Elderberry Lipophilic and Hydrophilic Bioactive Compounds: Characterization and Extract Encapsulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Invention Information
2.2. Chemicals and Reagents
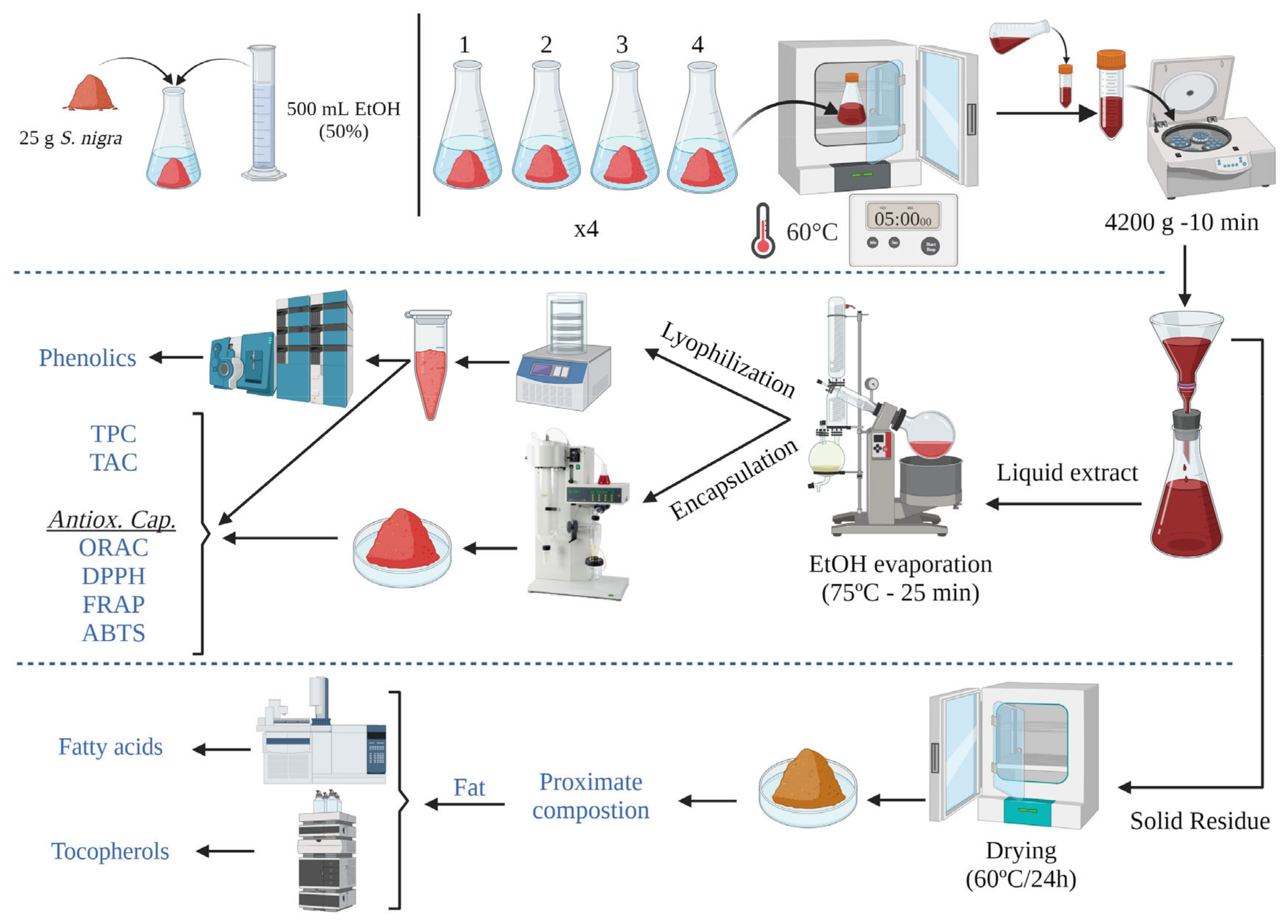
2.3. Elderberry Material and Experimental Design
2.4. Solid Residue Characterization
2.4.1. Proximate Composition
2.4.2. Fatty Acid Composition
2.4.3. Tocopherol Composition
2.5. Encapsulated and/or Lyophilized Extract Characterization
2.5.1. Determination of Total Phenolic Compounds (TPC) and Anthocyanins (TAC)
2.5.2. Characterization of Phenolic Compounds
2.5.3. Determination of In Vitro Antioxidant Capacity
2.6. Statistical Analysis
3. Results and Discussion
3.1. Proximate Composition and Lipophilic Bioactive Compounds of the Solid Residue
3.2. Main Bioactive Compounds and Antioxidant Capacity of Lyophilized and Encapsulated Elderberry Extracts
3.3. Phenolic Compounds of Lyophilized Elderberry Extracts
4. Conclusions
5. Patents
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Uhl, K.R.; Fyhrie, K.J.; Brodt, S.B.; Mitchell, A.E. Blue Elderberry (Sambucus nigra ssp. cerulea): A Robust and Underutilized Fruit for Value-Added Products. ACS Food Sci. Technol. 2022, 2, 347–358. [Google Scholar] [CrossRef]
- Costa, C.P.; Patinha, S.; Rudnitskaya, A.; Santos, S.A.O.; Silvestre, A.J.D.; Rocha, S.M. Sustainable Valorization of Sambucus nigra L. Berries: From Crop Biodiversity to Nutritional Value of Juice and Pomace. Foods 2022, 11, 104. [Google Scholar] [CrossRef]
- Domínguez, R.; Zhang, L.; Rocchetti, G.; Lucini, L.; Pateiro, M.; Munekata, P.E.S.; Lorenzo, J.M. Elderberry (Sambucus nigra L.) as potential source of antioxidants. Characterization, optimization of extraction parameters and bioactive properties. Food Chem. 2020, 330, 127266. [Google Scholar] [CrossRef] [PubMed]
- Młynarczyk, K.; Walkowiak-Tomczak, D.; Staniek, H.; Kidoń, M.; Łysiak, G.P. The Content of Selected Minerals, Bioactive Compounds, and the Antioxidant Properties of the Flowers and Fruit of Selected Cultivars and Wildly Growing Plants of Sambucus nigra L. Molecules 2020, 25, 876. [Google Scholar] [CrossRef] [PubMed]
- Kitryt, V.; Syrpas, M.; Pukalskas, A. Modeling and optimization of supercritical carbon dioxide extraction for isolation of valuable lipophilic constituents from elderberry (Sambucus nigra L.) pomace. J. CO2 Util. 2020, 35, 225–235. [Google Scholar] [CrossRef]
- Młynarczyk, K.; Walkowiak-Tomczak, D.; Łysiak, G.P. Bioactive properties of Sambucus nigra L. As a functional ingredient for food and pharmaceutical industry. J. Funct. Foods 2018, 40, 377–390. [Google Scholar] [CrossRef]
- Waswa, E.N.; Li, J.; Mkala, E.M.; Wanga, V.O.; Mutinda, E.S.; Nanjala, C.; Odago, W.O.; Katumo, D.M.; Gichua, M.K.; Gituru, R.W.; et al. Ethnobotany, phytochemistry, pharmacology, and toxicology of the genus Sambucus L. (Viburnaceae). J. Ethnopharmacol. 2022, 292, 115102. [Google Scholar] [CrossRef] [PubMed]
- Gull, A.; Sheikh, M.A.; Kour, J.; Zehra, B.; Zargar, I.A.; Wani, A.A.; Bhatia, S.; Lone, M.A. Anthocyanins. In Nutraceuticals and Health Care; Kour, J., Nayik, G.A., Eds.; Academic Press: Cambridge, MA, USA, 2022; Volume 1, pp. 317–329. ISBN 9780323897792. [Google Scholar]
- Domínguez, R.; Pateiro, M.; Munekata, P.E.S.; López, E.M.S.; Rodríguez, J.A.; Barros, L.; Lorenzo, J.M. Potential Use of Elderberry (Sambucus nigra L.) as Natural Colorant and Antioxidant in the Food Industry. A Review. Foods 2021, 10, 2713. [Google Scholar] [CrossRef]
- Baeza, R.; Sánchez, V.; Salierno, G.; Molinari, F.; López, P.; Chirife, J. Storage stability of anthocyanins in freeze-dried elderberry pulp using low proportions of encapsulating agents. Food Sci. Technol. Int. 2021, 27, 135–144. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Silva, P.; Silva, A.M.; Nunes, F.M. Effect of harvesting year and elderberry cultivar on the chemical composition and potential bioactivity: A three-year study. Food Chem. 2020, 302, 125366. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Todorovic, B.; Veberic, R.; Stampar, F.; Ivancic, A. Investigation of anthocyanin profile of four elderberry species and interspecific hybrids. J. Agric. Food Chem. 2014, 62, 5573–5580. [Google Scholar] [CrossRef] [PubMed]
- Fazio, A.; Plastina, P.; Meijerink, J.; Witkamp, R.F.; Gabriele, B. Comparative analyses of seeds of wild fruits of Rubus and Sambucus species from Southern Italy: Fatty acid composition of the oil, total phenolic content, antioxidant and anti-inflammatory properties of the methanolic extracts. Food Chem. 2013, 140, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; He, X.Q.; Wu, D.T.; Li, H.B.; Feng, Y.B.; Zou, L.; Gan, R.Y. Elderberry (Sambucus nigra L.): Bioactive Compounds, Health Functions, and Applications. J. Agric. Food Chem. 2022, 70, 4202–4220. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, R.; Orsat, V. Spray Drying of Elderberry (Sambucus nigra L.) Juice to Maintain Its Phenolic Content. Dry. Technol. 2011, 29, 1729–1740. [Google Scholar] [CrossRef]
- Neves, C.M.B.; Pinto, A.; Gonçalves, F.; Wessel, D.F. Changes in Elderberry (Sambucus nigra L.) Juice Concentrate Polyphenols during Storage. Appl. Sci. 2021, 11, 6941. [Google Scholar] [CrossRef]
- Busso Casati, C.; Baeza, R.; Sánchez, V. Physicochemical properties and bioactive compounds content in encapsulated freeze-dried powders obtained from blueberry, elderberry, blackcurrant and maqui berry. J. Berry Res. 2019, 9, 431–447. [Google Scholar] [CrossRef]
- Mattson, M.L.G.; Corfield, R.; Bajda, L.; Pérez, O.E.; Schebor, C.; Salvatori, D. Potential bioactive ingredient from elderberry fruit: Process optimization for a maximum phenolic recovery, physicochemical characterization, and bioaccesibility. J. Berry Res. 2021, 11, 51–68. [Google Scholar] [CrossRef]
- Gagneten, M.; Corfield, R.; Mattson, M.G.; Sozzi, A.; Leiva, G.; Salvatori, D.; Schebor, C. Spray-dried powders from berries extracts obtained upon several processing steps to improve the bioactive components content. Powder Technol. 2019, 342, 1008–1015. [Google Scholar] [CrossRef]
- Ribeiro, A.M.; Estevinho, B.N.; Rocha, F. Spray Drying Encapsulation of Elderberry Extract and Evaluating the Release and Stability of Phenolic Compounds in Encapsulated Powders. Food Bioprocess Technol. 2019, 12, 1381–1394. [Google Scholar] [CrossRef]
- Aksu, M.İ.; Turan, E.; Gülbandılar, A.; Tamtürk, F. Utilization of spray-dried raspberry powder as a natural additive to improve oxidative stability, microbial quality and overcome the perception of discoloration in vacuum-packed ground beef during chilled storage. Meat Sci. 2023, 197, 109072. [Google Scholar] [CrossRef]
- Anusha Siddiqui, S.; Redha, A.A.; Esmaeili, Y.; Mehdizadeh, M. Novel insights on extraction and encapsulation techniques of elderberry bioactive compounds. Crit. Rev. Food Sci. Nutr. 2023, 63, 5937–5952. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, T.; Ferreira, T.; Ferreira, J.; Teixeira, F.; Ferreira, D.; Silva-Reis, R.; Neuparth, M.J.; Pires, M.J.; Pinto, M.d.L.; Gil da Costa, R.M.; et al. Supplementation of an Anthocyanin-Rich Elderberry (Sambucus nigra L.) Extract in FVB/n Mice: A Healthier Alternative to Synthetic Colorants. Appl. Sci. 2022, 12, 11928. [Google Scholar] [CrossRef]
- Rocchetti, G.; Becchi, P.P.; Lucini, L.; Cittadini, A.; Munekata, P.E.S.; Pateiro, M.; Domínguez, R.; Lorenzo, J.M. Elderberry (Sambucus nigra L.) Encapsulated Extracts as Meat Extenders against Lipid and Protein Oxidation during the Shelf-Life of Beef Burgers. Antioxidants 2022, 11, 2130. [Google Scholar] [CrossRef]
- ISO 1442; International Standards Meat and Meat Products—Determination of Moisture Content. International Organization for Standarization: Geneva, Switzerland, 1997.
- ISO 937; International Standards Meat and Meat Products—Determination of Nitrogen Content. International Organization for Standarization: Geneva, Switzerland, 1978.
- ISO 936; International Standards Meat and Meat Products—Determination of Ash Content. International Organization for Standarization: Geneva, Switzerland, 1998.
- AOCS. AOCS Official Procedure Am5-04. Rapid Determination of Oil/Fat Utilizing High Temperature Solvent Extraction; American Oil Chemists Society: Urbana, IL, USA, 2005. [Google Scholar]
- Barros, J.C.; Munekata, P.E.S.; De Carvalho, F.A.L.; Pateiro, M.; Barba, F.J.; Domínguez, R.; Trindade, M.A.; Lorenzo, J.M. Use of tiger nut (Cyperus esculentus L.) oil emulsion as animal fat replacement in beef burgers. Foods 2020, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Bessada, S.M.F.; Barreira, J.C.M.; Barros, L.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P. Phenolic profile and antioxidant activity of Coleostephus myconis (L.) Rchb.f.: An underexploited and highly disseminated species. Ind. Crops Prod. 2016, 89, 45–51. [Google Scholar] [CrossRef]
- Franco, D.; Munekata, P.E.S.; Agregán, R.; Bermúdez, R.; López-Pedrouso, M.; Pateiro, M.; Lorenzo, J.M. Application of pulsed electric fields for obtaining antioxidant extracts from fish residues. Antioxidants 2020, 9, 90. [Google Scholar] [CrossRef]
- Sidor, A.; Gramza-Michałowska, A. Advanced research on the antioxidant and health benefit of elderberry (Sambucus nigra) in food—A review. J. Funct. Foods 2015, 18, 941–958. [Google Scholar] [CrossRef]
- Künsch, U.; Temperli, A. Changes in free and protein-bound amino acids in elderberry fruit (Sambucus nigra) during maturation. J. Sci. Food Agric. 1978, 29, 1037–1040. [Google Scholar] [CrossRef]
- Câmara, J.S.; Albuquerque, B.R.; Aguiar, J.; Corrêa, R.C.G.; Gonçalves, J.L.; Granato, D.; Pereira, J.A.M.; Barros, L.; Ferreira, I.C.F.R. Food Bioactive Compounds and Emerging Techniques for Their Extraction: Polyphenols as a Case Study. Foods 2021, 10, 37. [Google Scholar] [CrossRef]
- Duymuş, H.G.; Göger, F.; Başer, K.H.C. In vitro antioxidant properties and anthocyanin compositions of elderberry extracts. Food Chem. 2014, 155, 112–119. [Google Scholar] [CrossRef]
- Silva, P.; Ferreira, S.; Nunes, F.M. Elderberry (Sambucus nigra L.) by-products a source of anthocyanins and antioxidant polyphenols. Ind. Crops Prod. 2017, 95, 227–234. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Domingues, M.R.; Barros, C.; Santos, S.A.O.; Silvestre, A.J.D.; Silva, A.M.; Nunes, F.M. Major anthocyanins in elderberry effectively trap methylglyoxal and reduce cytotoxicity of methylglyoxal in HepG2 cell line. Food Chem. X 2022, 16, 100468. [Google Scholar] [CrossRef] [PubMed]
- Mikulic-Petkovsek, M.; Ivancic, A.; Todorovic, B.; Veberic, R.; Stampar, F. Fruit Phenolic Composition of Different Elderberry Species and Hybrids. J. Food Sci. 2015, 80, C2180–C2190. [Google Scholar] [CrossRef] [PubMed]
- Senica, M.; Stampar, F.; Veberic, R.; Mikulic-Petkovsek, M. Processed elderberry (Sambucus nigra L.) products: A beneficial or harmful food alternative? LWT Food Sci. Technol. 2016, 72, 182–188. [Google Scholar] [CrossRef]
- Veberic, R.; Jakopic, J.; Stampar, F.; Schmitzer, V. European elderberry (Sambucus nigra L.) rich in sugars, organic acids, anthocyanins and selected polyphenols. Food Chem. 2009, 114, 511–515. [Google Scholar] [CrossRef]

| Solid Residue | |||
|---|---|---|---|
| Values | Min | Max | |
| Proximate Composition (g/100 g) | |||
| Moisture | 18.34 ± 6.10 | 10.31 | 23.93 |
| Fat † | 17.07 ± 1.25 | 15.28 | 18.75 |
| Protein † | 15.66 ± 0.55 | 14.78 | 16.43 |
| Ash † | 1.36 ± 0.10 | 1.23 | 1.51 |
| Carbohydrates † | 65.91 ± 1.25 | 64.42 | 68.32 |
| Tocopherols (µg/g of oil) | |||
| α-Tocopherol | 317 ± 37.5 | 260.53 | 360.30 |
| β-Tocopherol | 6.24 ± 0.72 | 5.25 | 7.44 |
| δ-Tocopherol | 463 ± 48.2 | 396.36 | 518.96 |
| γ-Tocopherol | 4.61 ± 0.49 | 3.99 | 5.29 |
| Total Tocopherols | 791 ± 81.4 | 667.06 | 877.70 |
| Fatty acids * (g/100 g of oil) | |||
| C16:0 (palmitic acid) | 6.12 ± 0.25 | 5.70 | 6.43 |
| C18:0 (stearic acid) | 1.56 ± 0.03 | 1.52 | 1.61 |
| C18:1 n-9 (oleic acid) | 10.72 ± 0.21 | 10.33 | 11.07 |
| C18:1 n-7 (vaccenic acid) | 0.68 ± 0.03 | 0.63 | 0.71 |
| C18:2 n-6 (linoleic acid) | 32.88 ± 0.43 | 32.04 | 33.46 |
| C18:3 n-3 (α-linolenic acid) | 32.56 ± 0.47 | 31.68 | 33.19 |
| 9c,11t-C18:2 (conjugated linoleic acid) | 0.17 ± 0.02 | 0.13 | 0.21 |
| C20:0 (arachidic acid) | 0.17 ± 0.01 | 0.15 | 0.18 |
| C20:1 n-9 (cis-11-eicosenoic acid) | 0.13 ± 0.003 | 0.13 | 0.14 |
| C22:0 (behenic acid) | 0.15 ± 0.01 | 0.13 | 0.18 |
| SFA | 8.50 ± 0.32 | 7.92 | 8.85 |
| MUFA | 11.80 ± 0.27 | 11.30 | 12.20 |
| PUFA | 65.75 ± 0.91 | 63.98 | 66.98 |
| Σ n-3 | 32.59 ± 0.47 | 31.72 | 33.23 |
| Σ n-6 | 32.98 ± 0.43 | 32.14 | 33.57 |
| n-6/n-3 | 1.01 ± 0.00 | 1.01 | 1.02 |
| Total fatty acids | 86.05 ± 1.45 | 83.21 | 88.02 |
| Extracts | Sig. | ||
|---|---|---|---|
| Lyophilized | Encapsulated | ||
| Bioactive compounds | |||
| Total phenolic compounds (mg GAE/100 g) | 7486 ± 395 | 1132 ± 168 | *** |
| Anthocyanins (mg/100 g) | 520 ± 39.2 | 60.0 ± 7.55 | *** |
| Antioxidant Capacity | |||
| DPPH (mg Trolox/g) | 39.92 ± 1.73 | 3.84 ± 0.47 | *** |
| ABTS (mg ascorbic acid/g) | 145.9 ± 7.4 | 12.91 ± 1.66 | *** |
| FRAP (mmol Fe2+/100 g) | 177.7 ± 8.76 | 12.27 ± 1.98 | *** |
| ORAC (mg Trolox/g) | 286 ± 23.3 | 46.0 ± 13.1 | *** |
| IC50 (mg/mL) | 4.24 ± 0.44 | 34.5 ± 7.81 | *** |
| Non-Anthocyanin | ||||||
|---|---|---|---|---|---|---|
| Peak No. | Rt (min) | λ Max (nm) | [M-H]− (m/z) | MS2 | Tentative Identification | Quantification (mg/100 g of Extract) |
| 1 | 5.83 | 325 | 353 | 191(100), 179(9), 173(19), 161(5) | cis 5-O-Caffeoylquinic acid | 218 ± 8.30 |
| 2 | 6.63 | 327 | 353 | 191(100), 179(6), 173(21), 161(9) | trans 5-O-Caffeoylquinic acid | 286 ± 17.0 |
| 3 | 16.52 | 353 | 609 | 301(100) | Quercetin-3-O-rutinoside | 218 ± 14.5 |
| Total non-anthocyanin | 722 ± 31.5 | |||||
| Anthocyanin | ||||||
| 4 | 27.44 | 515 | 743 | 581(72), 449(100), 287(78) | Cyanidin-O-sambubioside-O-hexoside | 525 ± 57.1 |
| 5 | 27.89 | 515 | 743 | 581(72), 449(100), 287(78) | Cyanidin-3-O-sambubioside-5-O-glucoside | 943 ± 53.8 |
| 6 | 30.97 | 518 | 581 | 287(100) | Cyanidin-3-O-sambubioside | 4827 ± 546 |
| 7 | 31.92 | 517 | 449 | 287(100) | Cyanidin-3-O-glucoside | 4926 ± 756 |
| Total anthocyanin | 11,221 ± 1143 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domínguez-Valencia, R.; Cittadini, A.; Pateiro, M.; Munekata, P.E.S.; Lorenzo, J.M. Elderberry Lipophilic and Hydrophilic Bioactive Compounds: Characterization and Extract Encapsulation. Foods 2023, 12, 4233. https://doi.org/10.3390/foods12234233
Domínguez-Valencia R, Cittadini A, Pateiro M, Munekata PES, Lorenzo JM. Elderberry Lipophilic and Hydrophilic Bioactive Compounds: Characterization and Extract Encapsulation. Foods. 2023; 12(23):4233. https://doi.org/10.3390/foods12234233
Chicago/Turabian StyleDomínguez-Valencia, Rubén, Aurora Cittadini, Mirian Pateiro, Paulo E. S. Munekata, and José M. Lorenzo. 2023. "Elderberry Lipophilic and Hydrophilic Bioactive Compounds: Characterization and Extract Encapsulation" Foods 12, no. 23: 4233. https://doi.org/10.3390/foods12234233
APA StyleDomínguez-Valencia, R., Cittadini, A., Pateiro, M., Munekata, P. E. S., & Lorenzo, J. M. (2023). Elderberry Lipophilic and Hydrophilic Bioactive Compounds: Characterization and Extract Encapsulation. Foods, 12(23), 4233. https://doi.org/10.3390/foods12234233










