Synergistic Microbial Inhibition and Quality Preservation for Grapes through High-Voltage Electric Field Cold Plasma and Nano-ZnO Antimicrobial Film Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials and Chemicals
2.2. Apparatus and Analytical Tools
2.3. Methods
2.3.1. Sample Groups
2.3.2. Co-Processing of Grapes
2.3.3. Optimizing the HVEF-CP Parameters
Single-Factor Experiments
Response Surface Experiment
2.3.4. Measurement Methods
2.3.5. Statistical Analysis
3. Results and Discussion
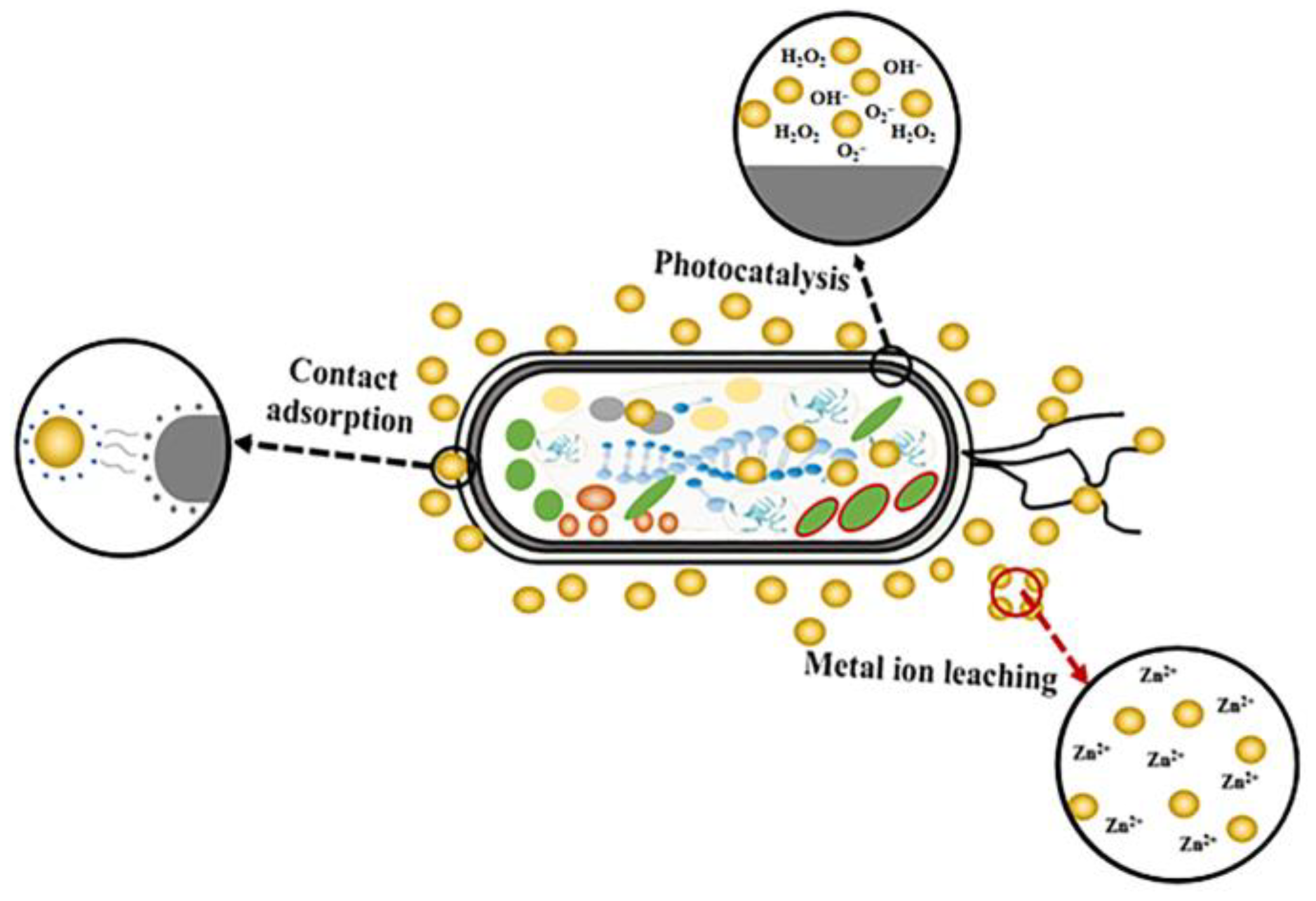
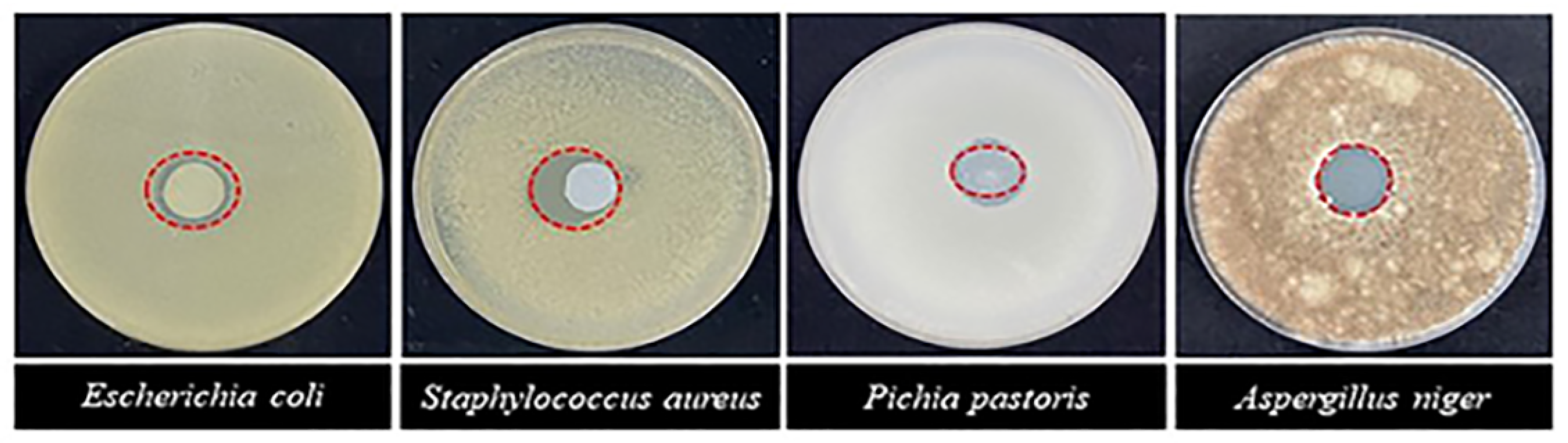
3.1. Performance of Nano-ZnO Antimicrobial Film
3.2. Optimization of HVEF-CP Processing Parameters
3.2.1. Single-Factor Experimental Results
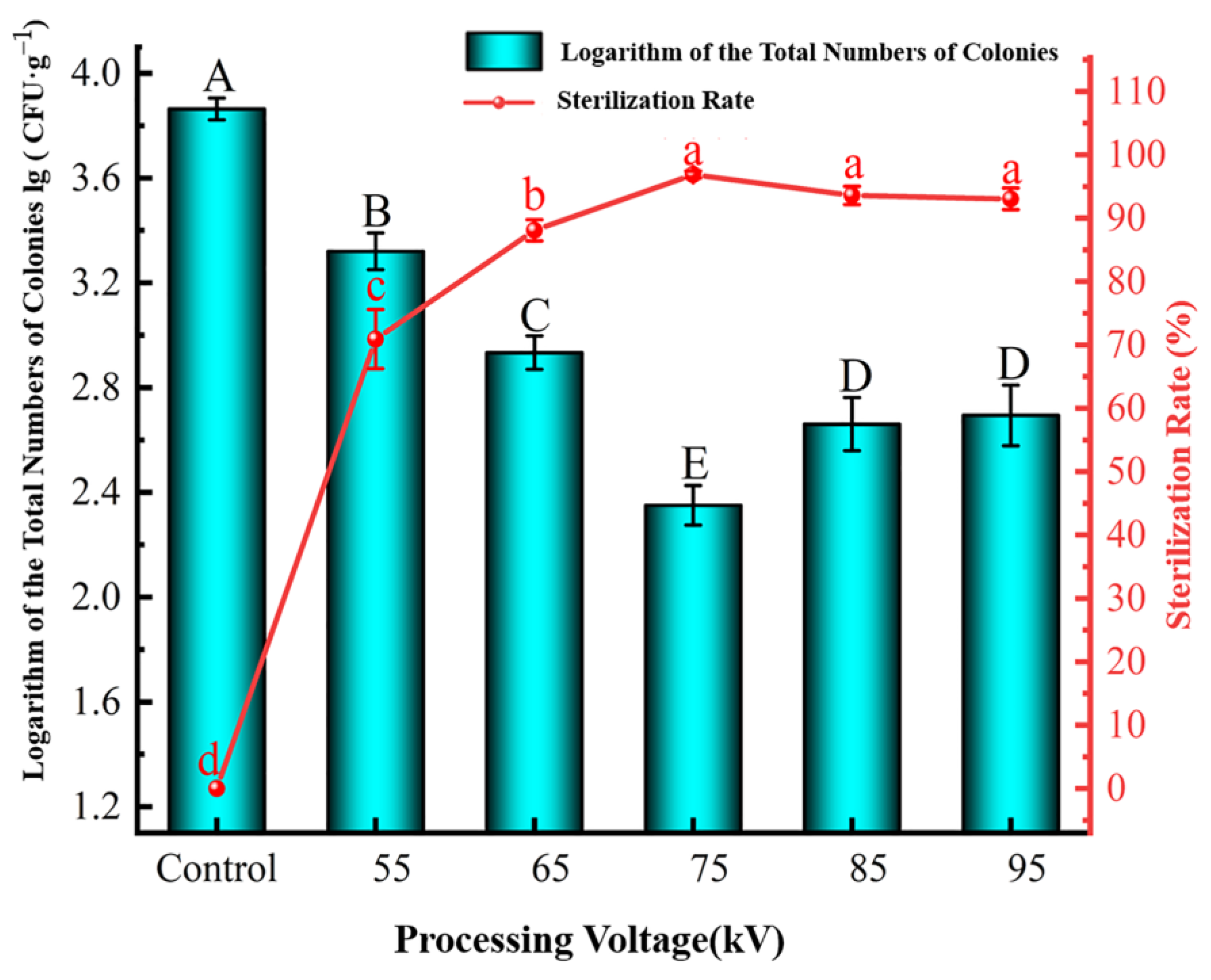
Effect of Processing Voltage
Effect of Processing Frequency
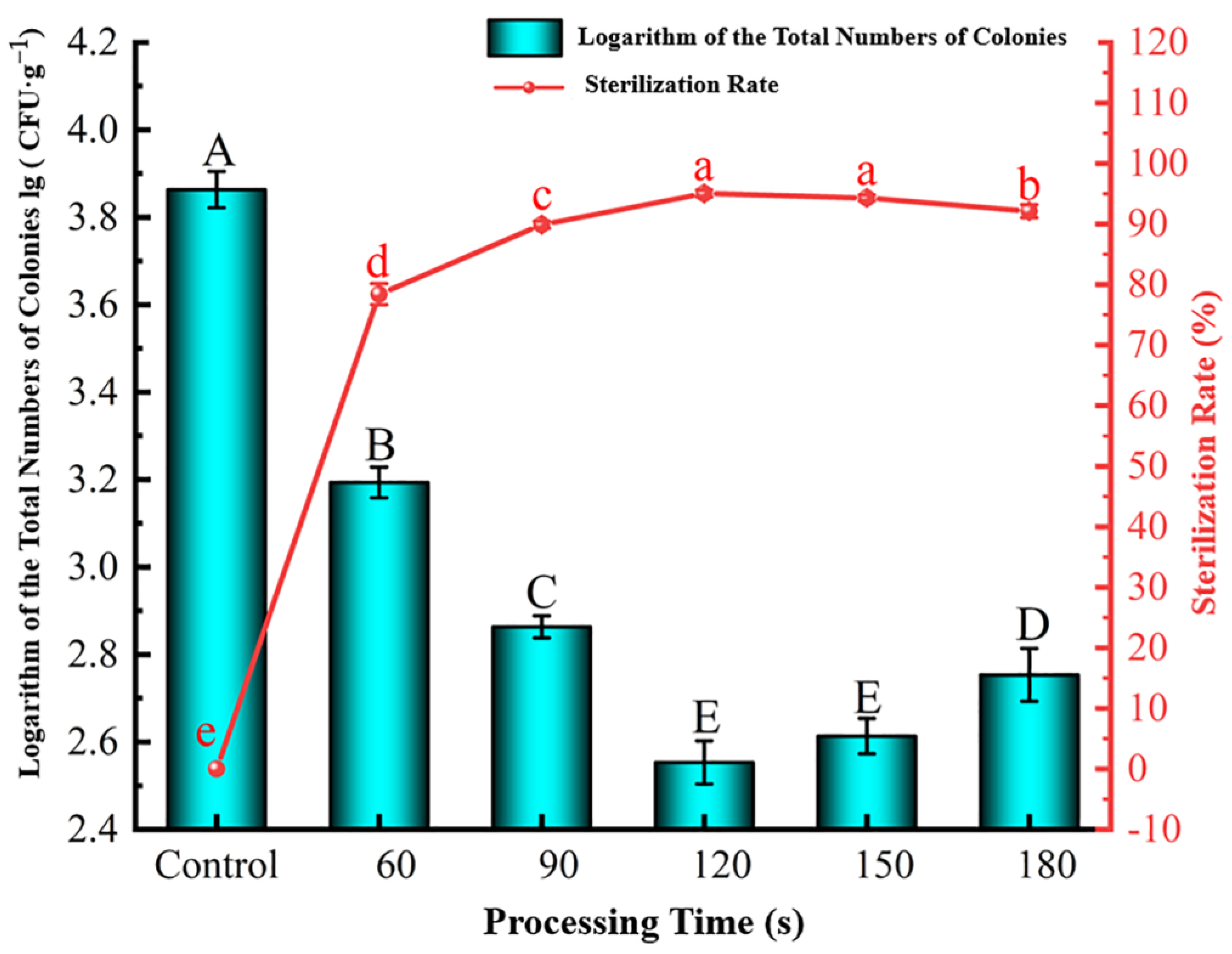
Effect of Processing Time
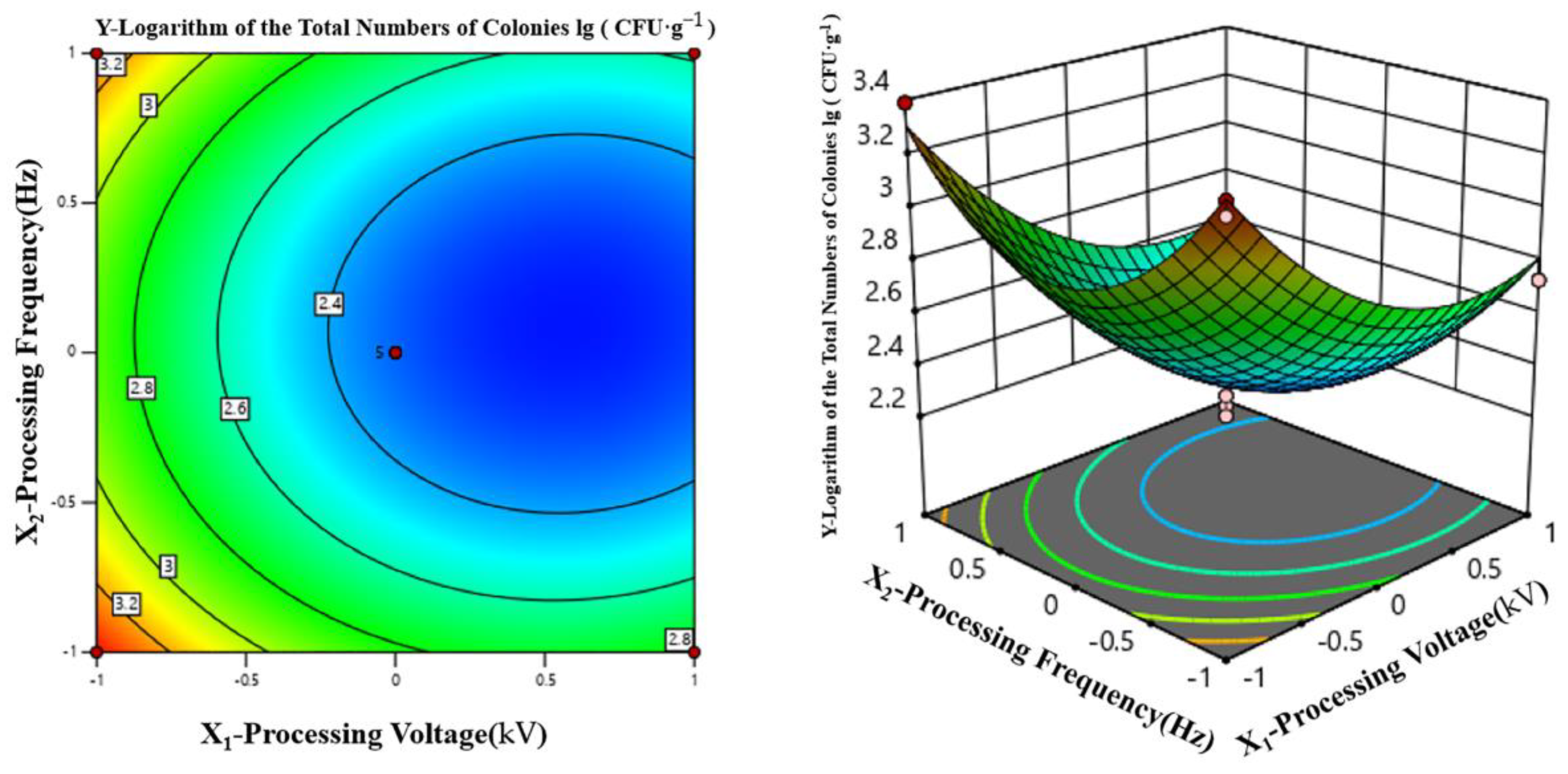
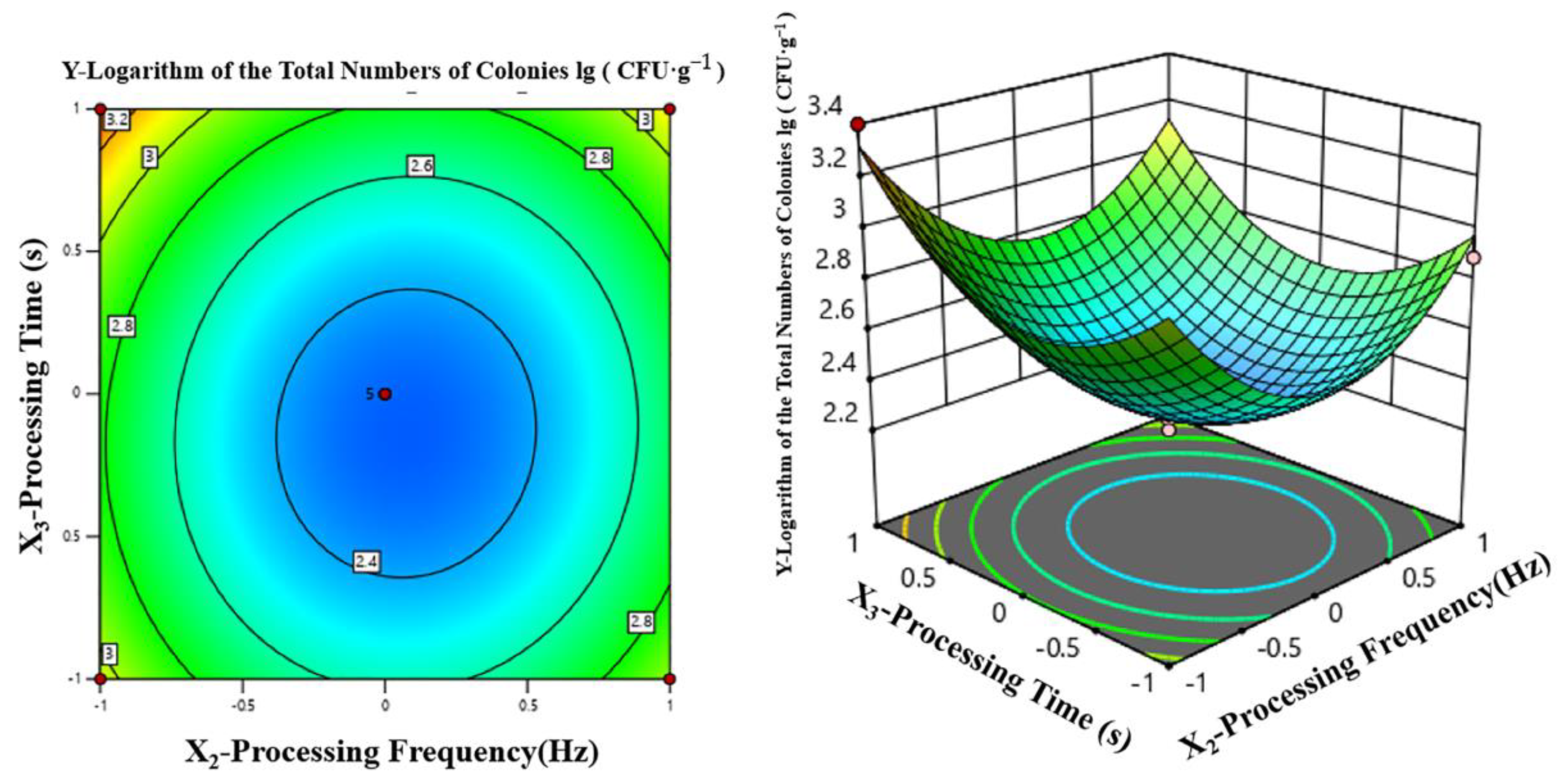
3.2.2. Response Surface Test
Experimental Design and Results
Two-Factor Interaction Analysis
Determination and Verification of the Optimal Processing Parameters
3.3. Antibacterial Effect and Quality of Grapes Co-Processed by HVEF-CP + Nano-ZnO Antimicrobial Film
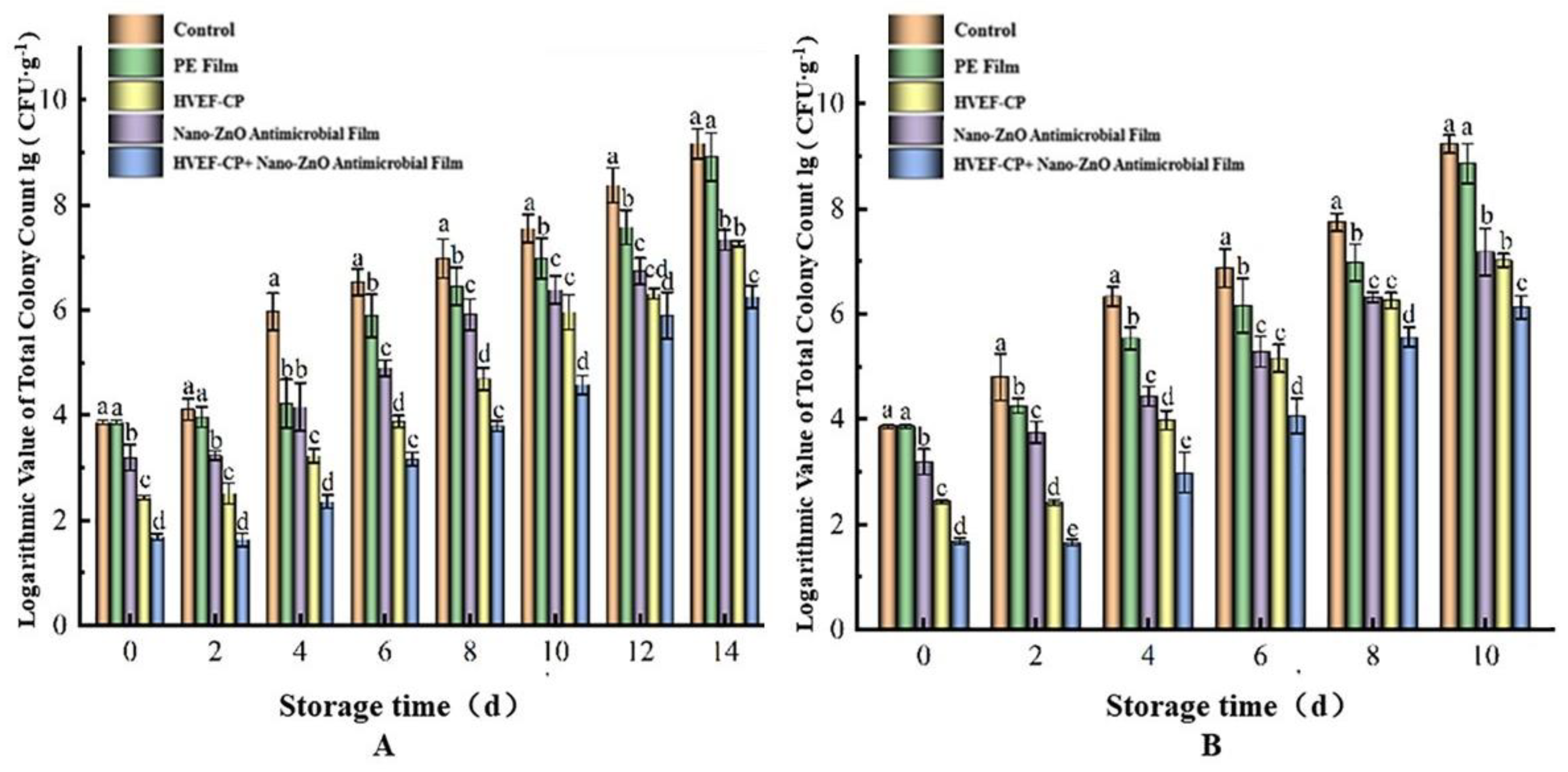
3.3.1. Effects of Different Sterilization Approaches on lg (CFU·g−1) Value
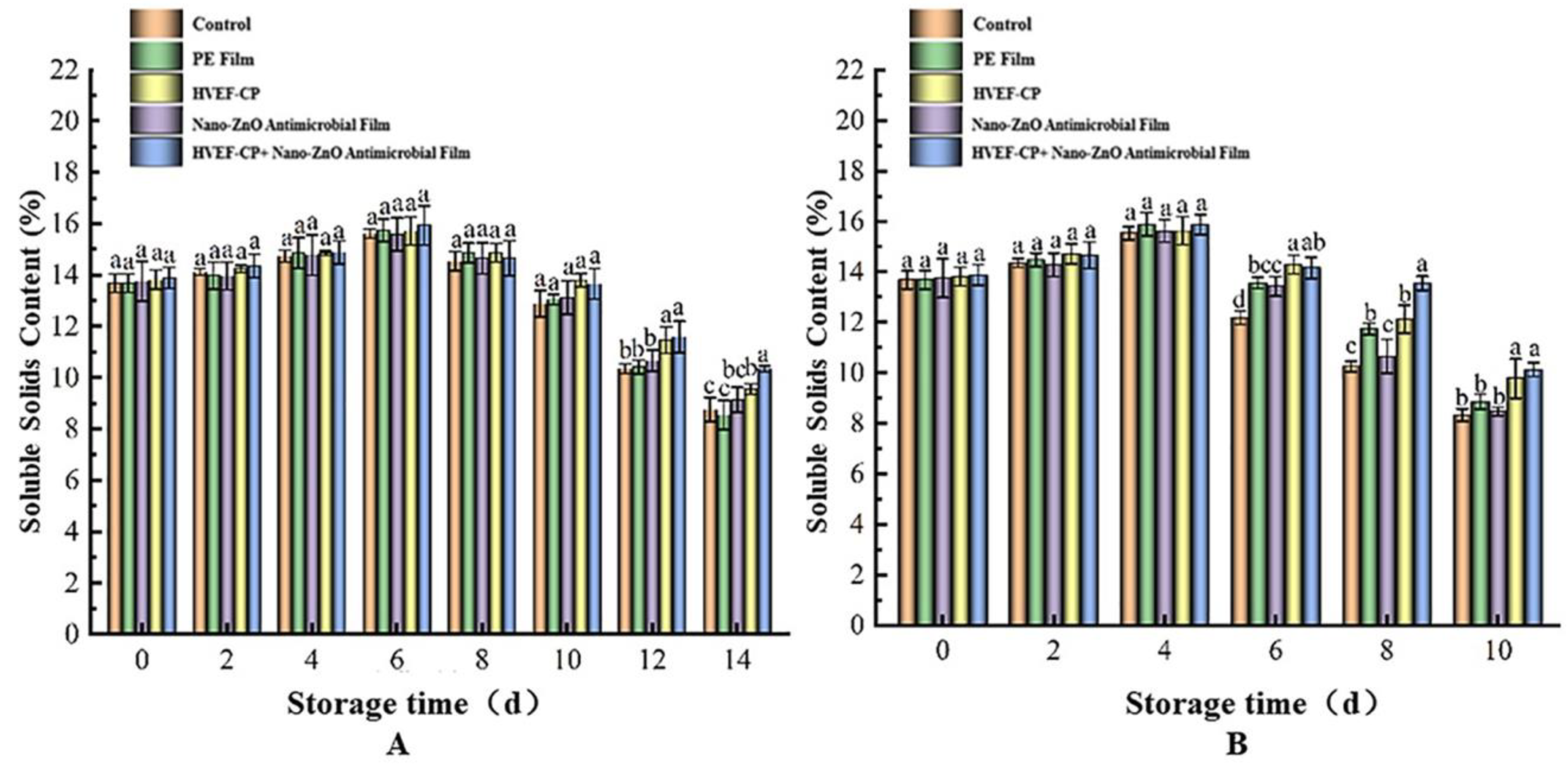
3.3.2. Effects of Different Sterilization Methods on TSS Content
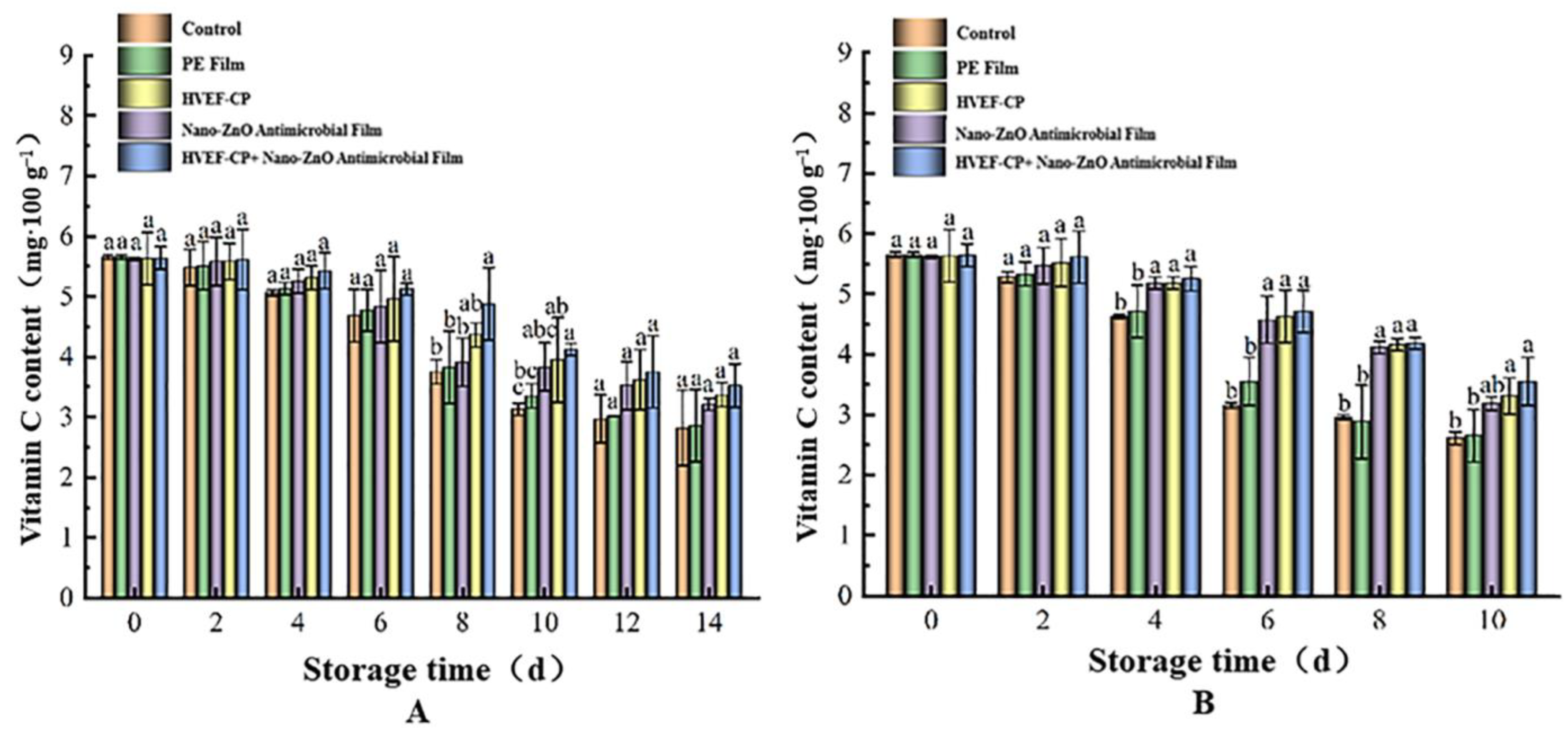
3.3.3. Effects of Different Sterilization Methods on VC Content
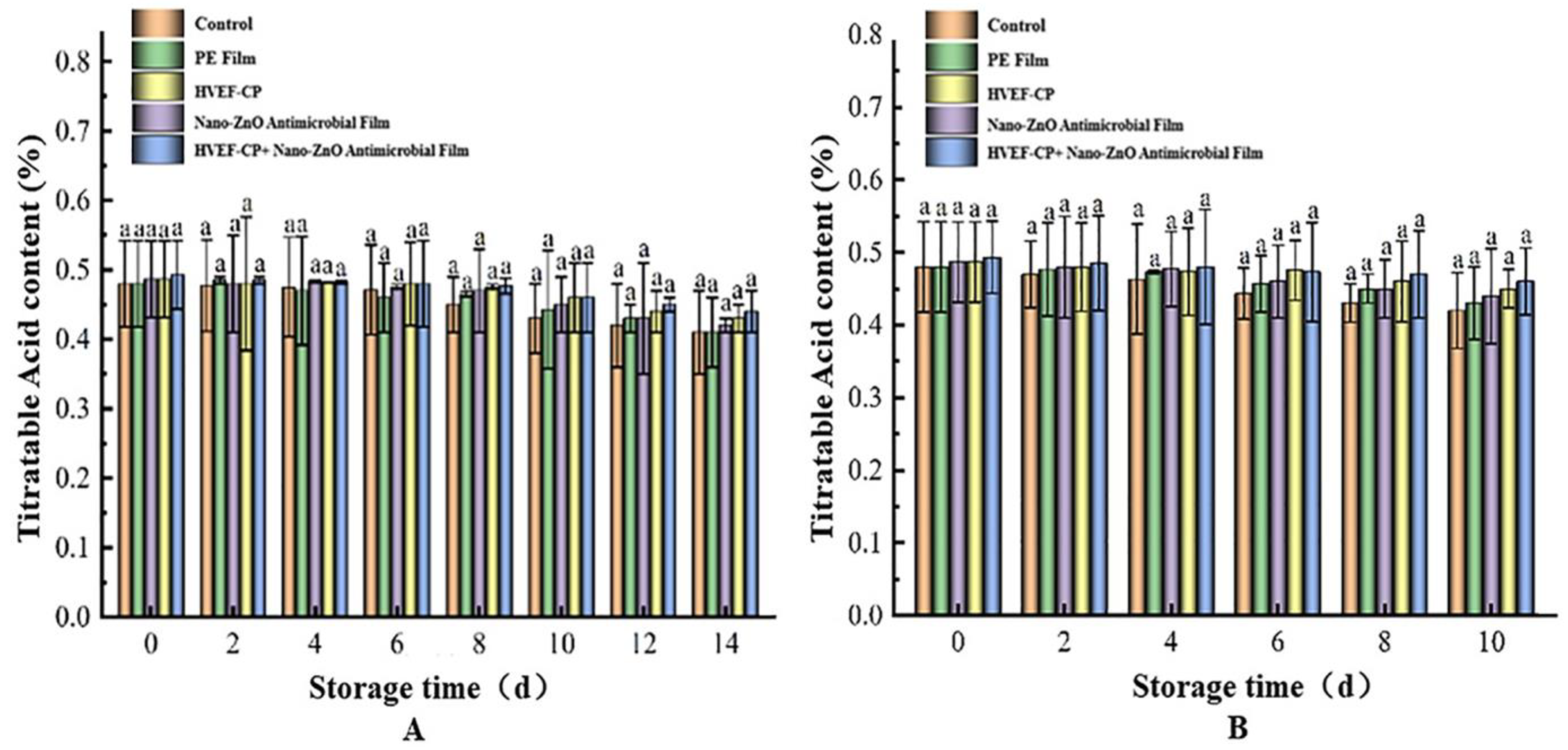
3.3.4. Effects of Different Sterilization Methods on TA Content
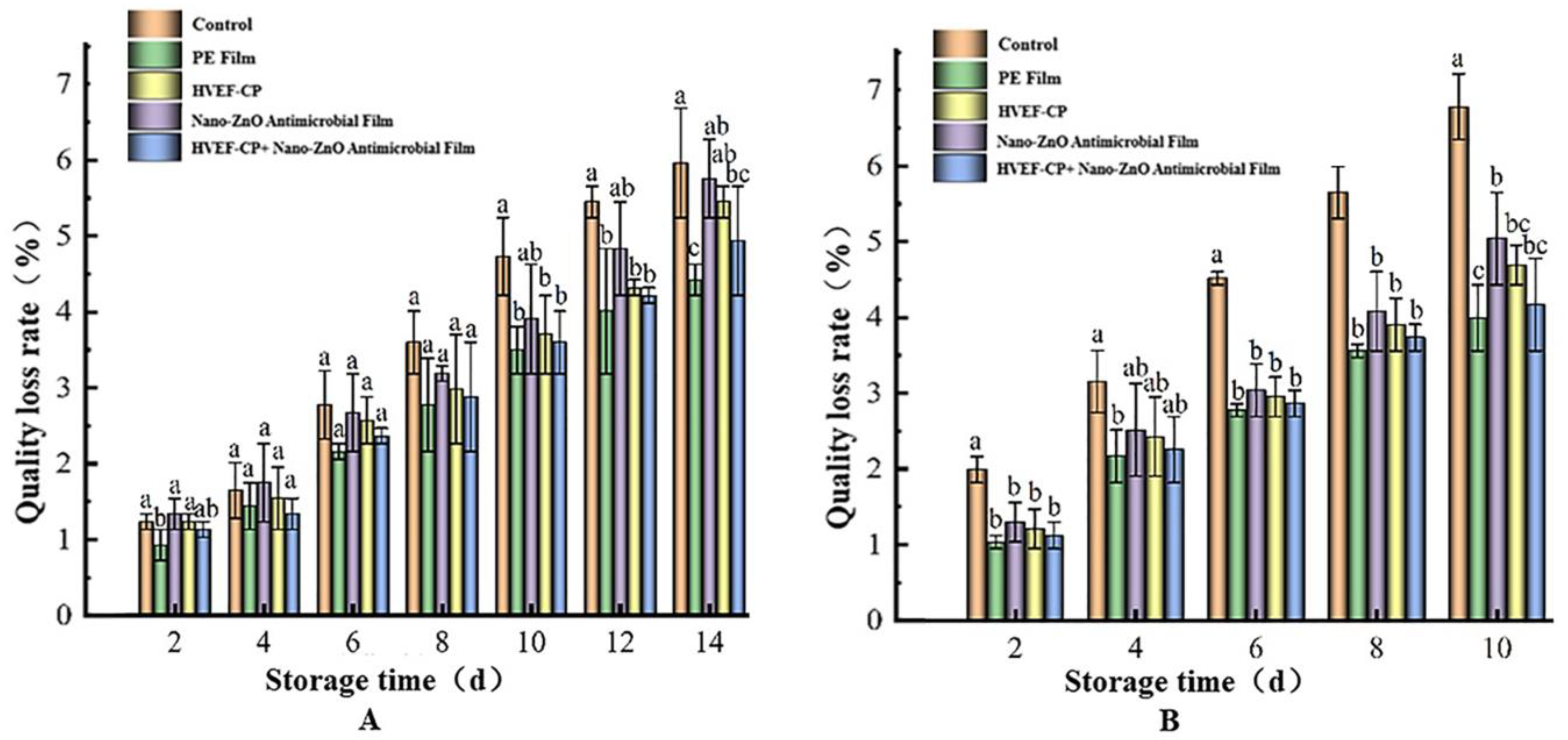
3.3.5. Effects of Different Sterilization Methods on Mass Loss
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Al-Lami, H.F.D.; You, M.P.; Barbetti, M.J. Incidence, pathogenicity and diversity of Alternaria spp. associated with alternaria leaf spot of canola (Brassica napus) in Australia. Plant Pathol. 2019, 68, 492–503. [Google Scholar] [CrossRef]
- Rotondo, F.; Hong, S.G.; Peever, T.L.; Pryor, B.M. Molecular diversity and allergenic profiles of Alternaria spp. from desert environments in Arizona. Fungal Biol. 2018, 122, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Lü, X.G.; Li, F.M.; Wang, P. Research progress on sterilization of cold plasma in the field of food processing. Chin. Agric. Sci. Technol. Trib. 2017, 19, 96–102. [Google Scholar]
- Ge, H.; Chen, Q.; Kong, B.H. Recent advances in application of cold plasma technology in meat preservation and processing. Food Sci. 2019, 40, 286–292, (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Jiménez-Sánchez, C.; Lozano-Sánchez, J.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Alternatives to conventional thermal treatments in fruit-juice processing. Part 1: Techniques and applications. Crit. Rev. Food Sci. Nutr. 2017, 57, 501–523. [Google Scholar] [CrossRef] [PubMed]
- Shiekh, K.A.; Benjakul, S.; Qi, H.; Zhang, B.; Deng, S. Combined hurdle effects of pulsed electric field and vacuum impregnation of Chamuang leaf extract on quality and shelf-life of Pacific white shrimp subjected to high voltage cold atmospheric plasma. Food Packag. Shelf Life 2021, 28, 100660. [Google Scholar] [CrossRef]
- Ekonomou, S.I.; Boziaris, I.S. Non-thermal methods for ensuring the microbiological quality and safety of seafood. Appl. Sci. 2021, 11, 833. [Google Scholar] [CrossRef]
- Coutinho, N.M.; Silveira, M.R.; Rocha, R.S.; Moraes, J.; Ferreira, M.V.S.; Pimentel, T.C.; Freitas, M.Q.; Silva, M.C.; Raices, R.S.L.; Ranadheera, C.S.; et al. Cold plasma processing of milk and dairy products. Trends Food Sci. Technol. 2018, 74, 56–68. [Google Scholar] [CrossRef]
- Lacombe, A.; Niemira, B.A.; Gurtler, J.B.; Fan, X.; Sites, J.; Boyd, G.; Chen, H. Atmospheric cold plasma inactivation of aerobic microorganisms on blueberries and effects on quality attributes. Food Microbiol. 2015, 46, 479–484. [Google Scholar] [CrossRef]
- Dasan, B.G.; Boyaci, I.H.; Mutlu, M. Nonthermal plasma treatment of Aspergillus spp. spores on hazelnuts in an atmospheric pressure fluidized bed plasma system: Impact of process parameters and surveillance of the residual viability of spores. J. Food Eng. 2017, 196, 139–149. [Google Scholar] [CrossRef]
- Huang, Y.; Ye, X.P.; Doona, C.J.; Feeherry, F.E.; Radosevich, M.; Wang, S. An investigation of inactivation mechanisms of Bacillus amyloliquefaciens spores in non-thermal plasma of ambient air. J. Sci. Food Agric. 2019, 99, 368–378. [Google Scholar] [CrossRef]
- Bourke, P.; Ziuzina, D.; Boehm, D.; Cullen, P.J.; Keener, K. The potential of cold plasma for safe and sustainable food production. Trends Biotechnol. 2018, 36, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Umair, M.; Jabbar, S.; Senan, A.M.; Sultana, T.; Nasiru, M.M.; Shah, A.A.; Zhuang, H. Influence of combined effect of ultra-sonication and high-voltage cold plasma treatment on quality parameters of carrot juice. Foods 2019, 8, 593. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Wang, T.C.; Sun, Y.H.; Zhang, Y.; Wei, J.; Cai, R.; Guo, C.; Yuan, Y.; Yue, T. Role and mechanism of cold plasma in inactivating Alicyclobacillus acidoterrestris in apple juice. Foods 2023, 12, 1531. [Google Scholar] [CrossRef] [PubMed]
- Umair, M.; Jabbar, S.; Nasiru, M.M.; Senan, A.M.; Zhuang, H.; Zhang, J. Sequential application of high-voltage electric field cold plasma treatment and acid blanching improves the quality of fresh carrot juice (Daucus carota L.). J. Agric. Food Chem. 2020, 68, 15311–15318. [Google Scholar] [CrossRef]
- Li, X.; Li, M.; Ji, N.; Jin, P.; Zhang, J.; Zheng, Y.; Zhang, X.; Li, F. Cold plasma treatment induces phenolic accumulation and enhances antioxidant activity in fresh-cut pitaya (Hylocereus undatus) fruit. LWT 2019, 115, 108447. [Google Scholar] [CrossRef]
- Umair, M.; Sultana, T.; Xun, S.; Jabbar, S.; Rajoka, M.S.R.; Albahi, A.; Abid, M.; Ranjha, M.M.A.N.; El-Seedi, H.R.; Xie, F.; et al. Advances in the application of functional nanomaterial and cold plasma for the fresh-keeping active packaging of meat. Food Sci. Nutr. 2023, 11, 5753–5772. [Google Scholar] [CrossRef]
- Misra, N.N.; Jo, C. Applications of cold plasma technology for microbiological safety in meat industry. Trends Food Sci. Technol. 2017, 64, 74–86. [Google Scholar] [CrossRef]
- Rana, S.; Mehta, D.; Bansal, V.; Shivhare, U.S.; Yadav, S.K. Atmospheric cold plasma (ACP) treatment improved in-package shelf-life of strawberry fruit. J. Food Sci. Technol. 2020, 57, 102–112. [Google Scholar] [CrossRef]
- Park, N.S.; Yun, S.E.; Lee, H.Y.; Lee, H.J.; Choi, J.H.; Kim, G.C. No-ozone cold plasma can kill oral pathogenic microbes in H2O2-dependent and independent manner. Sci. Rep. 2022, 12, 7597. [Google Scholar] [CrossRef]
- Lee, S.Y.; Park, H.H.; Min, S.C. Pulsed light plasma treatment for the inactivation of Aspergillus flavus spores, Bacillus pumilus spores, and Escherichia coli O157:H7 in red pepper flakes. Food Control 2020, 118, 107401. [Google Scholar] [CrossRef]
- Ahmed, H.; Maunula, L.; Korhonen, J. Reduction of norovirus in foods by nonthermal treatments: A review. J. Food Prot. 2020, 83, 2053–2073. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Lin, Q.; Shi, W.Q.; Yu, X.; Wang, S. Food preservation by cold plasma from dielectric barrier discharges in agri-food industries. Front. Nutr. 2022, 9, 1015980. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xue, T.; Wang, S.; Jia, X.; Cao, S.; Niu, B.; Guo, R.; Yan, H. Preparation, characterization and food packaging application of nano ZnO@Xylan/quaternized xylan/polyvinyl alcohol composite films. Int. J. Biol. Macromol. 2022, 215, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Bhadra, P.; Sengupta, S.; Ratchagar, N.P.; Achar, B.; Chadha, A.; Bhattacharya, E. Selective transportation of charged ZnO nanoparticles and microorganism dialysis through silicon nanoporous membranes. J. Membr. Sci. 2016, 503, 16–24. [Google Scholar] [CrossRef]
- Zhang, H.; Hortal, M.; Jordá-Beneyto, M.; Rosa, E.; Lara-Lledo, M.; Lorente, I. ZnO-PLA nanocomposite coated paper for antimicrobial packaging application. LWT 2017, 78, 250–257. [Google Scholar] [CrossRef]
- Qi, K.Z.; Cheng, B.; Wang, Y.Y.; Ho, W. Review on the improvement of the photocatalytic and antibacterial activities of ZnO. J. Alloys Compd. 2017, 727, 792–820. [Google Scholar] [CrossRef]
- Goktas, A.; Modanlı, S.; Tumbul, A.; Kilic, A. Facile synthesis and characterization of ZnO, ZnO:Co, and ZnO/ZnO:Co nano rod-like homojunction thin films: Role of crystallite/grain size and microstrain in photocatalytic performance. J. Alloys Compd. 2022, 893, 162334. [Google Scholar] [CrossRef]
- Ma, H.J.; Hao, B.F.; Wei, S.; Guo, J.; Li, M.; Zhang, L. A high-efficiency TiO2/ZnO nano-film with surface oxygen vacancies for dye degradation. Materials 2021, 14, 3299. [Google Scholar] [CrossRef]
- Li, J.; Zhang, G.T.; Zhang, D.J. Structural optimization of sorghum straw powder/ZnO/PVA nanocomposite films. Coatings 2022, 12, 1226. [Google Scholar] [CrossRef]
- Wang, L.; Lin, L.Z.; Chen, X.H.; Tong, C.; Pang, J. Synthesis and characteristics of konjac glucomannan films incorporated with functionalized microcrystalline cellulose. Colloids Surf. A Physicochem. Eng. Asp. 2019, 563, 237–245. [Google Scholar] [CrossRef]
- GB 4789.2-2016; Standardization Administration of China. Determination of Total Colony Count. Standards Press of China: Beijing, China, 2016.
- Nerdy, N. Determination of vitamin C in various colours of bell pepper (Capsicum annuum L.) by titration method. ALCHEMY J. Penelit. Kim. 2018, 14, 164–177. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, Z.; Cai, H.; Wang, L.; Hu, C.; Li, D.; Chen, Y.; Kang, Y.; Li, L. Controlled moisture permeability of thermoplastic starch/polylactic acid/poly butylene adipate-co-terephthalate film for the autolysis of straw mushroom Volvariella volvacea. Food Chem. 2022, 373, 131409. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.X.; Zhou, H.J.; Guo, G.Y.; Tan, J.; Wang, Q.; Tang, J.; Liu, W.; Shen, H.; Li, J.; Zhang, X. Enhanced anti-infective efficacy of ZnO nanoreservoirs through a combination of intrinsic anti-biofilm activity and reinforced innate defense. ACS Appl. Mater. Interfaces 2017, 9, 33609–33623. [Google Scholar] [CrossRef] [PubMed]
- Kairyte, K.; Kadys, A.; Luksiene, Z. Antibacterial and antifungal activity of photoactivated ZnO nanoparticles in suspension. J. Photochem. Photobiol. B Biol. 2013, 128, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhao, W.; Zhang, Q.; Gao, G.; Meng, Y. Effect of cold plasma treatment on sterilizing rate and quality of kiwi turbid juice. J. Food Process Eng. 2021, 44, e13711. [Google Scholar] [CrossRef]
- Yi, F.; Wang, J.M.; Xiang, Y.; Wang, J.M.; Xiang, Y. Physiological and quality changes in fresh-cut mango fruit as influenced by cold plasma. Postharvest Biol. Technol. 2022, 194, 112105. [Google Scholar] [CrossRef]
- Wei, Q.Y.; Yuan, Y.; Gao, T.; Zhang, J.H.; Yan, W.J. Optimization of cold sterilization process and its effect on quality of dried jujube by high voltage electric field cold plasma. J. Sci. Technol. Food Indus. 2021, 42, 317–324, (In Chinese with English abstract). [Google Scholar]
- Liu, Y.N.; Sun, Y.Y.; Wang, Y.H.; Zhao, Y.; Duan, M.; Wang, H.; Dai, R.; Liu, Y.; Li, X.; Jia, F. Inactivation mechanisms of atmospheric pressure plasma jet on Bacillus cereus spores and its application on low-water activity foods. Food Res. Int. 2023, 169, 112867. [Google Scholar] [CrossRef]
- Choi, M.S.; Jeon, E.B.; Kim, J.Y.; Choi, E.H.; Lim, J.S.; Choi, J.; Park, S.Y. Impact of non-thermal dielectric barrier discharge plasma on Staphylococcus aureus and Bacillus cereus and quality of dried blackmouth angler (Lophiomus setigerus). J. Food Eng. 2020, 278, 109952. [Google Scholar] [CrossRef]
- Zhuang, H.; Rothrock, M.J., Jr.; Hiett, K.L.; Lawrence, K.C.; Gamble, G.R.; Bowker, B.C.; Keener, K.M. In-package air cold plasma treatment of chicken breast meat: Treatment time effect. J. Food Qual. 2019, 2019, 1837351. [Google Scholar] [CrossRef]
- Lee, J.Y.; Park, H.J.; Lee, C.Y.; Choi, W.Y. Extending shelf-life of minimally processed apples with edible coatings and antibrowning agents. LWT 2003, 36, 323–329. [Google Scholar] [CrossRef]
- Kongboonkird, M.; Chuesiang, P.; Ryu, V.; Siripatrawan, U. Effects of argon dielectric barrier discharge (DBD) cold plasma treatment on mechanical, barrier and antimicrobial properties of chitosan film. Int. J. Food Sci. Technol. 2023, 58, 995–1006. [Google Scholar] [CrossRef]
- Du, Y.H.; Mi, S.N.; Wang, H.H.; Yang, F.W.; Yu, H.; Xie, Y.F.; Guo, Y.H.; Cheng, Y.L.; Yao, W.R. Inactivation mechanism of Alternaria alternata by dielectric barrier discharge plasma and its quality control on fresh wolfberries. Food Control 2023, 148, 109620. [Google Scholar] [CrossRef]
- Sarangapani, C.; O’Toole, G.; Cullen, P.J.; Bourke, P. Atmospheric cold plasma dissipation efficiency of agrochemicals on blueberries. Innov. Food Sci. Emerg. Technol. 2017, 44, 235–241. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, R.; Gan, Z.; Shao, T.; Zhang, X.; He, M.; Sun, A. Effect of cold plasma on blueberry juice quality. Food Chem. 2019, 290, 79–86. [Google Scholar] [CrossRef]
- Dvoracek, V.; Janovska, D.; Papouskova, L.; Bicanová, E. Post-harvest content of free titratable acids in the grain of proso millet varieties (Panicum milliaceum L.), and changes during grain processing and storage. Czech. J. Genet. Plant Breed. 2010, 46, S90–S95. [Google Scholar] [CrossRef]
- Jia, S.T.; Zhang, N.; Ji, H.P.; Zhang, X.J.; Dong, C.H.; Yu, J.Z.; Yan, S.J.; Chen, C.K.; Liang, L.Y. Effects of atmospheric cold plasma treatment on the storage quality and chlorophyll metabolism of postharvest tomato. Foods 2022, 11, 4088. [Google Scholar] [CrossRef]
- Li, F.; Min, D.; Song, B.; Shao, S.; Zhang, X. Ethylene effects on apple fruit cuticular wax composition and content during cold storage. Postharvest Biol. Technol. 2017, 134, 98–105. [Google Scholar] [CrossRef]
- Bozkurt, F.; Tornuk, F.; Toker, O.S.; Karasu, S.; Arici, M.; Durak, M.Z. Effect of vaporized ethyl pyruvate as a novel preservation agent for control of postharvest quality and fungal damage of strawberry and cherry fruits. LWT 2016, 65, 1044–1049. [Google Scholar] [CrossRef]




| Level | Factor | ||
|---|---|---|---|
| Processing Voltage (kV) | Processing Frequency (Hz) | Processing Time (s) | |
| 1 | 55 | 55 | 60 |
| 2 | 65 | 75 | 90 |
| 3 | 75 | 95 | 120 |
| 4 | 85 | 115 | 150 |
| 5 | 95 | 135 | 180 |
| Factor | Level | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| X1/processing voltage (kV) | 65 | 75 | 85 |
| X2/processing frequency (Hz) | 75 | 95 | 115 |
| X3/processing time (s) | 90 | 120 | 150 |
| Microbial Species | Inhibition Zone Diameter (mm) |
|---|---|
| Escherichia coli | 16.05 ± 0.56 b |
| Staphylococcus aureus | 18.57 ± 0.86 a |
| Pichia pastoris | 13.84 ± 0.72 c |
| Aspergillus niger | 13.96 ± 0.94 c |
| Test Number | X1 | X2 | X3 | lg (CFU·g−1) | |
|---|---|---|---|---|---|
| Actual Value | Predicted Value | ||||
| 1 | −1 | −1 | 0 | 3.33 | 3.39 |
| 2 | 1 | −1 | 0 | 2.73 | 2.82 |
| 3 | −1 | 1 | 0 | 3.39 | 3.30 |
| 4 | 1 | 1 | 0 | 2.68 | 2.62 |
| 5 | −1 | 0 | −1 | 3.20 | 3.20 |
| 6 | 1 | 0 | −1 | 2.52 | 2.50 |
| 7 | −1 | 0 | 1 | 3.30 | 3.32 |
| 8 | 1 | 0 | 1 | 2.78 | 2.78 |
| 9 | 0 | −1 | −1 | 3.12 | 3.06 |
| 10 | 0 | 1 | −1 | 2.89 | 2.97 |
| 11 | 0 | −1 | 1 | 3.40 | 3.32 |
| 12 | 0 | 1 | 1 | 3.06 | 3.12 |
| 13 | 0 | 0 | 0 | 2.37 | 2.32 |
| 14 | 0 | 0 | 0 | 2.24 | 2.32 |
| 15 | 0 | 0 | 0 | 2.20 | 2.32 |
| 16 | 0 | 0 | 0 | 2.50 | 2.32 |
| 17 | 0 | 0 | 0 | 2.28 | 2.32 |
| Source | Sum of Squares | df | Mean Square | F Value | p-Value |
|---|---|---|---|---|---|
| Model | 2.78 | 9 | 0.3093 | 21.25 | 0.0003 ** |
| X1/processing voltage (kV) | 0.7875 | 1 | 0.7875 | 54.12 | 0.0002 ** |
| X2/processing frequency (Hz) | 0.0392 | 1 | 0.0392 | 2.69 | 0.1447 |
| X3/processing time (s) | 0.0820 | 1 | 0.0820 | 5.64 | 0.0493 * |
| X1X2 | 0.0030 | 1 | 0.0030 | 0.2079 | 0.6622 |
| X1X3 | 0.0064 | 1 | 0.0064 | 0.4398 | 0.5284 |
| X2X3 | 0.0030 | 1 | 0.0030 | 0.2079 | 0.6622 |
| X12 | 0.3150 | 1 | 0.3150 | 21.65 | 0.0023 ** |
| X22 | 0.8189 | 1 | 0.8189 | 56.28 | 0.0001 ** |
| X32 | 0.5411 | 1 | 0.5411 | 37.19 | 0.0005 ** |
| Residual | 0.1019 | 7 | 0.0146 | ||
| Lack of fit | 0.0446 | 3 | 0.0149 | 1.04 | 0.4663 |
| Pure error | 0.0573 | 4 | 0.0143 | ||
| Cor total | 2.89 | 16 | |||
| CV = 4.27% | |||||
| R2 = 0.9647 | |||||
| R2Adj = 0.9193 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Zhang, G.; Zhang, Z.; Zhang, Y.; Zhang, D. Synergistic Microbial Inhibition and Quality Preservation for Grapes through High-Voltage Electric Field Cold Plasma and Nano-ZnO Antimicrobial Film Treatment. Foods 2023, 12, 4234. https://doi.org/10.3390/foods12234234
Li J, Zhang G, Zhang Z, Zhang Y, Zhang D. Synergistic Microbial Inhibition and Quality Preservation for Grapes through High-Voltage Electric Field Cold Plasma and Nano-ZnO Antimicrobial Film Treatment. Foods. 2023; 12(23):4234. https://doi.org/10.3390/foods12234234
Chicago/Turabian StyleLi, Juan, Guantao Zhang, Zitong Zhang, Yuan Zhang, and Dongjie Zhang. 2023. "Synergistic Microbial Inhibition and Quality Preservation for Grapes through High-Voltage Electric Field Cold Plasma and Nano-ZnO Antimicrobial Film Treatment" Foods 12, no. 23: 4234. https://doi.org/10.3390/foods12234234
APA StyleLi, J., Zhang, G., Zhang, Z., Zhang, Y., & Zhang, D. (2023). Synergistic Microbial Inhibition and Quality Preservation for Grapes through High-Voltage Electric Field Cold Plasma and Nano-ZnO Antimicrobial Film Treatment. Foods, 12(23), 4234. https://doi.org/10.3390/foods12234234





