Abstract
Fermented vegetable-based foods, renowned for their unique flavors and human health benefits, contain probiotic organisms with reported in vitro antioxidative effects. This study investigates the probiotic properties of Latilactobacillus sakei MS103 (L. sakei MS103) and its antioxidant activities using an in vitro oxidative stress model based on the hydrogen peroxide (H2O2)-induced oxidative damage of RAW 264.7 cells. L. sakei MS103 exhibited tolerance to extreme conditions (bile salts, low pH, lysozyme, H2O2), antibiotic sensitivity, and auto-aggregation ability. Moreover, L. sakei MS103 co-aggregated with pathogenic Porphyromonas gingivalis cells, inhibited P. gingivalis-induced biofilm formation, and exhibited robust hydrophobic and electrostatic properties that enabled it to strongly bind to gingival epithelial cells and HT-29 cells for enhanced antioxidant effects. Additionally, L. sakei MS103 exhibited other antioxidant properties, including ion-chelating capability and the ability to effectively scavenge superoxide anion free radicals, hydroxyl, 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid, and 2,2-diphenyl-1-picrylhydrazyl. Furthermore, the addition of live or heat-killed L. sakei MS103 cells to H2O2-exposed RAW 264.7 cells alleviated oxidative stress, as reflected by reduced malondialdehyde levels, increased glutathione levels, and the up-regulated expression of four antioxidant-related genes (gshR2, gshR4, Gpx, and npx). These findings highlight L. sakei MS103 as a potential probiotic capable of inhibiting activities of P. gingivalis pathogenic bacteria and mitigating oxidative stress.
1. Introduction
Fermentation, a time-honored method of food preservation, profoundly influences both nutritional quality and flavor [1]. Across the globe, this technique is widely embraced to enhance the taste and maintain the nutritional value of vegetables. China, renowned for its culinary diversity, produces iconic products, such as Sichuan pickles and Suancai, that are crafted using unique regional methods. Various cultures have developed their own traditional vegetable pickles. Korea’s celebrated kimchi, for instance, involves the salting of cabbages and radishes, rinsing to remove excess salt, and fermentation of the vegetables in kimchi paste, while Nepal’s gundruk is fermented without added salt [2]. Turkey’s tursu is a beloved traditional pickle created by submerging vegetables in brine and allowing them to ferment at room temperature, which is a similar method to that used to prepare Chinese pickles (paocai).
Garlic, a global natural spice and ingredient of fermented foods, has been extensively studied for its diverse health benefits. These attributes include antimicrobial and anticancer properties, immune system-enhancing effects, antioxidant effects, and various other beneficial activities [3] that have stimulated the development of a broad array of garlic-based products, such as garlic oil, flakes, salt, and paste. Moreover, the addition of garlic during the pickling process not only prevents the growth of unwanted microorganisms but also encourages the proliferation of beneficial Lactiplantibacillus species [4]. In northeastern China, sweet pickled garlic, with its rich contents of protein, healthy fats, sugar, and other essential nutrients, is considered a wholesome delicacy [4,5,6,7,8]. The sweet pickled garlic is always eaten directly, which may be beneficial for preventing oral disease.
The Food and Agriculture Organization of the United Nations (FAO)/World Health Organization (WHO) defined probiotics as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host”. Lactic acid bacteria (LAB), a prominent group of probiotic organisms [2], were initially isolated from milk and have since been detected in many fermented products with ancient origins [9]. Due to their longstanding role in food preservation, LAB are currently viewed as generally recognized as safe (GRAS) when used for this purpose. Furthermore, LAB possess essential characteristics associated with enhanced food safety and shelf life. These benefits have been linked to their abilities to inhibit pathogen growth during antimicrobial compounds production, including diacetyl, hydrogen peroxide and others. LAB also compete with pathogens for nutrients, further contributing to food safety [10,11].
LAB and its fermentates provide health benefits in several studies. For example, Fasano and Budelli reported that LAB fermentates improved both gut health and overall health, while studies conducted by a group of Food for Health Ireland (FHI) researchers revealed that the consumption of LAB fermentates of reconstituted skim milk (RSM) curbed weight gain by regulating appetite. In an independent study, Casey and colleagues demonstrated that using a mixture of five LAB strains isolated from milk fermentation on pigs infected with Salmonella serovar Typhimurium resulted in a significant reduction in Salmonella counts in the pigs’ feces, contributing to the overall enhanced health of the animals. Furthermore, LAB fermentates have been shown to mitigate the effects of food-borne pathogens, as reported by Morgan and colleagues [12].
It is worth noting that the health impacts of LAB also extend to oral health, where they may exert harmful effects by contributing to the development of oral disorders, such as dental caries and periodontitis [13]. P. gingivalis is a common pathogen, which was considered associated with periodontal diseases [14]. Given the multifaceted benefits of LAB, understanding their specific characteristics and functions is essential when exploring their various potential applications in promoting health—especially, the LAB effect on the P. gingivalis with biofilm formation.
Oxidative stress, driven by reactive oxygen species (ROS) overproduction in oxidative chemical processes, can irreversibly damage cells and tissues. Several diseases including heart disease, cancer and so on were closely associated with ROS damage phenomena [15,16,17]. Recent evidence from in vivo and in vitro studies has demonstrated significant antioxidant capabilities of probiotic bacteria linked to valuable health benefits. In particular, certain strains of lactobacilli and bifidobacteria have gained recognition for their impressive antioxidative properties, which is a crucial factor in strengthening the body’s antioxidant defenses and mitigating oxidative stress [18,19].
Within the gut environment, probiotic bacteria exert their antioxidant effects by releasing antioxidant molecules, secreting antioxidant enzymes, directly scavenging ROS, and chelating iron to prevent iron-catalyzed oxidation [20,21]. As a result, the use of probiotics with their antioxidant abilities holds promise as a reliable approach to mitigating the damage caused by oxidative stress.
Latilactobacillus sakei, a Gram-positive lactic acid bacillus, was initially isolated from Japanese sake in 1934 by Katagiti et al. It has since been found in other foods, including meat products and various plant-derived foods, such as fermented cabbage. In particular, L. sakei isolated from kimchi has been associated with various potential health-related benefits, including the alleviation of obesity, inflammatory bowel disease, and atopic dermatitis [22].
In 2021, genomic information was obtained for 56 diverse L. sakei strains, revealing several LAB genes associated with antioxidative capabilities and potential health benefits [23,24]. In our preliminary study, we demonstrated that L. sakei MS103 isolated from a traditional Chinese fermented food (sweet pickle garlic) possessed potent abilities in scavenging 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2’-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid (ABTS) radicals (Table S1). This finding led us to speculate that L. sakei MS103 may possess antioxidative properties capable of counteracting the harmful effects of pathogenic bacteria.
Here, we investigated the potential probiotic properties of L. sakei MS103 in vitro, including environmental tolerance, surface characteristics, antioxidative activities, antibiotic resistance, and beneficial effects on P. gingivalis biofilm formation. L. sakei MS103 antioxidant activities were assessed using the H2O2-Induced Oxidative Damage RAW 264.7 Cell Model. Our preliminary research results highlight the significant observed antioxidant benefits of L. sakei MS103 that justify its further evaluation as a potential health-enhancing ingredient for incorporation in dietary supplements, cosmetics, pharmaceuticals, and nutraceuticals.
2. Materials and Methods
2.1. Latilactobacillus sakei MS103 Strains and Sample Preparation
Latilactobacillus sakei MS103 (L. sakei MS103) was initially isolated from sweet pickled garlic, which is a traditional Chinese fermented food product. The L. sakei MS103 used in our experiment was deposited with the No. 21,362 at the China General Microbiological Culture Collection Center (CGMCC, Beijing, China). The de Man, Rogosa and Sharpe (MRS) broth (Hopebio Co., Qingdao, China) was used to inoculate the L. sakei MS103 (3%, v/v). The L. sakei MS103 was cultured for 16 h with 37 °C temperature in an anaerobic incubator. The anaerobic incubator was under the same conditions (85% N2, 10% H2, and 5% CO2) in all experiments. For cells and strains’ incubation, without special explanation, the temperature was maintained at 37 °C. After centrifuging at 1500× g for 10 min at a temperature of 4 °C, the L. sakei MS103 were collected. The phosphate-buffered saline (PBS) was used to wash the pellet three times. Before conducting any further experiments, the L. sakei MS103 was adjusted to 9 Log CFU/mL, 8 Log CFU/mL, or 7 Log CFU/mL with the PBS buffer. For the L. sakei MS103 culture medium group, the L. sakei MS103 was collected with medium and adjusted to the suitable concentration. For the live L. sakei MS103, the L. sakei MS103 culture medium was centrifuged for 10 min at the temperature of 4 °C (1500× g). Then, the bacterial supernatant was collected by removing the bacterial cell using a 0.22 M membrane filter [17].
To prepare heat-killed L. sakei MS103, bacterial cells were collected after culture, centrifuged, and washed with PBS, as described above, and then incubated at 100 °C for 15 min. Thereafter, the heat-killed cells were centrifuged at 4 °C for 10 min with 1500× g. After removing the supernatant, the cells were adjusted to 9 Log CFU/mL, 8 Log CFU/mL, or 7 Log CFU/mL in DMEM prior to use in experiments.
Porphyromonas gingivalis (P. gingivalis) (ATCC 3327), which was obtained from the CGMCC, was propagated on Columbia Blood Agar (Hopebio Co.) with the temperature of 37 °C for 48 h in an anaerobic incubator condition. After culture, P. gingivalis cells were resuspended in Brain–Heart Infusion (BHI) broth (3% v/v, Hopebio Co.) and incubated at the temperature of 37 °C for an additional 48 h.
From Otwo Biotech (Shenzhen, China), three kinds of cells, RAW 264.7 (mouse mononuclear macrophage-derived) cells, human gingival epithelial cells (HGE) and human colorectal adenocarcinoma-derived cells (HT-29), were obtained. DMEM (HyClone, Logan, UT, USA) was used to culture the cells with 10% (v/v) fetal bovine serum (FBS; HyClone). In DMEM culture medium,100 U/mL penicillin/streptomycin (Sigma-Aldrich, St. Louis, MO, USA) was added. All cells were cultured at the temperature of 37 °C in an incubator. Then, every other day, the fresh medium was used to replace the spent medium; on intervening days, cells were subcultured by removing spent medium from culture flasks and adding 0.25% trypsin–EDTA solution (Sigma-Aldrich), tapping the flasks to dislodge adherent cells from flask walls, and then diluting the resuspended cells 1:3 (by volume) in fresh medium and transferring cell suspensions to new flasks.
2.2. Physiological Functional Properties of L. sakei MS103
2.2.1. L. sakei MS103 Tolerance to Pepsin, Bile, Lysozyme, and H2O2
L. sakei MS103 16 h cultures were cultured with pepsin (0.3% (w/v)) of MRS broth at pH 2.0, pH 3.0 and pH 5.0. Different concentrations with bovine bile (0.3% (w/v) and 0.5%(w/v)) and MRS broth were used. Lysozymes (100 μg/mL or 200 μg/mL) were added into the MRS broth. MRS broths with 0.08 mM H2O2 or 0.8 mM H2O2 were prepared. Then, cultures were incubated under aerobic conditions for 20 h (37 °C). During the culture period, every 2 h until 20 h, a UV-VIS spectrophotometer (UV-2700, Shimadzu, Kyoto, Japan) were used to measure the optical density (OD) values at 600 nm.
2.2.2. L. sakei MS103 Auto-Aggregation Activity
L. sakei MS103 16 h cultures were centrifuged at 4 °C for10 min with 1500× g. The PBS at pH 7.0 was used to wash the pellet 3 times. Then, the pellet was resuspended in PBS buffer, and we adjusted the OD600 values to 1.0. Next, the suspension of OD600 values was measured every 2 h after being incubated at 37 °C until reaching 8 h [25,26].
The auto-aggregation ability of L. sakei MS103 was calculated as:
where AT denotes the OD600 value every 2 h until 8 h, and A0 denotes the OD600 value at 0 h.
2.2.3. L. sakei MS103 Co-Aggregation Activity
L. sakei MS103 was cultured for 18 h, and P. gingivalis cells were harvested for 24 h. Then, cells were resuspended in sterile PBS at pH 7.0 after being washed three times. The L. sakei MS103 concentration was adjusted to 0.50 by OD600 values; the P. gingivalis concentration was adjusted to 0.60 at OD600. Then, 2 mL of L. sakei MS103 and 2 mL of P. gingivalis suspensions were shaken for 5 min (200 r/min). Afterwards, without shaking, the suspensions were incubated for 8 h at the temperature of 37 °C. The OD600 values were measured every 2 h until incubation 8 h [27]. The co-aggregation ability was calculated as follows:
where Ax denotes OD600 values of L. sakei MS103 at 0 h, Ay denotes OD600 values of P. gingivalis at 0 h, and Amix denotes the OD600 value of the mixture for both strains after incubation every 2 h.
2.2.4. L. sakei MS103 Surface Hydrophobicity Characteristics
The microbial adhesion to hydrocarbons (MATH) method was used to determine the surface hydrophobicity of L. sakei MS103 in the hydrophobic organic solvent xylene, while surface charge characteristics were assessed using chloroform and ethyl acetate as the Lewis acid and Lewis base, respectively [28]. L. sakei MS103 was prepared using the abovementioned method. First, 0.1 mol/L KNO3 buffer at pH 6.2 was used to resuspend the strain pellet, which was adjusted to an OD600 absorbance value of 0.60. For the surface hydrophobicity assay, 1 mL of xylene, ethyl acetate, or chloroform was added to L. sakei MS103 (3 mL) suspension separately. For preincubating, the two-phase mixture was kept at room temperature (10 min) and vortexed (2 min). Then, the mixture was incubated at room temperature (30 min). Thereafter, the aqueous phase absorbance at OD600 was observed for use in calculating the cell surface hydrophobicity as based on the adherence of L. sakei MS103 to hydrocarbons as follows:
where Ax and Ay denote the OD600 value before and after the organic solvent treatment, respectively.
2.2.5. Adhesion of L. sakei MS103 to HT-29 and HGE Cells
Both HT-29 and HGE cells were used to study the adhesion of L. sakei MS103 to host cells. First, 1 × 105 cells/well of HT-29 and HGE cells were transferred into a tissue culture plate (12 wells). Then, with the cells adhered to the plate well and about 70–80% cell confluence, fresh high-glucose DMEM medium (high glucose) was used to wash and replace the medium in the well. The plates were incubated at a temperature of 37 °C for 24 h. Then, PBS buffer was used to wash the monolayers of cells for three times. For each well, the cell concentrations with 1 × 108 CFU/mL were adjusted by adding 500 μL of L. sakei MS103. After incubating the plate for 2 h (37 °C), each well had PBS added and was washed gently three times, after which non-adherent bacteria were removed. Then, we added 2 mL of 1% (v/v) Triton X-100 (Sigma-Aldrich, USA) to each well and incubated them for 10 min. The adherent cells detached from the well surfaces [29]. The viable bacteria were counted by plate-counting method in wells. The L. sakei MS103 adhesion ability was calculated using the following equation:
where Vx and Vy denote the L. sakei MS103 cells of the total adherent count and the initial added number, respectively.
2.2.6. Antibiotic Resistance
The disk diffusion method was used to detect the antibiotic susceptibility of L. sakei MS103 [30,31]. L. sakei MS103 with 8 Log CFU/mL for 1 mL was spread on the MRS agar-solidified plated surface evenly. Next, the plates were incubated for 48 h at the temperature of 37 °C with the antibiotic-containing disks placed. Vernier calipers (Aladdin Biochemical Technology Ltd., Shanghai, China; minimum resolution = 0.02 mm) were used to measure the diameter for the inhibition zone from the disk. Then, the results were expressed as susceptible (S), intermediate (I), or resistant (R) as specified, following antibiotic discs instruction (BKMAM, Changde, China). All analyses were repeat three times.
2.3. Antibacterial Activity of L. sakei MS103 as Based on Inhibition of P. gingivalis Biofilm Formation
P. gingivalis cultures were adjusted to 6 Log CFU/mL with BHI broth after being incubated (37 °C) for 48 h. After L. sakei MS103 was cultured for 16 h, the strains with medium were collected directly without centrifuging to form the culture medium group. Next, the 2 transwell 24-well plates (0.4 μm, Corning, NY, USA) were used for culturing the L. sakei MS103 and P. gingivalis cells. The L. sakei MS103 included the culture group and supernatant group. The L. sakei MS103 and P. gingivalis cells of 1:1 (v/v) were added to the transwell plate. The L. sakei MS103 cells were added into the upper chambers, while the P. gingivalis cells were added into lower chambers. After the transwell plates were incubated for 48 h, culture supernatants within lower chambers were removed and discarded gently. Next, PBS was gently pipetted into the bottom chambers, which were then gently aspirated to remove planktonic P. gingivalis cells from the biofilm at the bottom of each chamber. Thereafter, in the lower chamber, 2.5% (v/v) glutaraldehyde solution was used to fix the P. gingivalis biofilm at 4 °C overnight. Then, the chamber was dried at room temperature. Next, staining at room temperature in each lower transwell chamber proceeded by using 100 μL of 0.1% (w/v) crystal violet for 15 min (room temperature). Afterwards, 75% ethanol was used to remove the unbound crystal violet of the stained biofilm. Then, the chamber was allowed to dry at room temperature [32].
A phase contrast fluorescence microscope (IX73, Olympus, Kyoto, Japan) was used to observe the P. gingivalis biofilm structure. A microplate reader was used to measure the biofilm absorbance at 540 nm. The biofilm inhibition rate of L. sakei MS103 to P. gingivalis was calculated as:
where AS and AC represent the OD540 value of the P. gingivalis biofilm in the absence and presence of treated L. sakei MS103, respectively.
2.4. Antioxidant Activity In Vitro
Live and heat-killed L. sakei MS103 were prepared as described in Section 2.1. The antioxidant activities of 1 × 108 CFU/mL live and heat-killed L. sakei MS103 in culture medium were measured for all groups. For the culture medium group, the L. sakei MS103 strains were collected with medium to adjust the concentration without centrifuge.
2.4.1. DPPH Free Radical-Scavenging Activity (RSA)
A DPPH Free Radical Scavenging Capacity Assay Kit (BC4750, Solarbio, Beijing, China) was used to measure the scavenging of DPPH radicals. L. sakei MS103 was cultured, collected, and then washed with PBS at pH 7.0 twice. The L. sakei MS103 concentration was adjusted to 1 × 108 CFU/mL, and then L. sakei MS103 was mixed with 100% ethanolic DPPH solution (0.2 mM) in a 1:2 ratio (v/v). After the mixture was incubated at 25 °C in the dark for 30 min, it was centrifuged for 10 min at 6000× g. The same volume of deionized water was added in the control group instead of the bacterial sample. For the blank group, the DPPH radical solution were replaced by ethanol with the same volume. The absorbance at 517 nm was measured.
DPPH-free RSA was calculated as follows:
where As, Ac and Ab denote the absorbance of the test sample, control group and blank group, respectively.
2.4.2. ABTS Radical Scavenging Assay (ABTS-RSA)
The decreasing of absorbance at 405 nm shows the ability of an antioxidant to scavenge the ABTS radical cation (ABTS•+). The ABTS radical-scavenging activity was assessed using an ABTS Radical Scavenging Assay Kit (BC4770, Solarbio).
The probiotic ABTS-RSA (%) was calculated as:
where As, Ac and Ab denote the absorbance of the test sample, control group and blank group, respectively.
2.4.3. Hydroxyl Radical Scavenging Assay
The hydroxyl free radical-scavenging activity was measured using a Hydroxyl Free Radical Scavenging Capacity Assay Kit (BC1329, BC4770, Solarbio). Briefly, the sample suspension (0.5 mL) was mixed with PBS (1 mL), phenanthroline (2.5 mL, 2.5 mM), FeSO4 (0.5 mL, 2.5 mM), and H2O2 (0.5 mL, 2.5 mM). Then, the mixture was incubated for 1 h with the temperature at 37 °C. Then, the mixture was centrifugated for 10 min (6000× g), and the absorbance at 536 nm was observed.
The hydroxyl free radical-scavenging activity was calculated as follows:
where As, Ac and Ab denote the absorbance of the test sample, the control group without the sample and the blank group (without the sample and H2O2), respectively.
2.4.4. Superoxide Anion Radical Scavenging Assay
The superoxide anion radical scavenging assay was conducted using a previously reported method [33,34]. First, 3.0 mL of Tris-HCl solution at pH 8.2 was mixed with 1.0 mL of sample and incubated for 20 min under 25 °C. Next, the mixture was incubated for 4 min (room temperature) after 0.4 mL of pyrogallol (25 mM) was added. Thereafter, to stop the reaction, HCl (0.5 mL) was added. The absorbance at 325 nm was observed. The control group contained an equivalent volume of deionized water instead of sample. The superoxide anion radical scavenging of the L. sakei MS103 was calculated as:
where AS and AC denote the absorbance at 325 nm for the test sample and control, respectively.
2.4.5. Ferrous Ion-Chelating (FIC) Assay
First, 1 mL of L. sakei MS103 suspensions at different cell concentrations was mixed with FeCl2 solution (0.05 mL, 2 mM) for 5 min. Next, phenanthroline (0.2 mL, 5 mM) was added to each sample. Thereafter, 2.75 mL of distilled water was added to each sample; then, each sample was gently mixed for 10 min. Next, each sample was centrifuged at 2300× g for 10 min at 4 °C; then, the absorbances of samples were measured at 562 nm. For the control group, the sample (1 mL) and distilled water (3 mL) was mixed. For the blank group, 1 mL of water (instead of sample) was mixed with 0.05 mL FeCl2 (2 mM) solution, 0.2 mL of phenanthroline (5 mM) and 2.75 mL of distilled water. The ferrous ion-chelating (FIC) ability was calculated as:
where As, Ac and Ab denote the absorbance of the test sample, the control group and the blank group, respectively.
2.5. Antioxidant Properties of L. sakei MS103 as Assessed Using the H2O2-Induced Oxidative Damage RAW 264.7 Cell Model
2.5.1. Cytotoxicity Text
As a reported method, a Cell Counting Kit-8 (CCK-8) assay (APExBIO, Houston, TX, USA) was used to observed the cytotoxicity of L. sakei MS103 against RAW 264.7 cells [35]. Briefly, 200 µL of a cell suspension of RAW 264.7 cells at a density of 5 × 104/mL was added to individual wells of 96-well plates. After the adherent RAW 264.7 cells confluence reached 70–80% at 1 × 106 cells/well, the live or heat-killed L. sakei MS103 cells at 0 Log CFU/mL, 7 Log CFU/mL, 8 Log CFU/mL, and 9 Log CFU/mL concentration were added to wells independently. After incubating the plate for 6 h, 300 μL of CCK-8 working reagent was added after the culture medium was removed. The multi-function microplate reader (F200 Pro, Tecan Infinite, Männedorf, Switzerland) was used to observe the OD identity at 450 nm for each well after the plates had been incubating for 4 h.
Cell viability was calculated as:
where AS and AC denote the OD450 absorbance of RAW264.7 cells co-incubated with L. sakei MS103 cells and without L. sakei MS103 cells, respectively.
2.5.2. Assays for Measuring Glutathione (GSH) and Malondialdehyde (MDA) Levels
First, 5 × 104 cells were added to individual wells of 96-well plates in 200 µL of medium. Then, the plates were incubated under standard culture conditions until the cells grew to 80–90% confluence. Next, supernatants were discarded, using PBS to wash the cells; then, 200 μL of DMEM containing 0.1 mmol/L H2O2 was added to wells to generate the H2O2-Induced Oxidative Damage RAW 264.7 Cell Model. Thereafter, live or heat-killed L. sakei MS103 cells were co-incubated with H2O2-exposed RAW 264.7 cells for 6 h. Concurrently, controls that included the blank (DMEM alone) and H2O2-damage positive control (DMEM containing 0.1 mmol/L H2O2) groups were incubated in the same way as the sample group. Concentrations of GSH and MDA were selected according to the instructions provided with the GSH assay kit (BC1175, Solarbio, Beijing, China) and MDA assay kit (BC0020, Solarbio). For GSH and MDA, the absorbance was measured at 412 nm and 532 nm with a spectrometer, respectively.
2.6. RNA Extraction and gshR4, Gpx, npx, and gshR2 Gene Expression
After RAW 264.7 cells were co-cultured with either live L. sakei MS103 or heat-killed L. sakei MS103 in medium including 0.l mM H2O2, treated RAW 264.7 cells were transferred to centrifuged tubes for RNA extraction. The total RNA was extracted from cells with the RNA Extraction Kit (TaKaRa 9767, Kyoto, Japan). The reverse transcription of total RNA (approximately 0.5 μg) into cDNA was completed by the Primescript™ RT reagent Kit (TaKaRa) [36,37]. The RNA subjected for reverse transcription are listed in Table S2. The relative transcriptional-level expression of gshR4, Gpx, npx, and gshR2 were determined by cDNA, which served as a template. Real-time -PCR was performed by a TB Green® Premix Ex Taq™ II Kit (TaKaRa, RR820A). The β-actin were served and normalized as the reference gene. The qRT-PCR results were calculated by the 2−ΔΔCt method. The specificity of the reaction was evaluated by the melting curve analysis. In Table 1, the primer sequences are listed.

Table 1.
Primer sequences.
2.7. Statistical Analysis
Statistical calculations and computational data analysis were performed using GraphPad Prism 8.0 software (GraphPad Software Inc., San Diego, CA, USA). All data are expressed as the mean ± SD. Comparisons between groups were conducted using t-tests. One-way Analysis of Variance (ANOVA) was conducted to assess the statistical significance of differences in results obtained between groups receiving different treatments [38]. Values of p < 0.05 were considered statistically significant.
3. Results and Discussion
3.1. Characterization of L. sakei MS103
Probiotic bacterial strains play a crucial role in promoting health primarily by exerting clinically relevant effects within the gastrointestinal tract. To attain probiotic status, these strains must tolerate extreme conditions while passing through the stomach and other harsh environments within the human body.
In Figure 1A, we present the results of experiments conducted to assess L. sakei MS103 survival and proliferation during exposure to 0.3% pepsin at pH < 5.0. The results demonstrate that L. sakei MS103 survived and showed continued growth with these conditions. Similarly, when cultured in bile acids (0.3% and 0.5% ox gall, w/v), L. sakei MS103 maintained high growth activity throughout the 20-h period. However, when exposed to 0.3% pepsin at pH 2.0 or 3.0, the L. sakei MS103 exhibited slower growth and only survived for approximately 10 h.
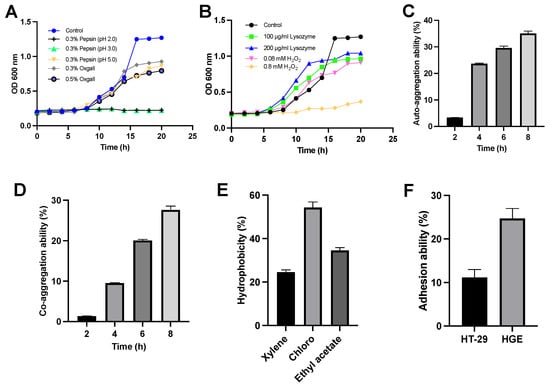
Figure 1.
Characterization of L. sakei MS103 (A): growth curves of L. sakei MS103 exposed to different MRS broth for 20 h. The control group was only MRS broth. The MRS broth contained 0.3% pepsin at pH 2.0, pH 3.0, pH 5.0, 0.3% oxgall and 0.5% oxgall, individually. (B) Growth curves of L. sakei MS103 at different MRS broth for 20 h. The MRS broth contained 100 μg/mL lysozyme, 200 μg/mL lysozyme, 0.08 mM H2O2 and 0.8 mM H2O2, individually. (C) The L. sakei MS103 auto-aggregation ability. (D) The L. sakei MS103 co-aggregation ability. (E) The L. sakei MS103 surface hydrophobicity. (F) The L. sakei MS103 adhesion ability to HT-29 and HE cells. Values represent the mean ± SEM (n = 3).
When exposed to H2O2 or lysozyme, L. sakei MS103 cells demonstrated tolerance to 0.08 mM H2O2 and 200 g/mL lysozyme, maintaining steady growth throughout the 20 h period of exposure (Figure 1B). However, when exposed to 0.8 mM H2O2 for 20 h, L. sakei MS103 cells exhibited slower growth. However, surviving L. sakei MS103 cells were detected, indicating some degree of tolerance to H2O2, which is a weak oxidant known to cause oxidative damage. This result suggests that L. sakei MS103 possesses antioxidant abilities, which is a trait observed in other probiotics as well. For instance, studies by Xie et al. observed strong Lactiplantibacillus plantarum GXL94 resistance to H2O2, indicating antioxidant activity [39]. Similarly, research by Angmo’s group and Chul’s group revealed that certain LAB strains isolated from fermented foods survived exposure to low pH, bile salts, and lysozyme [40,41]. Based on these results, we speculate that L. sakei MS103 possesses antioxidant activity, enabling this strain to survive extreme environmental conditions.
Importantly, the auto-aggregate tendency was closely related to the adherence to the host cells of LAB. Here, L. sakei MS103 cells exhibited a 3.3% auto-aggregation rate in the first 2 h, which increased to 35% after 8 h (Figure 1C). When L. sakei MS103 was mixed with P. gingivalis, its co-aggregation rate at 2 h was low (1.3%); then, it gradually rose and reached a peak of 27.6% at 8 h (Figure 1D), indicating the agglutination of L. sakei MS103 with P. gingivalis. Notably, LAB strains with strong auto-aggregation abilities can efficiently colonize mucosal surfaces, enhancing their probiotic functions relative to those of strains lacking this ability [42]. Additionally, probiotics with co-aggregation abilities can produce antimicrobial substances that can inhibit activities near targeted organisms [40].
In this study, P. gingivalis served as a model pathogen that was used to assess L. sakei MS103 cells’ co-aggregation ability. Importantly, there was a progressive enhancement in L. sakei MS103 cells’ auto-aggregation capability with increasing incubation time along with robust L. sakei MS103 cells’ co-aggregation capability when co-incubated with P. gingivalis, underscoring the potential of this probiotic to serve as a treatment for combating bacterial infections, warranting further study.
Cell surface hydrophobicity is one of several key factors involved in non-specific cellular adhesion [43]. Bacterial colonization, which reflects cell surface hydrophobicity, is influenced by interactions between LAB and the environment or host. The results obtained in our study (Figure 1E) revealed a hydrophobicity value for L. sakei MS103 in xylene of 24.57%, indicating significant hydrophobicity. Changes in the L. sakei MS103 surface hydrophobicity were noted when it was exposed to acidic solvent (chloroform) and alkaline solvent (ethyl acetate), as reflected by probiotic hydrophobicity rates in chloroform and ethyl acetate of 54.31% and 34.53%, respectively. These results suggest the presence of numerous charges on L. sakei MS103 cell surfaces that exert electrostatic forces. Importantly, L. sakei MS103 exhibited a higher affinity for chloroform than for ethyl acetate, which was consistent with the published results for other LAB and Latilactobacillus strains [44,45].
Adhesion is a crucial property that shields probiotics from immediate elimination, granting them a competitive edge [31,46]. In this study, we found that an adhesion rate of L. sakei MS103 was 11.15% to HT-29 cells and 24.7% to HGE cells. This demonstrates that L. sakei MS103 has a higher adhesion ability to HGE cells compared to HT-29 cells. Pathogenic bacteria and viruses often rely on adhesion to host tissues to exert their pathogenic effects [47]. Therefore, Lactiplantibacillus strains with strong adhesion abilities to the same tissues may inhibit pathogen invasion and host cell-binding activities effectively [48,49]. Based on our results (Figure 1F), L. sakei MS103 shows promise as a potential treatment for oral diseases caused by bacterial strains with high adhesion to HGE cells.
Before combining antibiotic therapy with a probiotic, it is essential to assess the probiotic’s antibiotic sensitivity. This ensures that antibiotics do not inactivate the probiotic organisms and that antibiotic genes within the probiotic are not horizontally transferred to other bacterial species or disseminated to the environment. Therefore, the antibiotic sensitivity of a probiotic is a crucial factor that should be considered before administering it to patients.
In this study, the agar diffusion method was used to assess the sensitivity of L. sakei MS103 to 18 antibiotics following the Clinical and Laboratory Standards Institute (CLSI) guidelines (2020) [50]. The results in Table 2 indicate that L. sakei MS103 is sensitive to minocycline, gentamicin, polymyxin B, ceftazidime, and vancomycin, resistant to tetracycline, piperacillin, ampicillin, chloramphenicol, rifampicin, cefazolin, ceftazidime, and ceftriaxone, and moderately sensitive to erythromycin, doxycycline, streptomycin, and cefoperazone.

Table 2.
L. sakei MS103 antibiotic sensitive ability.
Notably, Özden et al. reported that a probiotic L. sakei strain isolated from fermented Turkish sausage exhibited sensitivity to vancomycin and gentamicin, which was consistent with our results for L. sakei MS103 [51]. Other research groups have reported that numerous Limosilactobacillus and Lactiplantibacillus strains are resistant to ceftriaxone and cefazolin, which is in alignment with our findings for L. sakei MS103 [52]. While strains from different sources may possess varying antibiotic susceptibility profiles, our results suggest that L. sakei MS103 exhibits suitable antibiotic sensitivity for use as a probiotic.
3.2. Antibacterial Activity of L. sakei MS103 against P. gingivalis Biofilm Formation
P. gingivalis is a key pathogen that participates in the formation of mature dental plaque. It can form biofilms along with various pathogenic and non-pathogenic bacterial species, contributing to the development of periodontal disease. Investigating biofilm formation by P. gingivalis in the presence of a probiotic can provide valuable insights for preventing periodontitis.
In our experiments, we observed crystal violet-stained P. gingivalis biofilms under a microscope. Highly structured and irregular biofilm structures containing numerous P. gingivalis organisms were observed in the control group with P. gingivalis cultured 48 h along with several mushroom-like microcolonies (Figure 2A).
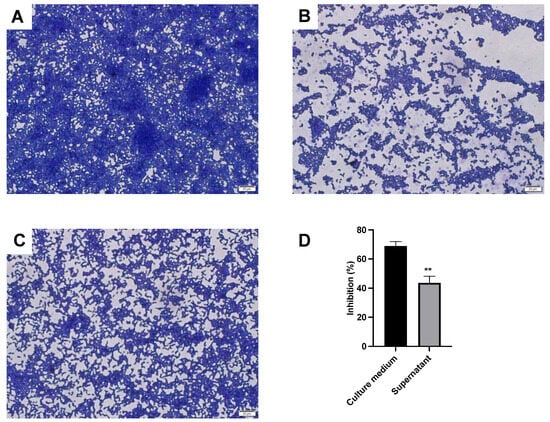
Figure 2.
The L. sakei MS103-treated P. gingivalis biofilm. After being cultured for 48 h, the P. gingivalis biofilm was stained with crystal violet. (A) Control group without L. sakei MS103. (B) L. sakei MS103 culture medium treated for P. gingivalis. (C) L. sakei MS103 supernatant treated for P. gingivalis. (D) Inhibition rate of L. sakei MS103 on biofilm formation of P. gingivalis. Values represent the mean ± SEM (n = 3). ** p < 0.01.
In contrast, P. gingivalis biofilm formation was significant inhibited by adding L. sakei MS103 culture medium, resulting in the formation of biofilm consisting of only a single layer of P. gingivalis cells with a loose structure lacking membranous characteristics (Figure 2B). When P. gingivalis was treated with cell-free L. sakei MS103 supernatant, which was an unevenly distributed biofilm formed that contained large gaps in its structure and loosely arranged P. gingivalis cells accompanied by smaller mushroom-shaped microcolonies (Figure 2C). Furthermore, the addition of L. sakei MS103 culture medium or bacteria-free culture supernatant to P. gingivalis cultures inhibited biofilm formation by 65.29% and 38.62%, respectively (Figure 2D). In culture medium groups, after L. sakei MS103 was cultured for 16 h, the strains with medium were collected directly without centrifuging. These results indicate that the L. sakei MS103 culture medium significantly outperformed cell-free L. sakei MS103 supernatant in inhibiting P. gingivalis biofilm formation.
Numerous probiotics have been reported to interfere with biofilm formation by oral pathogens, aligning with our findings. Consequently, the screening of probiotic candidates for capabilities in inhibiting activities of human oral bacteria may be a valuable approach for preventing dental caries and other oral diseases.
Notably, Lactiplantibacillus strains have been reported to effectively prevent biofilm formation by Salmonella spp. [53]. Additionally, lipoteichoic acids produced by lactobacilli have been shown to inhibit E. faecalis biofilm formation and disrupt preformed biofilm structures [54,55]. Therefore, with inhibiting the biofilm formation of P. gingivalis, the L. sakei MS103 provides an advantage for making some oral health products in the future. Our results imply that L. sakei MS103 possesses probiotic characteristics that may enable it to inhibit biofilm formation by pathogenic bacteria.
3.3. Results of In Vitro Assays of Antioxidant Activity Induced by L. sakei MS103 Treatment
In biological systems, DNA can be damaged by ROS such as superoxide and hydroxyl radicals. To mitigate these effects, probiotics with antioxidative activities have been developed to safeguard cells from oxidative damage. This study assesses L. sakei MS103 antioxidative activities, including ferrous ion-chelating (FIC) ability and L. sakei MS103 activities related to the scavenging of the superoxide anion, DPPH, hydroxyl radical free radicals, and ABTS, by comparing activities of L. sakei MS103 culture medium, live L. sakei MS103, and heat-killed L. sakei MS103 to controls (Figure 3).
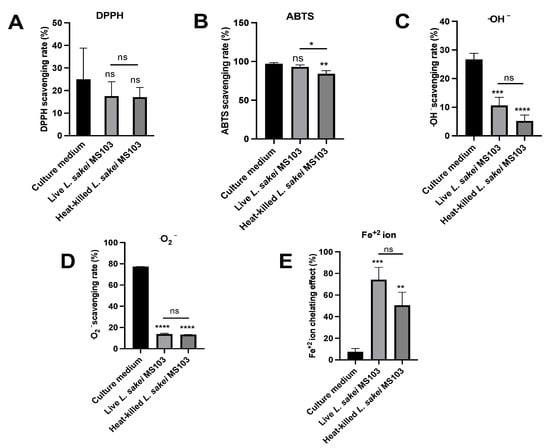
Figure 3.
Antioxidant activity induced by L. sakei MS103 treatment in culture medium, live L. sakei MS103 and heat-killed L. sakei MS103. (A) DPPH-free RSA. (B) ABTS-RSA. (C) Hydroxyl radical RSA. (D) Superoxide anion scavenging activities. (E) Fe2+-chelating capacity. Values represent the mean ± SEM (n = 3). * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
DPPH radical scavenging assays are commonly employed to assess antioxidant activities that are primarily related to hydrogen-donating capabilities. In Figure 3A, it is evident that the live L. sakei MS103 group (39.15%) exhibited higher DPPH radical-scavenging activity as compared that of the L. sakei MS103 culture medium group (24.96%) and heat-killed L. sakei MS103 group (17.01%).
In contrast, when assessing the total antioxidant capacities based on ABTS radical-scavenging ability, all three groups, L. sakei MS103 culture medium (97.16%), live L. sakei MS103 (93.06%), and heat-killed L. sakei MS103 (87.39%), exhibited excellent ABTS radical-scavenging abilities (Figure 4). These results collectively indicate that L. sakei MS103 possesses robust antioxidant capacity that is comparable to that reported for L. sakei KU15041 and Latilactobacillus curvatus KU15003 [56].
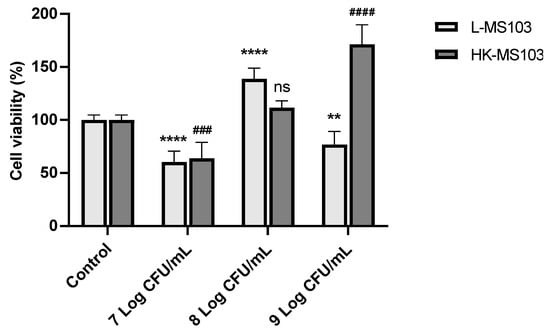
Figure 4.
Effect of L. sakei MS103 on the cell viability of RAW264.7 cells. Values represent the mean ± SEM (n = 3). ** p < 0.01, **** p < 0.0001, live L. sakei MS103 compared to control group; ### p < 0.001, #### p <0.0001, heat-killed L. sakei MS103 compared to control group.
Primary ROS generated during cellular metabolism typically include superoxide anion (•O2−) and hydroxyl radical (•OH), among other types of free radicals. Among these, (•OH) poses a substantial threat to biological macromolecules, resulting in adverse effects on cell health. Therefore, (•OH)-scavenging capacity, an indicator of antioxidant activity, is a crucial factor to consider when selecting probiotics with resistance to ROS-induced damage.
In this study, we assessed the (•OH)-scavenging capacities of the L. sakei MS103 culture medium, live L. sakei MS103, and heat-killed L. sakei MS103 (Figure 3C). Our findings demonstrated that the L. sakei MS103 culture medium exhibited the highest (•OH)-scavenging capacity (26.69%), which was followed by live L. sakei MS103 cells (10.62%). Heat-killed L. sakei MS103 cells displayed the lowest overall (•OH) scavenging capacity (5.20%). Notably, these findings are consistent with results reported for other LAB strains showing that both live and heat-killed LAB possess (•OH)-scavenging capacity [57].
Another significant ROS, superoxide anion (•O2−), can lead to oxidative stress and biomolecular damage and thus was included in our analysis of L. sakei MS103 superoxide anion-scavenging abilities. Our findings revealed that both live and heat-killed L. sakei MS103 had similar (•O2−)-scavenging capacities of 13.77% and 13.27%, respectively (Figure 3D). Of note, the L. sakei MS103 culture medium exhibited significantly higher (•O2−)-scavenging ability (77.26%). In summary, our results demonstrate that L. sakei MS103 possesses remarkable superoxide anion scavenging capacity that is likely related to substances present on L. sakei MS103 cell surfaces.
Metal-chelating assays, particularly those used to assess Fe2+-chelating capacity [58], are valuable for understanding antioxidant mechanisms and mitigating Fenton reaction-induced free radical formation. Notably, Fe2+ chelation is an effective strategy for protecting cells from oxidative damage [59]. In this study, we found that live L. sakei MS103 exhibited an Fe2+-chelating capacity of 74.11%, while heat-killed L. sakei MS103 had a slightly lower capacity of 50.46%. Notably, the L. sakei MS103 culture medium displayed the lowest Fe2+-chelating capacity (7.57% (Figure 3E)). Previous studies have suggested that the strong (•OH)-scavenging activities of LAB strains may be linked to their abilities to bind to metal ions such as Fe2+ [60,61]. Additionally, some LAB species have shown antioxidant activities associated with the elimination of transition metal ions [62,63]. In summary, our results demonstrate the significant Fe2+-chelating capacity of L. sakei MS103, suggesting it may exert antioxidant effects by inhibiting metal ion-catalyzed free radical generation.
From our results, the L. sakei MS103 existed antioxidative activities but not significant differences between live and heat-killed L. sakei MS103 in the scavenging of DPPH, ABTS, superoxide anion, and hydroxyl radical free radicals and FIC ability.
3.4. RAW 264.7 Cell-Protective L. sakei MS103 Antioxidant Properties
3.4.1. Effect of L. sakei MS103 on RAW 264.7 Cell Viability and Proliferation
Macrophages are important immune system cells that phagocytize pathogenic microorganisms [64]. However, the presence of bacteria and viruses can lead to increased macrophage ROS production that could lead to macrophage apoptosis when ROS levels become elevated [65,66,67].
The cytotoxic effects of L. sakei MS103 on RAW 264.7 cells were assessed. We examined RAW 264.7 cell viability following exposure to varying concentrations of L. sakei MS103. As shown in Figure 4, as compared to the control group, the addition of 7 Log CFU/mL live or heat-killed L. sakei MS103 led to reduced RAW 264.7 cell viability to only 60.4% and 63.98%, respectively. Intriguingly, at an L. sakei MS103 concentration of 8 Log CFU/mL, the cell viability increased significantly to 138.75%, which is a viability rate that was considerably higher than that of the control group. Similarly, RAW 264.7 cell viability increased after the addition of 8 Log CFU/mL heat-killed L. sakei MS103 (111.54%).
In contrast, 9 Log CFU/mL of live L. sakei MS103 added to RAW 264.7 cells led to decreased viability and the proliferation of RAW 264.7 cells, although no such inhibition was observed after the addition of 9 Log CFU/mL heat-killed organisms. These results indicate that both 7 Log CFU/mL of live or heat-killed L. sakei MS103 and 9 Log CFU/mL of live L. sakei MS103 exerted cytotoxic effects against RAW 264.7 cells, resulting in partial cell death and reduced RAW 264.7 cell viability. Therefore, the live or heat-killed L. sakei MS103 at 8 Log CFU/mL was used in further experiments.
3.4.2. L. sakei MS103 Antioxidant Effects on RAW 264.7 Cell GSH and MDA Levels
H2O2 plays a pivotal role in the generation of highly diffusible and long-lasting active oxygen molecules, such as hydroxyl radicals, which have the potential to induce cellular oxidative damage through specific interactions [68]. Excessive exogenous H2O2 disrupts cellular equilibrium, leading to ROS overproduction and oxidative damage.
While ROS can damage cells, macrophages utilize ROS to directly eliminate invading pathogens, although this defense mechanism can lead to macrophage injury and cell death. To assess the potential antioxidant effect of L. sakei MS103, we employed the widely used H2O2-Induced Oxidative Damage RAW 264.7 Cell Model. This model measures cellular responses to oxidative stress based on intracellular GSH and MDA levels, whereby elevated GSH and reduced MDA levels indicate increased cellular antioxidant activity that can protect cells from H2O2-induced damage.
GSH concentrations in the following groups were observed: control (32.71 μg/mL), H2O2-exposed (15.89 μg/mL), live L. sakei MS103-treated H2O2-exposed (18.4 μg/mL), and heat-killed L. sakei MS103-treated H2O2-exposed (17.2 μg/mL) cells. The results indicate that the treatment of H2O2-exposed RAW 264.7 cells with either live or heat-killed L. sakei MS103 cells resulted in decreased intracellular GSH concentration (Figure 5A).
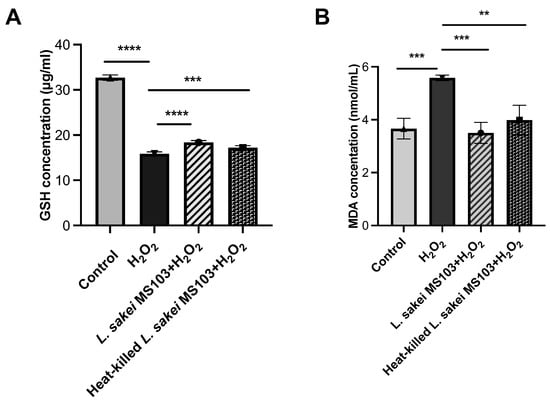
Figure 5.
Concentration of GSH and MDA for RAW 264.7 cells after treatment with live and heat-killed L. sakei MS103. RAW264.7 cells were treated with live L. sakei MS103 or heat-killed L. sakei MS103 and effect with H2O2 (0.1 mmol/L) for 6 h. (A): GSH concentration changing results. (B): MDA concentration changing results. Values represent the mean ± SEM (n = 3). ** p < 0.01, *** p < 0.001, **** p < 0.0001, compared to the control group.
Furthermore, the treatment of H2O2-exposed RAW 264.7 cells with either live or heat-killed L. sakei MS103 cells led to increased intracellular MDA concentration as compared to that of the control group. These findings collectively demonstrate that both live and heat-killed L. sakei MS103 exhibit antioxidant capabilities that may reduce the H2O2-induced oxidative damage of macrophages (Figure 5B).
3.5. Gene Expression Changes in H2O2-Exposed RAW 264.7 Cells after L. sakei MS103 Treatment
The above-mentioned results demonstrate that L. sakei MS103 cells’ antioxidative effects can protect H2O2-exposed RAW 264.7 cells from oxidative damage. To investigate the transcription-level mechanisms responsible for observed L. sakei MS103-induced increases in RAW 264.7 cell antioxidant activity, we assessed changes in the expression of four antioxidant response-related genes (gshR4, Gpx, npx, and gshR2). From the published results, these four genes were all related to the antioxidant effects [33]. These experiments were conducted using H2O2-exposed RAW 264.7 cells as a cellular oxidative damage model with and without added L. sakei MS103. Table S1 provides the RT-qPCR amplification parameters, with the results presented in Figure 6. Our findings revealed that H2O2 exposure down-regulated the mRNA-level expression of all four genes in RAW 264.7 cells. In contrast, the treatment of RAW 264.7 cells with live or heat-killed L. sakei MS103 significantly increased the mRNA-level expression of all these genes.
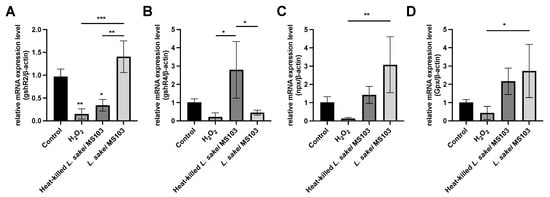
Figure 6.
The mRNA expression for the H2O2 effect of RAW 264.7 cells treated with L. sakei MS103. Live or heat-killed L. sakei MS103 treated with RAW264.7 cells (effect with 0.1 mmol/L H2O2) for 6 h. The β-actin gene used to determine the mRNA levels of (A): gshR2, (B): gshR4, (C): npx and (D): Gpx. Values represent the mean ± SEM (n = 3). Comparison between H2O2 effect group and L. sakei MS103 treatment. * p < 0.05, ** p < 0.01, *** p < 0.001.
Genes encoding one or two glutathione reductases, referred to as gshR genes, are crucial components of the oxidative stress response mechanisms in various probiotic organisms, including Streptococcus thermophilus and E. faecalis [69]. In our study, these genes were found to play pivotal roles in the oxidative stress response of RAW 264.7 cells. When H2O2-exposed RAW 264.7 cells were treated with either live or heat-killed L. sakei MS103, we observed 2.6-fold and 9.2-fold increases in gshR2 expression, respectively (Figure 6A). In contrast, both treatments led to increased gshR4 expression (0.44-fold and 2.7-fold up-regulation, respectively), indicating heat-killed L. sakei MS103 induced a greater increase in gshR4 expression as compared that induced by live L. sakei MS103 (Figure 6B). Taken together, these results indicate that the addition of either live or heat-killed L. sakei MS103 to oxidatively stressed RAW 264.7 cells induced up-regulated RAW 264.7 cell expression of both gshR2 and gshR4 relative to controls. However, a greater up-regulation of gshR4 expression was induced by treatment with heat-killed organisms, while live organisms induced a greater up-regulation of gshR2 expression.
Furthermore, npx gene expression, which is associated with NADH oxidase-NADH peroxidase system function [70], showed significant up-regulation in model cells following treatment with either live L. sakei MS103 (23.9-fold) or heat-killed L. sakei MS103 (11.1-fold) (Figure 6C). These findings, along with the above-mentioned gshR results, strongly suggest that the L. sakei MS103 treatment-induced up-regulated expression of gshR and npx plays a crucial role in mitigating the oxidative damage of H2O2-exposed RAW 264.7 cells.
Similarly, treatment of the oxidative stress model with either live or heat-killed L. sakei MS103 induced an up-regulation of Gpx gene expression (2.7-fold and 2.1-fold increases, respectively (Figure 6D), thus underscoring the antioxidant potential of L. sakei MS103 in reducing the effects of oxidative stress. Taken together, the above-mentioned results demonstrate that most of the key genes were up-regulated in L. sakei MS103-treated model cells. While further research is needed to fully understand the mechanisms underlying these antioxidant effects, our results support L. sakei MS103 as a promising antioxidant probiotic for use in various applications.
4. Conclusions
In this study, we assessed the antioxidant potential of L. sakei MS103, which is a probiotic strain isolated from a traditional fermented food known as sweet pickled garlic. This remarkable biosafe strain exhibits robust survivability in harsh environmental conditions, including hydrogen peroxide, lysozyme exposure, low pH levels and bile salts. Additionally, it possesses the abilities of auto-aggregation, co-aggregation, and adhesion, facilitating its adhesion and colonization within specific microenvironments. Furthermore, our in vitro experiments demonstrated that L. sakei MS103 effectively inhibited P. gingivalis-induced biofilm formation. Additionally, L. sakei MS103 exhibited antioxidant properties with antioxidative activities. Notably, the addition of L. sakei MS103 to H2O2-exposed RAW 264.7 cells led to increased RAW 264.7 cell antioxidative activity, as reflected by the significant regulated expression of four RAW 264.7 cell genes associated with oxidative stress responses following L. sakei MS103 treatment. Collectively, these findings strongly suggest that L. sakei MS103 holds promise as a potent candidate treatment for conditions related to oxidative stress.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/foods12234276/s1, Table S1: Antioxidant ability of the lactic acid bacteria; Table S2: The RNA concentration and RNA concentration.
Author Contributions
Conceptualization, C.L. (Chen Li) and C.L. (Chang Luan); Data curation, H.L., C.C. and Y.L.; Methodology, H.L., C.C. and Y.L.; Project administration, C.L. (Chen Li) and C.L. (Chang Luan); Supervision, Z.L.; Validation, H.L. and C.C.; Writing—original draft, H.L. and Z.L.; Writing—review and editing, C.L. (Chen Li) and C.L. (Chang Luan). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data is contained within the article or Supplementary Material.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kumar, M.; Ghosh, M.; Ganguli, A. Mitogenic response and probiotic characteristics of lactic acid bacteria isolated from indigenously pickled vegetables and fermented beverages. World J. Microbiol. Biotechnol. 2012, 28, 703–711. [Google Scholar] [CrossRef]
- Mir, S.; Raja, J.; Masoodi, F.A. Fermented Vegetables, a Rich Repository of Beneficial Probiotics—A Review. Ferment. Technol. 2018, 7, 1–6. [Google Scholar] [CrossRef]
- Rahman, M.S. Allicin and Other Functional Active Components in Garlic: Health Benefits and Bioavailability. Int. J. Food Prop. 2007, 10, 245–268. [Google Scholar] [CrossRef]
- Huang, T.-T.; Wu, Z.; Wenxue, Z. Effects of garlic addition on bacterial communities and the conversions of nitrate and nitrite in a simulated pickle fermentation system. Food Control. 2020, 113, 107215. [Google Scholar] [CrossRef]
- Li, X.; Ning, Y.; Liu, D.; Yan, A.; Wang, Z.; Wang, S.; Miao, M.; Zhu, H.; Jia, Y. Metabolic mechanism of phenyllactic acid naturally occurring in Chinese pickles. Food Chem. 2015, 186, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.S.; El Sheikha, A.F.; Hammami, R.; Kumar, A. Traditionally fermented pickles: How the microbial diversity associated with their nutritional and health benefits? J. Funct. Foods 2020, 70, 103971. [Google Scholar] [CrossRef]
- An, F.; Sun, H.; Wu, J.; Zhao, C.; Li, T.; Huang, H.; Fang, Q.; Mu, E.; Wu, R. Investigating the core microbiota and its influencing factors in traditional Chinese pickles. Food Res. Int. 2021, 147, 110543. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Chen, H.; Wang, X.; Lin, X.; Ji, C.; Li, S.; Liang, H. Effects of different temperatures on bacterial diversity and volatile flavor compounds during the fermentation of suancai, a traditional fermented vegetable food from northeastern China. Lwt Food Sci. Technol. 2020, 118, 108773. [Google Scholar] [CrossRef]
- Modi, M. Role of Lactic Acid Bacteria as Probiotics in Health and Disease. La Prensa Med. 2015, 100, 1–9. [Google Scholar] [CrossRef]
- Gálvez, A.; Abriouel, H.; López, R.L.; Ben Omar, N. Bacteriocin-based strategies for food biopreservation. Int. J. Food Microbiol. 2007, 120, 51–70. [Google Scholar] [CrossRef]
- Mathur, H.; Beresford, T.P.; Cotter, P.D. Health Benefits of Lactic Acid Bacteria (LAB) Fermentates. Nutrients 2020, 12, 1679. [Google Scholar] [CrossRef] [PubMed]
- Morgan, S.M.; Galvin, M.; Kelly, J.; Ross, R.P.; Hill, C. Development of a Lacticin 3147–Enriched Whey Powder with Inhibitory Activity against Foodborne Pathogens. J. Food Prot. 1999, 62, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Fugaban, J.I.I.; Holzapfel, W.H.; Todorov, S.D. Selection of Beneficial Bacterial Strains with Potential as Oral Probiotic Candidates. Probiotics Antimicrob. Proteins 2022, 14, 1077–1093. [Google Scholar] [CrossRef] [PubMed]
- Chow, Y.C.; Yam, H.C.; Gunasekaran, B.; Lai, W.Y.; Wo, W.Y.; Agarwal, T.; Ong, Y.Y.; Cheong, S.L.; Tan, S.-A. Implications of Porphyromonas gingivalis peptidyl arginine deiminase and gingipain R in human health and diseases. Front. Cell. Infect. Microbiol. 2022, 12, 1456. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Shah, C.; Mokashe, N.; Chavan, R.; Yadav, H.; Prajapati, J. Probiotics as potential antioxidants: A systematic review. J. Agric. Food Chem. 2015, 63, 3615–3626. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Wang, Y.; Xu, H.; Mei, X.; Yu, D.; Wang, Y.; Li, W. Antioxidant Properties of Probiotic Bacteria. Nutrients 2017, 9, 521. [Google Scholar] [CrossRef]
- Kim, K.T.; Kim, J.W.; Kim, S.I.; Kim, S.; Nguyen, T.H.; Kang, C.H. Antioxidant and Anti-Inflammatory Effect and Probiotic Properties of Lactic Acid Bacteria Isolated from Canine and Feline Feces. Microorganisms 2021, 9, 1971. [Google Scholar] [CrossRef]
- Amaretti, A.; di Nunzio, M.; Pompei, A.; Raimondi, S.; Rossi, M.; Bordoni, A. Antioxidant properties of potentially probiotic bacteria: In vitro and in vivo activities. Appl. Microbiol. Biotechnol. 2013, 97, 809–817. [Google Scholar] [CrossRef]
- Feng, T.; Wang, J. Oxidative stress tolerance and antioxidant capacity of lactic acid bacteria as probiotic: A systematic review. Gut Microbes 2020, 12, 1801944. [Google Scholar] [CrossRef]
- Ismail, A.S.; Hooper, L.V. Epithelial cells and their neighbors. IV. Bacterial contributions to intestinal epithelial barrier integrity. Am. J. Physiol. Gastrointest Liver Physiol. 2005, 289, G779–G784. [Google Scholar] [CrossRef]
- Prado, C.; Michels, M.; Ávila, P.; Burger, H.; Milioli, M.V.M.; Dal-Pizzol, F. The protective effects of fecal microbiota transplantation in an experimental model of necrotizing enterocolitis. J. Pediatr. Surg. 2019, 54, 1578–1583. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.-F.; Kim, H.; Kim, H.R.; Gim, M.G.; Chung, D.K. Different immune regulatory potential of Lactobacillus plantarum and Lactobacillus sakei isolated from kimchi. J. Microbiol. Biotechnol. 2014, 24, 1629–1635. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Chen, Y.; Duan, H.; Qiao, N.; Wang, G.; Zhao, J.; Zhai, Q.; Tian, F.; Chen, W. Latilactobacillus sakei: A candidate probiotic with a key role in food fermentations and health promotion. Crit. Rev. Food Sci. Nutr. 2022, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Champomier-Vergès, M.C.; Chaillou, S.; Cornet, M.; Zagorec, M. Erratum to ”Lactobacillus sakei: Recent developments and future prospects“: [Research in Microbiology 152 (2001) 839]. Res. Microbiol. 2002, 153, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Aslim, B.; Onal, D.; Beyatli, Y. Factors influencing autoaggregation and aggregation of Lactobacillus delbrueckii subsp. bulgaricus isolated from handmade yogurt. J. Food Prot. 2007, 70, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Del Re, B.; Sgorbati, B.; Miglioli, M.; Palenzona, D. Adhesion, autoaggregation and hydrophobicity of 13 strains of Bifidobacterium longum. Lett. Appl. Microbiol. 2000, 31, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Vlková, E.; Rada, V.; Šmehilová, M.; Killer, J. Auto-aggregation and co-aggregation ability in bifidobacteria and clostridia. Folia Microbiol. 2008, 53, 263–269. [Google Scholar] [CrossRef]
- Bautista-Gallego, J.; Arroyo-López, F.N.; Rantsiou, K.; Jiménez-Díaz, R.; Garrido-Fernández, A.; Cocolin, L. Screening of lactic acid bacteria isolated from fermented table olives with probiotic potential. Food Res. Int. 2013, 50, 135–142. [Google Scholar] [CrossRef]
- Chen, S.; Chen, L.; Chen, L.; Ren, X.; Ge, H.; Li, B.; Ma, G.; Ke, X.; Zhu, J.; Li, L.; et al. Potential probiotic characterization of Lactobacillus reuteri from traditional Chinese highland barley wine and application for room-temperature-storage drinkable yogurt. J. Dairy Sci. 2018, 101, 5780–5788. [Google Scholar] [CrossRef]
- Kahlmeter, G.; Brown, D.F.; Goldstein, F.W.; MacGowan, A.P.; Mouton, J.W.; Odenholt, I.; Rodloff, A.; Soussy, C.J.; Steinbakk, M.; Soriano, F.; et al. European Committee on Antimicrobial Susceptibility Testing (EUCAST) Technical Notes on antimicrobial susceptibility testing. Clin. Microbiol. Infect. 2006, 12, 501–503. [Google Scholar] [CrossRef]
- Sui, L.; Zhu, X.; Wu, D.; Ma, T.; Tuo, Y.; Jiang, S.; Qian, F.; Mu, G. In vitro assessment of probiotic and functional properties of Bacillus coagulans T242. Food Biosci. 2020, 36, 100675. [Google Scholar] [CrossRef]
- Song, Y.J.; Yu, H.H.; Kim, Y.J.; Lee, N.K.; Paik, H.D. Anti-Biofilm Activity of Grapefruit Seed Extract against Staphylococcus aureus and Escherichia Coli. J. Microbiol. Biotechnol. 2019, 29, 1177–1183. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Xing, Z.; Li, C.; Wang, J.; Wang, Y. Molecular mechanisms and in vitro antioxidant effects of Lactobacillus plantarum MA2. Food Chem. 2017, 221, 1642–1649. [Google Scholar] [CrossRef] [PubMed]
- Li, X. Improved pyrogallol autoxidation method: A reliable and cheap superoxide-scavenging assay suitable for all antioxidants. J Agric. Food Chem. 2012, 60, 6418–6424. [Google Scholar] [CrossRef]
- Duan, Y.; Ying, Z.; He, F.; Ying, X.; Jia, L.; Yang, G. A new skeleton flavonoid and a new lignan from Portulaca oleracea L. and their activities. Fitoterapia 2021, 153, 104993. [Google Scholar] [CrossRef]
- Luan, C.; Jiang, N.; Zhou, X.; Zhang, C.; Zhao, Y.; Li, Z.; Li, C. Antibacterial and anti-biofilm activities of probiotic Lactobacillus curvatus BSF206 and Pediococcus pentosaceus AC1-2 against Streptococcus mutans. Microb. Pathog. 2022, 164, 105446. [Google Scholar] [CrossRef]
- Luan, C.; Yan, J.; Jiang, N.; Zhang, C.; Geng, X.; Li, Z.; Li, C. Leuconostoc mesenteroides LVBH107 Antibacterial Activity against Porphyromonas gingivalis and Anti-Inflammatory Activity against P. gingivalis Lipopolysaccharide-Stimulated RAW 264.7 Cells. Nutrients 2022, 14, 2584. [Google Scholar] [CrossRef]
- Lee, S.W. Regression analysis for continuous independent variables in medical research: Statistical standard and guideline of Life Cycle Committee. Life Cycle 2022, 2, 1–8. [Google Scholar] [CrossRef]
- Zhou, Y.; Gong, W.; Xu, C.; Zhu, Z.; Peng, Y.; Xie, C. Probiotic assessment and antioxidant characterization of Lactobacillus plantarum GXL94 isolated from fermented chili. Front. Microbiol. 2022, 13, 997940. [Google Scholar] [CrossRef]
- Angmo, K.; Kumari, A.; Savitri; Bhalla, T.C. Probiotic characterization of lactic acid bacteria isolated from fermented foods and beverage of Ladakh. LWT Food Sci. Technol. 2016, 66, 428–435. [Google Scholar] [CrossRef]
- Lee, C.S.; Kim, S.H. Anti-inflammatory and Anti-osteoporotic Potential of Lactobacillus plantarum A41 and L. fermentum SRK414 as Probiotics. Probiotics Antimicrob. Proteins 2020, 12, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, N.C.; Silva de Ruiz, C.; Claudia Otero, M.; Sesma, F.; Elena Nader-Macias, M. Lactic acid bacteria isolated from young calves—Characterization and potential as probiotics. Res. Vet. Sci. 2012, 92, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Rijnaarts, H.H.; Norde, W.; Bouwer, E.J.; Lyklema, J.; Zehnder, A.J. Bacterial Adhesion under Static and Dynamic Conditions. Appl Environ. Microbiol 1993, 59, 3255–3265. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Chen, Y.; Chen, Z.; Zhou, Q.; Wei, F.; Li, P.; Gu, Q. Antioxidant properties and molecular mechanisms of Lactiplantibacillus plantarum ZJ316: A potential probiotic resource. LWT 2023, 187, 115269. [Google Scholar] [CrossRef]
- Dlamini, Z.C.; Langa, R.L.S.; Aiyegoro, O.A.; Okoh, A.I. Safety Evaluation and Colonisation Abilities of Four Lactic Acid Bacteria as Future Probiotics. Probiotics Antimicrob. Proteins 2019, 11, 397–402. [Google Scholar] [CrossRef]
- Boirivant, M.; Strober, W. The mechanism of action of probiotics. Curr. Opin. Gastroenterol. 2007, 23, 679–692. [Google Scholar] [CrossRef]
- Kakisu, E.; Bolla, P.; Abraham, A.; de Urraza, P.; De Antoni, G. Lactobacillus plantarum isolated from kefir: Protection of cultured Hep-2 cells against Shigella invasion. Int. Dairy J. 2013, 33, 22–26. [Google Scholar] [CrossRef]
- Mack, D.R.; Michail, S.; Wei, S.; McDougall, L.; Hollingsworth, M.A. Probiotics inhibit enteropathogenic E. coli adherence in vitro by inducing intestinal mucin gene expression. Am. J. Physiol. 1999, 276, G941–G950. [Google Scholar] [CrossRef]
- Servin, A.L.; Coconnier, M.H. Adhesion of probiotic strains to the intestinal mucosa and interaction with pathogens. Best Pract. Res. Clin. Gastroenterol. 2003, 17, 741–754. [Google Scholar] [CrossRef]
- CLSL. Performance Standards for Antimicrobial Susceptibility Testing, 30th ed.; CLSI supplement M100; Clinical and Laboratory Standards Institute: Wayne, NY, USA, 2020. [Google Scholar]
- Yüceer, Ö.; Özden Tuncer, B. Determination of Antibiotic Resistance and Biogenic Amine Production of Lactic Acid Bacteria Isolated from Fermented T urkish Sausage (Sucuk). J. Food Saf. 2015, 35, 276–285. [Google Scholar] [CrossRef]
- Anisimova, E.A.; Yarullina, D.R. Antibiotic Resistance of Lactobacillus Strains. Curr. Microbiol. 2019, 76, 1407–1416. [Google Scholar] [CrossRef] [PubMed]
- Merino, L.; Trejo, F.M.; De Antoni, G.; Golowczyc, M.A. Lactobacillus strains inhibit biofilm formation of Salmonella sp. isolates from poultry. Food Res. Int. 2019, 123, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.R.; Kang, M.; Yoo, Y.-J.; Yun, C.-H.; Perinpanayagam, H.; Kum, K.-Y.; Han, S.H. Lactobacillus plantarum lipoteichoic acid disrupts mature Enterococcus faecalis biofilm. J. Microbiol. 2020, 58, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Park, O.-J.; Kim, A.R.; Ahn, K.B.; Lee, D.; Kum, K.-Y.; Yun, C.-H.; Han, S.H. Lipoteichoic acids of lactobacilli inhibit Enterococcus faecalis biofilm formation and disrupt the preformed biofilm. J. Microbiol. 2019, 57, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Hyun, J.-H.; Woo, I.-K.; Kim, K.-T.; Park, Y.-S.; Kang, D.-K.; Lee, N.-K.; Paik, H.-D. Antioxidant and immunostimulatory effect of heat-treated paraprobiotics Latilactobacillus sakei KU15041 and Latilactobacillus curvatus KU15003. Res. Sq. 2023, 43, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Qiao, Y.; Peng, Q.; Dia, V.P.; Shi, B. Probiotic activity of ropy Lactiplantibacillus plantarum NA isolated from Chinese northeast sauerkraut and comparative evaluation of its live and heat-killed cells on antioxidant activity and RAW 264.7 macrophage stimulation. Food Funct. 2023, 14, 2481–2495. [Google Scholar] [CrossRef]
- Liu, Q.; Xie, F.; Rolston, R.; Moreira, P.I.; Nunomura, A.; Zhu, X.; Smith, M.A.; Perry, G. Prevention and treatment of Alzheimer disease and aging: Antioxidants. Mini Rev. Med. Chem. 2007, 7, 171–180. [Google Scholar] [CrossRef]
- Rival, S.G.; Boeriu, C.G.; Wichers, H.J. Caseins and casein hydrolysates. 2. Antioxidative properties and relevance to lipoxygenase inhibition. J. Agric. Food Chem. 2001, 49, 295–302. [Google Scholar] [CrossRef]
- DÜz, M.; DoĞan, Y.N.; DoĞan, İ. Antioxidant activitiy of Lactobacillus plantarum, Lactobacillus sake and Lactobacillus curvatus strains isolated from fermented Turkish Sucuk. An. Acad. Bras. Cienc. 2020, 92, e20200105. [Google Scholar] [CrossRef]
- Rwubuzizi, R.; Kim, H.; Holzapfel, W.H.; Todorov, S.D. Beneficial, safety, and antioxidant properties of lactic acid bacteria: A next step in their evaluation as potential probiotics. Heliyon 2023, 9, e15610. [Google Scholar] [CrossRef]
- Lin, M.Y.; Yen, C.L. Antioxidative ability of lactic acid bacteria. J. Agric. Food Chem. 1999, 47, 1460–1466. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-F.; Pan, T.-M. In vitro effects of lactic acid bacteria on cancer cell viability and antioxidant activity. J. Food Drug Anal. 2010, 18, 8. [Google Scholar] [CrossRef]
- Tur, J.; Pereira-Lopes, S.; Vico, T.; Marín, E.A.; Muñoz, J.P.; Hernández-Alvarez, M.; Cardona, P.J.; Zorzano, A.; Lloberas, J.; Celada, A. Mitofusin 2 in Macrophages Links Mitochondrial ROS Production, Cytokine Release, Phagocytosis, Autophagy, and Bactericidal Activity. Cell Rep. 2020, 32, 108079. [Google Scholar] [CrossRef] [PubMed]
- Robinson, N.; Ganesan, R.; Hegedűs, C.; Kovács, K.; Kufer, T.A.; Virág, L. Programmed necrotic cell death of macrophages: Focus on pyroptosis, necroptosis, and parthanatos. Redox Biol. 2019, 26, 101239. [Google Scholar] [CrossRef]
- Almeida, L.T.; Ferraz, A.C.; da Silva Caetano, C.C.; da Silva Menegatto, M.B.; Dos Santos Pereira Andrade, A.C.; Lima, R.L.S.; Camini, F.C.; Pereira, S.H.; da Silva Pereira, K.Y.; de Mello Silva, B.; et al. Zika virus induces oxidative stress and decreases antioxidant enzyme activities in vitro and in vivo. Virus Res. 2020, 286, 198084. [Google Scholar] [CrossRef]
- Grom, A.A.; Mellins, E.D. Macrophage activation syndrome: Advances towards understanding pathogenesis. Curr. Opin. Rheumatol. 2010, 22, 561–566. [Google Scholar] [CrossRef]
- Li, Q.; Qiu, Z.; Wang, Y.; Guo, C.; Cai, X.; Zhang, Y.; Liu, L.; Xue, H.; Tang, J. Tea polyphenols alleviate hydrogen peroxide-induced oxidative stress damage through the Mst/Nrf2 axis and the Keap1/Nrf2/HO-1 pathway in murine RAW264.7 cells. Exp. Ther. Med. 2021, 22, 1473. [Google Scholar] [CrossRef]
- Jänsch, A.; Korakli, M.; Vogel, R.F.; Gänzle, M.G. Glutathione reductase from Lactobacillus sanfranciscensis DSM20451T: Contribution to oxygen tolerance and thiol exchange reactions in wheat sourdoughs. Appl. Environ. Microbiol. 2007, 73, 4469–4476. [Google Scholar] [CrossRef]
- Kang, T.S.; Korber, D.R.; Tanaka, T. Influence of oxygen on NADH recycling and oxidative stress resistance systems in Lactobacillus panis PM1. Amb Express 2013, 3, 1–9. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).