Effects of Konjac Glucomannan/ε-Polylysine Hydrochloride/Ferulic Acid Composite Coating on the Freshness Preservation Performance and Flavor of Refrigerated Sea Bass Fillets
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Composite Coating
2.3. Treatment of Sea Bass Fillets
2.4. Sensory Evaluation
2.5. Color Difference Measurement
2.6. Texture Determination
2.7. Determination of Volatile Components
2.8. Statistical Analysis
3. Results and Analysis
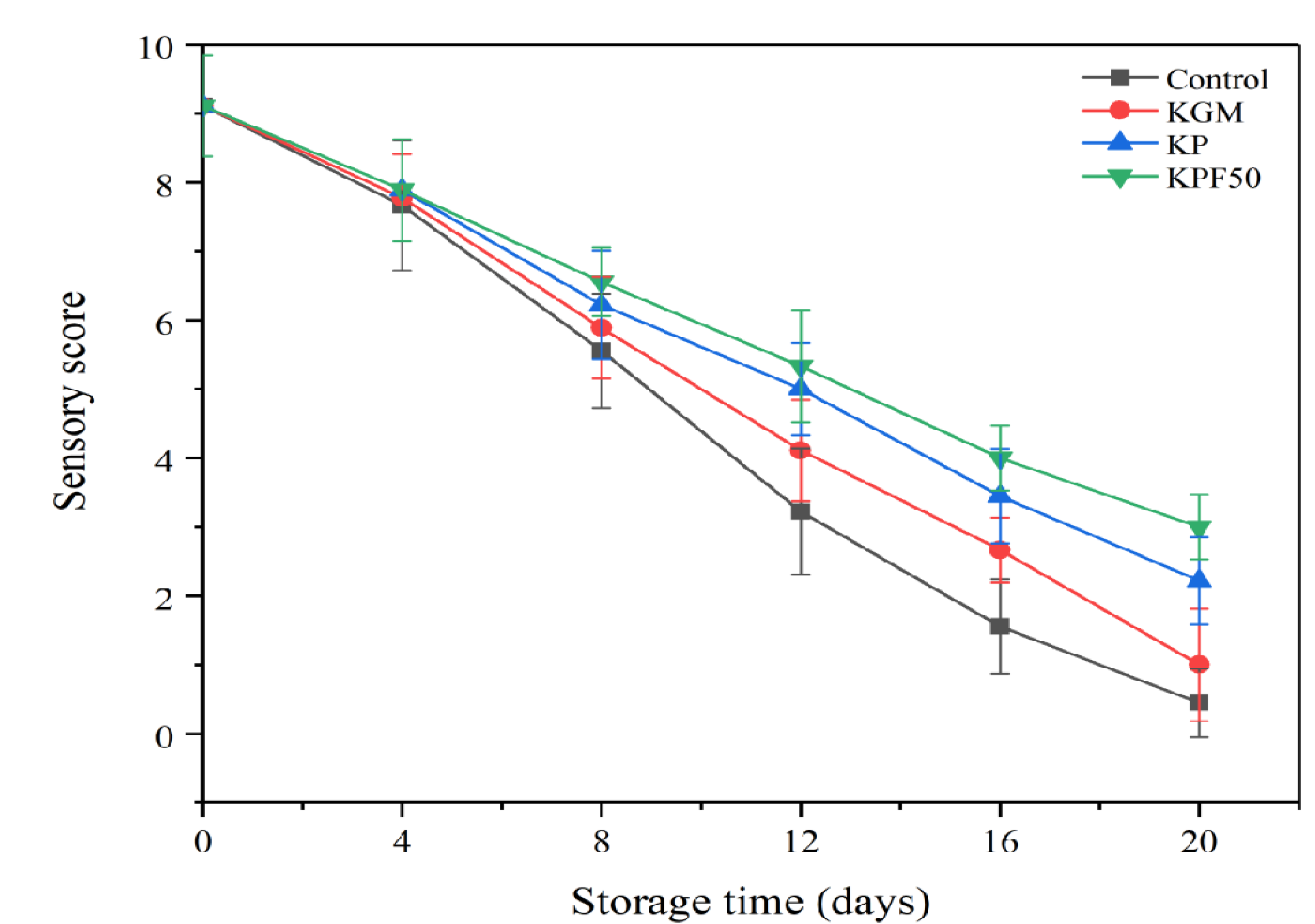
3.1. Effect of Composite Coating on the Sensory Score of Sea Bass Fillets
3.2. Effect of Composite Coating on the Color of Sea Bass Fillets
3.3. Effect of Composite Coating on the Texture of Sea Bass Fillets
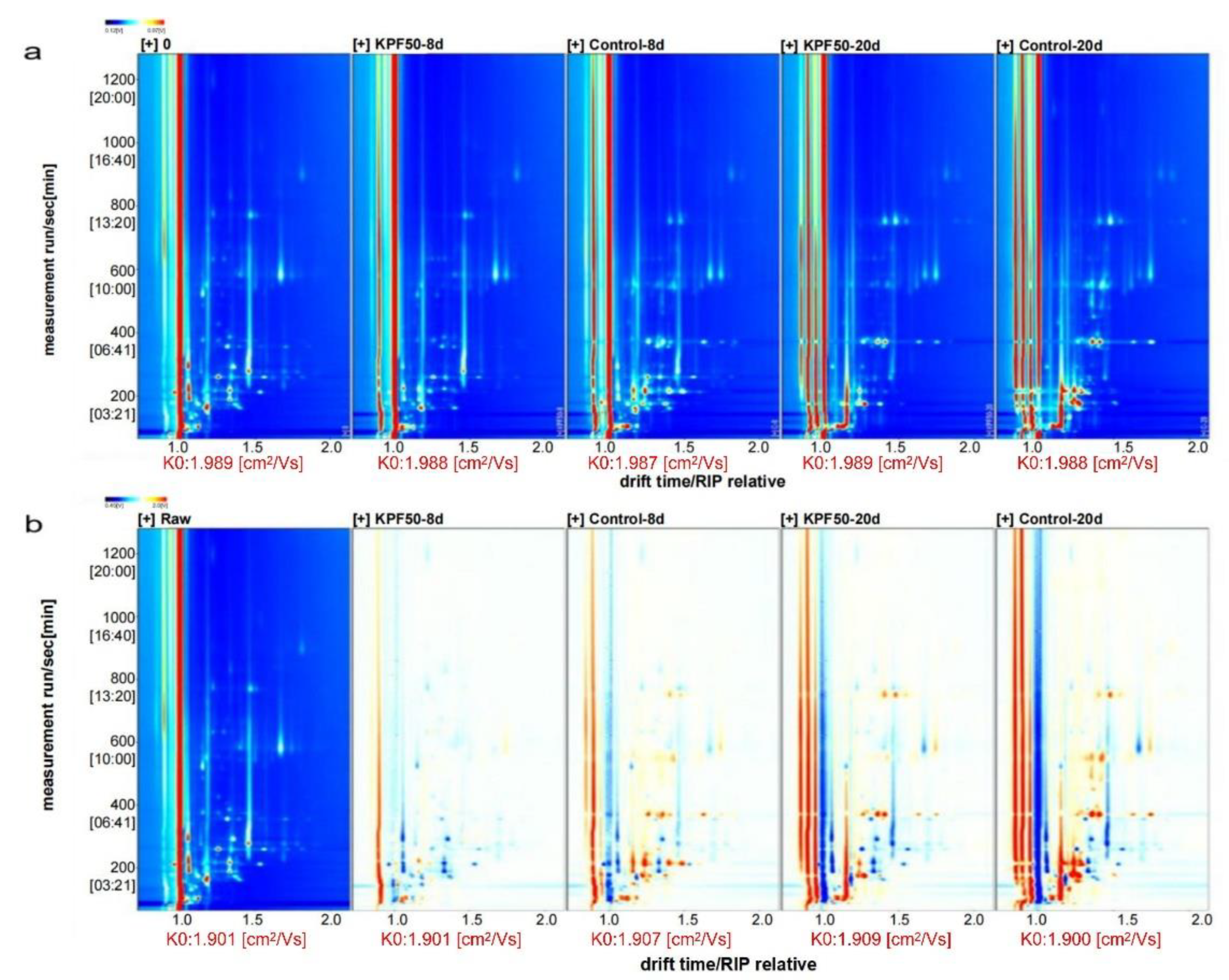
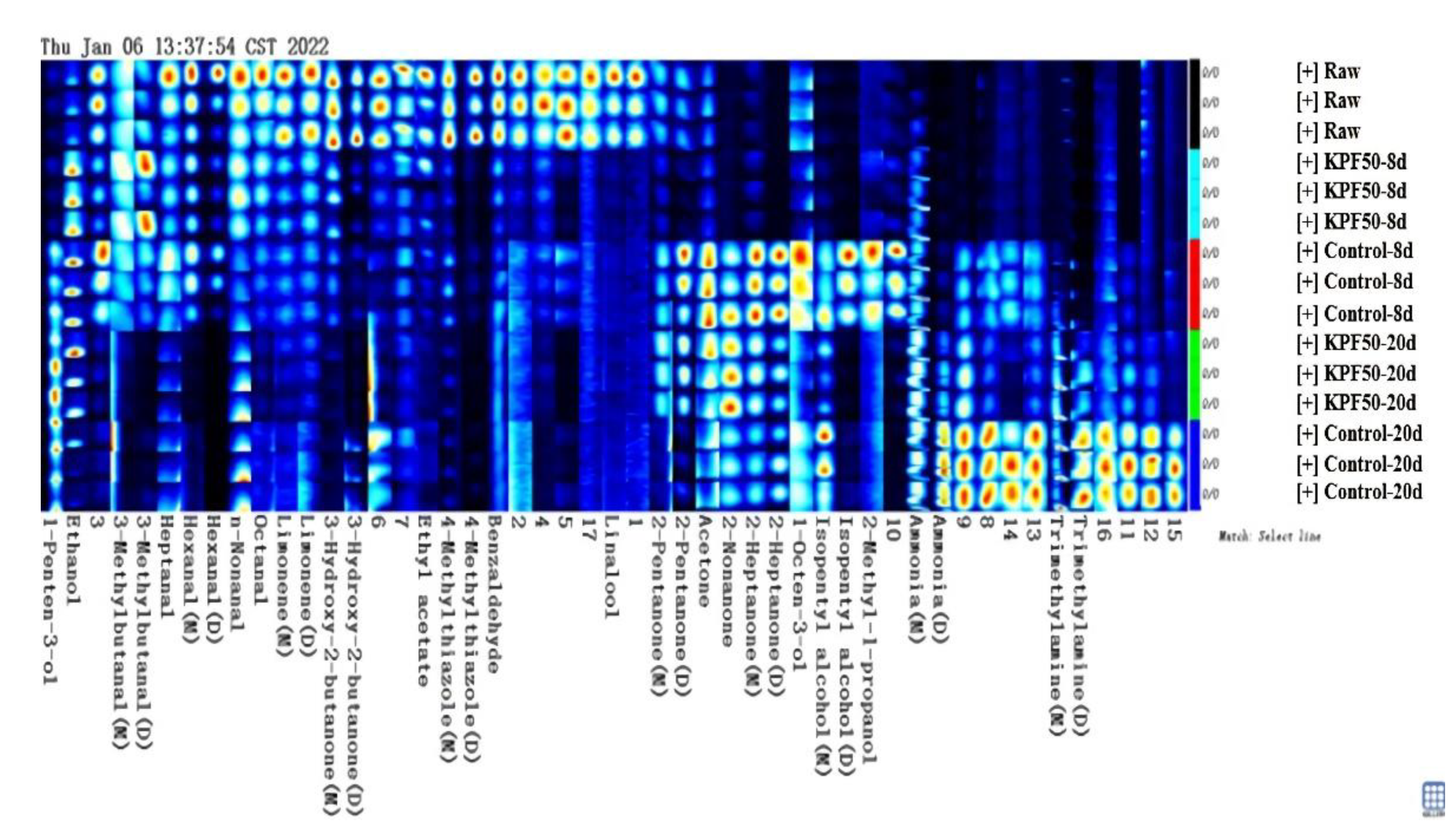
3.4. Effect of Composite Coating on Volatile Components of Sea Bass Fillets
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, L.-M.; Shi, X.-K.; Cui, K.-P.; Su, Y.-P. Commercial transport technology of sea bass live fish. Jiangxi Aquat. Prod. Sci. Technol. 2018, 1, 31–32. (In Chinese) [Google Scholar]
- Cai, L.; Wu, X.; Li, X.; Zhong, K.; Li, Y.; Li, J. Effects of different freezing treatments on physicochemical responses and microbial characteristics of Japanese sea bass (Lateolabrax japonicas) fillets during refrigerated storage. LWT Food Sci. Technol. 2014, 59, 122–129. [Google Scholar] [CrossRef]
- Wang, Q.; Mei, J.; Xie, J. The effect of cold preservation and live transportation on the stress and quality of sea bass. Chin. J. Food 2022, 22, 203–213. [Google Scholar] [CrossRef]
- Wu, J.; Yang, H.; Zhang, J.-T.; Zhang, R.; Sun, T.; Xie, J.; Guo, X.-H.; Yu, J.-Y.; Li, J.-R. Effect of in situ synthetic nano-SiOx/Lysozyme/Tea Polyphenols/Chitosan composite plastic coating on the preservation performance of sea bass fillets. Food Sci. 2020, 41, 181–189. [Google Scholar]
- Bekhit, E.; Holman, B.; Giteru, S.G.; Hopkins, D.L. Total volatile basic nitrogen (TVB-N) and its role in meat spoilage: A review. Trends Food Sci. Technol. 2021, 109, 280–302. [Google Scholar] [CrossRef]
- Ali, M.Y.; Hasan, M.N.; Moyaduzzaman, M.F.; Faruque, M.O. Post mortem variation in total volatile base nitrogen (TVB-N) and trimethylamine nitrogen (TMA-N) of Vetki (Lates calcarifer) according to size. Khulna Univ. Stud. 2008, 9, 257–264. [Google Scholar]
- Ning, Q.; Guo, Y.-H.; Wu, C.-H.; Pang, J.; Wu, X.-H. Effect of carboxylated cellulose nanocrystalline whiskers on properties of Konjac glucomannan composite gel. J. Food Sci. Technol. 2020, 38, 7. [Google Scholar]
- Ni, Y.; Liu, Y.; Zhang, W.; Shi, S.; Zhu, W.; Wang, R.; Zhang, L.; Chen, L.; Sun, J.; Pang, J. Advanced konjac glucomannan-based films in food packaging: Classification, preparation, formation mechanism and function. LWT Food Sci. Technol. 2021, 152, 112338. [Google Scholar] [CrossRef]
- Jia, S.; Liu, Y.; Zhuang, S.; Sun, X.; Li, Y.; Hong, H.; Lv, Y.; Luo, Y. Effect of ε-polylysine and ice storage on microbiota composition and quality of Pacific white shrimp (Litopenaeus vannamei) stored at 0 °C. Food Microbiol. 2019, 83, 27–35. [Google Scholar] [CrossRef]
- Cai, L.; Cao, A.; Bai, F.; Li, J. Effect of ε-polylysine in combination with alginate coating treatment on physicochemical and microbial characteristics of Japanese sea bass (Lateolabrax japonicas) during refrigerated storage. LWT Food Sci. Technol. 2015, 62, 1053–1059. [Google Scholar] [CrossRef]
- Jl, A.; Wlab, C.; Yi, W.A.; Xsa, D.; Jmab, C.; Yc, A.; Jing, X. The preservation effects of chitosan copolymers (gallic acid and protocatechuic acid) on sea bass (Lateolabrax japonicus) fillets—ScienceDirect. Aquac. Fish. 2023, 8, 305–315. [Google Scholar]
- Fan, K.; Zhang, M.; Bhandari, B.; Jiang, F. A combination treatment of ultrasound and ε-polylysine to improve microorganisms and storage quality of fresh-cut lettuce. LWT Food Sci. Technol. 2019, 113, 108315. [Google Scholar] [CrossRef]
- Alirezalu, K.; Movlan, H.S.; Yaghoubi, M.; Pateiro, M.; Lorenzo, J.M. ε-polylysine coating with stinging nettle extract for fresh beef preservation. Meat Sci. 2021, 176, 108474. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.M.; Gonalves, M.P.; Rocha, C.M.R. Effect of ferulic acid on the performance of soy protein isolate-based edible coatings applied to fresh-cut apples. Food Sci. Technol. Zur. 2017, 80, 409–415. [Google Scholar] [CrossRef]
- Nicolau-Lapea, I.; Aguiló-Aguayo, I.; Kramer, B.; Abadias, M.; Muranyi, P. Combination of ferulic acid with Aloe vera gel or alginate coatings for shelf-life prolongation of fresh-cut apples. Food Packag. Shelf Life 2021, 27, 100620. [Google Scholar] [CrossRef]
- Esteves, E.; Anibal, J. Sensory evaluation of seafood freshness using the quality index method: A meta-analysis. Int. J. Food Microbiol. 2021, 337, 108934. [Google Scholar] [CrossRef]
- Wang, L.-Z.; Wang, S.-Y.; Jian, C.; Cao, R.; Du, Y.-J. Progress on lipid oxidation and its impact on quality during processing and storage of aquatic products. J. Jiangsu Ocean. Univ. Nat. Sci. Ed. 2021, 30, 42–49. [Google Scholar]
- Cao, T.L.; Song, K.B. Development of bioactive Bombacaceae gum films containing cinnamon leaf essential oil and their application in packaging of fresh salmon fillets. LWT Food Sci. Technol. 2020, 131, 109647. [Google Scholar] [CrossRef]
- Smaoui, S.; Ben Hlima, H.; Tavares, L.; Ben Braïek, O.; Ennouri, K.; Abdelkafi, S.; Mellouli, L.; Mousavi Khaneghah, A. Application of eco-friendly active films and coatings based on natural antioxidant in meat products: A review—ScienceDirect. Prog. Org. Coat. 2022, 166, 106780. [Google Scholar]
- Li, B.-X.; Yu, Y.-L.; He, Y.; Guo, L.-R.; Ren, D.; Xu, D. Effect of chitosan-nanocellulose composite coating on the preservation effect of sugar orange. Food Sci. 2021, 42, 185–192. [Google Scholar]
- Ghorai, T.; Dora, K. Quality evaluation of fish protein soluble (FPS) injected seabass (Lates calcarifer) fillets through texture profile analysis during chilled storage. Indian J. Fish. 2021, 68, 105–110. [Google Scholar] [CrossRef]
- Szymczak, M.; Kamiński, P.; Felisiak, K.; Szymczak, B.; Sawicki, T. Effect of constant and fluctuating temperatures during frozen storage on quality of marinated fillets from Atlantic and Baltic herrings (Clupea harengus). LWT Food Sci. Technol. 2020, 133, 109961. [Google Scholar] [CrossRef]
- Alvarez-Perez, O.B.; Ventura-Sobrevilla, J.M.; Torres-Leon, C.; Rojas-Molina, R.; Rodriguez-Herrera, R.; Aguilar-Gonzalez, M.A.; Aguilar, C.N. Development and characterization of whey protein films incorporated with tarbush polyphenols and candelilla wax. Food Biosci. 2022, 45, 101505. [Google Scholar] [CrossRef]
- Xu, Y.-X.; Bai, X.-T.; Feng, R.; Zhao, H.-L.; Li, X.-P.; Li, J.-R.; Yi, S.-M.; Xie, J.; Guo, X.-H. Based on GC-IMS, analysis of flavor substance changes during sea bass meat steaming and stoichiometry. Food Sci. 2021, 42, 270–275. [Google Scholar]
- Wang, Y.; Li, J.; Wu, Y.; Yang, S.; Wang, D.; Liu, Q. Analysis of Volatile Compounds in Sea Bass (Lateolabrax japonicus) Resulting from Different Slaughter Methods Using Electronic-Nose (E-Nose) and Gas Chromatography-Ion Mobility Spectrometry. Molecules 2021, 26, 5889. [Google Scholar] [CrossRef] [PubMed]
- Aune, T.F.; Olsen, R.L.; Akse, L.; Ytterstad, E.; Esaiassen, M. Influence of different cold storage temperatures during the Rigor mortis phase on fillet contraction and longer-term quality changes of Atlantic cod fillets. LWT Food Sci. Technol. 2014, 59, 583–586. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, X.; Wang, Y.; Pan, D.; Sun, Y.; Cao, J. Study on the volatile compounds generated from lipid oxidation of Chinese bacon (unsmoked) during processing. Eur. J. Lipid Sci. Technol. 2017, 119, 1600512. [Google Scholar] [CrossRef]
- Li, M.-Q.; Liu, D.-Y.; Wang, X.; Wu, J.-C.; Zhao, Z.-N.; Zou, Y.-F. Effects of Different Smoking Materials on Volatile Flavor Compounds in Smoked Chicken Thighs. Food Sci. 2019, 40, 220–227. [Google Scholar] [CrossRef]
- Zhao, T.; Benjakul, S.; Sanmartin, C.; Ying, X.; Ma, L.; Xiao, G.; Yu, J.; Liu, G.; Deng, S. Changes of Volatile Flavor Compounds in Large Yellow Croaker (Larimichthys crocea) during Storage, as Evaluated by Headspace Gas Chromatography-Ion Mobility Spectrometry and Principal Component Analysis. Foods 2021, 10, 2917. [Google Scholar] [CrossRef]


| Quality Parameters | Score | ||
|---|---|---|---|
| 10–8 | 7–4 | 3–0 | |
| Appearance (0.2) | Bright color, full of luster | Dim color, slightly shiny | Dark color, dull surface |
| Smell (0.3) | Strong umami | Light smell, slightly peculiar smell | Strong stink |
| Texture (0.5) | Muscles elastic, dense, clear texture | Muscles are more elastic, less dense but not loose | Muscle tissue inelastic, not dense, loose |
| Sample | 0 d | 4 d | 8 d | 12 d | 16 d | 20 d | |
|---|---|---|---|---|---|---|---|
| L* | Control | 64.42 ± 0.49 aA | 63.66 ± 0.85 aB | 61.21 ± 0.82 bC | 58.91 ± 0.19 bC | 56.02 ± 0.19 cC | 53.66 ± 0.57 dC |
| KGM | 64.42 ± 0.49 aA | 63.37 ± 0.70 aB | 61.9 ± 10.32 bBC | 59.25 ± 0.76 cBC | 55.05 ± 0.64 dD | 52.93 ± 0.71 eC | |
| KP | 64.42 ± 0.49 aA | 64.92 ± 0.66 aAB | 62.25 ± 0.70 bAB | 60.82 ± 0.60 cB | 57.75 ± 0.41 dB | 55.08 ± 0.16 eB | |
| KPF50 | 64.42 ± 0.49 bA | 65.51 ± 0.58 aA | 63.68 ± 0.59 cA | 61.83 ± 0.47 dA | 59.08 ± 0.17 eA | 56.72 ± 0.40 fA | |
| a* | Control | 0.65 ± 0.03 aA | 0.43 ± 0.05 bB | 0.38 ± 0.02 bC | 0.19 ± 0.02 cBC | 0.03 ± 0.08 dB | −0.21 ± 0.03 eB |
| KGM | 0.65 ± 0.03 aA | 0.46 ± 0.04 bB | 0.39 ± 0.02 bC | 0.13 ± 0.03 cC | −0.08 ± 0.09 dAB | −0.24 ± 0.08 eB | |
| KP | 0.65 ± 0.03 aA | 0.52 ± 0.02 bA | 0.45 ± 0.03 bB | 0.23 ± 0.05 cB | 0.08 ± 0.03 dA | −0.07 ± 0.11 eAB | |
| KPF50 | 0.65 ± 0.03 aA | 0.55 ± 0.02 bA | 0.51 ± 0.02 bA | 0.32 ± 0.05 cA | 0.14 ± 0.05 dA | 0.33 ± 0.07 eA | |
| b* | Control | −0.49 ± 0.02 fA | −0.21 ± 0.02 eA | −0.1 ± 0.06 dA | 0.01 ± 0.05 cA | 0.15 ± 0.04 bA | 0.30 ± 0.04 aA |
| KGM | −0.49 ± 0.02 fA | −0.24 ± 0.04 eA | −0.07 ± 0.02 dA | 0.05 ± 0.04 cA | 0.17 ± 0.04 bA | 0.32 ± 0.05 aA | |
| KP | −0.49 ± 0.02 fA | −0.33 ± 0.04 eB | −0.23 ± 0.03 dB | −0.11 ± 0.02 cB | 0.04 ± 0.05 bB | 0.19 ± 0.02 aB | |
| KPF50 | −0.49 ± 0.02 eA | −0.39 ± 0.03 dC | −0.29 ± 0.02 cB | −0.17 ± 0.04 bB | −0.09 ± 0.06 bB | 0.14 ± 0.06 aB |
| Category | Name of Compound | Molecular Formula | Retention Index | Retention Time/s | Travel Time/ms | Peak Area | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Raw | KPF50—8 d | Control—8 d | KPF50—20 d | Control—20 d | ||||||
| Aldehydes | nonanal | C9H18O | 1105.00 | 774.35 | 1.47 | 358.16 | 318.64 | 191.00 | 229.85 | 253.49 |
| caprylic aldehyde | C8H16O | 1008.30 | 581.51 | 1.40 | 268.00 | 162.07 | 103.94 | 54.94 | 71.47 | |
| benzaldehyde | C7H6O | 977.50 | 521.54 | 1.15 | 829.05 | 343.45 | 384.80 | 471.61 | 481.45 | |
| heptanal | C7H14O | 900.70 | 387.24 | 1.33 | 201.95 | 154.55 | 161.50 | 94.06 | 108.94 | |
| Hexanal monomer | C6H12O | 790.20 | 261.50 | 1.26 | 1233.83 | 810.41 | 937.81 | 128.73 | 290.11 | |
| Hexanal dimer | C6H12O | 789.80 | 261.13 | 1.57 | 624.64 | 270.46 | 442.75 | 38.48 | 27.60 | |
| 3-methylbutylaldehyde | C5H10O | 647.60 | 163.60 | 1.17 | 278.67 | 532.50 | 283.53 | 43.13 | 79.14 | |
| 3-methylbutyraldehyde dimer | C5H10O | 647.60 | 163.60 | 1.40 | 6885.45 | 8586.27 | 18,518.25 | 44,054.62 | 44,637.79 | |
| 1-Pentan-3-ol | C5H10O | 682.80 | 179.07 | 0.95 | 374.45 | 358.44 | 1458.22 | 1912.24 | 1600.50 | |
| ethyl alcohol | C2H6O | 440.70 | 96.23 | 1.05 | 1106.71 | 2668.58 | 1865.12 | 1811.71 | 799.76 | |
| linalool | C10H18O | 1105.60 | 775.77 | 1.23 | 115.46 | 87.26 | 197.13 | 133.51 | 162.63 | |
| Alcohols | 1-octen-3-ol | C8H16O | 994.80 | 557.86 | 1.17 | 745.18 | 654.59 | 465.54 | 296.75 | 485.14 |
| 2-Methyl-1-propanol | C4H10O | 618.50 | 151.83 | 1.36 | 446.34 | 182.43 | 196.80 | 211.66 | 205.66 | |
| Isoamyl alcohol monomer | C5H12O | 737.60 | 216.67 | 1.23 | 65.74 | 56.01 | 971.36 | 65.46 | 173.03 | |
| Isoamyl alcohol dimer | C5H12O | 734.70 | 214.39 | 1.50 | 14.18 | 16.07 | 55.18 | 21.23 | 26.32 | |
| 2-Methyl-1-propanol | C4H10O | 618.50 | 151.83 | 1.36 | 446.34 | 182.43 | 196.80 | 211.66 | 205.66 | |
| Ketone | 3-hydroxy-2-butanone monomer | C4H8O2 | 742.70 | 220.68 | 1.06 | 3126.38 | 1537.63 | 949.28 | 294.66 | 360.76 |
| 3-hydroxy-2-butanone dimer | C4H8O2 | 740.90 | 219.23 | 1.33 | 1594.56 | 387.69 | 405.27 | 189.13 | 784.18 | |
| 2-pentanone monomer | C5H10O | 674.50 | 175.29 | 1.12 | 548.24 | 242.98 | 561.07 | 794.15 | 404.77 | |
| 2-pentanone dimer | C5H10O | 675.10 | 175.58 | 1.37 | 493.73 | 140.50 | 930.19 | 676.32 | 235.54 | |
| acetone | C3H6O | 470.10 | 103.79 | 1.12 | 1198.01 | 883.27 | 3419.88 | 2928.52 | 3006.84 | |
| 2-nonanone | C9H18O | 1095.50 | 752.94 | 1.41 | 78.20 | 84.19 | 492.57 | 586.55 | 363.25 | |
| 2-heptanone monomer | C7H14O | 891.10 | 373.15 | 1.26 | 32.89 | 27.97 | 532.21 | 296.55 | 336.05 | |
| 2-heptanone dimer | C7H14O | 890.80 | 372.74 | 1.63 | 293.49 | 403.06 | 1875.56 | 1078.46 | 2163.75 | |
| Ammonia monomer | NH3 | 780.00 | 252.21 | 0.89 | 13,733.51 | 38,369.74 | 50,283.61 | 64,843.40 | 62,940.28 | |
| Ammonia | Ammonia dimer | NH3 | 781.00 | 253.09 | 0.85 | 2453.99 | 3979.86 | 9303.66 | 26,329.55 | 61,693.84 |
| Trimethylamine monomer | C3H9N | 519.80 | 117.90 | 0.96 | 295.66 | 284.48 | 799.37 | 4278.75 | 9233.84 | |
| Trimethylamine dimer | C3H9N | 483.40 | 107.39 | 1.15 | 134.21 | 169.61 | 761.16 | 592.38 | 392.69 | |
| Limonene monomer | C10H16 | 1037.5 | 633.95 | 1.2276 | 143.54 | 94.92 | 70.08 | 57.52 | 71.03 | |
| Alkanes | Limonene dimer | C10H16 | 1035.5 | 630.36 | 1.29389 | 199.76 | 97.39 | 112.17 | 87.24 | 70.15 |
| Esters | ethyl acetate | C4H8O2 | 590.40 | 141.28 | 1.10 | 399.01 | 232.80 | 145.29 | 83.30 | 96.60 |
| Else | 4-Methylthiazole monomer | C4H5NS | 825.60 | 296.29 | 1.06 | 2888.51 | 775.18 | 899.53 | 380.03 | 437.78 |
| 4-Methylthiazole dimer | C4H5NS | 823.00 | 293.58 | 1.36 | 1147.53 | 171.03 | 235.98 | 93.57 | 114.17 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, H.; Liao, J.; Chen, Y.; Tong, X.; Sun, X.; Yan, J.; Pang, J. Effects of Konjac Glucomannan/ε-Polylysine Hydrochloride/Ferulic Acid Composite Coating on the Freshness Preservation Performance and Flavor of Refrigerated Sea Bass Fillets. Foods 2023, 12, 517. https://doi.org/10.3390/foods12030517
Xiao H, Liao J, Chen Y, Tong X, Sun X, Yan J, Pang J. Effects of Konjac Glucomannan/ε-Polylysine Hydrochloride/Ferulic Acid Composite Coating on the Freshness Preservation Performance and Flavor of Refrigerated Sea Bass Fillets. Foods. 2023; 12(3):517. https://doi.org/10.3390/foods12030517
Chicago/Turabian StyleXiao, Huibao, Jun Liao, Yongshi Chen, Xiuping Tong, Xiangyun Sun, Jiqiang Yan, and Jie Pang. 2023. "Effects of Konjac Glucomannan/ε-Polylysine Hydrochloride/Ferulic Acid Composite Coating on the Freshness Preservation Performance and Flavor of Refrigerated Sea Bass Fillets" Foods 12, no. 3: 517. https://doi.org/10.3390/foods12030517
APA StyleXiao, H., Liao, J., Chen, Y., Tong, X., Sun, X., Yan, J., & Pang, J. (2023). Effects of Konjac Glucomannan/ε-Polylysine Hydrochloride/Ferulic Acid Composite Coating on the Freshness Preservation Performance and Flavor of Refrigerated Sea Bass Fillets. Foods, 12(3), 517. https://doi.org/10.3390/foods12030517





