 9.2684 | 9.10343 | 9.10343 | 9.1931 | 9.1931 |
| Type of Bacteria | Feces | Bile | Crop Content | Grease |
|---|---|---|---|---|
| Total aerobic count | 0% | 0% | 0% | 0% |
| Enterobacteriaceae | 5.1% | 0% | 0% | 0% |
| E. coli | 16.7% | 0% | 0% | 0% |
| Campylobacter spp. | 4.1% | 0% | 0.2% | 0% |
| Type of Bacteria | Feces | Bile | Crop Content | Grease |
|---|---|---|---|---|
| Total aerobic count | 0% | 0% | 0% | 0% |
| Enterobacteriaceae | 5.6% | 0% | 0% | 0% |
| E. coli | 17.9% | 0% | 0% | 0% |
| Campylobacter spp. | 4.3% | 0% | 0.2% | 0% |
| Type of Bacteria | Feces | Bile | Crop Content | Grease |
|---|---|---|---|---|
| Total aerobic count | 0.001% | 0.122% | 0.02311% | <0.001% |
| Enterobacteriaceae | 2.031% | <0.001% | 0.00143% | <0.001% |
| E. coli | 3.851% | 0.002% | 0.00615% | <0.001% |
| Campylobacter spp. | 1.077% | 0.121% | 0.11128% | <0.001% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Libera, K.; Lipman, L.; Berends, B.R. Small Contaminations on Broiler Carcasses Are More a Quality Matter than a Food Safety Issue. Foods 2023, 12, 522. https://doi.org/10.3390/foods12030522
Libera K, Lipman L, Berends BR. Small Contaminations on Broiler Carcasses Are More a Quality Matter than a Food Safety Issue. Foods. 2023; 12(3):522. https://doi.org/10.3390/foods12030522
Chicago/Turabian StyleLibera, Kacper, Len Lipman, and Boyd R. Berends. 2023. "Small Contaminations on Broiler Carcasses Are More a Quality Matter than a Food Safety Issue" Foods 12, no. 3: 522. https://doi.org/10.3390/foods12030522
APA StyleLibera, K., Lipman, L., & Berends, B. R. (2023). Small Contaminations on Broiler Carcasses Are More a Quality Matter than a Food Safety Issue. Foods, 12(3), 522. https://doi.org/10.3390/foods12030522





