Machine Learning Algorithms Applied to Semi-Quantitative Data of the Volatilome of Citrus and Other Nectar Honeys with the Use of HS-SPME/GC–MS Analysis, Lead to a New Index of Geographical Origin Authentication
Abstract
1. Introduction
2. Materials and Methods
2.1. Honey Samples
2.2. Reagents and Solutions
2.3. Determination of Volatile Compounds
2.3.1. Extraction of Volatile Compounds
2.3.2. Instrumentation and Analytical Conditions
2.3.3. Identification of Volatile Compounds
2.4. Statistical Analysis: Machine Learning Theory
3. Results
3.1. Volatile Compounds of Citrus and Other Nectar Honeys
3.2. Geographical Origin Indication of Citrus and Other Nectar Honeys Based on Volatile Compounds and Machine Learning Algorithms
3.2.1. Estimation of Sample Size: Power Analysis
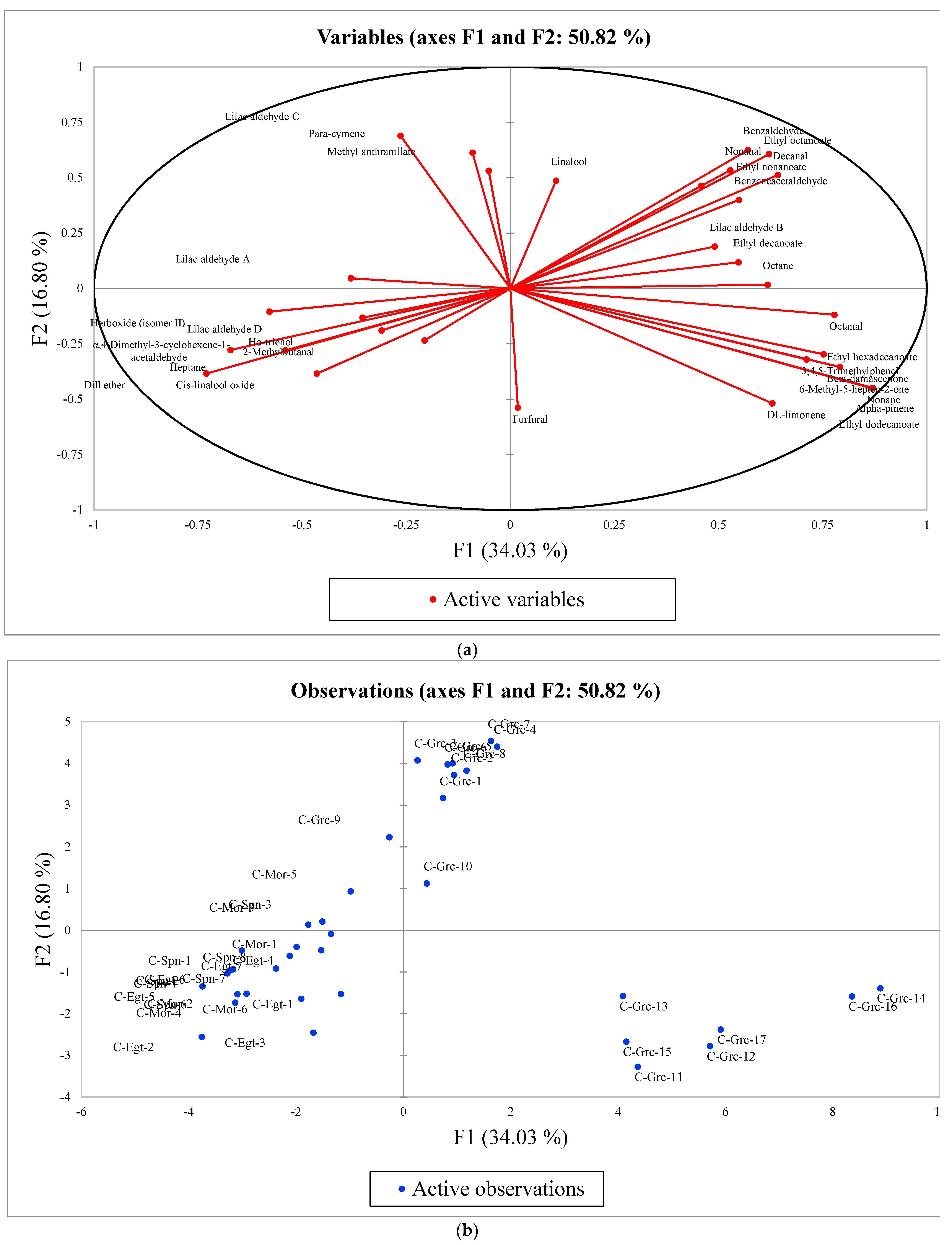
3.2.2. PCA: Characterization of Citrus Honey Samples of Different Geographical Origin (Part I)
3.2.3. PCA: Characterization of Citrus and Other Nectar Honey Samples of Different Geographical Origin (Part II)
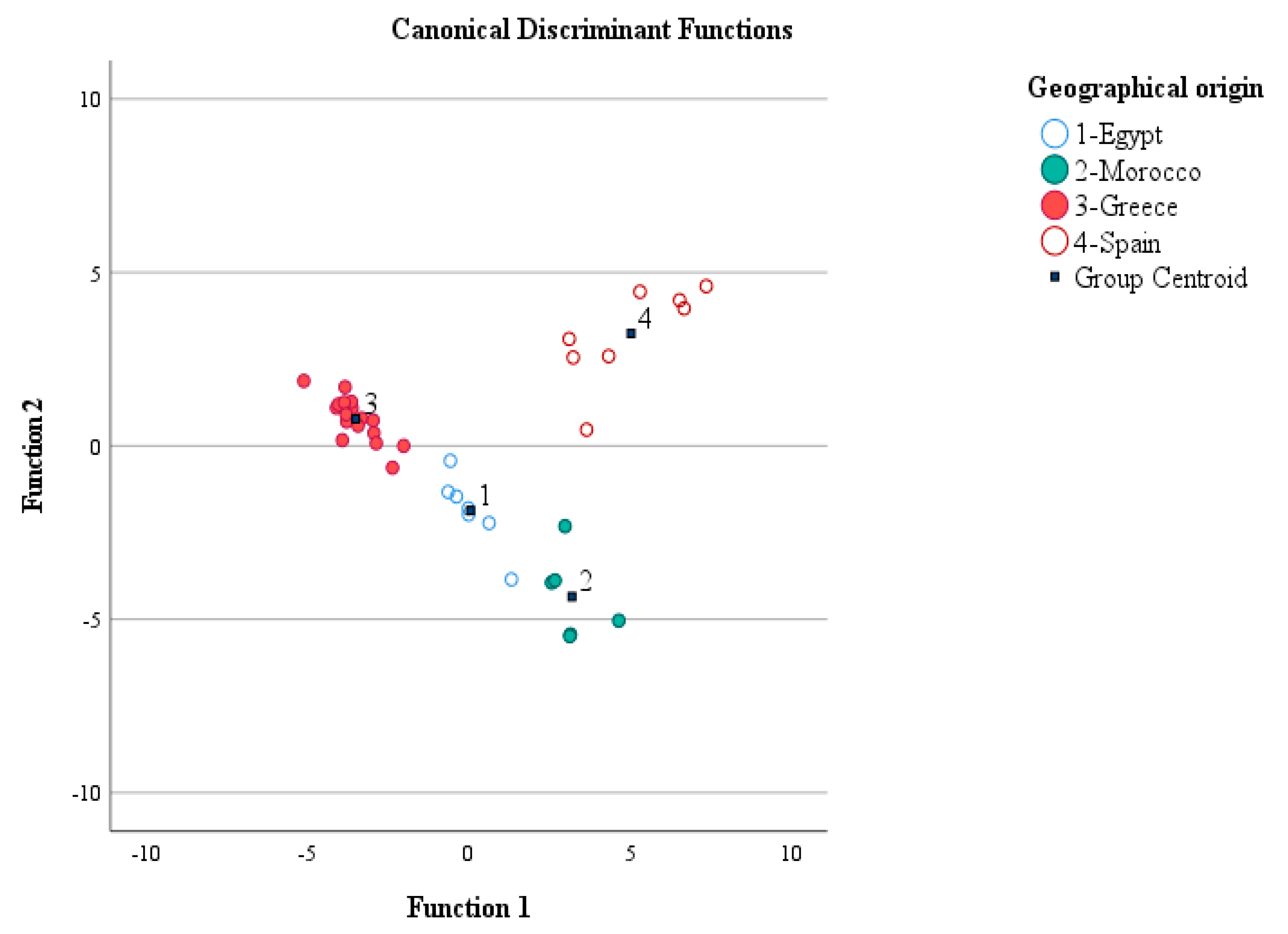
3.2.4. LDA: Geographical Origin Discrimination of Citrus Honey (Part I)
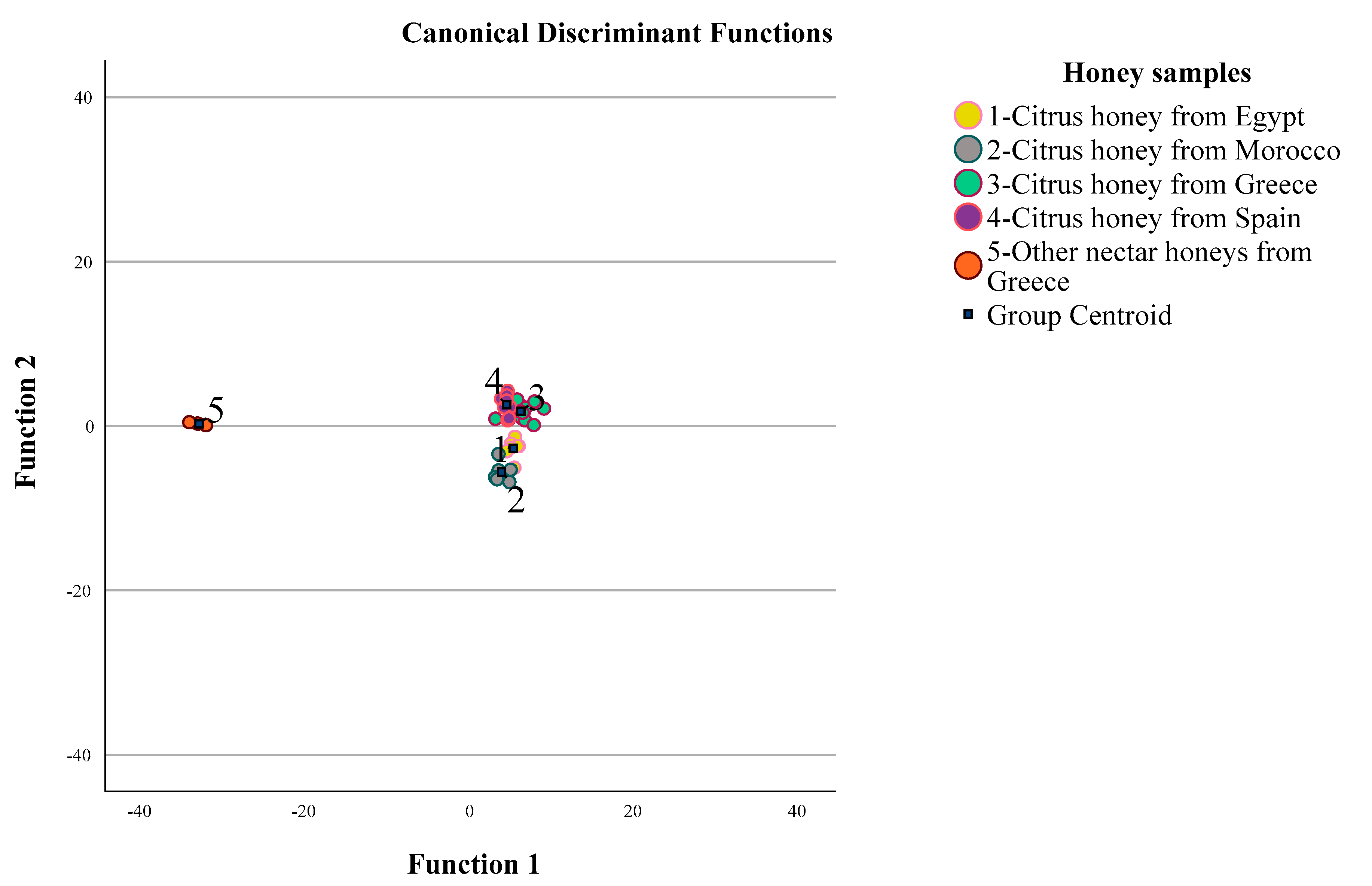
3.2.5. LDA: Discrimination of Citrus and other Nectar Honeys (Part II)
4. Discussion
5. A New Index for the Geographical Origin Discrimination of Citrus Honey Based on the Ratio of Semi-Quantitative Data of Specific Volatile Compounds
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Council Directive 2001/110/EC of 20 December 2001 relating to honey. Off. J. Eur. Communities 2002, 10, 47–52.
- Von Der Ohe, W.; Persano Oddo, L.; Piana, M.L.; Morlot, M.; Martin, P. Harmonized methods of melissopalynology. Apidologie 2004, 35, S18–S25. [Google Scholar] [CrossRef]
- Alissandrakis, E.; Tarantilis, P.A.; Harizanis, P.C.; Polissiou, M. Aroma investigation of unifloral Greek citrus honey using solid-phase microextraction coupled to gas chromatographic-mass spectrometric analysis. Food Chem. 2007, 100, 396–404. [Google Scholar] [CrossRef]
- Kadar, M.; Juan-Borrás, M.; Carot, M.J.; Domenech, E.; Escriche, I. Volatile fraction composition and physicochemical parameters as tools for the differentiation of lemon blossom honey and orange blossom honey. J. Sci. Food Agric. 2011, 91, 2768–2776. [Google Scholar] [CrossRef] [PubMed]
- Karabagias, I.K.; Louppis, P.A.; Karabournioti, S.; Kontakos, S.; Papastephanou, C.; Kontominas, M.G. Characterization and geographical discrimination of commercial Citrus spp. honeys produced in different Mediterranean countries based on minerals, volatile compounds and physicochemical parameters, using chemometrics. Food Chem. 2017, 217, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Escriche, I.; Juan-Borrás, M.; Visquert, M.; Asensio-Grau, A.; Valiente, J.M. Volatile profile of Spanish raw citrus honey: The best strategy for its correct labeling. J. Food Process. Preserv. 2021, 46, e16200. [Google Scholar] [CrossRef]
- Castro-Vázquez, L.; Díaz-Maroto, M.C.; Pérez-Coello, M.S. Aroma composition and new chemical markers of Spanish citrus honeys. Food Chem. 2007, 103, 601–606. [Google Scholar] [CrossRef]
- Castro-Vázquez, L.; Díaz-Maroto, M.C.; González-Viñas, M.A.; Pérez-Coello, M.S. Differentiation of monofloral citrus, rosemary, eucalyptus, lavender, thyme, and heather honeys based on volatile composition and sensory descriptive analysis. Food Chem. 2009, 112, 1022–1030. [Google Scholar] [CrossRef]
- Rodriguez, I.; Salud, S.; Hortensia, G.; Luis, U.J.; Jodral, M. Characterisation of Sierra Morena citrus blossom honey (Citrus sp). Int. J. Food Sci. Technol. 2010, 45, 2008–2015. [Google Scholar] [CrossRef]
- Juan-Borrás, M.; Domenech, E.; Hellebrandova, M.; Escriche, I. Effect of country origin on physicochemical, sugar and volatile composition of acacia, sunflower and tilia honeys. Food Res. Int. 2014, 60, 86–94. [Google Scholar] [CrossRef]
- Patrignani, M.; Fagúndez, G.A.; Tananaki, C.; Thrasyvoulou, A.; Lupano, C.E. Volatile compounds of Argentinean honeys: Correlation with floral and geographical origin. Food Chem. 2018, 246, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Pawliszyn, J. Solid Phase Microextraction: Theory and Practice; John Wiley & Sons: Hoboken, NJ, USA, 1997; p. 264. [Google Scholar]
- Kataoka, K.; Lord, H.L.; Pawliszyn, J. Applications of solid-phase microextraction in food analysis. J. Chromatogr. A 2000, 880, 35–62. [Google Scholar] [CrossRef] [PubMed]
- Karabournioti, S.; Eleftheriou, E.P.; Thrasyvoulou, A.; Fasseas, C. Pollen polymorphism in Thymus capitatus (Lamiaceae). Can. J. Bot. 2007, 85, 493–500. [Google Scholar] [CrossRef]
- Karabagias, I.K. Volatile metabolites or pollen characteristics as regional markers of monofloral thyme honey? Sep. Sci. Plus 2018, 1, 83–92. [Google Scholar] [CrossRef]
- Field, A. Discovering Statistics Using SPSS, 3rd ed.; Sage Publications Ltd.: London, UK, 2009. [Google Scholar]
- Huberty, C.J.; Olejnik, S. Applied MANOVA and Discriminant Analysis, 2nd ed.; John Wiley and Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Jolliffe, I.T. Principal Component Analysis. Springer Series in Statistics; Springer: New York, NY, USA, 2002. [Google Scholar]
- Duru, M.E.; Çayan, M.; Taş, F.; Küçükaydın, S.; Tel-Çayan, G. Characterization of volatile compounds of Turkish pine honeys from different regions and classification with chemometric studies. Eur. Food Res. Technol. 2021, 247, 2533–2544. [Google Scholar] [CrossRef]
- Soria, A.C.; Martínez-Castro, I.; Sanz, J. Some aspects of dynamic headspace analysis of volatile components in honey. Food Res. Int. 2008, 41, 838–848. [Google Scholar] [CrossRef]
- Escriche, I.; Kadar, M.; Juan-Borrás, M.; Domenech, E. Using flavonoids, phenolic compounds and headspace volatile profile for botanical authentication of lemon and orange honeys. Food Res. Int. 2011, 44, 1504–1513. [Google Scholar] [CrossRef]
- Juan-Borrás, M.; Periche, A.; Domenech, E.; Escriche, I. Correlation between methyl anthranilate level and percentage of pollen in Spanish citrus honey. Int. J. Food Sci. Technol. 2015, 50, 1690–1696. [Google Scholar] [CrossRef]

| Volatile Compounds | RT (min) | RI | Citrus Honey from Egypt Avg (±SD) | Citrus Honey from Morocco Avg (±SD) | Citrus Honey from Greece Avg (±SD) | Citrus Honey from Spain Avg (±SD) | Nectar Honey from Greece Avg (±SD) | F |
|---|---|---|---|---|---|---|---|---|
| Alcohols | ||||||||
| 1-Octanol | 18.92 | 1069 | nd | nd | nd | nd | 0.003 (0.005) | 4.21 ** |
| 1-Nonanol | 21.04 | 1170 | nd | nd | nd | nd | 0.01 (0.01) | 24.27 *** |
| Aldehydes | ||||||||
| 2-Methylbutanal | 6.79 | <800 | 0.07 (0.09) | nd | nd | nd | nd | 5.11 ** |
| Furfural | 13.25 | 837 | 0.13 (0.17) | 0.14 (0.22) | 0.01(0.01) | 0.004 (0.01) | 0.02 (0.02) | 3.22 * |
| Benzaldehyde | 16.75 | 978 | nd | 0.04 (0.05) | 0.04(0.02) | 0.004 (0.01) | 0.07 (0.09) | 3.65 * |
| Octanal | 17.45 | 1005 | nd | 0.01 (0.02) | 0.02(0.03) | nd | 0.01 (0.01) | 1.58 ns |
| Benzeneacetaldehyde | 18.58 | 1059 | nd | 0.10 (0.09) | 0.10(0.11) | 0.003 (0.01) | 0.46 (0.50) | 5.88 ** |
| Nonanal | 19.64 | 1107 | nd | 0.09 (0.05) | 0.15(0.11) | 0.03 (0.06) | nd | 7.58 *** |
| Lilac aldehyde (isomer I, A) | 20.57 | 1147 | 0.13 (0.23) | 0.06 (0.09) | 0.05(0.14) | 0.10 (0.09) | nd | 0.89 ns |
| Lilac aldehyde (isomer II, B) | 20.63 | 1149 | 0.06 (0.08) | 0.06 (0.11) | 0.24(0.22) | 0.06 (0.06) | 0.04 (0.06) | 3.89 ** |
| Lilac aldehyde (isomer III, C) | 20.76 | 1156 | 0.14 (0.07) | 0.27 (0.09) | 0.31(0.35) | 0.09 (0.08) | nd | 2.75 * |
| Lilac aldehyde (isomer IV, D) | 21.17 | 1176 | nd | nd | nd | 0.06 (0.02) | nd | 49.19 *** |
| Decanal | 21.77 | 1209 | nd | nd | 0.09(0.13) | nd | 0.03 (0.02) | 3.48 * |
| α,4-Dimethyl-3 cyclohexene-1-acetaldehyde | 22.31 | 1234 | 0.05 (0.07) | 0.07 (0.04) | 0.003(0.01) | 0.06 (0.04) | 0.003(0.005) | 6.41 *** |
| Hydrocarbons | ||||||||
| Heptane | 9.49 | <800 | 0.09 (0.05) | 0.05 (0.06) | nd | 0.04 (0.06) | nd | 7.92 *** |
| Octane | 12.26 | 800 | 0.01 (0.02) | 0.01 (0.02) | 0.03(0.02) | 0.01 (0.01) | 0.04 (0.02) | 3.18 * |
| Nonane | 14.94 | 900 | nd | nd | 0.02(0.03) | nd | 0.001 (0.01) | 1.56 ns |
| Delta-3-carene | 17.94 | 1024 | nd | nd | nd | nd | 0.007 (0.005) | 16.84 *** |
| Undecane | 19.59 | 1099 | nd | nd | nd | nd | 0.007 (0.005) | 16.84 *** |
| Ethers | ||||||||
| Dill ether | 21.72 | 1203 | 0.11 (0.08) | 0.13 (0.05) | nd | 0.07 (0.03) | 0.003 (0.005) | 22.42 *** |
| Esters | ||||||||
| Ethyl hexanoate | 17.25 | 993 | nd | nd | nd | nd | 0.003 (0.005) | 4.21 ** |
| Ethyl malonate | 18.67 | 1044 | nd | nd | nd | nd | 0.003 (0.005) | 4.21 ** |
| Ethyl heptanoate | 19.44 | 1092 | nd | nd | nd | nd | 0.007 (0.005) | 16.84 *** |
| Ethyl octanoate | 21.36 | 1191 | nd | 0.01 (0.01) | 0.03 (0.02) | 0.003 (0.009) | 0.03 (0.02) | 7.94 *** |
| Ethyl nonanoate | 23.26 | 1290 | 0.03 (0.05) | 0.01 (0.02) | 0.04 (0.03) | 0.02 (0.02) | 0.07 (0.05) | 2.89 * |
| Methyl anthranilate | 24.74 | 1366 | 0.03 (0.05) | nd | 0.02 (0.03) | nd | nd | 2.06 ns |
| Ethyl decanoate | 25.05 | 1389 | 0.04 (0.09) | nd | 0.02 (0.02) | 0.09 (0.02) | 0.04 (0.01) | 1.02 ns |
| Ethyl dodecanoate | 28.42 | 1588 | nd | nd | 0.003 (0.004) | nd | 0.01 (0.00) | 12.42 *** |
| Ethyl hexadecanoate | 34.94 | 1990 | nd | nd | 0.002 (0.004) | nd | nd | 1.29 ns |
| Ketones | ||||||||
| 6-Methyl-5-hepten-2-one | 17.06 | 986 | nd | nd | 0.001 (0.002) | nd | nd | 2.85 * |
| β-Damascenone | 25.37 | 1401 | nd | nd | 0.003 (0.06) | nd | 0.003 (0.005) | 1.75 ns |
| Phenolic compounds | ||||||||
| Benzeneethanol | 20.20 | 1129 | nd | nd | nd | nd | 0.07 (0.09) | 7.16 *** |
| Benzeneacetonitrile | 20.71 | 1154 | nd | nd | nd | nd | 0.04 (0.05) | 7.35 *** |
| 3,4,5-Trimethylphenol | 24.10 | 1330 | nd | nd | 0.003 (0.005) | nd | 0.002 (0.004) | 1.16 ns |
| Terpenes | ||||||||
| α-Pinene | 16.18 | 949 | nd | nd | 0.01 (0.09) | nd | 0.01 (0.004) | 3.38 * |
| Herboxide isomer II | 17.55 | 1007 | 0.05 (0.05) | 0.10 (0.13) | 0.01 (0.01) | 0.08 (0.06) | 0.003 (0.01) | 4.92 ** |
| Para-cymene | 18.13 | 1038 | nd | 0.03 (0.05) | 0.03 (0.03) | 0.03 (0.05) | nd | 2.24 ns |
| dL-Limonene | 18.25 | 1044 | 0.01 (0.02) | 0.01 (0.01) | 0.002 (0.003) | nd | nd | 0.86 ns |
| cis-Linalool oxide | 19.11 | 1077 | 0.08 (0.08) | 0.11 (0.04) | 0.02 (0.01) | 0.04 (0.02) | 0.003 (0.01) | 9.02 *** |
| Linalool | 19.54 | 1103 | 0.04 (0.05) | 0.02 (0.02) | 0.07 (0.03) | 0.06 (0.02) | 0.02 (0.03) | 4.29 ** |
| Hotrienol | 19.63 | 1104 | nd | 0.01 (0.02) | nd | 0.02 (0.03) | 0.03 (0.08) | 1.47 ns |
| TSQVC | 1.07 | 1.33 | 1.33 | 0.87 | 1.05 | |||
| Rch, Rnh (Karabagias-Nayik index) | 0.35a | 0.29b | 0.04c | 0.27d | 0.08e |
| Principal Component Analysis | Ethyl Dodecanoate (F1) | Lilac Aldehyde C (F2) | Alpha-4-Dimethyl-3-Cyclohexene-1-Acetaldehyde (F3) | Lilac Aldehyde D (F4) | 2-Methylbutanal (F5) | Methyl Anthranilate (F6) | Para-Cymene (F7) |
|---|---|---|---|---|---|---|---|
| Eigenvalue | 10.889 | 5.374 | 2.348 | 1.976 | 1.602 | 1.298 | 1.225 |
| Variability (%) | 34.029 | 16.795 | 7.339 | 6.174 | 5.006 | 4.056 | 3.829 |
| Cumulative % | 34.029 | 50.825 | 58.164 | 64.338 | 69.344 | 73.400 | 77.230 |
| LDA | Prediction Rate | Geographical Origin | Predicted Group Membership | Total Citrus and Nectar Honey Samples | ||||
|---|---|---|---|---|---|---|---|---|
| Method | Citrus Honey from Egypt | Citrus Honey from Morocco | Citrus Honey from Greece | Citrus Honey from Spain | Other Nectar Honeys from Greece | |||
| Original a | Count | Citrus honey from Egypt | 7 | 0 | 0 | 0 | 0 | 7 |
| Citrus honey from Morocco | 0 | 6 | 0 | 0 | 0 | 6 | ||
| Citrus honey from Greece | 0 | 0 | 17 | 0 | 0 | 17 | ||
| Citrus honey from Spain | 0 | 0 | 1 | 7 | 0 | 8 | ||
| Other nectar honeys from Greece | 0 | 0 | 0 | 0 | 6 | 6 | ||
| % | Citrus honey from Egypt | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | 100.0 | |
| Citrus honey from Morocco | 0.0 | 100.0 | 0.0 | 0.0 | 0.0 | 100.0 | ||
| Citrus honey from Greece | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 | 100.0 | ||
| Citrus honey from Spain | 0.0 | 0.0 | 12.5 | 87.5 | 0.0 | 100.0 | ||
| Other nectar honeys from Greece | 0.0 | 0.0 | 0.0 | 0.0 | 100.0 | 100.0 | ||
| Cross-validated b,c | Count | Citrus honey from Egypt | 5 | 1 | 1 | 0 | 0 | 7 |
| Citrus honey from Morocco | 1 | 4 | 1 | 0 | 0 | 6 | ||
| Citrus honey from Greece | 3 | 1 | 12 | 1 | 0 | 17 | ||
| Citrus honey from Spain | 0 | 0 | 1 | 7 | 0 | 8 | ||
| Other nectar honeys from Greece | 0 | 0 | 0 | 0 | 6 | 6 | ||
| % | Citrus honey from Egypt | 71.4 | 14.3 | 14.3 | 0.0 | 0.0 | 100.0 | |
| Citrus honey from Morocco | 16.7 | 66.7 | 16.7 | 0.0 | 0.0 | 100.0 | ||
| Citrus honey from Greece | 17.6 | 5.9 | 70.6 | 5.9 | 0.0 | 100.0 | ||
| Citrus honey from Spain | 0.0 | 0.0 | 12.5 | 87.5 | 0.0 | 100.0 | ||
| Other nectar honeys from Greece | 0.0 | 0.0 | 0.0 | 0.0 | 100.0 | 100.0 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karabagias, I.K.; Nayik, G.A. Machine Learning Algorithms Applied to Semi-Quantitative Data of the Volatilome of Citrus and Other Nectar Honeys with the Use of HS-SPME/GC–MS Analysis, Lead to a New Index of Geographical Origin Authentication. Foods 2023, 12, 509. https://doi.org/10.3390/foods12030509
Karabagias IK, Nayik GA. Machine Learning Algorithms Applied to Semi-Quantitative Data of the Volatilome of Citrus and Other Nectar Honeys with the Use of HS-SPME/GC–MS Analysis, Lead to a New Index of Geographical Origin Authentication. Foods. 2023; 12(3):509. https://doi.org/10.3390/foods12030509
Chicago/Turabian StyleKarabagias, Ioannis Konstantinos, and Gulzar Ahmad Nayik. 2023. "Machine Learning Algorithms Applied to Semi-Quantitative Data of the Volatilome of Citrus and Other Nectar Honeys with the Use of HS-SPME/GC–MS Analysis, Lead to a New Index of Geographical Origin Authentication" Foods 12, no. 3: 509. https://doi.org/10.3390/foods12030509
APA StyleKarabagias, I. K., & Nayik, G. A. (2023). Machine Learning Algorithms Applied to Semi-Quantitative Data of the Volatilome of Citrus and Other Nectar Honeys with the Use of HS-SPME/GC–MS Analysis, Lead to a New Index of Geographical Origin Authentication. Foods, 12(3), 509. https://doi.org/10.3390/foods12030509








