Antiviral Activity of Beebread, Bee-Collected Pollen and Artificially Fermented Pollen against Influenza A Virus
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples and Sample Preparation
2.1.1. Isolation and Identification of Microorganisms Applied in BCP Fermentation
2.1.2. Starter and Additional Cultures
- A total of 11.5 g of AF1 inoculated with 108 CFUs of Z. siamensis was resuspended in 2.3 mL sterile saline. The sample was mixed with sterile spatula and incubated for 24 h at 33 °C.
- A total of 2.5 g of the above product (A) inoculated with 108 CFUs of A. kunkeei was resuspended in 250 μL sterile saline. The sample was mixed with sterile spatula and incubated for 3 days at 33 °C.
- A total of 11.5 g of AF1 was added to B, mixed and inoculated with 108 CFUs of Bacillus licheniformis, 108 CFUs of Bacillus sp. and 108 CFUs of Bacillus subtilis resuspended in 1 mL sterile saline. The sample was mixed with sterile spatula and incubated for 24 h at 33 °C, then pressed using sterile pestle and incubated for 28 days at 33 °C. Bacillus strains were selected after screening for their ability to produce diverse enzymes (unpublished data).
2.1.3. Proteinaceous Fractions of BCP, BB, Fermented BCP and Proteinase-K Treatment
2.1.4. Ethyl Acetate, n-Boutanol and Aqueous Fractions
2.2. Chemical Analysis of BCP and BB Fractions
2.2.1. Nuclear Magnetic Resonance (NMR) Spectroscopy
2.2.2. Liquid Chromatography High-Resolution Quadrupole Time-of-Flight Mass Spectrometry (LC-Q-TOF-MS/MS)
2.2.3. Gas Chromatography–Mass Spectrometry (GC-MS) Analysis
2.3. Cytotoxicity and Antiviral Assays
2.3.1. Cytotoxicity Assay
2.3.2. Influenza A Virus and Cell Culture
2.3.3. RNA Extraction and cDNA Synthesis
2.3.4. Real-Time PCR Assay
2.3.5. CC50, IC50 and SI Calculation
3. Results and Discussion
3.1. Experimental Design
3.2. Cytotoxicity Levels of Tested Samples
3.3. Real-Time PCR and Antiviral Activity
3.4. Selectivity Index Values
3.5. Chemical Analysis of BCP and BB Samples
3.5.1. LC-MS Analysis
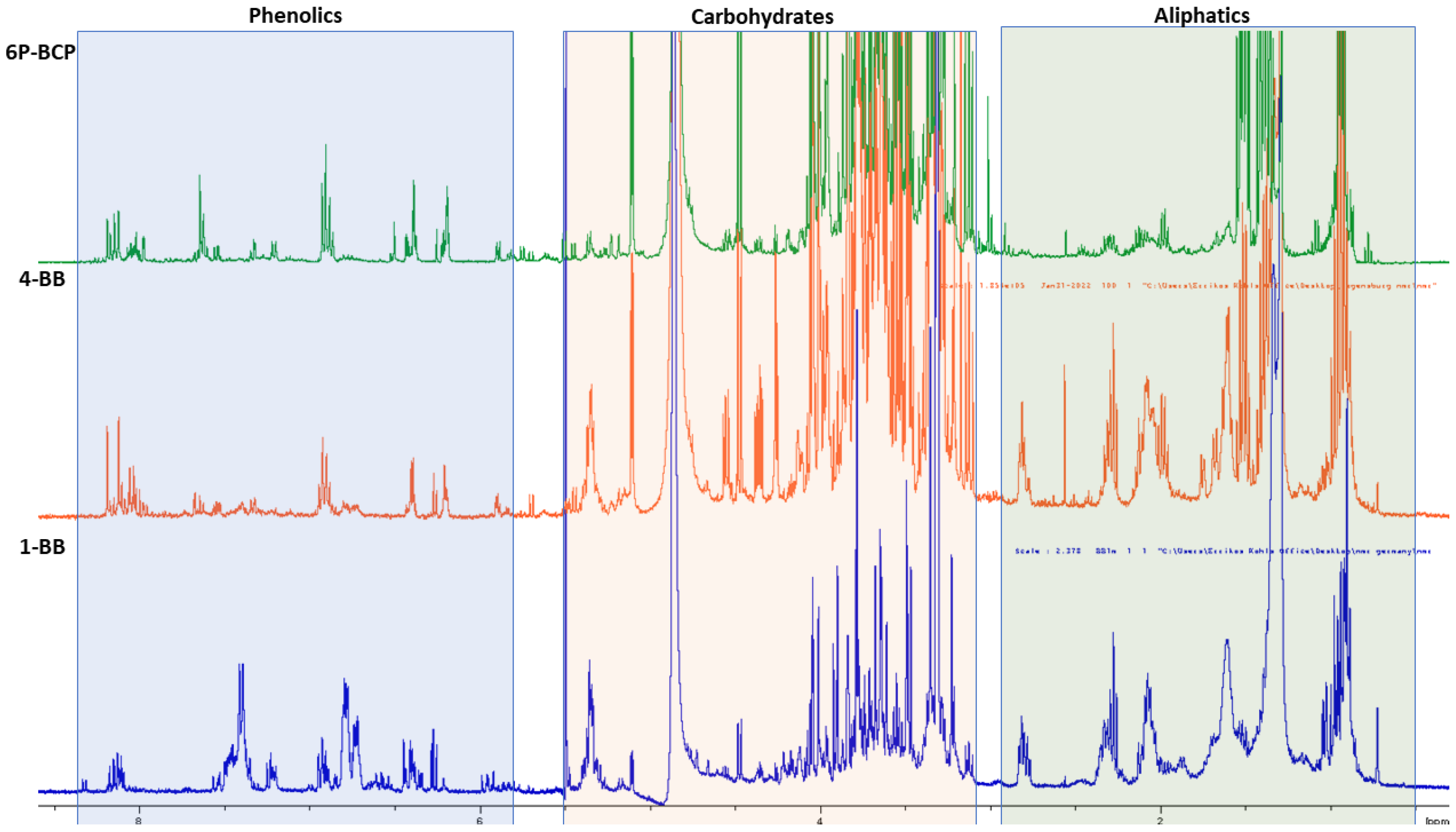
3.5.2. NMR and GC-MS Characterization of 1-BB, 4-BB and 6P-BCP Fractions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. NMR Characterization of Selected Bee-Derived n-Butanol Residues
References
- Mărgăoan, R.; Stranț, M.; Varadi, A.; Topal, E.; Yücel, B.; Cornea-Cipcigan, M.; Campos, M.G.; Vodnar, D.C. Bee Collected Pollen and Bee Bread: Bioactive Constituents and Health Benefits. Antioxidants 2019, 8, 568. [Google Scholar] [CrossRef] [PubMed]
- Kostić, A.Ž.; Milinčić, D.D.; Barać, M.B.; Ali Shariati, M.; Tešić, Ž.L.; Pešić, M.B. The Application of Pollen as a Functional Food and Feed Ingredient—The Present and Perspectives. Biomolecules 2020, 10, 84. [Google Scholar] [CrossRef] [PubMed]
- El Ghouizi, A.; Bakour, M.; Laaroussi, H.; Ousaaid, D.; El Menyiy, N.; Hano, C.; Lyoussi, B. Bee Pollen as Functional Food: Insights into Its Composition and Therapeutic Properties. Antioxidants 2023, 12, 557. [Google Scholar] [CrossRef] [PubMed]
- Laaroussi, H.; Ferreira-Santos, P.; Genisheva, Z.; Bakour, M.; Ousaaid, D.; El Ghouizi, A.; Teixeira, J.A.; Lyoussi, B. Unveiling the techno-functional and bioactive properties of bee pollen as an added-value food ingredient. Food Chem. 2023, 405, 134958. [Google Scholar] [CrossRef]
- Didaras, N.A.; Karatasou, K.; Dimitriou, T.G.; Amoutzias, G.D.; Mossialos, D. Antimicrobial Activity of Bee-Collected Pollen and Beebread: State of the Art and Future Perspectives. Antibiotics 2020, 9, 811. [Google Scholar] [CrossRef]
- Vásquez, A.; Olofsson, T.C. The lactic acid bacteria involved in the production of bee pollen and bee bread. J. Apic. Res. 2009, 48, 189–195. [Google Scholar] [CrossRef]
- Didaras, N.A.; Kafantaris, I.; Dimitriou, T.G.; Mitsagga, C.; Karatasou, K.; Giavasis, I.; Stagos, D.; Amoutzias, G.D.; Hatjina, F.; Mossialos, D. Biological Properties of Bee Bread Collected from Apiaries Located across Greece. Antibiotics 2021, 10, 555. [Google Scholar] [CrossRef]
- Bakour, M.; Laaroussi, H.; Ousaaid, D.; El Ghouizi, A.; Es-Safi, I.; Mechchate, H.; Lyoussi, B. Bee Bread as a Promising Source of Bioactive Molecules and Functional Properties: An Up-To-Date Review. Antibiotics 2022, 11, 203. [Google Scholar] [CrossRef]
- Pełka, K.; Otłowska, O.; Worobo, R.W.; Szweda, P. Bee Bread Exhibits Higher Antimicrobial Potential Compared to Bee Pollen. Antibiotics 2021, 10, 125. [Google Scholar] [CrossRef]
- Kontogiannis, T.; Dimitriou, T.G.; Didaras, N.A.; Mossialos, D. Antiviral Activity of Bee Products. Curr. Pharm. Des. 2022, 28, 2867–2878. [Google Scholar] [CrossRef]
- Asma, S.T.; Bobiş, O.; Bonta, V.; Acaroz, U.; Shah, S.R.A.; Istanbullugil, F.R.; Arslan-Acaroz, D. General Nutritional Profile of Bee Products and Their Potential Antiviral Properties against Mammalian Viruses. Nutrients 2022, 14, 3579. [Google Scholar] [CrossRef] [PubMed]
- Didaras, N.A.; Dimitriou, T.G.; Daskou, M.; Karatasou, K.; Mossialos, D. In Vitro Assessment of the Antiviral Activity of Greek Bee Bread And Bee Collected Pollen Against Enterovirus D68. J. Microbiol. Biotechnol. Food Sci. 2022, 11, e4859. [Google Scholar] [CrossRef]
- Lee, I.-K.; Hwang, B.; Kim, D.-W.; Kim, J.-Y.; Woo, E.-E.; Lee, Y.-J.; Choi, H.; Yun, B.-S. Characterization of Neuraminidase Inhibitors in Korean Papaver rhoeas Bee Pollen Contributing to Anti-Influenza Activities In Vitro. Planta Med. 2016, 82, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, R.P.; Gordon, M.L. An overview of influenza A virus genes, protein functions, and replication cycle highlighting important updates. Virus Genes 2022, 58, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, Y.; Liu, S. Emerging antiviral therapies and drugs for the treatment of influenza. Expert Opin. Emerg. Drugs 2022, 27, 389–403. [Google Scholar] [CrossRef]
- Govorkova, E.A.; Takashita, E.; Daniels, R.S.; Fujisaki, S.; Presser, L.D.; Patel, M.C.; Huang, W.; Lackenby, A.; Nguyen, H.T.; Pereyaslov, D.; et al. Global update on the susceptibilities of human influenza viruses to neuraminidase inhibitors and the cap-dependent endonuclease inhibitor baloxavir, 2018–2020. Antivir. Res. 2022, 200, 105281. [Google Scholar] [CrossRef]
- Pfannebecker, J.; Schiffer-Hetz, C.; Fröhlich, J.; Becker, B. Culture medium optimization for osmotolerant yeasts by use of a parallel fermenter system and rapid microbiological testing. J. Microbiol. Methods 2016, 130, 14–22. [Google Scholar] [CrossRef]
- Tsadila, C.; Nikolaidis, M.; Dimitriou, T.G.; Kafantaris, I.; Amoutzias, G.D.; Pournaras, S.; Mossialos, D. Antibacterial Activity and Characterization of Bacteria Isolated from Diverse Types of Greek Honey against Nosocomial and Foodborne Pathogens. Appl. Sci. 2021, 11, 5801. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Wingfield, P. Protein Precipitation Using Ammonium Sulfate. In Current Protocols in Protein Science; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1998; Volume Appendix 3, pp. A.3F.1–A.3F.8. [Google Scholar]
- Acar Şahin, A.; Aslım, B.; Tan, S.; Alan, Ş.; Pınar, N.M. Differences in structure, allergenic protein content and pectate lyase enzyme activity of some Cupressaceae pollen. Turk. J. Biochem. 2018, 43, 435–446. [Google Scholar] [CrossRef]
- Tsami, K.; Barda, C.; Ladopoulos, G.; Didaras, N.A.; Grafakou, M.-E.; Heilmann, J.; Mossialos, D.; Rallis, M.C.; Skaltsa, H. Chemical Profile and In Vitro Evaluation of the Antibacterial Activity of Dioscorea communis Berry Juice. Sci 2022, 4, 21. [Google Scholar] [CrossRef]
- van Meerloo, J.; Kaspers, G.J.L.; Cloos, J. Cell Sensitivity Assays: The MTT Assay. In Cancer Cell Culture. Methods in Molecular Biology (Methods and Protocols); Cree, I., Ed.; Humana Press: Totowa, NJ, USA, 2011; pp. 237–245. [Google Scholar]
- Casas, I.; Powell, L.; Klapper, P.E.; Cleator, G.M. New method for the extraction of viral RNA and DNA from cerebrospinal fluid for use in the polymerase chain reaction assay. J. Virol. Methods 1995, 53, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Eisfeld, A.J.; Neumann, G.; Kawaoka, Y. Influenza A virus isolation, culture and identification. Nat. Protoc. 2014, 9, 2663–2681. [Google Scholar] [CrossRef] [PubMed]
- Pritchett, J.C.; Naesens, L.; Montoya, J. Treating HHV-6 Infections: The Laboratory Efficacy and Clinical Use of Anti-HHV-6 Agents. In Human Herpesviruses HHV-6A, HHV-6B, and HHV-7; Flamand, L., Lautenschlager, I., Krueger, G.R.F., Ablashi, D.V., Eds.; Elsevier B.V.: Amsterdam, The Netherlands, 2014; pp. 311–331. ISBN 9780444627032. [Google Scholar]
- Cavalli, R.; Donalisio, M.; Bisazza, A.; Civra, A.; Ranucci, E.; Ferruti, P.; Lembo, D. Enhanced Antiviral Activity of Acyclovir Loaded into Nanoparticles. In Methods in Enzymology; Düzgüneş, N., Ed.; Academic Press Inc.: Cambridge, MA, USA, 2012; Volume 509, pp. 1–19. [Google Scholar]
- Di Cagno, R.; Filannino, P.; Cantatore, V.; Gobbetti, M. Novel solid-state fermentation of bee-collected pollen emulating the natural fermentation process of bee bread. Food Microbiol. 2019, 82, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Kaškonienė, V.; Adaškevičiūtė, V.; Kaškonas, P.; Mickienė, R.; Maruška, A. Antimicrobial and antioxidant activities of natural and fermented bee pollen. Food Biosci. 2020, 34, 100532. [Google Scholar] [CrossRef]
- Adaškevičiūtė, V.; Kaškonienė, V.; Barčauskaitė, K.; Kaškonas, P.; Maruška, A. The Impact of Fermentation on Bee Pollen Polyphenolic Compounds Composition. Antioxidants 2022, 11, 645. [Google Scholar] [CrossRef] [PubMed]
- Gilliam, M. Microbiology of pollen and bee bread: The yeasts. Apidologie 1979, 10, 43–53. [Google Scholar] [CrossRef]
- Saksinchai, S.; Suzuki, M.; Lumyong, S.; Ohkuma, M.; Chantawannakul, P. Two new species of the genus Candida in the Zygoascus clade, Candida lundiana sp. nov. and Candida suthepensis sp. nov., isolated from raw honey in Thailand. Antonie Van Leeuwenhoek 2012, 101, 633–640. [Google Scholar] [CrossRef]
- Poyraz, F.; Yalmanci, D.; İspirli, H.; Dertli, E. Characterization of Bee Bread Produced with Defined Starter Cultures Mimicking the Natural Fermentation Process. Fermentation 2023, 9, 174. [Google Scholar] [CrossRef]
- Gilliam, M. Microbiology of pollen and bee bread: The genus Bacillus. Apidologie 1979, 10, 269–274. [Google Scholar] [CrossRef]
- Pełka, K.; Worobo, R.W.; Walkusz, J.; Szweda, P. Bee Pollen and Bee Bread as a Source of Bacteria Producing Antimicrobials. Antibiotics 2021, 10, 713. [Google Scholar] [CrossRef]
- Watanabe, K.; Rahmasari, R.; Matsunaga, A.; Haruyama, T.; Kobayashi, N. Anti-influenza Viral Effects of Honey In Vitro: Potent High Activity of Manuka Honey. Arch. Med. Res. 2014, 45, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Barda, C.; Anastasiou, K.; Tzara, A.; Grafakou, M.-E.; Kalpoutzakis, E.; Heilmann, J.; Rallis, M.; Kourounakis, A.P.; Skaltsa, H. A Bio-Guided Screening for Antioxidant, Anti-Inflammatory and Hypolipidemic Potential Supported by Non-Targeted Metabolomic Analysis of Crepis spp. Molecules 2022, 27, 6173. [Google Scholar] [CrossRef] [PubMed]
- Peršurić, Ž.; Pavelić, S.K. Bioactives from Bee Products and Accompanying Extracellular Vesicles as Novel Bioactive Components for Wound Healing. Molecules 2021, 26, 3770. [Google Scholar] [CrossRef]
- Alotaibi, M.; Ali, A.; Bakhshwin, D.; Alatawi, Y.; Alotaibi, S.; Alhifany, A.; Alharthi, B.; Alharthi, N.; Alyazidi, A.; Alharthi, Y.; et al. Effectiveness and Safety of Favipiravir Compared to Hydroxychloroquine for Management of Covid-19: A Retrospective Study. Int. J. Gen. Med. 2021, 14, 5597–5606. [Google Scholar] [CrossRef] [PubMed]
- Mabry, T.J.; Markham, K.R. Mass Spectrometry of Flavonoids BT. In The Flavonoids; Harborne, J.B., Mabry, T.J., Mabry, H., Eds.; Springer: Boston, MA, USA, 1975; pp. 78–126. ISBN 978-1-4899-2909-9. [Google Scholar]
- Papachristoforou, A.; Termentzi, A.; Halabalaki, M.; Koutouvela, E. The application of highly centrifuged honey as an improved diet for experimentally caged honey bees. J. Apic. Res. 2013, 52, 179–183. [Google Scholar] [CrossRef]
- Mazzei, P.; Piccolo, A.; Brescia, M.; Caprio, E. Assessment of geographical origin and production period of royal jelly by NMR metabolomics. Chem. Biol. Technol. Agric. 2020, 7, 24. [Google Scholar] [CrossRef]
| Sample | Type | Description |
|---|---|---|
| AF1 | Bee collected pollen | Multifloral Thessaly, Greece, Spring 2022 |
| AF4 | Fermented AF1 BCP | |
| AF5 | Fermented AF1 BCP | |
| AF17 | Fermented AF1 BCP | |
| AF1-P | Bee collected pollen | Proteinaceous fraction of AF1 |
| AF4-P | Fermented BCP fraction | Proteinaceous fraction of AF4 |
| AF5-P | Fermented BCP fraction | Proteinaceous fraction of AF5 |
| AF17-P | Fermented BCP fraction | Proteinaceous fraction of AF17 |
| 1-BB | Beebread | Multifloral Thessaly, Greece, Spring 2019 |
| 4-BB | Beebread | Multifloral Thessaly, Greece, Spring 2019 |
| 6P-BCP | Bee-collected pollen | Multifloral Thessaly, Greece, Spring 2019 |
| 1-BB-P | Beebread fraction | Proteinaceous fraction of 1-BB |
| 4-BB-P | Beebread fraction | Proteinaceous fraction of 4-BB |
| 6P-BCP-P | Bee-collected pollen fraction | Proteinaceous fraction of 6-BCP |
| 1-BB-Bu | Beebread fraction | n-butanol fraction of 1-BB |
| 4-BB-Bu | Beebread fraction | n-butanol fraction of 4-BB |
| 6P-BCP-Bu | Bee-collected pollen fraction | n-butanol fraction of 6-BCP |
| 4-BB-H | Beebread fraction | Aqueous fraction of 4-BB |
| 6P-BCP-H | Bee-collected pollen fraction | Aqueous fraction of 6-BCP |
| Scheme 50 | CC50 | IC50 | SI |
|---|---|---|---|
| AF1 | 11.57 | 3.91 | 2.95 |
| AF4 | 10.63 | 10.04 | 1.06 |
| AF5 | 11.11 | 2.27 | 4.89 |
| AF17 | 12.00 | 2.47 | 4.85 |
| AF1-P | >64 | 3.14 | 20.38 |
| AF4-P | 51.18 | 2.71 | 18.88 |
| AF5-P | 47.78 | 3.37 | 14.17 |
| AF17-P | >64 | 0.46 | 139.13 |
| 1-BB | 4.88 | 0.16 | 31.28 |
| 4-BB | 0.83 | 0.18 | 4.60 |
| 6P-BCP | 0.47 | 0.42 | 1.12 |
| 1-BB-P | 32.00 | 0.097 | 329.89 |
| 4-BB-P | 7.45 | 0.022 | 338.64 |
| 6P-BCP-P | 3.92 | 0.052 | 75.38 |
| 1-BB-Bu | >64 | 2.91 | 21.99 |
| 4-BB-Bu | >64 | - | - |
| 6P-BCP-Bu | >64 | - | - |
| 4-BB-H | 7.70 | - | - |
| 6P-BCP-H | 6.97 | - | - |
| Rt | Positive Ion Mode | Negative Ion Mode | Mass | Molecular Formula | Proposed Compounds | 1-BB-Bu | 4-BB-Bu | 6P-BCP-Bu |
|---|---|---|---|---|---|---|---|---|
| Found | Found | |||||||
| 0.324 | 203.0529 [M+Na]+ | 179.0564 [M−H]− | 180.0634 | C6H12O6 | carbohydrates | • | • | • |
| 0.337 | 365.1056 [M+Na]+ | 341.1091 [M−H]− | 342.1165 | C12H22O11 | carbohydrates | • | • | • |
| 0.490 | 349.1118 [M+Na]+ | 371.1186 [M+HCOO]− | 326.1215 | C12H22O10 | carbohydrates | • | • | • |
| 0.493 | 139.0390 [M+Na]+ | 138.0318 | C7H6O3 | simple phenolic | • | • | ||
| 0.680 | 134.0470 [M−H]− | 135.0542 | C4H9NO4 | amino acid derivative | • | • | • | |
| 1.048 | 132.1018 [M+H]+ | C6H13NO2 | leucine isomer | • | • | • | ||
| 1.730 | 243.0620 [M−H]− | 244.0695 | C9H12N2O6 | uridine isomer | • | • | ||
| 1.850 | 166.0864 [M+H]+ | 165.079 | C9H11NO2 | amino acid | • | • | • | |
| 1.998 | 149.0241 [M+H]+ | 148.1583 | C9H8O2 | cinnamic acid isomer | • | |||
| 2.046 | 181.0498 [M+H]+ | 179.0350 [M−H]− | 180.0421 | C9H8O4 | caffeic acid isomer | • | • | |
| 2.426 | 195.0649 [M+H]+ | 194.0579, | C10H10O4 | ferulic acid isomer | • | • | ||
| 3.210 | 153.0546 [M+H]+ | 151.0400 [M−H]− | 152.0468 | C8H8O3 | methoxybenzoic acid | • | • | |
| 3.772 | 181.0496 [M+H]+ | 179.0349 [M−H]− | 180.0421 | C9H8O4 | caffeic acid isomer | • | • | |
| 4.936 | 627.1563 [M+H] + | 625.141 [M−H]− | 626.1482 | C27H30O17 | flavonoid di-glycoside | • | • | • |
| 4.981 | 611.1611 [M+H]+ | 595.1303 [M−H]− | 609.1462 | C27H30O16 | flavonoid di-glycoside | • | • | • |
| 5.000 | 641.1713 [M+H]+ | 639.1562 [M−H]− | 640.1633 | C28H32O17 | flavonoid di-glycoside | • | • | |
| 5.078 | 641.1721 [M+H]+ | 639.1570 [M−H]− | 640.1646 | C28H32O17 | flavonoid di-glycoside | • | • | |
| 5.182 | 597.1451 [M+H]+ | 595.1300 [M−H]− | 596.1377 | C26H28O16 | flavonoid di-glycoside | • | • | |
| 5.191 | 641.1722 [M+H]+ | 639.1568 [M−H]− | 640.1648 | C28H32O17 | flavonoid di-glycoside | • | ||
| 5.200 | 671.1820 [M+H]+ | 669.1665 [M−H]− | 670.1739 | C29H34O18 | flavonoid di-glycoside | • | ||
| 5.260 | 597.1460 [M+H]+ | 595.1303 [M−H]− | 596.1379 | C26H28O16 | flavonoid di-glycoside | • | • | |
| 5.266 | 773.2135 [M+H]+ | 771.1986 [M−H]− | 772.2062 | C33H40O21 | flavonoid tri-glycoside | • | • | |
| 5.320 | 611.1612 [M+H]+ | 609.1465 [M−H]− | 610.1539 | C27H30O16 | flavonoid di-glycoside | • | • | |
| 5.440 | 787.2298 [M+H]+ | 785.2148 [M−H]− | 786.2224 | C34H42O21 | flavonoid tri-glycoside | • | • | |
| 5.460 | 611.1618 [M+H]+ | 609.1452 [M−H]− | 610.1525 | C27H30O16 | flavonoid di-glycoside | • | ||
| 5.531 | 625.1763 [M+H]+ | 623.1616 [M−H]- | 624.1691 | C28H32O16 | flavonoid tri-glycoside | • | ||
| 5.570 | 611.1621 [M+H]+ | 609.1455 [M−H]− | 610.1529 | C27H30O16 | flavonoid di-glycoside | • | • | |
| 5.603 | 563.1759 [M+H]+ | 561.1614 [M−H]− | 562.1686 | C27H30O13 | flavonoid di-glycoside | • | ||
| 5.647 | 757.2190 [M+H]+ | 755.2041 [M−H]− | 756.2116 | C33H40O20 | flavonoid tri-glycoside | • | ||
| 5.741 | 595.1659 [M+H]+ | 593.1511 [M−H]− | 594.1585 | C27H30O15 | flavonoid di-glycoside | • | • | • |
| 5.790 | 581.1506 [M+H]+ | 579.1351 [M−H]− | 580.1433 | C26H28O15 | flavonoid di-glycoside | • | • | |
| 5.840 | 625.1770 [M+H]+ | 623.1616 [M−H]− | 624.1696 | C28H32O16 | flavonoid di-glycoside | • | • | • |
| 5.881 | 611.1617 [M+H]+ | 609.1454 [M−H]− | 610.1527 | C27H30O16 | flavonoid di-glycoside | • | • | |
| 6.258 | 625.1768 [M+H]+ | 623.1610 [M−H]− | 624.1682 | C28H32O16 | flavonoid di-glycoside | • | ||
| 6.355 | 449.1078 [M+H]+ | 447.0930 [M−H]− | 448.1005 | C21H20O11 | flavonoid glycoside | • | • | |
| 6.382 | 417.1008 [M+H]+ | 415.1033 [M−H]− | 416.3789 | C21H20O9 | flavonoid glycoside | • | ||
| 6.751 | 465.1028 [M+H]+ | 463.0946 [M−H]− | 464.0955 | C21H20O12 | flavonoid glycoside | • | • | |
| 7.033 | 433.1026 [M+H]+ | 431.0975 [M−H]− | 432.1048 | C21H20O10 | flavonoid glycoside | • | ||
| 7.403 | 653.1714 [M+H]+ | 651.1561 [M−H]− | 652.1642 | C29H32O17 | flavonoid tri-glycoside | • | ||
| 7.812 | 303.0500 [M+H]+ | 301.0352 [M−H]− | 302.0427 | C15H10O7 | quercetin | • | ||
| 7.841 | 255.0662 [M−H]− | 256.2568 | C15H12O4 | isoflavone derivative | • | |||
| 8.936 | 317.0658 [M+H]+ | 315.0511 [M−H]− | 316.0583 | C16H12O7 | methoxylated flavonoid | • | ||
| 8.995 | 287.0553 [M+H]+ | 285.0405 [M−H]− | 286.0478 | C15H10O6 | kaempferol | • | • | • |
| 8.999 | 255.0652 [M+H]+ | 253.0506 [M−H]− | 254.2423 | C15H10O4 | isoflavone derivative | • | ||
| 9.230 | 317.0658 [M+H]+ | 315.0508 [M−H]− | 316.0558 | C16H12O7 | methoxylated flavonoid | • | • |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimitriou, T.G.; Asoutis Didaras, N.; Barda, C.; Skopeliti, D.; Kontogianni, K.; Karatasou, K.; Skaltsa, H.; Mossialos, D. Antiviral Activity of Beebread, Bee-Collected Pollen and Artificially Fermented Pollen against Influenza A Virus. Foods 2023, 12, 1978. https://doi.org/10.3390/foods12101978
Dimitriou TG, Asoutis Didaras N, Barda C, Skopeliti D, Kontogianni K, Karatasou K, Skaltsa H, Mossialos D. Antiviral Activity of Beebread, Bee-Collected Pollen and Artificially Fermented Pollen against Influenza A Virus. Foods. 2023; 12(10):1978. https://doi.org/10.3390/foods12101978
Chicago/Turabian StyleDimitriou, Tilemachos G., Nikos Asoutis Didaras, Christina Barda, Dimitra Skopeliti, Katerina Kontogianni, Katerina Karatasou, Helen Skaltsa, and Dimitris Mossialos. 2023. "Antiviral Activity of Beebread, Bee-Collected Pollen and Artificially Fermented Pollen against Influenza A Virus" Foods 12, no. 10: 1978. https://doi.org/10.3390/foods12101978
APA StyleDimitriou, T. G., Asoutis Didaras, N., Barda, C., Skopeliti, D., Kontogianni, K., Karatasou, K., Skaltsa, H., & Mossialos, D. (2023). Antiviral Activity of Beebread, Bee-Collected Pollen and Artificially Fermented Pollen against Influenza A Virus. Foods, 12(10), 1978. https://doi.org/10.3390/foods12101978











