Abstract
Salmonella is one of the leading causes of foodborne disease worldwide, usually related to contaminated poultry or poultry products, such as eggs. Since egg contamination with Salmonella depends on multiple factors that make it challenging to control, consumers’ knowledge about food safety and the proper handling of eggs is crucial. The aims of the study were (1) to determine the prevalence of Salmonella in eggs from conventional and alternative production systems, (2) to characterize the Salmonella isolates according to phenotypic-genotypic and antimicrobial-resistant traits, and (3) to understand how consumers manage the hazards related to egg contamination in the household. A total of 426 egg samples were analyzed (conventional systems = 240; alternative systems = 186). Culture-based and molecular microbiological methods were used to identify Salmonella and bioinformatics analysis of whole genome sequences was used to determine the serotype and antimicrobial-resistant genes. Salmonella enterica serotype Enteritidis was detected only in eggs from alternative systems (1.1%, 2/186). Isolates showed resistance to nalidixic acid (100%, 2/2), and the aac(6′)-Iaa gene and a mutation in the gyrA gene were identified in both isolates. Overall, consumers demonstrated knowledge regarding food safety; however, many still engage in practices that pose a risk of acquiring foodborne illnesses.
1. Introduction
Foodborne illnesses are considered one of the most important public health concerns worldwide. Millions of cases and related chronic health complications are documented yearly [1]. According to the World Health Organization (WHO), almost one in 10 people fall ill each year after eating contaminated food. That represents around 600 million cases and 420,000 deaths. Also, foodborne diseases significantly impact national economies, tourism, and trade [2].
Globally, one of the most frequently documented causes of foodborne disease is non-typhoidal Salmonella. In the United States, it is the second most common cause of foodborne outbreaks, and around 20% of the illnesses caused by Salmonella are related to poultry and poultry products, such as eggs [3,4]. In Chile, egg consumption accounted for 10.7% of all foodborne outbreaks reported by the Ministry of Health in 2019, primarily linked to inappropriate food handling at home [5].
Non-typhoidal Salmonella usually causes uncomplicated gastroenteritis in humans and usually manifests with fever, chills, vomiting, and diarrhea. Nonetheless, the disease can be life-threatening for vulnerable groups, such as children, the elderly, and patients with compromised immunity [6].
Worldwide, the production and consumption of eggs are constantly increasing and between 1961 and 2021 egg production increased from 14 to 1633 million tons [7]. China is the world’s largest hen egg producer, followed by India, Indonesia, and the United States. Altogether, these countries produce more than 940 million tons of eggs. In Chile, for instance, national production contributed about 4.5 million tons of eggs during 2021 [7]. In the United States, egg consumption was projected to be around 277.5 eggs per person in 2022. By 2023, it was predicted to reach 288.6 eggs per person [8]. In Chile, the annual egg consumption per person in 2018 was estimated to be around 230 eggs [9].
Over the years, egg production systems have diversified, mainly due to the growing concern for animal welfare. The European Union banned the use of conventional battery cages in 2012. These changes lead to the implementation of alternative systems, such as cage-free systems, which enhance the welfare of hens by permitting them to freely express their natural behavior and make decisions based on their needs and desires [10]. Nonetheless, in the alternative systems, hens might still be exposed to environmental stressors, predators, pests, and negative behaviors, such as intra-species aggression [11,12,13]. By 2022, it was estimated that around 25% of United States flocks were kept in cage-free systems [14]
The presence of Salmonella in eggs obtained from conventional systems depends on different factors, including the country and sampling methodologies [3]. Overall, egg contamination from industrial systems has been reported to be 0.005% in the United States, 0.37% in Europe, and between 0.5% and 5.6% in China [3,15,16]. However, for other pathogens, such as Campylobacter spp., free-range nest box swabs from alternative systems have shown a greater prevalence than cage swabs from conventional systems. The current data regarding pathogen prevalence between conventional and alternative systems remain unclear [17]. Furthermore, antimicrobial-resistant Salmonella has been isolated from eggs and egg products, including multi-resistant bacterial strains against β-lactams, fluoroquinolones, and aminoglycosides [18].
Since eggs might be a source of foodborne pathogens, such as Salmonella, consumers should be aware of their risks [4]. Unfortunately, only some consumers are aware of the hazards of consuming or manipulating contaminated eggs [19]. Considering the preparation and management of egg-containing foods at home, several factors might lead to salmonellosis, such as the natural contamination of eggs, consumers’ lack of knowledge regarding food safety, cross-contamination, and the deliberate consumption of raw or undercooked eggs. For instance, 25% of foodborne outbreaks are related to poor hygiene practices, such as cross-contamination [3,20,21].
The objectives of this study were: (1) to determine the prevalence of Salmonella in eggs from conventional and alternative production systems, (2) to characterize the Salmonella isolates according to phenotypic-genotypic and antimicrobial resistant traits, and (3) to understand how Chilean consumers manage the hazards related to egg contamination in the household.
2. Materials and Methods
2.1. Egg Samples
A total of 426 samples of eggs from conventional (battery-cage production systems) (n = 240) and cage-free (n = 186) systems were analyzed. The sample size was determined according to the results obtained with R software (Version 4.1.0) [22], based on the greater estimated prevalence of Salmonella in eggs reported for Europe for the last five years and the historical prevalence reported in the literature for alternative systems [23,24]. The number of samples analyzed for each production system exceeded the minimum required. Following the suggestions established by Public Health England, each sample consisted of six eggs for a total of 2556 eggs analyzed [25]. Samples were obtained from large (≥500,000 laying hens), medium (between 100,000 and 500,000 laying hens), and small (≤100,000 laying hens) egg producers [26], transported to the laboratory in their original package and analyzed within the same day. Sampling was conducted between May 2022 and January 2023.
2.2. Isolation and Identification of Salmonella
Microbiological analyses were carried out for eggshells according to the ISO 6579-1:2017 methodology [27]. For the pre-enrichment step, intact eggs were placed inside a sterile plastic bag containing 100 mL of preheated (37 °C) buffered peptone water (BD DifcoTM, 218105, Franklin Lakes, NJ, USA) and gently rubbed for one minute. The eggs were removed from the bag in an aseptic way, and the bag was incubated at 37 °C for 24 h. Then, for the enrichment step, 0.1 mL of the pre-enrichments were inoculated onto Rappaport-Vassiliadis broth (OxoidTM, CM0669, Waltham, MA, USA) and incubated at 42 °C for 24 h and 1 mL was inoculated in tetrathionate broth (OxoidTM, CM0671, MA, USA) and incubated at 37 °C for 24 h. Ten microliters of the enrichments were plated onto Hektoen agar (BD DifcoTM, 285340, NJ, USA) and Xylose Lysine Desoxycholate agar (XLD) (BD DifcoTM, 278820, NJ, USA) and incubated at 37 °C for 24 h. At least three presumptive and atypical Salmonella colonies were analyzed and later confirmed through biochemical tests and polymerase chain reaction (PCR) for the invA gene [28]. Salmonella isolates were stored at −20 °C for further analysis.
2.3. Antibiotic Susceptibility Testing
Confirmed Salmonella isolates underwent antibiotic susceptibility testing using the Kirby–Bauer disk diffusion method for the following antibiotics: nalidixic acid (NAL = 30 µg), amoxicillin/clavulanate (AMC = 30 µg), amikacin (AMK = 30 µg), ampicillin (AMP = 10 µg), azithromycin (AZM = 15 µg), cefoxitin (FOX = 30 µg), ceftriaxone (CRO = 30 µg), chloramphenicol (C = 30 µg), ciprofloxacin (CIP = 5 µg), streptomycin (S = 10 µg), gentamicin (GE = 10 µg), imipenem (IMP = 10 µg), kanamycin (K = 30 µg), meropenem (MEM = 10 µg), tetracycline (TE = 30 µg), sulfonamide (S3 = 300 µg), trimethoprim-sulfamethoxazole (SXT = 1.25/23.75 µg), and florfenicol (FFC= 30 µg). Results were interpreted according to Clinical and Laboratory Standards Institute (CLSI) guidelines [29]. E. coli ATCC 25922 and Staphylococcus aureus ATCC 25923 were used as control strains (CLSI, 2020).
2.4. Salmonella Whole Genome Sequencing (WGS) Analysis
Overnight cultures of the two Salmonella isolates grown in tryptic soy broth (BD DifcoTM 22092, USA) were used for the genomic DNA extraction using the DNeasy Blood and tissue kit (Qiagen, Germantown, TN, USA) following the protocol for Gram-negative bacteria recommended by the manufacturer. Libraries were prepared with the Illumina DNA pep library preparation kit (Illumina Inc., San Diego, CA, USA). Paired-end sequencing (2 × 150 bp) was performed in a NextSeq instrument using the Illumina NextSeq Reagent Kit P2 (300 cycles) according to the manufacturer’s instructions.
The sequenced data was deposited in the National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA) under numbers SRR26050061 and SRR26050062. Read quality control, read trimming, de novo assembly, and assembly quality control were performed in the GalaxyTrakr platform [30] using the FastQC [31], Trimmomatic v0.36.4 [32], Spades v3.12.0 [33], and QUAST v5.0.2 [34] tools. Sequence type (ST) determination and serovar prediction were determined using the MLST 2.22.0 [35] and sistr_cmd [36] in GalaxyTrakr tools.
Phylogenetic relatedness among Salmonella isolates was analyzed through a single nucleotide polymorphism (SNP) calling in the CSI Phylogeny 1.4 from the Center for Genomic Epidemiology (CGE) at Denmark Technical University (https://cge.food.dtu.dk/services/CSIPhylogeny/, accessed on 12 September 2023) with default parameters [37]. Salmonella serotype Enteritidis strain 92-0392 (CP018657.1) was used as the reference genome, and a difference of <20 SNPs was used to infer genetic relatedness between the isolates [38]. Finally, the presence of antimicrobial resistance genes was determined with AMRFinder, also from CGE [39].
2.5. Survey Implementation
An online survey to analyze the consumers’ knowledge and practices regarding food handling at home was prepared according to previously published surveys [3,19,21,40,41,42,43]. A sample size of 385 respondents was calculated according to the sample size formula for infinite populations [44]. The survey sample was non-probabilistic based on quotas according to the age group (18–29, 30–59, and 60+) (Supplementary Materials S1).
Closed (n = 19) and multiple-choice (n = 16) questions were included, and responses were received from 17 January through 28 February 2023. The survey gathered personal and demographic data, consumer habits regarding the purchase, storage, handling, and consumption of eggs, and food safety knowledge. The study was approved by the Institute of Food Nutrition and Technology (INTA) Scientific and Ethical Committee (CEC) from the University of Chile (Number 004/2023). Each participant agreed to answer the survey by accepting the informed consent form.
2.6. Statistical Analysis
Fisher’s exact test was used to compare the prevalence of Salmonella depending on the origin of the analyzed eggs (conventional vs. alternative production system). A p-value of ≤0.05 was considered significant. Descriptive and inferential statistics using SPSS Statistics Software (IBM, version 21) were used to analyze the survey’s answers. The Chi-square test was employed to determine the relationship between age groups and the survey answer [45]. Cramer’s V test was used to estimate the intensity or strength of the association, where the significance of variables according to the Chi-square test was determined at a p-value < 0.05. According to Akoglu (2018), the interpretation of the Cramer’s V test strength of the association was categorized as very strong (>0.25), strong (0.15–0.25), moderate (0.10–0.14), weak (0.05–0.09), and no or very weak (0–0.04) [46,47]. Adjusted standardized residuals (ASR) were used to estimate the magnitude and directionality of the relationship of the differences. The ASRs were considered statistically significant when the value was greater than 1.96 or less than −1.96 [48].
3. Results
3.1. Prevalence of Salmonella in Eggs from Different Production Systems
Salmonella was not detected in eggs from conventional battery-cage systems (0/240), while 1.1% (2/186) of samples from alternative cage-free systems carried Salmonella (Figure 1). Samples carrying Salmonella came from different batches from the same egg producer and were obtained eight months apart. No statistical differences were found between the Salmonella prevalence from conventional and alternative egg production systems (p-value = 0.1).
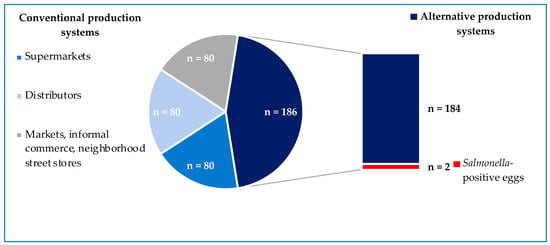
Figure 1.
Prevalence of Salmonella according to the egg-production system.
3.2. Phenotypic and Genomic Characterization of Salmonella Isolates
One Salmonella isolate was selected from each positive sample for further characterization, which was analyzed according to the Kirby–Bauer disc diffusion method and showed 100% (2/2) resistance to nalidixic acid while being susceptible to the remaining antibiotics tested. The bioinformatic analysis predicted that both isolates were Salmonella enterica subsp. enterica serotype Enteritidis (S. serotype Enteritidis), ST-11. Both genomes carried the aac(6′)-Iaa gene, which confers resistance to tobramycin and amikacin, and a mutation of the gyrA gene (TCC to TTC; Asp83Gly), conferring resistance to nalidixic acid. Nineteen SNPs were identified between both isolates, indicating a close phylogenetic relatedness, although the isolates were not clones [38,49].
3.3. Home Egg Management and Food Safety
A total of 385 answers were obtained from the online survey. A summary of the most relevant demographic data from the consumers is shown in Table S1. The survey was mainly answered by middle-aged (53.2%, 205/385) women (82.6%, 318/385). From the survey participants, 17% (65/385) declared to be related to the food handling and/or food safety field (Table S1). Table 1 shows the most representative responses regarding consumers’ habitual egg-purchasing behavior according to age, particularly the distinction between conventional and alternative egg production systems. Results showed that 40.8% of respondents prefer eggs from alternative systems, while 46.8% indicated that the production system was irrelevant to their purchasing decision.

Table 1.
Preference of the egg production system according to the age of the respondent.
The survey results revealed notable trends in household egg-handling practices (Table 2). Around 20% of respondents admitted buying cracked or broken eggs, a practice associated with potential food safety risks. The analysis revealed that this practice was less frequent in older adults than in other age groups (Cramer’s V test = 0.164). In addition, almost a quarter (24.9%) of respondents reported washing eggs before storing them, despite the widespread advice against such practice due to the risk of introducing contaminants through the pores of the eggshell (Table 2).

Table 2.
Most representative answers related to food handling habits at home and food safety knowledge of consumers.
The responses were strongly associated with middle-aged consumers who demonstrated safer hygiene practices (Cramer’s V test = 0.150), such as not washing eggs before storage. Furthermore, over half of the respondents showed awareness of proper egg storage practices, with the vast majority choosing to store eggs in the refrigerator (68.5%) (Table 3).

Table 3.
Egg Storage preferences in households.
Regarding preferences in egg consumption (Table 4), it is noteworthy that 46.3% of adults over 60 years old reported consuming raw or undercooked eggs in home-cooked dishes. Interestingly, within this demographic group, 93.9% manifested confidence that the proper cooking of eggs could prevent the onset of foodborne disease.

Table 4.
Consumer preferences and food safety awareness about egg consumption.
Consumers’ perception of food safety concerning different eating places and egg products was assessed. Most participants (76.5%) believe eating out increases the likelihood of becoming ill, particularly street food. In contrast, the prevailing consensus among respondents was that homemade food has minimal food safety risks (Table 5).

Table 5.
Places where consumers believe they are more likely to become ill after consuming raw or undercooked egg-containing foodstuffs.
4. Discussion
Poultry and poultry products have been identified as major sources of foodborne diseases and pathogen transmission [50,51], and S. Enterica is the primary pathogen related to eggs. The industry makes efforts to improve egg safety and lower the Salmonella prevalence through good manufacturing practices (GMP) and hazard analysis and critical control points (HACCP) systems. However, as bacteria keep genetically evolving, it is becoming more challenging to control this pathogen.
Egg production systems have evolved over the years adding another significant food safety challenge [50]. Due to political, commercial, and social pressure, egg production systems have changed from conventional caged systems to alternative methods, like cage-free or free-range, particularly in regions such as the United States and Europe [50]. Animal welfare is an important aspect that should be considered in every production system; nevertheless, since many food safety measures were designed for conventional production systems, which have a higher level of control, their efficacy might not be the same for alternative systems [50,51].
In the United States, for example, through the Egg Safety Final Rule issued in 2009, the Food and Drug Administration (FDA) highlighted essential measures to prevent the on-farm infection of hens with Salmonella, the potential production of contaminated eggs, and the proper actions that should be considered after laying eggs [52]. Nonetheless, due to different factors, such as the exposure of hens to vectors, these measures are incompatible with alternative systems that allow hens to roam outdoors [53].
As a result, in 2013 the FDA provided guidance for layers with access to areas outside the poultry house. However, the guidance document emphasizes that the provided information should be considered merely as a suggestion unless explicit regulatory or statutory obligations are referenced. Finally, only farms with 3000 or more laying hens are subjected to the Egg Rule [52,54].
In this study, we did not observe significant differences between the presence of Salmonella in conventional or alternative production systems (p = 0.1). However, we detected that two samples (1.1%, 2/186) obtained from cage-free hens that roam in open, less protected environments were contaminated with Salmonella serotype Enteritidis. These conditions could increase the likelihood of Salmonella contamination in egg production environments. Although the following factors were not analyzed in the study, egg safety, and thus the presence of Salmonella in eggs obtained from alternative systems, also depends on the flock density, the exposure to contaminated feces and substrate, dust, vectors, the stress level of the hens, and the location of the nesting boxes, among others. It must be considered that the internal content of eggs might also be contaminated and present different levels of Salmonella compared to the egg’s surface; therefore, the reported results can be underestimated.
Even though eggs can be contaminated on the surface and internally, the present study sought to determine the prevalence of Salmonella from eggshells due to the close relationship between eggshell contamination and the risk of cross-contamination during egg handling by consumers. In U.S.-based studies, for instance, no differences were reported in the contamination of egg contents with Salmonella between conventional and alternative egg production systems, while differences have been reported with respect to the prevalence of Salmonella in droppings related to the surface contamination of eggs [50].
The prevalence of Salmonella from the egg’s internal contents might be influenced by the measures implemented in the poultry industry, where control is based on a combination of strategies such as the purchase of replacement birds from free breeders, proper feed, biosecurity measures, management of gut microbiota, and vaccination [55]. Moreover, the contamination of the internal contents of eggs is considered a rare finding [56].
Although Salmonella was only related to alternative production systems, globally, data on the impact of different production systems on pathogen prevalence remains elusive [17,50,57]. Therefore, the way alternative systems are implemented raises concerns about the presence of pathogens and measures that should be applied.
To promote animal welfare, enriched cage systems might be a balanced alternative over conventional production systems since they combine the laying productivity of hens and improved animal welfare. These systems provide perches, scratch pads, nest boxes, and larger areas, which offer an improved environment over conventional cages [57,58].
In the United States, for instance, efforts have been made to establish more detailed measures to ensure the welfare of laying hens. As stated by the USDA, the U.S. egg industry has increasingly changed from conventional battery-caged systems to cage-free production, nearly doubling it in the 5-year span of 2017 to 2021 [59].
The Chilean Agricultural and Livestock Service created guidelines where important recommendations were established for both conventional and alternative systems to promote animal welfare. These guidelines promote welfare aspects regarding water and diet, health measures, housing, behavior, laying productivity, productive management practices, and training and education of welfare managers and staff [26].
Salmonella serotype Enteritidis is the predominant serovar found in eggs and has been linked to outbreaks worldwide. Recently, a study reported the involvement of Salmonella serotype Enteritidis ST11 in an outbreak of salmonellosis traced to a restaurant where eggs and chicken were considered the most likely cause of the outbreak [60]. This incident highlights the importance of solid control measures in egg handling and production. Vigilant monitoring, stringent hygiene practices, and strict temperature control throughout the egg supply chain are essential to reduce the risk of Salmonella contamination and protect public health.
Antibiotics in the poultry industry and other animal production systems are used for therapeutic and preventive control of diseases and to improve feed efficiency and productivity. However, their improper use might result in the dangerous accumulation of these chemicals in animal tissues and eggs and promote the selection of resistant bacteria [61]. The main concern regarding antimicrobial resistance is the failure to treat animal and human infections with already available antibiotics, thus reducing the chances of using complementary therapies, such as chemotherapy and surgery [62]. In this study, both S. serotype Enteritidis isolates were resistant to nalidixic acid, which has been previously reported in Salmonella isolated from eggs [18,63]. In Salmonella, quinolone resistance is usually related to mutations in the quinolone resistance determining region of the gyrA and parC genes [64,65]. Vuthy et al. (2017) described that over 75% of nalidixic acid-resistant Salmonella isolates had a mutation in the gyrA gene. On the other hand, our identified isolates were not phenotypically resistant to aminoglycoside amikacin [65]. Still, the bioinformatics analysis identified the gene aac(6′)-Iaa that confers resistance to tobramycin, amikacin, and kanamycin [66,67]. As shown in our results, it has been reported that the presence of the aac(6′)-Iaa gene is not necessarily related to phenotypic resistance to aminoglycosides since this gene is often weakly expressed or not expressed at all [68].
Survey results showed that consumers from every age range preferred alternative egg production systems (40.8%, 157/385) over conventional production systems (3.9%, 15/385). Nonetheless, no statistical relationship was demonstrated between this preference and the age group (p > 0.05) (Table 1). This is a significant result, considering that most eggs around the world are still produced in conventional systems and that alternative egg production systems in Chile only account for 1.2% of the available production systems [9,69]. Worldwide, as shown by Sinclair et al. (2022), consumers prefer to buy eggs from hens that have not been kept in cages [69].
Due to the complex nature of egg contamination with Salmonella and the multiple influencing factors contributing to its management, it remains critical to ensure that foods containing eggs from alternative production systems are handled and prepared appropriately. Our findings suggest that such systems are susceptible to Salmonella contamination, emphasizing the continued significance of such measures in preventing foodborne illnesses [51].
As stated by Cardoso et al. (2021) the first step for the safe handling of eggs the awareness of the potential biological dangers that might be present in eggs. To reduce the probability of acquiring salmonellosis from eggs, adequate practices must be focused on from the purchase to the consumption of egg-containing foods. Overall, consumers should examine eggs at the time of purchase, avoid washing the eggs before storage, and refrigerate the eggs. In addition, it is important to comply with the appropriate cooking temperature and time to eliminate Salmonella before consumption [3].
When analyzing consumer food handling habits at home, we observed that most consumers across all age groups avoid dangerous handling practices such as using cracked or dirty eggs (80.8%, 311/385), washing eggs before storage (75%, 289/385), preparing and consuming foods containing raw or undercooked eggs (59.5%, 229/385), among others; nevertheless, over 40% of the consumers do prepare and consume foods containing raw eggs (40.5%, 156/385) (Table 4).
It should be noted that Salmonella-associated foodborne outbreaks are mainly linked to contaminated eggs and raw egg products, such as sauces and dressings, like mayonnaise, hollandaise, aioli and egg butter mayonnaise, desserts such as tiramisu, mousse, and fried-ice cream batter, and drinks, such as raw egg protein drinks and eggnog [70,71]. Interestingly, regarding consumers’ knowledge about food safety, 96.6% (372/385) of respondents stated that proper cooking of eggs could prevent people from becoming ill (Table 4).
It is crucial to outline that although in some countries, such as the United States, all shell eggs and egg products packed for consumers must be refrigerated [72], in Chile, while the survey respondents preferred to refrigerate eggs, it is not a legal requirement [73], and the refrigeration of eggs at home is a consumer’s decision based on their food safety knowledge and practices.
In this survey, most respondents (76.5%, 254/385) think eating street food increases the likelihood of catching a foodborne illness, while only 4.8% (16/385) considered homemade food a risk. However, global evidence shows that most foodborne outbreaks typically occur in homes. Although results may vary among countries, Gargiulo et al. (2022) reported that household outbreaks accounted for 41.3% of all European cases in 2019, while restaurants, cafes, bars, and street food accounted for 28.6% [74].
One of the drawbacks of this research is that to determine the appropriate sample size for detecting Salmonella in eggs, we utilized European data rather than local information which was not available [23,24]. Furthermore, data on the prevalence of Salmonella from different egg production systems is not available in Chile. In addition, the recovery of Salmonella from eggs could increase with a bigger sample size. Nevertheless, this research produces publicly available data that can be utilized to develop studies with more precise estimations.
Overall, the safety of eggs and egg products remains a public health concern, particularly related to the contamination of Salmonella serotype Enteritidis. Our study showed the importance of addressing the challenges related to the shift from conventional egg production systems to alternative systems and the importance of understanding antimicrobial resistance mechanisms to develop tailored control approaches. Consumers’ education on food safety plays a crucial role in reducing foodborne illnesses since Salmonella-related disease is an ongoing threat that should be appropriately prevented. The cooperation and work between regulatory bodies and egg industry representatives must always prevail.
5. Future Perspective and Conclusions
Although the prevalence of Salmonella from eggs was low, this pathogen was only detected from alternative egg production systems. To our knowledge, this is the first Chilean study to compare the prevalence of Salmonella from conventional and alternative egg production systems. Efforts for reducing Salmonella are ongoing and should be adapted and improved for new alternative production systems since the effectiveness of existing egg safety measures developed for conventional production systems might not translate to the alternative ones. Responses to the survey, which complemented the Salmonella prevalence results, highlighted the need for increased food safety education and adequate food handling practices at home. The interplay between foodborne pathogens, the diversification of egg production systems, and consumer behavior represent challenging topics for ensuring food safety and culture. In addition, our findings suggest that more work should be done to prevent the selection of antibiotic-resistant strains arising from the inappropriate use of antibiotics in animal production systems, including poultry.
The future perspectives of this study include the comparison of the present results with the evaluation of the pathogens from the egg’s internal contents and determining the association between pathogen prevalence and the hygiene practices carried out by producers according to the production system.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/foods12234300/s1, Table S1: Demographic characteristics of survey participants (n = 385). Supplementary Materials S1: Survey.
Author Contributions
Conceptualization, N.C. and A.R.-J.; formal analysis, D.S., N.C., M.Q.-R., C.E.-A., M.T. and A.R.-J.; methodology, M.Q.-R. and C.E.-A.; writing—original draft, D.S., N.C. and A.R.-J.; writing—review and editing, D.S., N.C., M.Q.-R., C.E.-A., M.T., P.N. and A.R.-J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by “Sinergia Animal”.
Institutional Review Board Statement
The Institute of Food Nutrition and Technology (INTA) Scientific and Ethical Committee (CEC) from the University of Chile Number 004/2023.
Informed Consent Statement
Each participant agreed to answer the survey by accepting the informed consent form.
Data Availability Statement
Data is contained within the article or supplementary material.
Acknowledgments
A.R.-J.: acknowledges grant ANILLO ACT210004.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schirone, M.; Visciano, P.; Tofalo, R.; Suzzi, G. Foodborne pathogens: Hygiene and safety. Front. Microbiol. 2019, 10, 1974. [Google Scholar] [CrossRef]
- World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/food-safety (accessed on 14 September 2023).
- Cardoso, M.J.; Nicolau, A.I.; Borda, D.; Nielsen, L.; Maia, R.L.; Møretrø, T.; Ferreira, V.; Knøchel, S.; Langsrud, S.; Teixeira, P. Salmonella in eggs: From shopping to consumption-A review providing an evidence-based analysis of risk factors. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2716–2741. [Google Scholar] [CrossRef]
- O’Bryan, C.A.; Ricke, S.C.; Marcy, J.A. Public health impact of Salmonella spp. on raw poultry: Current concepts and future prospects in the United States. Food Control 2022, 132, 108539. [Google Scholar] [CrossRef]
- Departamento de Estadísticas e Información de Salud. Brotes de Enfermedades Transmitidas por Alimento (ETA). Chile, Periodo años 2011–2019. Available online: https://public.tableau.com/app/profile/deis4231/viz/BrotesdeEnfermedadesTransmitidasporAlimentoETA_Aos2011-2017/BrotesETAChile2011-2017 (accessed on 2 September 2023).
- Wu, L.J.; Luo, Y.; Shi, G.L.; Li, Z.Y. Prevalence, clinical characteristics and changes of antibiotic resistance in children with nontyphoidal Salmonella infections from 2009-2018 in Chongqing, China. Infect Drug Resist. 2021, 14, 1403–1413. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations. FAOSTAT Crops and Livestock. Available online: https://www.fao.org/faostat/es/#data/QCL/visualize (accessed on 22 August 2023).
- Shahbandeh, M. Per Capita Consumption of Eggs in the U.S. 2000–2023. Available online: https://www.statista.com/statistics/183678/per-capita-consumption-of-eggs-in-the-us-since-2000/ (accessed on 16 August 2023).
- Aguirre, R.; Pizarro, M.J. Panorama y Mercado del Huevo. 2018. Available online: https://www.odepa.gob.cl/wp-content/uploads/2018/04/Huevos.pdf (accessed on 23 August 2023).
- Bonnefous, C.; Collin, A.; Guilloteau, L.A.; Guesdon, V.; Filliat, C.; Réhault-Godbert, S.; Rodenburg, T.B.; Tuyttens, F.A.M.; Warin, L.; Steenfeldt, S.; et al. Welfare issues and potential solutions for laying hens in free range and organic production systems: A review based on literature and interviews. Front. Vet. Sci. 2022, 9, 952922. [Google Scholar] [CrossRef]
- Chousalkar, K.; Gole, V.; Caraguel, C.; Rault, J.L. Chasing Salmonella Typhimurium in free range egg production system. Vet. Microbiol. 2016, 192, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Gole, V.C.; Woodhouse, R.; Caraguel, C.; Moyle, T.; Rault, J.L.; Sexton, M.; Chousalkar, K. Dynamics of Salmonella shedding and welfare of hens in free-range egg production systems. Appl. Environ. Microbiol. 2017, 83, e03313-16. [Google Scholar] [CrossRef] [PubMed]
- Sibanda, T.Z.; Kolakshyapati, M.; Welch, M.; Schneider, D.; Boshoff, J.; Ruhnke, I. Managing free-range laying hens-part a: Frequent and non-frequent range users differ in laying performance but not egg quality. Animals 2020, 10, 991. [Google Scholar] [CrossRef]
- Alig, B.N.; Ferket, P.R.; Malheiros, R.D.; Anderson, K.E. The effect of housing environment on egg production, USDA egg size, and USDA grade distribution of commercial white egg layers. Poultry 2023, 2, 204–221. [Google Scholar] [CrossRef]
- Li, Y.; Yang, X.; Zhang, H.; Jia, H.; Liu, X.; Yu, B.; Zeng, Y.; Zhang, Y.; Pei, X.; Yang, D. Prevalence and antimicrobial susceptibility of Salmonella in the commercial eggs in China. Int. J. Food Microbiol. 2020, 325, 108623. [Google Scholar] [CrossRef]
- Li, W.; Li, H.; Zheng, S.; Wang, Z.; Sheng, H.; Shi, C.; Shi, X.; Niu, Q.; Yang, B. Prevalence, serotype, antibiotic susceptibility, and genotype of Salmonella in eggs from poultry farms and marketplaces in Yangling, Shaanxi Province, China. Front. Microbiol. 2020, 11, 1482. [Google Scholar] [CrossRef]
- Jones, D.R.; Anderson, K.E.; Guard, J.Y. Prevalence of coliforms, Salmonella, Listeria, and Campylobacter associated with eggs and the environment of conventional cage and free-range egg production. Poult. Sci. 2012, 91, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Castro-Vargas, R.E.; Herrera-Sánchez, M.P.; Rodríguez-Hernández, R.; Rondón-Barragán, I.S. Antibiotic resistance in Salmonella spp. isolated from poultry: A global overview. Vet. World 2020, 13, 2070–2084. [Google Scholar] [CrossRef] [PubMed]
- Mihalache, O.A.; Teixeira, P.; Nicolau, A.I. Raw-egg based-foods consumption and food handling practices: A recipe for foodborne diseases among Romanian and Portuguese consumers. Food Control 2022, 139, 109046. [Google Scholar] [CrossRef]
- Carrasco, E.; Morales-Rueda, A.; García-Gimeno, R.M. Cross-contamination and recontamination by Salmonella in foods: A review. Food Res. Int. 2012, 45, 545–556. [Google Scholar] [CrossRef]
- Nesbitt, A.; Thomas, M.K.; Marshall, B.; Snedeker, K.; Meleta, K.; Watson, B.; Bienefeld, M. Baseline for consumer food safety knowledge and behaviour in Canada. Food Control 2014, 38, 157–173. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- European Food Safety Authority (EFSA). The European Union One Health 2018 Zoonoses Report. EFSA J. 2019, 17, 5926. [Google Scholar] [CrossRef]
- Little, C.L.; Walsh, S.; Hucklesby, L.; Surman-Lee, S.; Pathak, K.; Gatty, Y.; Greenwood, M.; De Pinna, E.; Threlfall, E.J.; Maund, A.; et al. Survey of Salmonella contamination of non-United Kingdom-produced raw shell eggs on retail sale in the northwest of England and London, 2005 to 2006. J. Food Prot. 2007, 70, 2259–2265. [Google Scholar] [CrossRef]
- Public Health England. Detection of Salmonella species National Infection Service Food Water and Environmental Microbiology Standard Method. Available online: http://allcatsrgrey.org.uk/wp/wpfb-file/detection_of_salmonella_species-pdf/ (accessed on 16 August 2023).
- Servicio Agrícola y Ganadero (SAG). Guía de Buenas Prácticas sobre Bienestar Animal en los diferentes Sistemas de Producción de Huevos. Available online: https://www.sag.gob.cl/sites/default/files/gbp-ba_produccion_huevos_oct-2018.pdf (accessed on 14 September 2023).
- ISO 6579:2017-1; Microbiology of the Food Chain—Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella—Part 1: Detection of Salmonella spp. International Organization for Standardization (ISO): Geneva, Switzerland, 2017.
- Kim, J.S.; Lee, G.G.; Park, J.S.; Jung, Y.H.; Kwak, H.S.; Kim, S.B.; Nam, Y.S.; Kwon, S.T. A novel multiplex PCR assay for rapid and simultaneous detection of five pathogenic bacteria: Escherichia coli O157: H7, Salmonella, Staphylococcus aureus, Listeria monocytogenes, and Vibrio parahaemolyticus. J. Food Prot. 2007, 70, 1656–1662. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 30th ed.; CLSI Supplement M100; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2020; 332p. [Google Scholar]
- Gangiredla, J.; Rand, H.; Benisatto, D.; Payne, J.; Strittmatter, C.; Sanders, J.; Wolfgang, W.J.; Libuit, K.; Herrick, J.B.; Prarat, M.; et al. GalaxyTrakr: A distributed analysis tool for public health whole genome sequence data accessible to non-bioinformaticians. BMC Genom. 2021, 22, 114. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. 2010. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc (accessed on 17 September 2023).
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Antipov, D.; Hartwick, N.; Shen, M.; Raiko, M.; Lapidus, A.; Pevzner, P.A. plasmidSPAdes: Assembling plasmids from whole genome sequencing data. Bioinformatics 2016, 32, 3380–3387. [Google Scholar] [CrossRef]
- Gurevich, A.; Saveliev, V.; Vyahhi, N.; Tesler, G. QUAST: Quality assessment tool for genome assemblies. Bioinformatics 2013, 29, 1072–1075. [Google Scholar] [CrossRef] [PubMed]
- Jolley, K.A.; Bray, J.E.; Maiden, M.C.J. Open-access bacterial population genomics: BIGSdb software, the PubMLST.org website and their applications. Wellcome Open Res. 2018, 3, 124. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, C.E.; Kruczkiewicz, P.; Laing, C.R.; Lingohr, E.J.; Gannon, V.P.J.; Nash, J.H.E.; Taboada, E.N. The Salmonella in silico typing resource (SISTR): An open web-accessible tool for rapidly typing and subtyping draft Salmonella genome assemblies. PLoS ONE 2016, 11, e0147101. [Google Scholar] [CrossRef] [PubMed]
- Kaas, R.S.; Leekitcharoenphon, P.; Aarestrup, F.M.; Lund, O. Solving the problem of comparing whole bacterial genomes across different sequencing platforms. PLoS ONE 2014, 9, e104984. [Google Scholar] [CrossRef]
- Atxaerandio-Landa, A.; Arrieta-Gisasola, A.; Laorden, L.; Bikandi, J.; Garaizar, J.; Martinez-Malaxetxebarria, I.; Martinez-Ballesteros, I. A practical bioinformatics workflow for routine analysis of bacterial WGS data. Microorganisms 2022, 10, 2364. [Google Scholar] [CrossRef]
- Florensa, A.F.; Kaas, R.S.; Clausen, P.; Aytan-Aktug, D.; Aarestrup, F.M. ResFinder—an open online resource for identification of antimicrobial resistance genes in next-generation sequencing data and prediction of phenotypes from genotypes. Microb. Genom. 2022, 8, 748. [Google Scholar] [CrossRef]
- Lazou, T.; Georgiadis, M.; Pentieva, K.; McKevitt, A.; Iossifidou, E. Food safety knowledge and food-handling practices of Greek university students: A questionnaire-based survey. Food Control 2012, 28, 400–411. [Google Scholar] [CrossRef]
- Odeyemi, O.A.; Sani, N.A.; Obadina, A.O.; Saba, C.K.S.; Bamidele, F.A.; Abughoush, M.; Asghar, A.; Dongmo, F.F.D.; Macer, D.; Aberoumand, A. Food safety knowledge, attitudes and practices among consumers in developing countries: An international survey. Food Res. Int. 2019, 116, 1386–1390. [Google Scholar] [CrossRef]
- Kosa, K.M.; Cates, S.C.; Bradley, S.; Godwin, S.; Chambers, D. Consumer shell egg consumption and handling practices: Results from a national survey. J. Food Prot. 2015, 78, 1312–1319. [Google Scholar] [CrossRef]
- Whiley, H.; Clarke, B.; Ross, K. Knowledge and attitudes towards handling eggs in the home: An unexplored food safety issue? Int. J. Environ. Res. Public Health 2017, 14, 48. [Google Scholar] [CrossRef]
- Ahmad, H.; Halim, H. Determining Sample Size for Research Activities: The Case of Organizational Research. Selangor Bus. Rev. 2017, 2, 20–34. [Google Scholar]
- Kim, H.Y. Statistical notes for clinical researchers: Chi-squared test and Fisher’s exact test. Restor. Dent. Endod. 2017, 42, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Piochi, M.; Fontefrancesco, M.F.; Torri, L. Understanding Italian consumers’ perception of safety in animal food products. Foods 2022, 11, 3739. [Google Scholar] [CrossRef] [PubMed]
- Akoglu, H. User’s guide to correlation coefficients. Turk. J. Emerg. Med. 2018, 18, 91–93. [Google Scholar] [CrossRef]
- Estévez-Moreno, L.X.; Miranda-de la Lama, G.C.; Miguel-Pacheco, G.G. Consumer attitudes towards farm animal welfare in Argentina, Chile, Colombia, Ecuador, Peru and Bolivia: A segmentation-based study. Meat Sci. 2022, 187, 108747. [Google Scholar] [CrossRef]
- Jibril, A.H.; Okeke, I.N.; Dalsgaard, A.; Olsen, J.E. Prevalence and whole genome phylogenetic analysis reveal genetic relatedness between antibiotic resistance Salmonella in hatchlings and older chickens from farms in Nigeria. Poult. Sci. 2023, 102, 102427. [Google Scholar] [CrossRef]
- Ricke, S.C. Insights and challenges of Salmonella infection of laying hens. Curr. Opin. Food Sci. 2017, 18, 43–49. [Google Scholar] [CrossRef]
- Whiley, H.; Ross, K. Salmonella and eggs: From production to plate. Int. J. Environ. Res. Public Health 2015, 12, 2543–2556. [Google Scholar] [CrossRef]
- Food and Drug Administration (FDA). Prevention of Salmonella Enteritidis in Shell Eggs during Production, Storage, and Transportation (Layers with Access to Areas Outside the Poultry House): Questions and Answers Regarding the Final Rule: Guidance for Industry. 2022. Available online: https://www.fda.gov/media/86276/download (accessed on 18 August 2023).
- Holt, P.S. Centennial Review: A revisiting of hen welfare and egg safety consequences of mandatory outdoor access for organic egg production. Poult. Sci. 2021, 100, 101436. [Google Scholar] [CrossRef]
- Food and Drug Administration (FDA). Small Entity Compliance Guide: Prevention of Salmonella Enteritidis in Shell Eggs during Production, Transportation, and Storage. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/small-entity-compliance-guide-prevention-salmonella-enteritidis-shell-eggs-during-production#cov (accessed on 4 September 2023).
- Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; De Cesare, A.; Herman, L.; Hilbert, F.; Davies, R. Salmonella control in poultry flocks and its public health impact. EFSA J. 2019, 17, e05596. [Google Scholar] [CrossRef] [PubMed]
- Im, M.C.; Jeong, S.J.; Kwon, Y.K.; Jeong, O.M.; Kang, M.S.; Lee, Y.J. Prevalence and characteristics of Salmonella spp. isolated from commercial layer farms in Korea. Poult. Sci. 2015, 94, 1691–1698. [Google Scholar] [CrossRef] [PubMed]
- Ward, E.G. Impact of Egg Handling Practices on Salmonella Risk in Cage-Free Eggs. 2018. Available online: https://esploro.libs.uga.edu/esploro/outputs/graduate/Impact-of-egg-handling-practices-on/9949334202402959 (accessed on 18 August 2023).
- Philippe, X.F.; Mahmoudi, Y.; Cinq-Mars, D.; Lefrançois, M.; Moula, N.; Palacios, J.; Pelletier, F.; Godbout, S. Comparison of egg production, quality and composition in three production systems for laying hens. Livest. Sci. 2020, 232, 103917. [Google Scholar] [CrossRef]
- Ufer, D.J. State policies for farm animal welfare in production practices of U.S. livestock and poultry industries: An overview. Econ. Inf. Bull. 2022, 245, 1–24. [Google Scholar]
- Benson, H.E.; Reeve, L.; Findlater, L.; Vusirikala, A.; Pietzsch, M.; Olufon, O.; Matthews, E.; Hoban, A.; Painset, A.; Balasegaram, S.; et al. Local Salmonella Enteritidis restaurant outbreak investigation in England provides further evidence for eggs as source in widespread international cluster, March to April 2023. Euro. Surveill. 2023, 28, 2300309. [Google Scholar] [CrossRef]
- Ayalew, H.; Zhang, H.; Wang, J.; Wu, S.; Qiu, K.; Qi, G.; Tekeste, A.; Wassie, T.; Chanie, D. Potential feed additives as antibiotic alternatives in broiler production. Front Vet. Sci. 2022, 9, 916473. [Google Scholar] [CrossRef]
- Parkhill, J. Antimicrobial resistance exchange between humans and animals: Why we need to know more. Engineering 2022, 15, 11–12. [Google Scholar] [CrossRef]
- Xie, T.; Wu, G.; He, X.; Lai, Z.; Zhang, H.; Zhao, J. Antimicrobial resistance and genetic diversity of Salmonella Enterica from eggs. Food Sci. Nutr. 2019, 7, 2847–2853. [Google Scholar] [CrossRef]
- Izumiya, H.; Pei, Y.; Terajima, J.; Ohnishi, M.; Hayashi, T.; Iyoda, S.; Watanabe, H. New system for multilocus variable-number tandem-repeat analysis of the enterohemorrhagic Escherichia coli strains belonging to three major serogroups: O157, O26, and O111. Microbiol. Immunol. 2010, 54, 569–577. [Google Scholar] [CrossRef]
- Vuthy, Y.; Lay, K.S.; Seiha, H.; Kerleguer, A.; Aidara-Kane, A. Antibiotic susceptibility and molecular characterization of resistance genes among Escherichia coli and among Salmonella subsp. in chicken food chains. Asian Pac. J. Trop. Biomed. 2017, 7, 670–674. [Google Scholar] [CrossRef]
- Rakitin, A.L.; Yushina, Y.K.; Zaiko, E.V.; Bataeva, D.S.; Kuznetsova, O.A.; Semenova, A.A.; Ermolaeva, S.A.; Beletskiy, A.V.; Kolganova, T.V.; Mardanov, A.V.; et al. Evaluation of antibiotic resistance of Salmonella serotypes and whole-genome sequencing of multiresistant strains isolated from food products in Russia. Antibiotics 2021, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Casaux, M.L.; D’Alessandro, B.; Vignoli, R.; Fraga, M. Phenotypic and genotypic survey of antibiotic resistance in Salmonella Enterica isolates from dairy farms in Uruguay. Front Vet. Sci. 2023, 10, 1055432. [Google Scholar] [CrossRef]
- Habib, I.; Khan, M.; Mohamed, M.-Y.I.; Ghazawi, A.; Abdalla, A.; Lakshmi, G.; Elbediwi, M.; Al Marzooqi, H.M.; Afifi, H.S.; Shehata, M.G.; et al. Assessing the prevalence and potential risks of Salmonella infection associated with fresh salad vegetable consumption in the United Arab Emirates. Foods 2023, 12, 3060. [Google Scholar] [CrossRef]
- Sinclair, M.; Lee, N.Y.P.; Hötzel, M.J.; de Luna, M.C.T.; Sharma, A.; Idris, M.; Islam, M.A.; Iyasere, O.S.; Navarro, G.; Ahmed, A.A.; et al. Consumer attitudes towards egg production systems and hen welfare across the world. Front Anim. Sci. 2022, 3, 995430. [Google Scholar] [CrossRef]
- Keerthirathne, T.P.; Ross, K.; Fallowfield, H.; Whiley, H. A review of temperature, pH, and other factors that influence the survival of Salmonella in mayonnaise and other raw egg products. Pathogens 2016, 5, 63. [Google Scholar] [CrossRef]
- Government of South Australia. Raw Egg Products. Available online: https://www.sahealth.sa.gov.au/wps/wcm/connect/public+content/sa+health+internet/conditions/food+safety/keeping+your+food+safe/egg+safety/raw+egg+products#:~:text=Products%20that%20contain%20raw%20egg&text=Examples%20of%20raw%20egg%20products,egg%20protein%20drinks%2C%20egg%20nog (accessed on 25 August 2023).
- US Department of Agriculture (USDA). Shell Eggs from Farm to Table. Available online: https://www.fsis.usda.gov/food-safety/safe-food-handling-and-preparation/eggs/shell-eggs-farm-table#:~:text=FSIS%20verifies%20shell%20eggs%20packed,the%20safe%20handling%20of%20eggs. (accessed on 26 September 2023).
- Food Standards Chile. Reglamento Sanitario de los Alimentos (RSA). 2019. Available online: https://www.minsal.cl/reglamento-sanitario-de-los-alimentos/ (accessed on 22 August 2023).
- Gargiulo, A.H.; Duarte, S.G.; Campos, G.Z.; Landgraf, M.; Franco, B.; Pinto, U.M. Food safety issues related to eating in and eating out. Microorganisms 2022, 10, 2118. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).