Potential for Saccharina latissima Flour as a Functional Ingredient in the Baking Sector
Abstract
:1. Introduction
2. Materials and Methods
2.1. Flour Mixtures
2.2. Moisture Content Analysis
2.3. Ash Content Analysis of Studied Flours (S. latissima and Wheat Flour)
2.4. Protein Content Analysis of Studied Flours (S. latissima and Wheat Flour)
2.5. Total Fat Content Analysis of Studied Flours (S. latissima and Wheat Flour)
2.6. Crude Fiber Content Analysis of Studied Flours (S. latissima and Wheat Flour)
- α = mass of FibreBag (g);
- β = sample mass (g);
- χ = mass of crucible and dried FibreBag, after digestion (g);
- δ = mass of crucible and and ash (g);
- ζ = blank value of empty FibreBag (g);
- γ = mass of crucible and ash of the empty FibreBag (g);
- = mass of crucible (g).
2.7. Mineral Content Analysis of Studied Flours (S. latissima and Wheat Flour)
2.8. Total Polyphenol Content (TPC) of Studied Flours (S. latissima and Wheat Flour)
2.9. Antioxidant Activity of Studied Flours (S. latissima and Wheat Flour)
2.10. Rheological and Enzymatic Properties
2.11. Bread Making
2.12. Physicochemical Characteristics of the Experimental Bread
2.13. Sensory Analysis
2.14. Microbiological Analysis for Shelf Life
2.15. Texture Analysis
- -
- firmness (hardness), (N);
- -
- elasticity;
- -
- cohesiveness;
- -
- gumminess, (N).
2.16. Statistical Analysis
3. Results and Discussions
3.1. Chemical Composition Analysis
3.2. Rheological Properties of Doughs Obtained from Flour Mixtures
3.3. Bread Quality
3.4. Sensory Evaluation
3.5. Shelf Life Estimation Based on Microbiological Activity
3.6. Texture Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yuan, Y.; Zhang, J.; Fan, J.; Clark, J.; Shen, P.; Li, Y.; Zhang, C. Microwave assisted extraction of phenolic compounds from four economic brown macroalgae species and evaluation of their antioxidant activities and inhibitory effects on α-amylase, α-glucosidase, pancreatic lipase and tyrosinase. Food Res. Int. 2018, 113, 288–297. [Google Scholar] [CrossRef]
- Pirian, K.; Moein, S.; Sohrabipour, J.; Rabiei, R.; Blomster, J. Antidiabetic and antioxidant activities of brown and red macroalgae from the Persian Gulf. J. Appl. Phycol. 2017, 29, 3151–3159. [Google Scholar] [CrossRef]
- Jin, D.Q.; Lim, C.S.; Sung, J.Y.; Choi, H.G.; Ha, I.; Han, J.S. Ulva conglobata, a marine algae, has neuroprotective and anti-inflammatory effects in murine hippocampal and microglial cells. Neurosci. Lett. 2006, 402, 154–158. [Google Scholar] [CrossRef]
- Willcox, D.C.; Willcox, B.J.; Todoriki, H.; Suzuki, M. The okinawan diet: Health implications of a low-Calorie, nutrient-dense, antioxidant-rich dietary pattern low in glycemic load. J. Am. Coll. Nutr. 2009, 28, 500–516. [Google Scholar] [CrossRef]
- Kumari, P.; Kumar, M.; Gupta, V.; Reddy, C.R.K.; Jha, B. Tropical marine macroalgae as potential sources of nutritionally important PUFAs. Food Chem. 2010, 120, 749–757. [Google Scholar] [CrossRef]
- Leandro, A.; Pereira, L.; Goncalves, A.M.M. Diverse Applications of Marine Macroalgae. Mar. Drugs 2020, 18, 17. [Google Scholar] [CrossRef]
- Tavares, J.O.; Cotas, J.; Valado, A.; Pereira, L. Seaweed Superfoods: Bioactive Nutraceuticals for Coeliac Disease, Diabetes and Hyperglycemia Support. Preprints 2023. [Google Scholar] [CrossRef]
- Ciko, A.M.; Jokić, S.; Šubarić, D.; Jerković, I. Overview on the application of modern methods for the extraction of bioactive compounds from marine macroalgae. Mar. Drugs 2018, 16, 348. [Google Scholar] [CrossRef]
- Quitério, E.; Soares, C.; Ferraz, R.; Delerue-Matos, C.; Grosso, C. Marine Health-Promoting Compounds: Recent Trends for Their Characterization and Human Applications. Foods 2021, 10, 3100. [Google Scholar] [CrossRef]
- Tiwari, B.K.; Troy, D.J. Chapter 1—Seaweed Sustainability—Food and nonfood applications. In Seaweed Sustainability; Academic Press: Cambridge, MA, USA, 2015; pp. 1–6. [Google Scholar]
- Visch, W.; Kononets, M.; Hall, P.O.J.; Nylund, G.M.; Pavia, H. Environmental impact of kelp (Saccharina latissima) aquaculture. Mar. Pollut. Bull. 2020, 155, 110962. [Google Scholar] [CrossRef]
- Larrea-Marín, M.T.; Pomares-Alfonso, M.S.; Gómez-Juaristi, M.; Sánchez-Muniz, F.J.; de la Rocha, S.R. Validation of an ICP-OES method for macro and trace element determination in Laminaria and Porphyra seaweeds from four different countries. J. Food Compos. Anal. 2010, 23, 814–820. [Google Scholar] [CrossRef]
- López-López, I.; Cofrades, S.; Jiménez-Colmenero, F. Low-fat frankfurters enriched with n-3 PUFA and edible seaweed: Effects of olive oil and chilled storage on physicochemical, sensory and microbial characteristics. Meat Sci. 2009, 83, 148–154. [Google Scholar] [CrossRef]
- López-López, I.; Cofrades, S.; Cañeque, V.; Díaz, M.T.; López, O.; Jiménez-Colmenero, F. Effect of cooking on the chemical composition of low-salt, low-fat Wakame/olive oil added beef patties with special reference to fatty acid content. Meat Sci. 2011, 89, 27–34. [Google Scholar] [CrossRef]
- Cofrades, S.; López-López, I.; Solas, M.T.; Bravo, L.; Jiménez-Colmenero, F. Influence of different types and proportions of added edible seaweeds on characteristics of low-salt gel/emulsion meat systems. Meat Sci. 2008, 79, 767–776. [Google Scholar] [CrossRef]
- Cofrades, S.; López-López, I.; Ruiz-Capillas, C.; Triki, M.; Jiménez-Colmenero, F. Quality characteristics of low-salt restructured poultry with microbial transglutaminase and seaweed. Meat Sci. 2011, 87, 373–380. [Google Scholar] [CrossRef]
- Lo Turco, V.; Sgrò, B.; Albergamo, A.; Nava, V.; Rando, R.; Potortì, A.G.; Di Bella, G. Assessment of the Accuracy of Nutrition Label and Chemical Composition of Plant-Based Milks Available on the Italian Market. Foods 2023, 12, 3207. [Google Scholar] [CrossRef]
- Marigot Ltd. Aquamin Applications; Marigot Ltd.; Available online: https://aquamin.com/marine-minerals/ (accessed on 12 September 2022).
- Brewer, V.; Kussy, D.; Eckert, J. Calcium Fortification of Food Powders. U.S. Patent 11/893478, 4 December 2008. [Google Scholar]
- Weipeng, U. Seaweed Soybean Milk Powder. China Patent CN107410509A, 1 December 2017. [Google Scholar]
- ISO 712:2009; Cereals and Cereal Products. Determination of Moisture Content. Reference Method. ISO: Geneva, Switzerland, 2009.
- SR ISO 2171:2009; Cereale, Leguminoase şi Produse Derivate. Determinarea Conţinutului de Cenuşă Prin Calcinare. ASRO Publisher House: Bucharest, Romania, 2009.
- SR EN ISO 20483:2007; Cereale şi Leguminoase. Determinarea Conţinutului de Azot şi Calculul Conţinutului de Proteină Brută. Metoda Kjeldhal. ASRO Publisher House: Bucharest, Romania, 2007.
- SR 90:2007; Făină de Grâu. Metode de Analiză. ASRO Publisher House: Bucharest, Romania, 2007.
- Šne, E.; Segliòa, D.; Galoburda, R.; Krasnova, I. Content of phenolic compounds in various sea buckthorn parts. Proc. Latv. Acad. Sci. 2013, 67, 411–415. [Google Scholar] [CrossRef]
- Shekhar, S.; Liu, Y.; Wang, S.; Zhang, H.; Fang, X.; Zhang, J.; Fan, L.; Zheng, B.; Roman, R.J.; Wang, Z.; et al. Novel Mechanistic Insights and Potential Therapeutic Impact of TRPC6 in Neurovascular Coupling and Ischemic Stroke. Int. J. Mol. Sci. 2021, 22, 2074. [Google Scholar] [CrossRef]
- ICC Standard No.173; Determination of Rheological Behaviour as a Fuction of Mixing and Temperature Increase. International Association for Cereal Sciences and Technology: Vienna, Austria, 2010.
- Oprea, O.B.; Popa, M.E.; Apostol, L.; Gaceu, L. Research on the Potential Use of Grape Seed Flour in the Bakery Industry. Foods 2022, 11, 1589. [Google Scholar] [CrossRef]
- SR 91:2007; Pâine si Produse Proaspete de Patiserie. Metode de Analiza. ASRO Publisher House: Bucharest, Romania, 2007.
- AACC International. Approved Methods of the American Association of Cereal Chemists, 10th ed.; AACC International: St. Paul, MN, USA, 2000. [Google Scholar]
- ISO/TC 34/SC 12; Sensory Analysis—General Guidelines for the Selection, Trening and Monitoring of Selected Assessors and Expert Sensory Assessors. ISO 8586; International Standard Organization: Geneva, Switzerland, 2012.
- Ogunsakin, A.; Sanni, A.; Banwo, K. Effect of legume addition on the physiochemical and sensorial attributes of sorghum-based sourdough bread. LWT 2020, 118, 108769. [Google Scholar]
- Lawless, H.T.; Heymann, H. Sensory Evaluation of Food. Principles and Practices, 2nd ed.; Springer: New York, NY, USA, 2010. [Google Scholar]
- Ordinul nr. 27 din 2011 al ANSVA Pentru Matrici Produse de Panificație Simple. Monitorul Oficial, 22 June 2011.
- Commissiom Regulation (EU) No 432/2012 of 16 May 2012 Establishing a List of Permitted Health Claims Made on Foods, Other Than Those Referring to the Reduction of Disease Risk and to Children’s Development and Health; Official Journal of the European Union: Brussels, Belgium, 2012.
- Ruperez, P. Mineral content of edible marine seaweeds. Food Chem. 2002, 79, 23–26. [Google Scholar] [CrossRef]
- Paiva, L.; Lima, E.; Neto, A.I.; Marcone, M.; Baptista, J. Health-promoting ingredients from four selected Azorean macroalgae. Food Res. Int. 2016, 89, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Bo, S.; Pisu, E. Role of dietary magnesium in cardiovascular disease prevention, insulin sensitivity and diabetes. Curr. Opin. Lipidol. 2008, 19, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Ródenas de la Rocha, S.; Sánchez-Muniz, F.J.; Gómez-Juaristi, M.; Marín, M.T.L. Trace elements determination in edible seaweeds by an optimized and validated ICP-MS method. J. Food Compos. Anal. 2009, 22, 330–336. [Google Scholar] [CrossRef]
- Suzuki, N.; Iwata, Y. Determination of arsenic and other elemental abundances in marine macro-algae by photon activation analysis. Appl. Organomet. Chem. 1990, 4, 287–291. [Google Scholar] [CrossRef]
- Mišurcová, L.; Machů, L.; Orsavová, J. Seaweed Minerals as Nutraceuticals. In Advances in Food and Nutrition Research; WoodHead Publishing: Cambridge, UK, 2011; Volume 64, pp. 371–390. [Google Scholar]
- Osredkar, J. Copper and Zinc, biological role and significance of Copper/Zinc imbalance. J. Clin. Toxicol. 2011, 3, 0495. [Google Scholar] [CrossRef]
- Bocanegra, A.; Bastida, S.; Benedí, J.; Ródenas, S.; Sánchez-Muniz, F.J. Characteristics and nutritional and cardiovascular-health properties of seaweeds. J. Med. Food 2009, 12, 236–258. [Google Scholar] [CrossRef]
- Catarino, M.; Silva, A.; Cardoso, S. Phycochemical constituents and biological activities of Fucus spp. Mar. Drugs 2018, 16, 249. [Google Scholar] [CrossRef]
- Van Netten, C. Elemental and radioactive analysis of commercially available seaweed. Sci. Total Environ. 2000, 255, 169–175. [Google Scholar] [CrossRef]
- Schiener, P.; Black, K.D.; Stanley, M.S.; Green, D.H. The seasonal variation in the chemical composition of the kelp species Laminaria digitata, Laminaria hyperborea, Saccharina latissima and Alaria esculenta. J. Appl. Phycol. 2015, 27, 363–373. [Google Scholar] [CrossRef]
- Kupper, F.C.; Carpenter, L.J.; McFiggans, G.B.; Palmer, C.J.; Waite, T.J.; Boneberg, E.M.; Woitsch, S.; Weiller, M.; Abela, R.; Grolimund, D.; et al. Iodide accumulation provides kelp with an inorganic antioxidant impacting atmospheric chemistry. Proc. Natl. Acad. Sci. USA 2008, 105, 6954–6958. [Google Scholar] [CrossRef] [PubMed]
- Miyai, K.; Tokushige, T.; Kondo, M. Suppression of Thyroid Function during Ingestion of Seaweed “Kombu” (Laminaria japonoca) in Normal Japanese Adults. Endocr. J. 2008, 55, 1103–1108. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B. Iodine requirements and the risks and benefits of correcting iodine deficiency in populations. J. Trace Elem. Med. Biol. 2008, 22, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Stévant, P.; Marfaing, H.; Duinker, A.; Fleurence, J.; Rustad, T.; Sandbakken, I.; Chapman, A. Biomass soaking treatments to reduce potentially undesirable compounds in the edible seaweeds sugar kelp (Saccharina latissima) and winged kelp (Alaria esculenta) and health risk estimation for human consumption. J. Appl. Phycol. 2018, 30, 2047–2060. [Google Scholar] [CrossRef]
- Maehre, H.K.; Malde, M.K.; Eilertsen, K.-E.; Elvevoll, E.O. Characterization of protein, lipid and mineral contents in common Norwegian seaweeds and evaluation of their potential as food and feed: Biochemical composition of marine macroalgae. J. Sci. Food Agric. 2014, 94, 3281–3290. [Google Scholar] [CrossRef] [PubMed]
- Rey-Crespo, F.; López-Alonso, M.; Miranda, M. The use of seaweed from the Galician coast as a mineral supplement in organic dairy cattle. Animal 2014, 8, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Hernández, G.B.; Castillejo, N.; Carrión-Monteagudo, M.D.M.; Artés, F.; Artés-Hernández, F. Nutritional and bioactive compounds of commercialized algae powders used as food supplements. Food Sci. Technol. Int. 2018, 24, 172–182. [Google Scholar] [CrossRef]
- Capillo, G.; Savoca, S.; Costa, R.; Sanfilippo, M.; Rizzo, C.; Lo Giudice, A.; Albergamo, A.; Rando, R.; Bartolomeo, G.; Spanò, N.; et al. New insights into the culture method and antibacterial potential of Gracilaria gracilis. Mar. Drugs 2018, 16, 492. [Google Scholar] [CrossRef]
- Mironeasa, S.; Codina, G.G.; Mironeasa, C. Optimization of wheat-grape seed composite flour to improve alpha-amylase activity and dough rheological behavior. Int. J. Food Prop. 2016, 19, 859–872. [Google Scholar] [CrossRef]
- Apostol, L.; Belc, N.; Gaceu, L.; Vladut, V.; Oprea, O.B. Chemical composition and rheological parametrs of Helianthus Tuberosus flour used as a sources of bioactive compounds in bakery. Rev. Chim. 2019, 70, 2048–2053. [Google Scholar] [CrossRef]
- Baranzelli, J.; Hüttner Kringel, D.; Colussi, R.; Fernandes Paiva, F.; Camargo Aranha, B.; Zavariz de Miranda, M.; da Zavareze, E.R.; Guerra Diasa, A.R. Changes in enzymatic activity, technological quality and gamma-aminobutyric acid (GABA) content of wheat flour as affected by germination. LWT 2018, 90, 483–490. [Google Scholar] [CrossRef]
- Boita, E.R.F.; Oro, T.; Santetti, G.S.; Bertolin, T.E.; Gutkoski, L.C. Rheological properties of wheat flour dough and pan bread with wheat bran. J. Cereal Sci. 2016, 71, 177–182. [Google Scholar] [CrossRef]
- Bordei, D. Tehnologia Moderna a Panificatiei; Agir Publishing House: Bucharest, Romania, 2004. [Google Scholar]
- Mamat, H.; Matanjun, P.; Ibrahim, S.; Md Amin, S.F.; Abdul Hamid, M.; Rameli, A.S. The effect of seaweed composite flour on the textural properties of dough and bread. J. Appl. Phycol. 2014, 26, 1057–1062. [Google Scholar] [CrossRef]
- SR 878:1996; Pâine de Faina de Grâu. ASRO Publisher House: Bucharest, Romania, 1996.
- SR ISO 21527-2:2009; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Yeasts and Moulds—Part 2: Colony Count Technique in Products with Water Activity Less than or Equal to 0.95. ISO: Geneva, Switzerland, 2009.
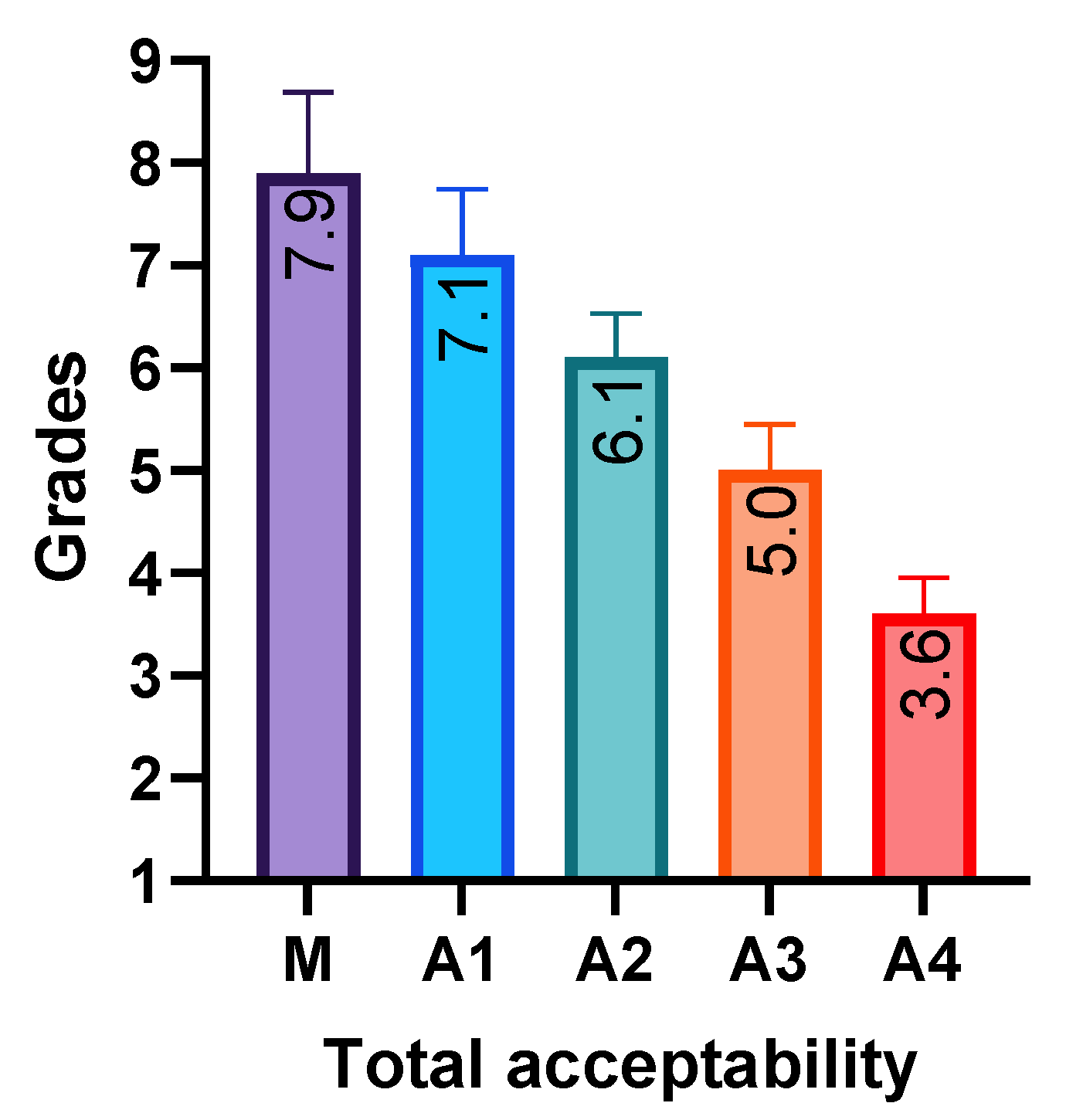
| Samples | Sample Composition (w/w) |
|---|---|
| M | Sample 0—100% wheat flour, type 650 |
| A1 | Sample 1—98.5% WF, type 650 + 1.5% dried S. latissima flour |
| A2 | Sample 2—97% WF, type 650 + 3% dried S. latissima flour |
| A3 | Sample 3—95.5% WF, type 650 + 4.5% dried S. latissima flour |
| A4 | Sample 4—94% WF, type 650 + 6% dried S. latissima flour |
| Control Sample (M) |
|---|
| Direct Method Recipe |
|
|
|
|
| Kneading: 2 min at 60 rpm followed by 8 min of 90 rpm in the mixer. Fermentation 1: 90 min at 30 °C, 70% relative humidity. Splitting into equal parts. Modeling into round shapes; second fermentation for 10 min; modeling into final shapes. Fermentation 2: 40 min at 30 °C, 70% relative humidity. Baking for 40 min at 240 °C, steaming the samples in the first 10 s. Cooling to 21 °C for 2 h. |
| Parameter | S. latissima Flour | CV (%) | Wheat Flour (WF) 650 | CV (%) | p-Value (t-Test) |
|---|---|---|---|---|---|
| Moisture content (%) | 7.170 ± 0.22 a | 1.534 | 13.1 ± 0.02 b | 0.076 | <0.0001 |
| Ash (%) | 38.92 ± 4.25 a | 5.515 | 0.660 ± 0.05 b | 4.009 | <0.0001 |
| Protein (%) | 14.34 ± 1.02 a | 3.825 | 11.37 ± 0.30 b | 1.366 | 0.0008 |
| Fat (%) | 0.706 ± 0.03 a | 2.162 | 1.167 ± 0.10 b | 4.949 | 0.0002 |
| Raw fiber (%) | 6.367 ± 0.46 a | 3.614 | 1.233 ± 0.05 b | 2.040 | <0.0001 |
| Potassium (mg/kg DM) | 62,088 ± 1949 a | 1.577 | 1877 ± 15.80 b | 0.420 | <0.0001 |
| Magnesium (mg/kg DM) | 6041 ± 166.0 a | 1.424 | 479.7 ± 10.41 b | 1.088 | <0.0001 |
| Calcium (mg/kg DM) | 8236 ± 636.0 a | 3.881 | 441.8 ± 7.810 b | 0.893 | <0.0001 |
| Iron (mg/kg DM) | 35.23 ± 6.20 a | 8.823 | 18.37 ± 1.610 b | 4.395 | <0.0001 |
| Na (mg/kg DM) | 15,205 ± 35.00 a | 0.117 | 20.98 ± 1.880 b | 4.483 | <0.0001 |
| Zinc (mg/kg DM) | 30.06 ± 0.33 a | 0.572 | 55.40 ± 2.50 b | 2.305 | <0.0001 |
| Copper (mg/kg DM) | 0.846 ± 0.03 a | 1.804 | 1.197 ± 0.030 b | 1.276 | <0.0001 |
| Selenium (mg/kg DM) | 0 a | 0 | 0.041 ± 0.006 b | 7.391 | <0.0001 |
| Manganese (mg/kg DM) | 3.967 ± 0.50 a | 6.344 | 5.183 ± 1.490 a | 14.370 | 0.0552 |
| Chromium (mg/kg DM) | 0 a | 0 | 0.079 ± 0.012 b | 7.595 | <0.0001 |
| Molybdenum (mg/kg DM) | 0 a | 0 | 0.1321 ± 0.002 b | 1.024 | <0.0001 |
| Phosphorus (mg/kg d.m.) | 2263 ± 129.0 a | 3.032 | 776.0 ± 88.00 b | 5.710 | <0.0001 |
| Iodine (mg/kg DM) | 12,530 ± 2076 a | 8.307 | 0.1 ± 0.020 b | 10.00 | <0.0001 |
| Total polyphenols (mg GAE/100 g) | 283.5 ± 13.79 a | 2.643 | 0 b | 0 | <0.0001 |
| DPPH (mg Trolox/100 g; µmol T/100 g) | 13.10 ± 0.20 a | 0.076 | 0 b | 0 | <0.0001 |
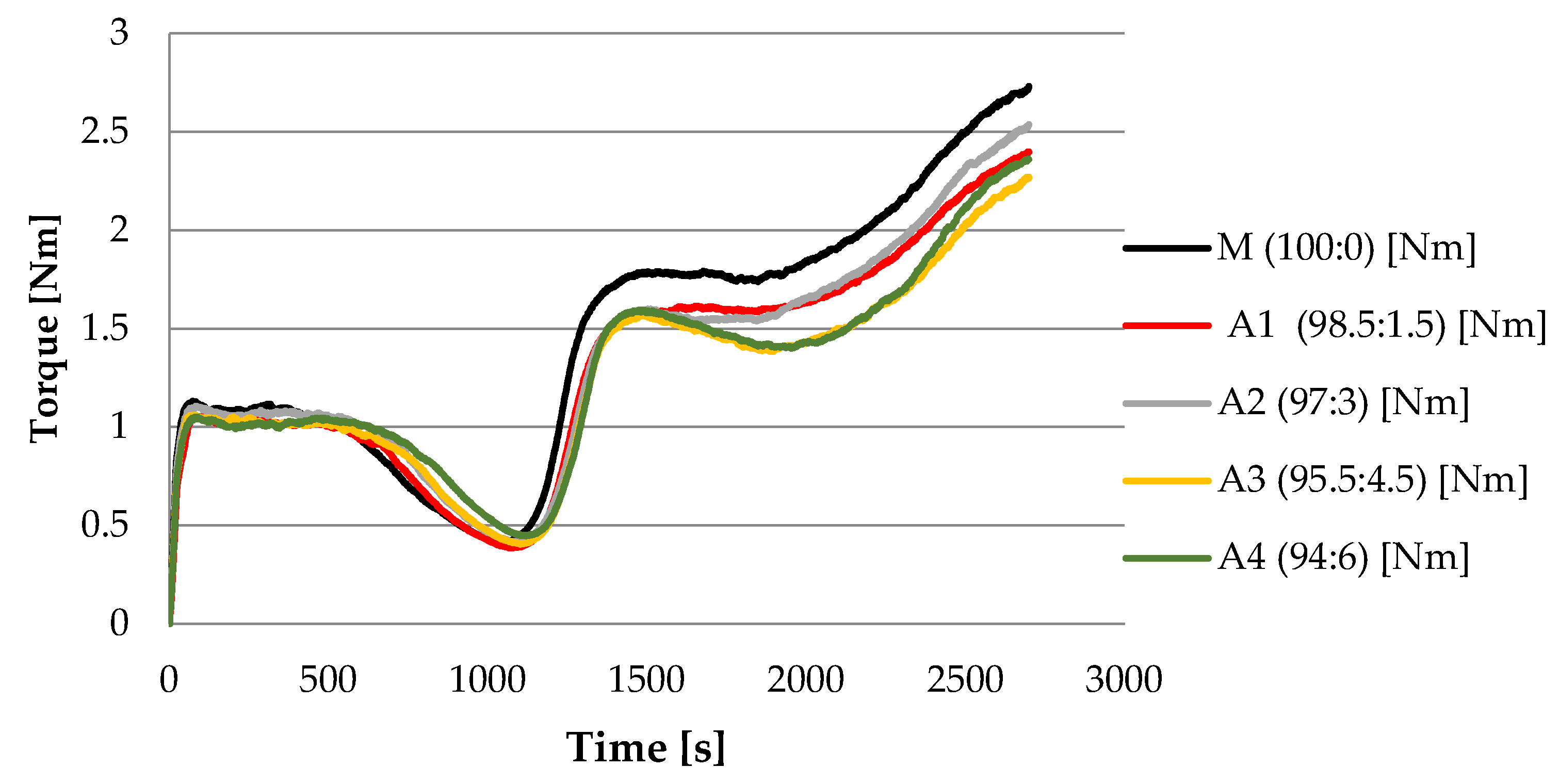
| Parameter | M (100% WF) | A1 (98.5% WF + 1.5% S.l.) | A2 (97% WF + 3% S.l.) | A3 (95.5% WF + 4.5% S.l.) | A4 (94% WF + 6% S.l.) |
|---|---|---|---|---|---|
| Water absorption (%) | 58.1 ± 0.05 a | 59.0 ± 0.06 b | 60.80 ± 0.05 c | 62.70 ± 0.01 d | 62.60 ± 0.01 d |
| Stability (min) | 8.78 ± 0.28 a | 10.33 ± 0.25 b | 10.37 ± 0.17 b | 10.83 ± 0.14 c | 11.83 ± 0.1 d |
| Amplitude (Nm) | 0.091 ± 0.01 a | 0.099 ± 0.01 b | 0.112 ± 0.01 c | 0.102 ± 0.01 b | 0.089 ± 0.01 a |
| Moisture (%) | 11.90 ± 0.5 a | 11.90 ± 0.4 a | 12.50 ± 0.5 c | 12.50 ± 0.2 c | 12.20 ± 0.1 b |
| α | −0.080 ± 0.002 a | −0. 098 ± 0.003 b | −0.120 ± 0.002 c | −0.098 ± 0.003 b | −0.044 ± 0.002 d |
| β | 0.112 ± 0.003 a | 0. 374 ± 0.003 b | 0.418 ± 0.004 c | 0.424 ± 0.003 d | 0.436 ± 0.004 e |
| γ | −0.024 ± 0.003 b | 0.030 ± 0.002 d | −0.010 ± 0.002 a | −0.036 ± 0.006 c | −0.026 ± 0.05 b |
| C1 | 1.132 ± 0.01 a | 1.056 ± 0.03 c,d | 1.107 ± 0.03 b | 1.063 ± 0.04 c | 1.052 ± 0.02 d |
| TC1 | 1.20 ± 0.1 a | 1.27 ± 0.08 a,b | 1.37 ± 0.06 c | 1.35 ± 0.07 c | 1.33 ± 0.05 b,c |
| C2 | 0.417 ± 0.01 a | 0.417 ± 0.02 a | 0.380 ± 0.02 b | 0.406 ± 0.01 a | 0.448 ± 0.01 c |
| TC2 | 17.37 ± 0.13 a | 17.93 ± 0.11 b | 17.93 ± 0.08 b | 18.37 ± 0.011 c | 18.65 ± 0.012 d |
| C3 | 1.793 ± 0.02 a | 1.699 ± 0.02 b | 1.601 ± 0.01 c | 1.576 ± 0.01 d | 1.594 ± 0.02 c,d |
| TC3 | 27.95 ± 0.32 a | 23.00 ± 0.45 d | 25.03 ± 0.28 b | 24.73 ± 0.51 b,c | 24.58 ± 0.29 c,d |
| C4 | 1.740 ± 0.01 a | 1.596 ± 0.01 b | 1.534 ± 0.01 c | 1.401 ± 0.01 d | 1.385 ± 0.01 e |
| TC4 | 30.82 ± 0.17 a | 30.60 ± 0.13 a | 30.48 ± 0.10 a | 31.62 ± 0.12 b | 32.57 ± 0.15 c |
| C5 | 2.731 ± 0.08 a | 2.540 ± 0.10 b | 2.397 ± 0.09 c | 2.276 ± 0.10 d | 2.362 ± 0.11 c,d |
| TC5 | 45.00 ± 0.01 a | 45 ± 0.01 a | 45.02 ± 0.01 a | 45.02 ± 0.01 a | 45.02 ± 0.01 a |
| Sample | Mass (kg) | Specific Volume (cm3/100 g) | Porosity (%) | Elasticity (%) | Humidity (%) | Acidity (Degree) |
|---|---|---|---|---|---|---|
| P0: 0% | 0.489 ± 0.01 a | 372 ± 4.46 a | 83 ± 1.66 a | 96 ± 0.96 a | 44.84 ± 0.90 a | 1.4 ± 0.04 a |
| A1 | 0.491 ± 0.03 a | 363 ± 4.36 b | 82 ± 1.64 b | 96 ± 0.96 a | 45.53 ± 0.91 b | 1.4 ± 0.04 a |
| A2 | 0.495 ± 0.02 a | 345 ± 4.14 c | 81.2 ± 1.62 c | 97 ± 0.97 b | 45.88 ± 0.92 c | 1.4 ± 0.03 a |
| A3 | 0.498 ± 0.02 a | 311 ± 3.73 d | 77 ± 1.54 d | 96 ± 0.96 a | 45.93 ± 0.92 d | 1.2 ± 0.02 b |
| A4 | 0.499 ± 0.02 a | 285 ± 3.42 e | 74.7 ± 1.49 e | 97 ± 0.97 b | 45.98 ± 0.92 e | 1.2 ± 0.01 b |
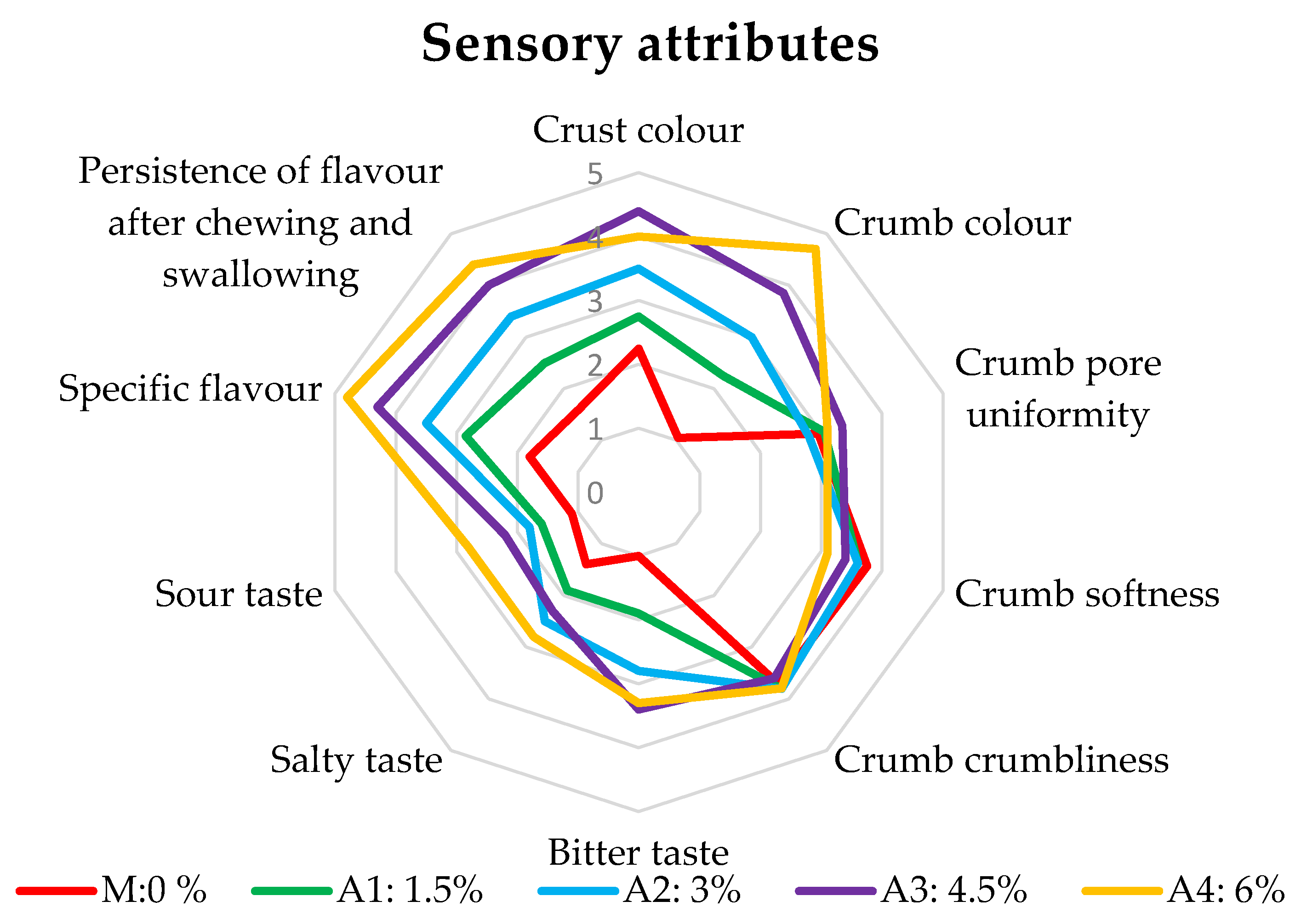
| Sensorial Attribute | Crust Color | Crumb Color | Crumb Pore Uniformity | Crumb Softness | Crumb Crumbliness | Bitter Taste | Salty Taste | Sour Taste | Specific Flavor | Persistence of Flavor after Chewing and Swallowing |
|---|---|---|---|---|---|---|---|---|---|---|
| Samples | ||||||||||
| M: 0% | 2.25 ± 0.05 a | 1.05 ± 0.02 a | 2.95 ± 0.21 a | 3.75 ± 0.08 d | 3.70 ± 0.07 a | 1.00 ± 0.02 a | 1.40 ± 0.03 a | 1.10 ± 0.02 a | 1.80 ± 0.04 a | 1.60 ± 0.03 a |
| A1: 1.5% | 2.75 ± 0.06 b | 2.25 ± 0.05 b | 3.05 ± 0.27 a | 3.60 ± 0.07 c | 3.80 ± 0.08 b | 1.90 ± 0.04 b | 1.90 ± 0.04 b | 1.60 ± 0.03 b | 2.85 ± 0.06 b | 2.50 ± 0.05 b |
| A2: 3% | 3.5 ± 0.07 c | 3.00 ± 0.06 c | 2.80 ± 0.39 a | 3.60 ± 0.07 c | 3.80 ± 0.08 b | 2.80 ± 0.06 c | 2.50 ± 0.05 c | 1.80 ± 0.04 c | 3.50 ± 0.07 c | 3.40 ± 0.07 c |
| A3: 4.5% | 4.4 ± 0.09 e | 3.85 ± 0.08 d | 3.35 ± 0.07 a | 3.40 ± 0.07 b | 3.60 ± 0.25 a,b | 3.40 ± 0.07 d | 2.30 ± 0.05 b, c,d,e | 2.20 ± 0.04 d | 4.30 ± 0.09 d | 4.00 ± 0.08 d |
| A4: 6% | 4 ± 0.08 d | 4.7 ± 0.09 e | 3.10 ± 0.25 a | 3.10 ± 0.06 a | 3.80 ± 0.08 b | 3.30 ± 0.20 c,d,e | 2.80 ± 0.06 e | 2.80 ± 0.06 e | 4.80 ± 0.10 e | 4.40 ± 0.09 e |
| Sample | Yeasts and Molds cfu/g | Water Activity Aw |
|---|---|---|
| Initial analysis | ||
| M | <10 | 0.968 ± 0.019 a |
| A1 | <10 | 0.974 ± 0.019 b |
| A2 | <10 | 0.972 ± 0.019 c |
| A3 | <10 | 0.972 ± 0.019 c,d |
| A4 | <10 | 0.972 ± 0.019 c,d,e |
| Analysis after 48 h | ||
| M | <10 | 0.960 ± 0.019 a |
| A1 | <10 | 0.971 ± 0.019 b |
| A2 | <10 | 0.969 ± 0.019 c |
| A3 | <10 | 0.970 ± 0.019 d |
| A4 | <10 | 0.969 ± 0.019 c,e |
| Analysis after 72 h | ||
| M | 3.0 × 101 | 0.917 ± 0.018 a |
| A1 | 4.0 × 101 | 0.965 ± 0.019 b |
| A2 | 4.0 × 101 | 0.951 ± 0.019 c |
| A3 | 3.0 × 101 | 0.963 ± 0.019 d |
| A4 | 7.0 × 101 | 0.950 ± 0.019 e |
| Analysis after 96 h | ||
| M | 6.0 × 101 | 0.907 ± 0.018 a |
| A1 | 6.0 × 101 | 0.944 ± 0.019 b |
| A2 | 6.0 × 101 | 0.947 ± 0.019 c |
| A3 | 7.0 × 101 | 0.953 ± 0.019 d |
| A4 | 9.0 × 101 | 0.940 ± 0.019 e |
| Samples | M | A1 | A2 | A3 | A4 | |
|---|---|---|---|---|---|---|
| Analysis | ||||||
| Firmness (Force 40%) (N) | Day 1 | 1.14 ± 0.07 a | 1.3 ± 0.17 a, b | 1.37 ± 0.09 b | 1.95 ± 0.18 c,d | 2.14 ± 0.04 d |
| Day 2 | 2.03 ± 0.12 a | 2.52 ± 0.02 b | 2.51 ± 0.27 a,b | 2.92 ± 0.22 b, c | 3.30 ± 0.35 b,c,d | |
| Day 3 | 2.21 ± 0.26 a | 1.87 ± 0.14 a | 2.55 ± 0.05 a,b | 2.87 ± 0.17 b | 3.67 ± 0.11 c | |
| Cohesiveness | Day 1 | 0.77 ± 0.02 a | 0.74 ± 0.04 a | 0.78 ± 0.04 a,b | 0.73 ± 0.00 a | 0.73 ± 0.03 a |
| Day 2 | 0.62 ± 0.07 a | 0.62 ± 0.08 a | 0.62 ± 0.01 a | 0.59 ± 0.03 a | 0.65 ± 0.03 a, b | |
| Day 3 | 0.58 ± 0.03 a | 0.52 ± 0.15 a | 0.58 ± 0.04 a | 0.59 ± 0.02 a | 0.56 ± 0.02 a, b | |
| Elasticity | Day 1 | 0.98 ± 0.00 a | 0.98 ± 0.00 a | 0.98 ± 0.00 a | 0.98 ± 0.00 a | 0.99 ± 0.02 a |
| Day 2 | 0.98 ± 0.01 a | 0.98 ± 0.00 a | 0.98 ± 0.00 a | 0.99 ± 0.00 a, b | 1 ± 0.01 b,c | |
| Day 3 | 0.98 ± 0.00 a | 0.98 ± 0.00 a | 0.98 ± 0.01 a | 0.98 ± 0.00 a | 0.97 ± 0.02 a | |
| Gumminess (N) | Day 1 | 0.87 ± 0.03 a | 0.94 ± 0.08 a | 1.05 ± 0.12 a | 1.39 ± 0.13 b | 1.55 ± 0.05 b |
| Day 2 | 1.23 ± 0.20 a | 1.53 ± 0.19 b | 1.52 ± 0.13 a,b,c | 1.69 ± 0.19 c | 2.12 ± 0.30 c | |
| Day 3 | 1.25 ± 0.08 a | 0.95 ± 0.20 a | 1.45 ± 0.14 a,b | 1.68 ± 0.16 c | 1.98 ± 0.01 b,c | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oprea, O.B.; Tolstorebrov, I.; Claussen, I.C.; Sannan, S.; Apostol, L.; Moșoiu, C.; Gaceu, L. Potential for Saccharina latissima Flour as a Functional Ingredient in the Baking Sector. Foods 2023, 12, 4498. https://doi.org/10.3390/foods12244498
Oprea OB, Tolstorebrov I, Claussen IC, Sannan S, Apostol L, Moșoiu C, Gaceu L. Potential for Saccharina latissima Flour as a Functional Ingredient in the Baking Sector. Foods. 2023; 12(24):4498. https://doi.org/10.3390/foods12244498
Chicago/Turabian StyleOprea, Oana Bianca, Ignat Tolstorebrov, Ingrid Camilla Claussen, Sigurd Sannan, Livia Apostol, Claudia Moșoiu, and Liviu Gaceu. 2023. "Potential for Saccharina latissima Flour as a Functional Ingredient in the Baking Sector" Foods 12, no. 24: 4498. https://doi.org/10.3390/foods12244498
APA StyleOprea, O. B., Tolstorebrov, I., Claussen, I. C., Sannan, S., Apostol, L., Moșoiu, C., & Gaceu, L. (2023). Potential for Saccharina latissima Flour as a Functional Ingredient in the Baking Sector. Foods, 12(24), 4498. https://doi.org/10.3390/foods12244498










