Varietal Influence on the Formation of Bioactive Amines during the Processing of Fermented Cocoa with Different Pulp Contents
Abstract
1. Introduction
2. Material and Methods
2.1. Material
2.2. Cocoa and Chocolate Processing
2.3. Determination of Free Bioactive Amines
2.4. Moisture Content
2.5. Data Analysis
3. Results
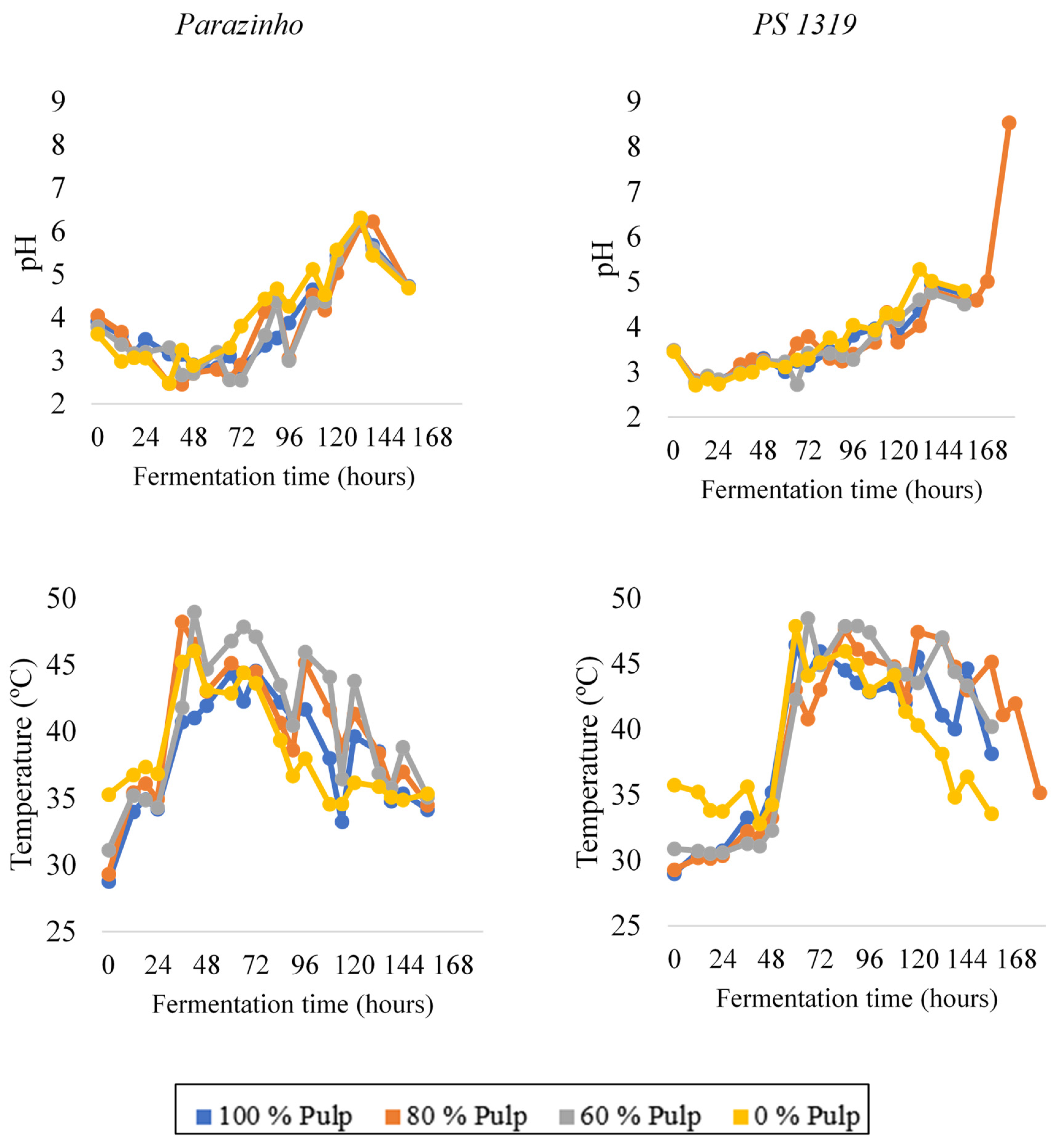
3.1. Temperature and pH Monitoring during Fermentation
3.2. Amine Contents in Cocoa Pulp and Seeds Prior to Fermentation
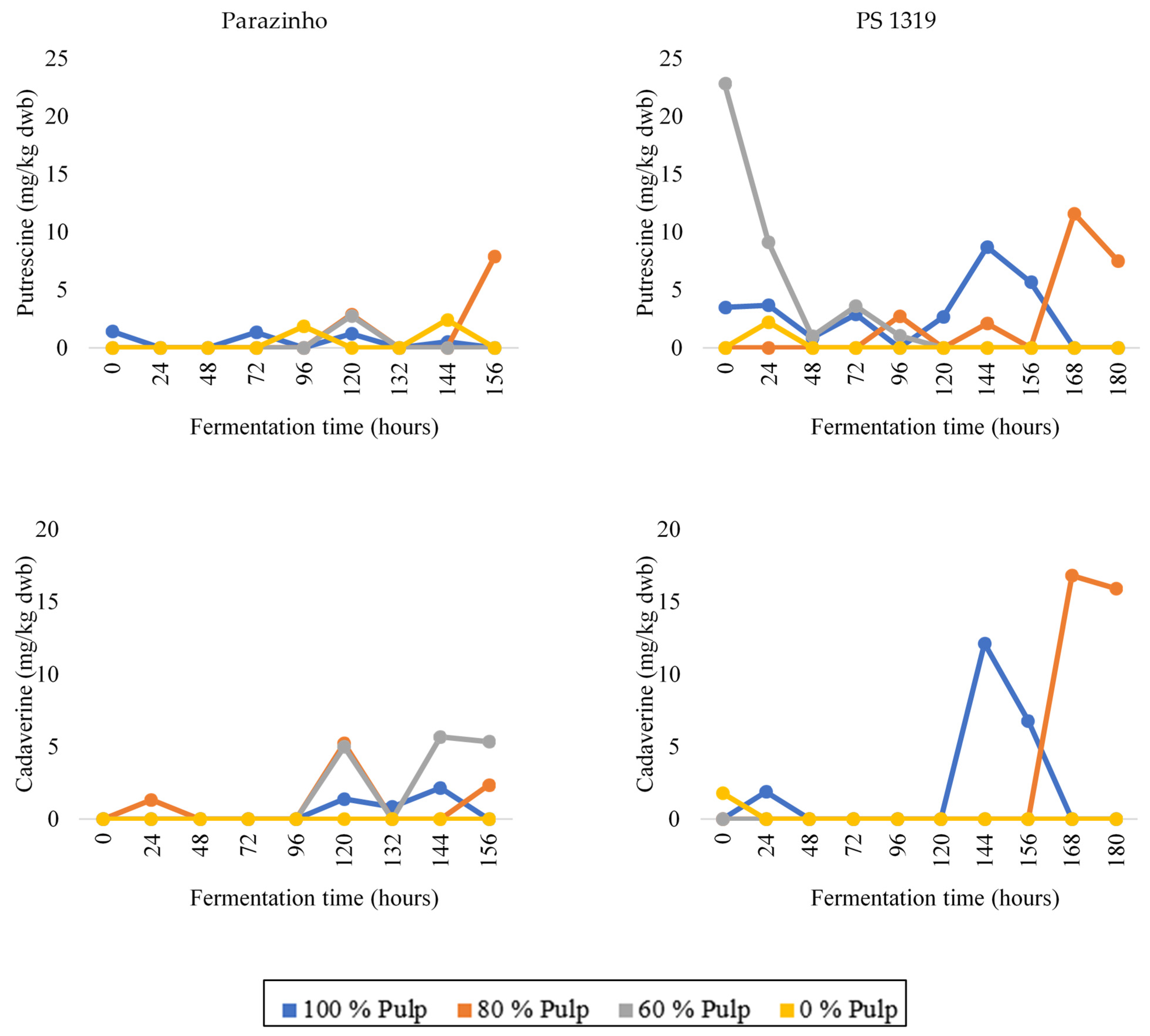
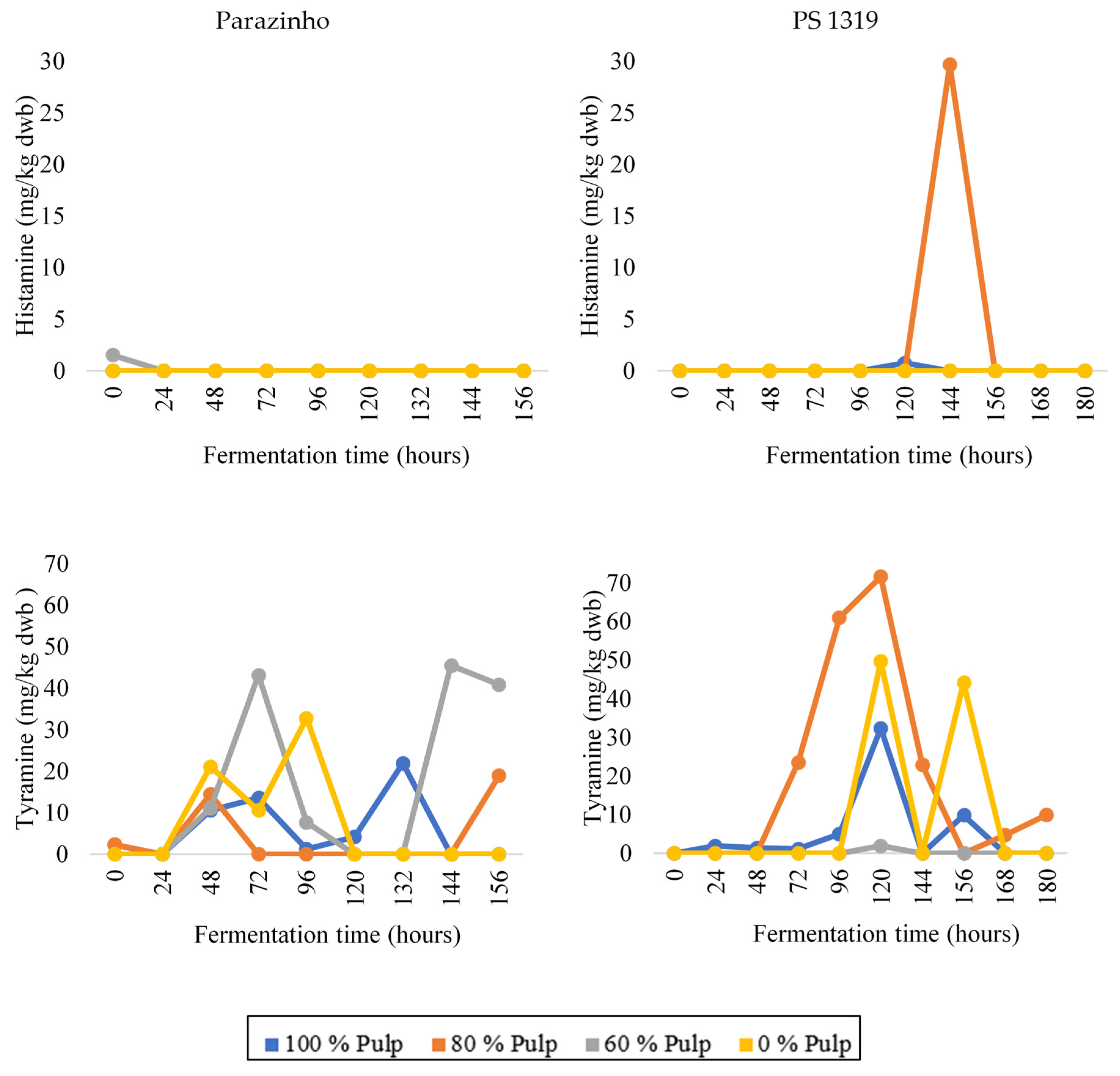
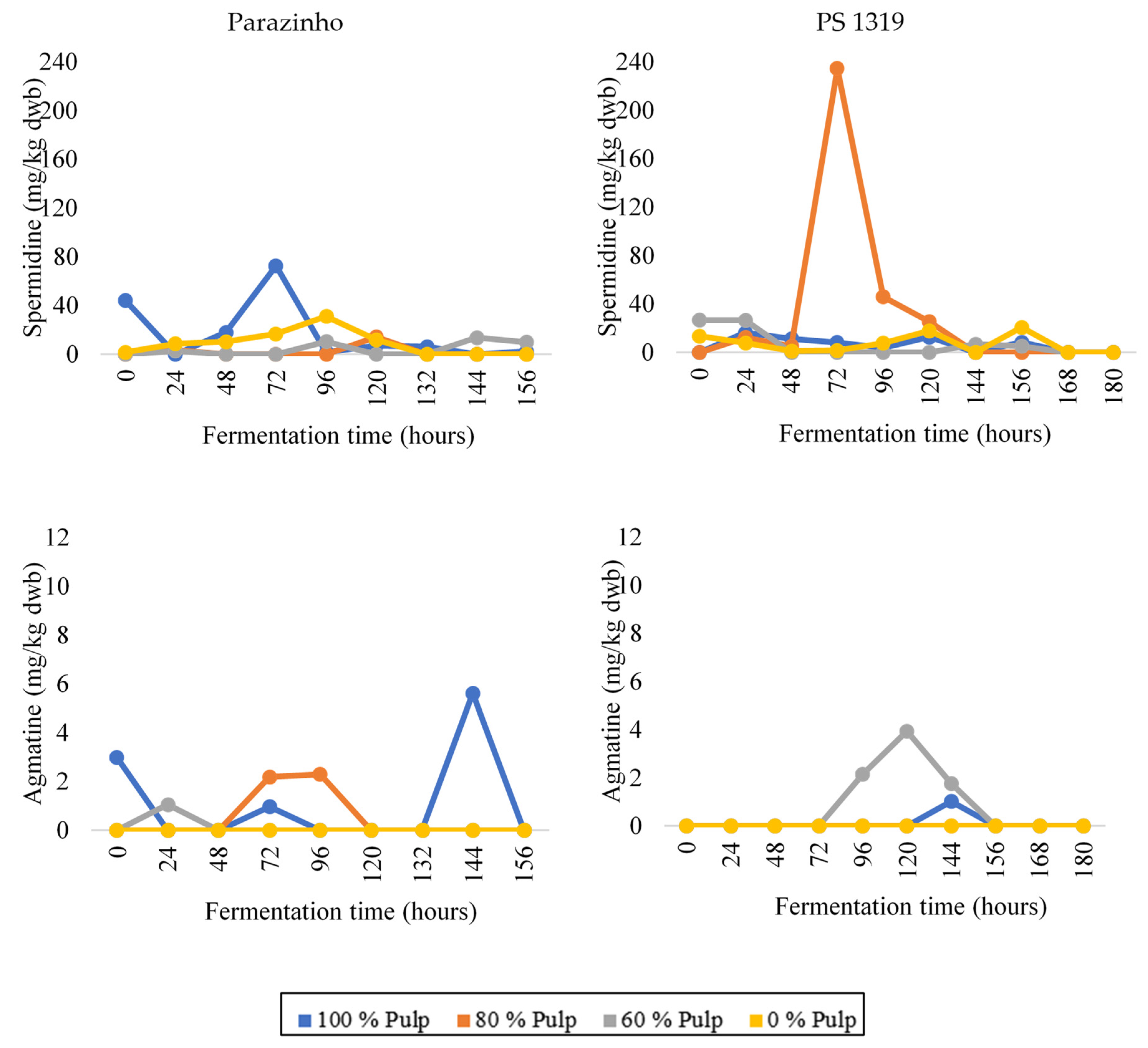
3.3. Changes in Bioactive Amines throughout Fermentation
3.4. Influence of Drying
3.5. Influence of Roasting
3.6. Influence of Liquor Production
3.7. Influence of Chocolate Production
3.8. Analysis of the Principal Components and Heatmap
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gloria, M.B.A. Amines. In Handbook of Food Science; Hui, H., Nollet, L.L., Eds.; Marcel Dekker: New York, NY, USA, 2005. [Google Scholar]
- Medina, M.A.; Urdiales, C.R.; Roríguez-Caso, C.; Ramirez, F.J.; Sanchézjiménez, F. Biogenic amines and polyamines: Similar biochemistry for different physiological missions and biochemical applications. Crit. Rev. Biochem. Mol. Biol. 2003, 38, 23–59. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Li, D.; Shi, R.; Wang, J. Effects of different co-cultures on the amino acid availability, biogenic amine concentrations and protein metabolism of fermented sufu and their relationships. LWT 2019, 113, 108323. [Google Scholar] [CrossRef]
- Gu, J.; Liu, T.; Hou, J.; Pan, L.; Sadiq, F.A.; Yuan, L.; Yang, H.; He, G. Analysis of bacterial diversity and biogenic amines content during the fermentation processing of stinky tofu. Food Res. Int. 2018, 111, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, M.A.; Moreno-Arribas, M.V. The problem of biogenic amines in fermented foods and the use of potential biogenic amine-degrading microorganisms as a solution. Trends Food Sci. Technol. 2014, 39, 146–155. [Google Scholar] [CrossRef]
- Hálasz, A.; Barath, A.; Simon-Sarkadi, L.; Holzapfel, W. Biogenic amines and their production by microorganisms in food. Trends Food Sci. Technol. 1994, 5, 42–49. [Google Scholar] [CrossRef]
- Ordonez, J.L.; Callejon, R.M.; Morales, M.L.; Garcia-Parrila, M.C. A survey of biogenic amines in vinegars. Food Chem. 2013, 141, 2713–2719. [Google Scholar] [CrossRef]
- Kalač, P.; Šavel, J.; Křížek, M.; Pelikánová, T.; Prokopová, M. Biogenic amine formation in bottled beer. Food Chem. 2002, 79, 431–434. [Google Scholar] [CrossRef]
- Deus, V.L.; Bispo, E.S.; Franca, A.S.; Glória, M.B.A. Understanding amino acids and bioactive amines changes during on-farm cocoa fermentation. J. Food Compos. Anal. 2021, 97, 103776. [Google Scholar] [CrossRef]
- Yilmaz, C.; Gökmen, V. Neuroactive compounds in foods: Occurrence, mechanism and potential health effects. Food Res. Int. 2020, 128, 108744. [Google Scholar] [CrossRef]
- Brito, B.D.N.C.; Chisté, R.C.; Pena, R.S.; Glória, M.B.A.; Lopes, A.S. Bioactive amines and phenolic compounds in cocoa beans are affected by fermentation. Food Chem. 2017, 228, 484–490. [Google Scholar] [CrossRef]
- Kalac, P.; Krausová, P. A review of dietary polyamines: Formation, implications for growth and health and occurrence in foods. Food Chem. 2005, 90, 219–230. [Google Scholar] [CrossRef]
- Moinard, C.; Cynober, L.; Bandt, J.P. Polyamines: Metabolism and implications in human diseases. Clin. Nutr. 2005, 24, 184–197. [Google Scholar] [CrossRef] [PubMed]
- Pastel, A.; Thompson, A.; Abdelmalek, L.; Adams-Huet, B.; Jialal, I. The relationship between tyramine levels and inflammation in metabolic syndrome. Horm. Mol. Biol. Clin. Investig. 2019, 6, 1–7. [Google Scholar]
- Bentz, E.N.; Lobayan, R.M.; Martínez, H.; Redondo, P.; Largo, A. Intrinsic antioxidant potential of the aminoindol structure: A computational kinetics study of tryptamine. J. Phys. Chem. 2018, 122, 6386–6395. [Google Scholar] [CrossRef] [PubMed]
- Tofalo, R.; Perpetuini, G.; Schirone, M.; Suzzi, G. Biogenic amines: Toxicology and health effect. In Encyclopedia of Food and Health; Academic Press: Cambridge, MA, USA, 2016; pp. 424–429. [Google Scholar]
- Kalac, P. Health effects and occurrence of dietary polyamines: A review for the period 2005-mid 2013. Food Chem. 2014, 161, 27–39. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Scientific opinion on risk based control of biogenic amines formation in fermented foods. EFSA J. 2011, 9, 2393. [Google Scholar] [CrossRef]
- Mozdzan, M.; Szemraj, J.; Rysz, J.; Stolarek, R.A.; Nowak, D. Antioxidant activity of spermine and spermidine re-evaluated with oxidizing systems involving iron and copper ions. Int. J. Biochem. Cell Biol. 2006, 38, 69–81. [Google Scholar] [CrossRef]
- Deus, V.L.; Bispo, E.S.; Franca, A.S.; Gloria, M.B.A. Influence of cocoa clones on the quality and functional properties of chocolate—Nitrogenous compounds. LWT 2020, 134, 110202. [Google Scholar] [CrossRef]
- Santos, I.C.; Almeida, A.A.F.; Anhert, D.; Conceição, A.S.; Pirovani, C.P.; Pires, J.L.; Valle, R.R.; Baligar, V.C. Molecular, Physiological and Biochemical Responses of Theobroma cacao L. Genotypes to Soil Water Deficit. PLoS ONE 2014, 9, e115746. [Google Scholar] [CrossRef]
- Barel, M. Influence sur les rendements et la qualité du cacao marchand et du cacao torréfié. Café Cacao Thé 1987, 31, 141–150. [Google Scholar]
- Association of Official Analytical Chemists. Official Methods of Analysis of AOAC International, 20th ed.; AOAC: Rockville, MD, USA, 2016. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Delgado-Ospina, J.; Di Mattia, C.D.; Paparella, A.; Mastrocola, D.; Martuscelli, M.; Chaves-Lopez, C. Effect of fermentation, drying and roasting on biogenic amines and other biocompounds in Colombian criollo cocoa beans and shells. Foods 2020, 9, 520. [Google Scholar] [CrossRef] [PubMed]
- Schwan, R.F.; Pereira, G.V.M.; Fleet, G.H. Microbial activities during cocoa fermentation. In Cocoa and Coffee Fermentations; Schwan, R.F., Fleet, G.H., Eds.; Taylor and Francis: London, UK, 2014; pp. 125–135. [Google Scholar]
- Pereira, I.; Silveira, P.T.S.; Martins, M.O.P.; Efraim, P. Caracterização da polpa de diferentes cultivares de cacau. In Proceedings of the XXVIII Congresso de Iniciação Científica da UNICAMP, Campinas, SP, Brazil; 2019. [Google Scholar]
- Alexandre, R.S.; Chagas, K.; Marques, H.I.P.; Costa, P.R.; Filho, J.C. Caracterização de frutos de clones de cacaueiros na região litorânea de São Mateus, ES. Rev. Bras. Eng. Agrícola Ambient. 2015, 19, 785–790. [Google Scholar] [CrossRef]
- Hwang, O.J.; Back, K. Simultaneous suppression of two distinct serotonin N-acetyltransferase isogenes by RNA interference leads to severe decreases in melatonin and accelerated seed deterioration in rice. Biomolecules 2020, 10, 141. [Google Scholar] [CrossRef] [PubMed]
- Handa, A.K.; Fatima, T.; Mattoo, A.K. Polyamines: Biomolecules with diverse functions in plant and human health and disease. Front. Chem. 2018, 6, 10. [Google Scholar] [CrossRef]
- Tufariello, M.; Anglana, C.; Crupi, P.; Virtuosi, I.; Fiume, P.; Di Terlizzi, B.; Moselhy, N.; Attay, H.A.; Pati, S.; Logrieco, A.F.; et al. Efficacy of yeast starters to drive and improve Picual, Manzanilla and Kalamàta table olive fermentation. J. Sci. Food Agric. 2019, 99, 2504–2512. [Google Scholar] [CrossRef] [PubMed]
- Pérez, C.; Colmenero, F. Aminas biógenas: Importância toxicológica. Electron. J. Biomed. 2010, 3, 58–60. [Google Scholar]
- Silveira, N.F.A.; Leitão, M.F.F.; Baldini, V.L.S.; Teixeira Filho, A.R. Bactérias produtoras de histamina e potencial para sua formação em peixes de origem fluvial e lacustre. Braz. J. Food Technol. 2001, 4, 19–25. [Google Scholar]
- Oracz, J.; Nebesny, E. Influence of roasting conditions on the biogenic amine content in cocoa beans of different Theobroma cacao cultivars. Food Res. Int. 2014, 55, 1–10. [Google Scholar] [CrossRef]
- Castri-Alayo, E.M.; Idrogo-Vasquez, G.; Siche, R.; Cardenas-Toro, F.P. Formation of aromatic compounds precursors during fermentation of Criollo and Forastero cocoa. Heliyon 2019, 5, e01157. [Google Scholar] [CrossRef]
- Nascimento, M.S.; Pena, P.O.; Brum, D.M.; Imazaki, F.T.; Tucci, M.L.S.; Efraim, P. Behavior of Salmonella during fermentation, drying and storage of cocoa beans. Int. J. Food Microbiol. 2013, 167, 363–368. [Google Scholar] [CrossRef]
- Cirilo, M.P.G.; Coelho, A.F.S.; Araujo, C.M.; Goncalves, F.R.B.; Nogueira, F.D.; Gloria, M.B.A. Profile and levels of bioactive amines in green and roasted coffee. Food Chem. 2003, 82, 397–402. [Google Scholar] [CrossRef]
- Zamora, R.; Delgado, R.M.; Hidalgo, F.J. Formation of β-phenylethylamine as a consequence of lipid oxidation. Food Res. Int. 2012, 46, 321–325. [Google Scholar] [CrossRef]
- Granvogl, M.; Bugan, S.; Schieberle, P. Formation of amines and aldehydes from parent amino acids during thermal processing of cocoa and model systems: New insights into pathways of the Strecker reaction. J. Agric. Food Chem. 2006, 54, 1730–1739. [Google Scholar] [CrossRef] [PubMed]
- Di Mattia, C.; Martuscelli, M.; Sacchetti, G.; Beheydt, B.; Mastrocola, D.; Pittia, P. Effect of different conching processes on procyanidin content and antioxidant properties of chocolate. Food Res. Int. 2014, 63, 367–372. [Google Scholar] [CrossRef]
- Reis, D.J.; Regunathan, S. Is agmatine a novel neurotransmitter in brain? Trends Pharmacol. Sci. 2000, 21, 187–193. [Google Scholar] [CrossRef]
- Afoakwa, E.O. Cocoa and chocolate consumption—Are there aphrodisiac and other benefits for human health? S. Afr. J. Clin. Nutr. 2008, 21, 107–113. [Google Scholar] [CrossRef]
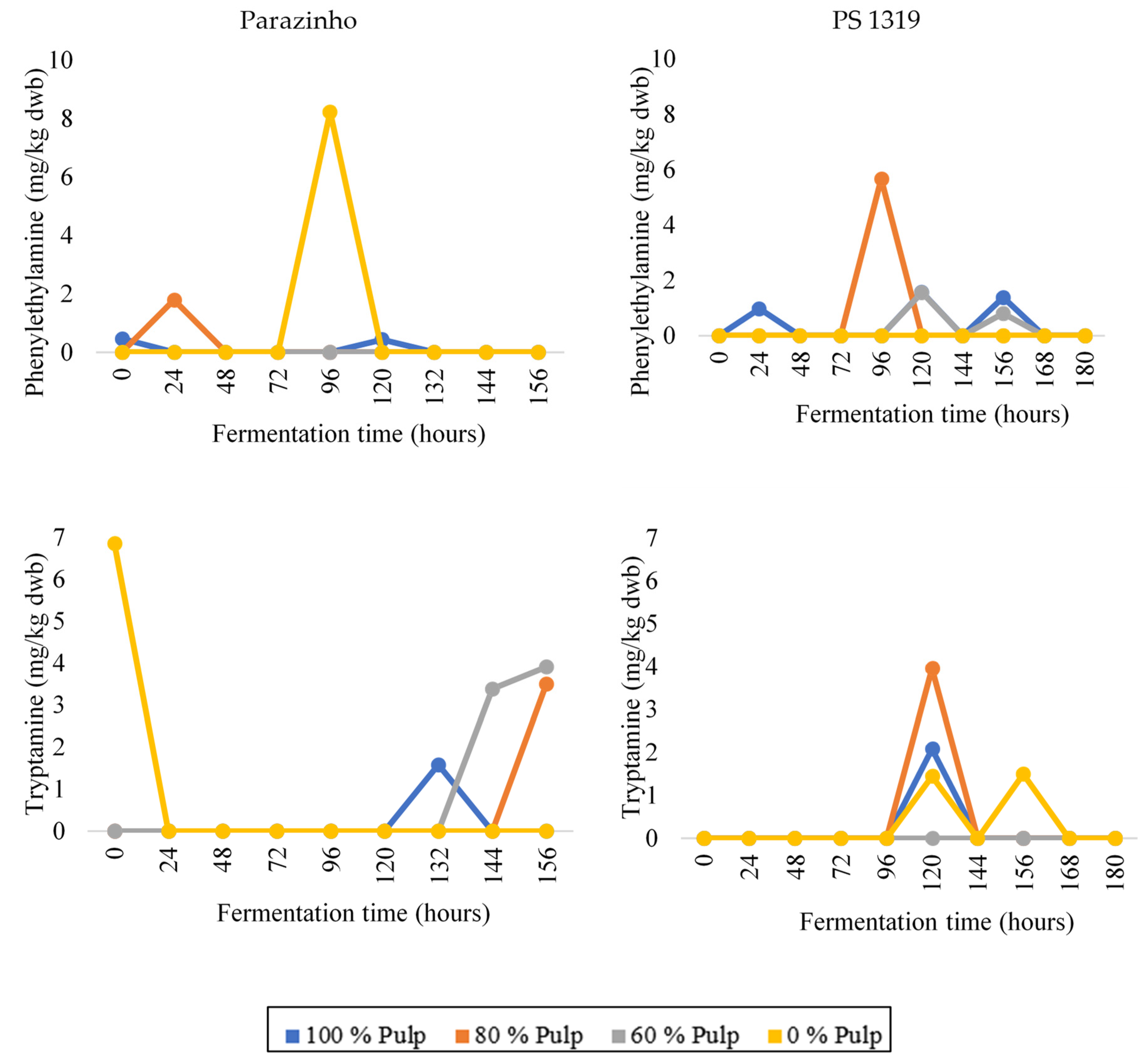
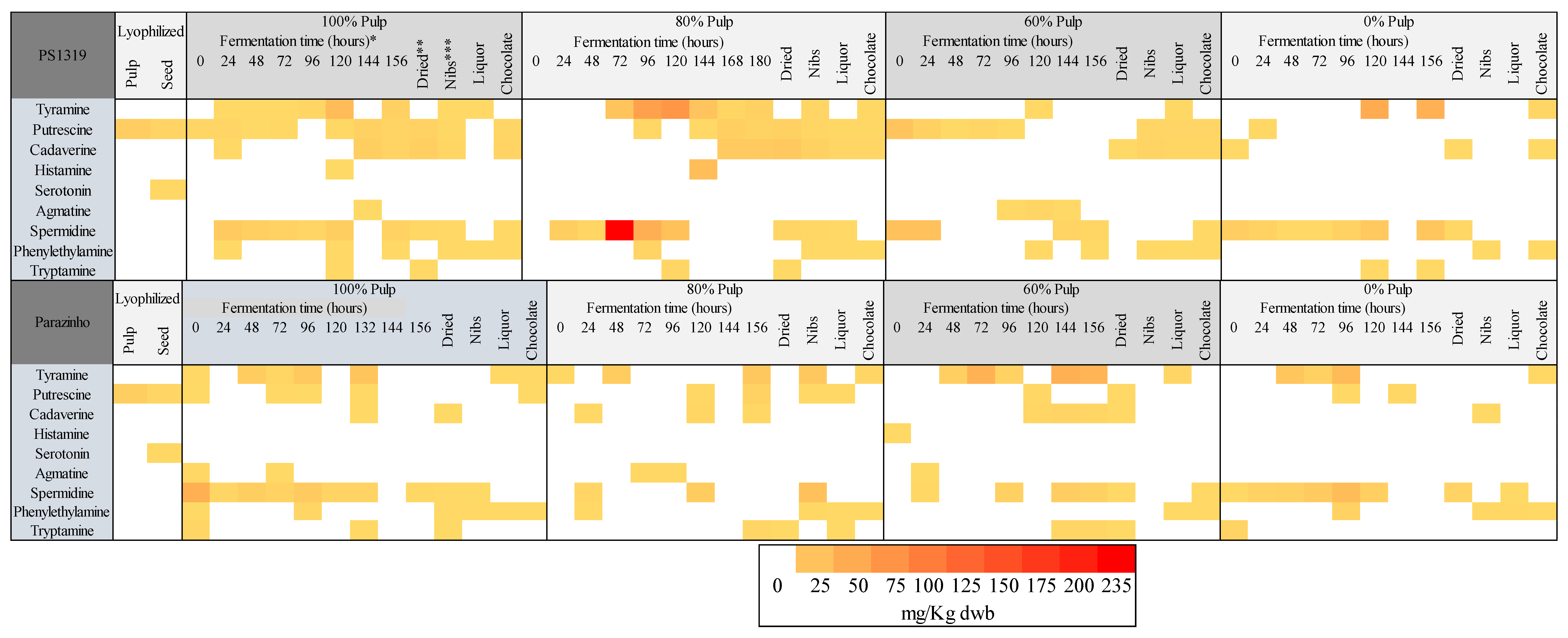
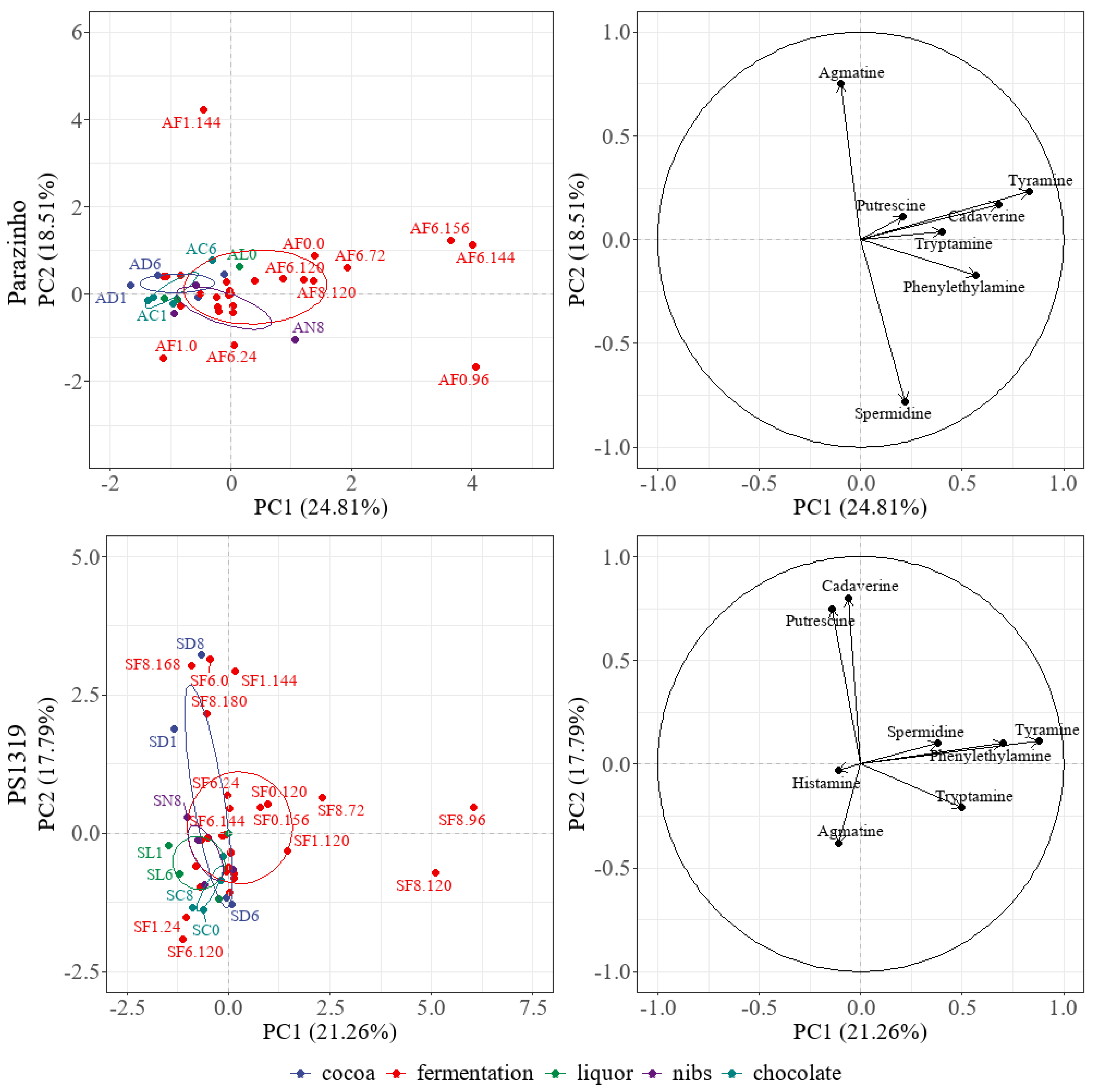
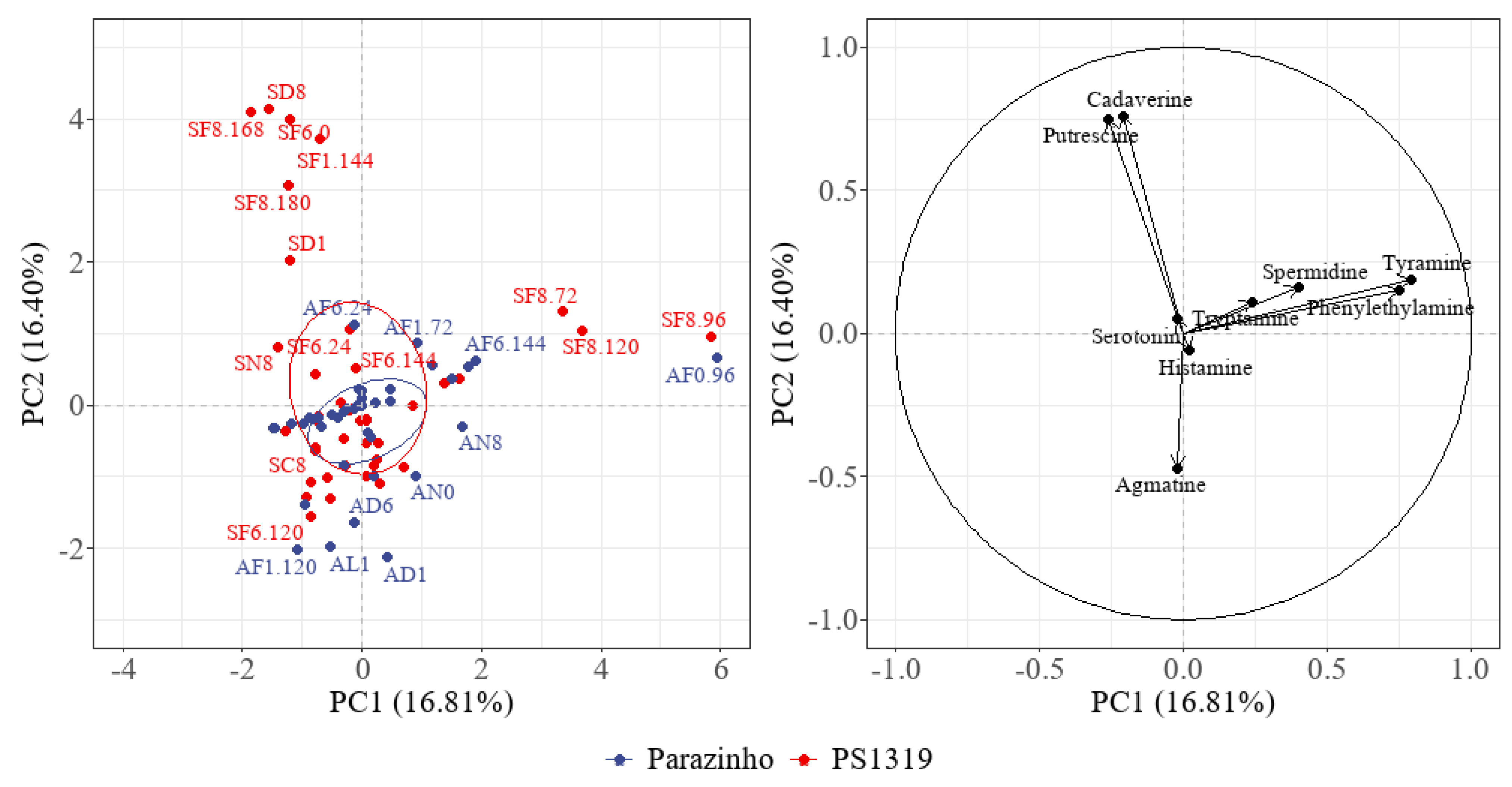
| Variety | % Pulp | Cocoa | Biogenic Amines (mg/kg dwb) | |||||
|---|---|---|---|---|---|---|---|---|
| Tym | Put | Cad | Spd | Phm | Trm | |||
| Parazinho | 100 | Fermented and dried | ND | 0.23 | 0.61 | 3.71 | ND | 0.51 |
| Nibs (roasted) * | ND | ND | ND | ND | ND | ND | ||
| 80 | Fermented and dried | ND | ND | ND | ND | ND | 0.94 | |
| Nibs (roasted) | ND | ND | ND | ND | ND | 0.94 | ||
| 60 | Fermented and dried | ND | 1.67 | 2.07 | 2.02 | ND | 1.04 | |
| Nibs (roasted) | ND | ND | ND | ND | ND | ND | ||
| 0 | Fermented and dried | ND | ND | ND | 5.72 | ND | ND | |
| Nibs (roasted) | ND | ND | 0.66 | ND | 2.47 | ND | ||
| PS 1319 | 100 | Fermented and dried | ND | 8.43 | 11.29 | 2.24 | ND | 1.10 |
| Nibs (roasted) | 1.56 | 3.09 | 5.05 | 1.24 | 1.73 | ND | ||
| 80 | Fermented and dried | ND | 10.48 | 17.96 | 3.38 | ND | 1.,79 | |
| Nibs (roasted) | 4.30 | 5.19 | 9.39 | 2.56 | 1.20 | ND | ||
| 60 | Fermented and dried | ND | ND | 0.66 | ND | ND | ND | |
| Nibs (roasted) | ND | 3.47 | 5.48 | ND | 1.51 | ND | ||
| 0 | Fermented and dried | ND | ND | 1.42 | 2.93 | ND | ND | |
| Nibs (roasted) | ND | ND | ND | ND | 0.57 | ND | ||
| Variety | % Pulp | Sample | Biogenic Amines (mg/kg dwb) | |||||
|---|---|---|---|---|---|---|---|---|
| Tym | Put | Cad | Spd | Phm | Trm | |||
| Parazinho | 100 | Liquor | 1.46 | 0.95 | 1.26 | ND | 0.95 | ND |
| Chocolate | 2.32 | ND | ND | ND | 1.26 | ND | ||
| 80 | Liquor | ND | 1.68 | ND | ND | 1.62 | 0.59 | |
| Chocolate | 3.39 | ND | ND | ND | 0.52 | ND | ||
| 60 | Liquor | 3.88 | ND | ND | ND | 0.88 | ND | |
| Chocolate | ND | ND | ND | 1.16 | 1.49 | ND | ||
| 0 | Liquor | ND | ND | ND | 0.86 | 2.60 | ND | |
| Chocolate | 2.49 | ND | ND | ND | 1.47 | ND | ||
| PS 1319 | 100 | Liquor | 1.30 | ND | ND | ND | 0.64 | ND |
| Chocolate | ND | 3.99 | 6.46 | 2.02 | 1.43 | ND | ||
| 80 | Liquor | ND | 2.77 | 3.94 | 1.45 | 1.19 | ND | |
| Chocolate | 2.61 | 2.38 | 3.83 | ND | 1.08 | ND | ||
| 60 | Liquor | 3.05 | 4.40 | 5.17 | ND | 0.77 | ND | |
| Chocolate | ND | 3.66 | 4.66 | 1.50 | 1.31 | ND | ||
| 0 | Liquor | ND | ND | ND | ND | ND | ND | |
| Chocolate | 3.58 | ND | 0.62 | ND | 1.41 | ND | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silveira, P.T.d.S.; Glória, M.B.A.; Tonin, I.P.; Martins, M.O.P.; Efraim, P. Varietal Influence on the Formation of Bioactive Amines during the Processing of Fermented Cocoa with Different Pulp Contents. Foods 2023, 12, 495. https://doi.org/10.3390/foods12030495
Silveira PTdS, Glória MBA, Tonin IP, Martins MOP, Efraim P. Varietal Influence on the Formation of Bioactive Amines during the Processing of Fermented Cocoa with Different Pulp Contents. Foods. 2023; 12(3):495. https://doi.org/10.3390/foods12030495
Chicago/Turabian StyleSilveira, Paulo Túlio de Souza, Maria Beatriz Abreu Glória, Isabela Portelinha Tonin, Marina Oliveira Paraíso Martins, and Priscilla Efraim. 2023. "Varietal Influence on the Formation of Bioactive Amines during the Processing of Fermented Cocoa with Different Pulp Contents" Foods 12, no. 3: 495. https://doi.org/10.3390/foods12030495
APA StyleSilveira, P. T. d. S., Glória, M. B. A., Tonin, I. P., Martins, M. O. P., & Efraim, P. (2023). Varietal Influence on the Formation of Bioactive Amines during the Processing of Fermented Cocoa with Different Pulp Contents. Foods, 12(3), 495. https://doi.org/10.3390/foods12030495






