Effects of Allium macrostemon Bunge Extract on Adipose Tissue Inflammation and Hepatic Endoplasmic Reticulum Stress in High-Fat Diet-Fed and Bisphenol A-Treated C57BL/6N Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Allium macrostemon Bunge (AM) Preparation and Chemical Analysis
2.1.1. Preparation of AM Extract
2.1.2. Mass Spectrometry Data Acquisition for Chemical Analysis of AM Extract
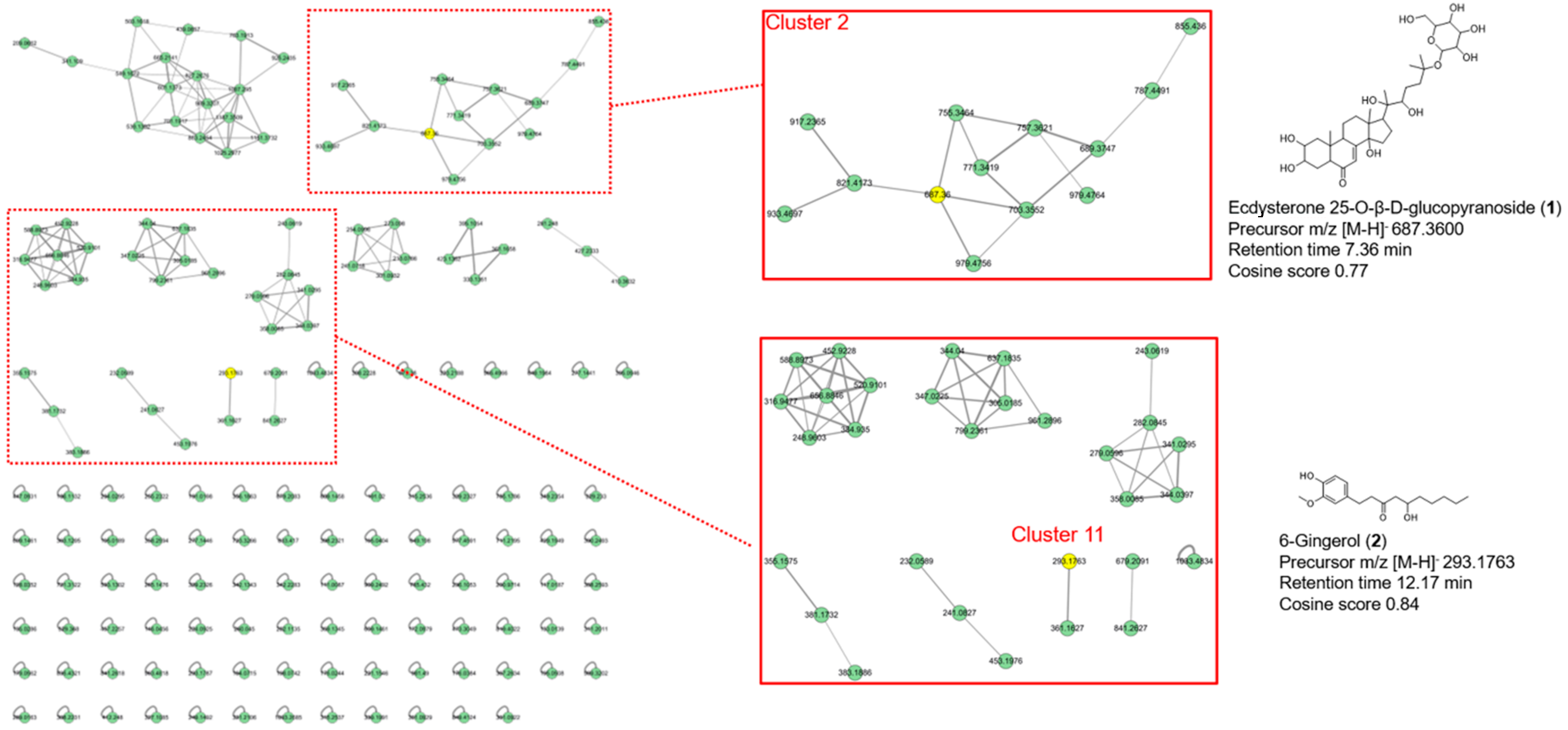
2.1.3. Feature-Based Molecular Networking Analysis of AM Extract
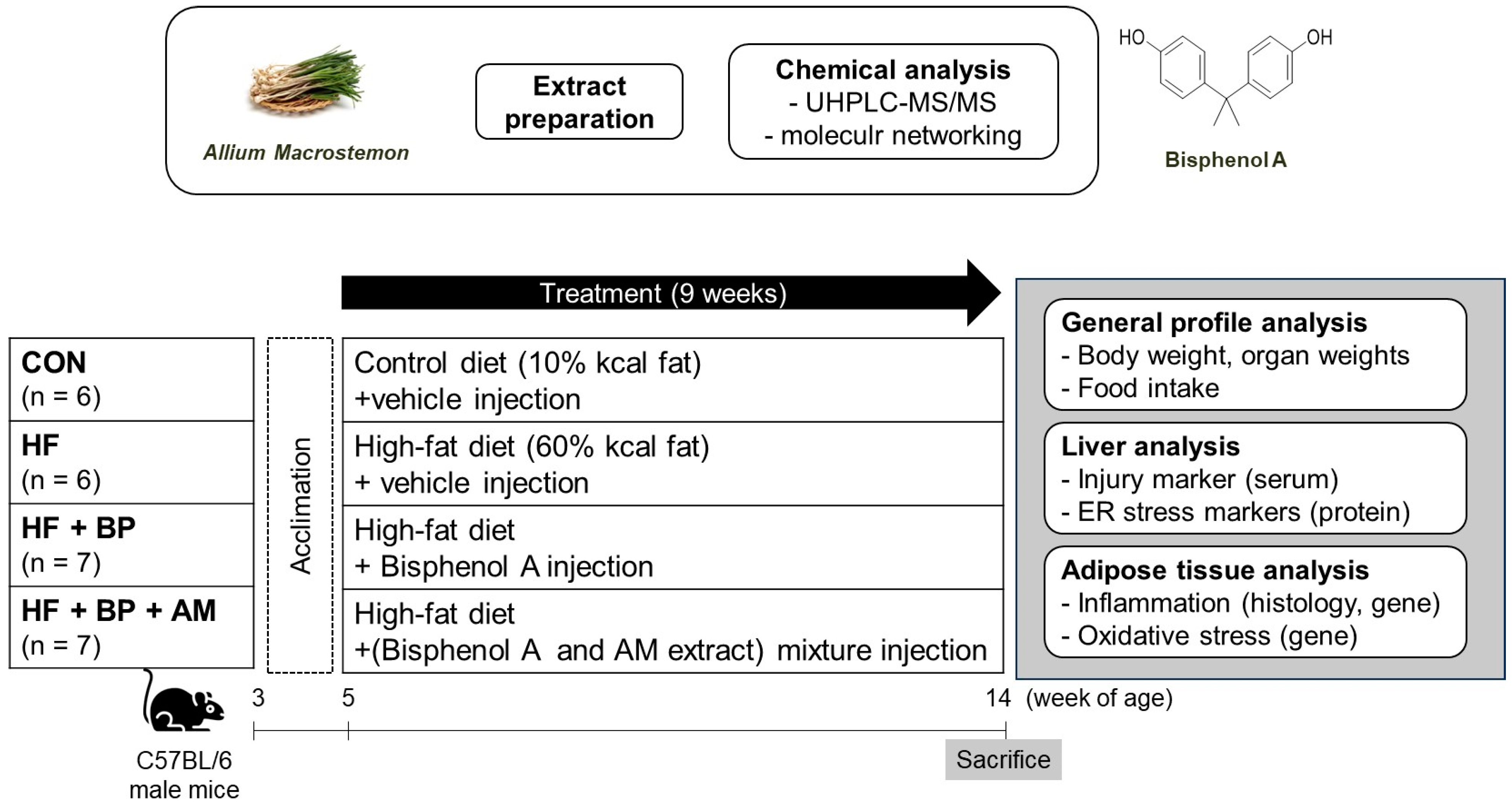
2.2. Animal Experiment
2.2.1. Animals and Experimental Design
- (i)
- the control (CON) group fed a control diet (n = 6);
- (ii)
- the high-fat (HF) group fed an HF diet (n = 6);
- (iii)
- the HF + BP group fed an HF diet and treated with BPA (n = 7);
- (iv)
- the HF + BP + AM group fed an HF diet and treated with BPA and AM extract (n = 7).
2.2.2. Serum and Tissue Isolation
2.2.3. Histological Analysis for Adipose Tissue
2.2.4. RNA Extraction, cDNA Synthesis, and Real-Time Polymerase Chain Reaction for Adipose Tissue
2.2.5. Protein Extraction and Western Blotting Analysis for Liver Tissue
2.3. Statistical Analysis
3. Results
3.1. Effects of AM Supplementation on Body Weight Gain, Energy Intake, Organ Weights, and Serum Analysis
3.2. Effects of AM Supplementation on Adipose Tissue Inflammation and Oxidative Stress
3.3. Effects of AM Supplementation on Hepatic ER Stress and Autophagy
3.4. Identification of Compounds in AM Extract by UHPLC-MS/MS and Molecular Network Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Nguyen, T.H.D.; Vu, D.C. Food Delivery Service During Social Distancing: Proactively Preventing or Potentially Spreading Coronavirus Disease-2019? Disaster Med. Public Health Prep. 2020, 14, e9–e10. [Google Scholar] [CrossRef]
- Manzoor, M.F.; Tariq, T.; Fatima, B.; Sahar, A.; Tariq, F.; Munir, S.; Khan, S.; Nawaz Ranjha, M.M.A.; Sameen, A.; Zeng, X.A.; et al. An insight into bisphenol A, food exposure and its adverse effects on health: A review. Front. Nutr. 2022, 9, 1047827. [Google Scholar] [CrossRef]
- Rochester, J.R. Bisphenol A and human health: A review of the literature. Reprod. Toxicol. 2013, 42, 132–155. [Google Scholar] [CrossRef]
- Luo, S.; Li, Y.; Lu, X.; Jiang, J.; Zhu, Q.; Shen, T. Effects of bisphenol A on inflammation and Th17 cells in adipose tissue of high-fat fed mice. Wei Sheng Yan Jiu 2017, 46, 7–12. [Google Scholar] [PubMed]
- Cusi, K. The role of adipose tissue and lipotoxicity in the pathogenesis of type 2 diabetes. Curr. Diabetes Rep. 2010, 10, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Guzik, T.J.; Skiba, D.S.; Touyz, R.M.; Harrison, D.G. The role of infiltrating immune cells in dysfunctional adipose tissue. Cardiovasc. Res. 2017, 113, 1009–1023. [Google Scholar] [CrossRef]
- Jackson, E.; Shoemaker, R.; Larian, N.; Cassis, L. Adipose Tissue as a Site of Toxin Accumulation. Compr. Physiol. 2017, 7, 1085–1135. [Google Scholar] [CrossRef] [PubMed]
- Geens, T.; Neels, H.; Covaci, A. Distribution of bisphenol-A, triclosan and n-nonylphenol in human adipose tissue, liver and brain. Chemosphere 2012, 87, 796–802. [Google Scholar] [CrossRef]
- Figueiredo, L.S.; Oliveira, K.M.; Freitas, I.N.; Silva, J.A., Jr.; Silva, J.N.; Favero-Santos, B.C.; Bonfleur, M.L.; Carneiro, E.M.; Ribeiro, R.A. Bisphenol-A exposure worsens hepatic steatosis in ovariectomized mice fed on a high-fat diet: Role of endoplasmic reticulum stress and fibrogenic pathways. Life Sci. 2020, 256, 118012. [Google Scholar] [CrossRef]
- Pirozzi, C.; Lama, A.; Annunziata, C.; Cavaliere, G.; Ruiz-Fernandez, C.; Monnolo, A.; Comella, F.; Gualillo, O.; Stornaiuolo, M.; Mollica, M.P.; et al. Oral Bisphenol A worsens liver immune-metabolic and mitochondrial dysfunction induced by high-fat diet in adult mice: Cross-talk between oxidative stress and inflammasome pathway. Antioxidants 2020, 9, 1201. [Google Scholar] [CrossRef]
- Asahi, J.; Kamo, H.; Baba, R.; Doi, Y.; Yamashita, A.; Murakami, D.; Hanada, A.; Hirano, T. Bisphenol A induces endoplasmic reticulum stress-associated apoptosis in mouse non-parenchymal hepatocytes. Life Sci. 2010, 87, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Malhi, H.; Kaufman, R.J. Endoplasmic reticulum stress in liver disease. J. Hepatol. 2011, 54, 795–809. [Google Scholar] [CrossRef] [PubMed]
- B’Chir, W.; Maurin, A.C.; Carraro, V.; Averous, J.; Jousse, C.; Muranishi, Y.; Parry, L.; Stepien, G.; Fafournoux, P.; Bruhat, A. The eIF2alpha/ATF4 pathway is essential for stress-induced autophagy gene expression. Nucleic Acids Res. 2013, 41, 7683–7699. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S.; Scafuro, M.; Meccariello, R. BPA and Nutraceuticals, Simultaneous Effects on Endocrine Functions. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 594–604. [Google Scholar] [CrossRef]
- Rameshrad, M.; Razavi, B.M.; Imenshahidi, M.; Hosseinzadeh, H. Vitis vinifera (grape) seed extract and resveratrol alleviate bisphenol-A-induced metabolic syndrome: Biochemical and molecular evidences. Phytother. Res. 2019, 33, 832–844. [Google Scholar] [CrossRef]
- Elgawish, R.A.; El-Beltagy, M.A.; El-Sayed, R.M.; Gaber, A.A.; Abdelrazek, H.M.A. Protective role of lycopene against metabolic disorders induced by chronic bisphenol A exposure in rats. Environ. Sci. Pollut. Res. Int. 2020, 27, 9192–9201. [Google Scholar] [CrossRef]
- Geng, S.; Wang, S.; Zhu, W.; Xie, C.; Li, X.; Wu, J.; Zhu, J.; Jiang, Y.; Yang, X.; Li, Y.; et al. Curcumin suppresses JNK pathway to attenuate BPA-induced insulin resistance in LO2 cells. Biomed. Pharmacother. 2018, 97, 1538–1543. [Google Scholar] [CrossRef]
- Fadishei, M.; Ghasemzadeh Rahbardar, M.; Imenshahidi, M.; Mohajeri, A.; Razavi, B.M.; Hosseinzadeh, H. Effects of Nigella sativa oil and thymoquinone against bisphenol A-induced metabolic disorder in rats. Phytother. Res. 2021, 35, 2005–2024. [Google Scholar] [CrossRef]
- Mahmoudi, A.; Hadrich, F.; Feki, I.; Ghorbel, H.; Bouallagui, Z.; Marrekchi, R.; Fourati, H.; Sayadi, S. Oleuropein and hydroxytyrosol rich extracts from olive leaves attenuate liver injury and lipid metabolism disturbance in bisphenol A-treated rats. Food Funct. 2018, 9, 3220–3234. [Google Scholar] [CrossRef]
- Mohsenzadeh, M.S.; Razavi, B.M.; Imenshahidi, M.; Mohajeri, S.A.; Rameshrad, M.; Hosseinzadeh, H. Evaluation of green tea extract and epigallocatechin gallate effects on bisphenol A-induced vascular toxicity in isolated rat aorta and cytotoxicity in human umbilical vein endothelial cells. Phytother. Res. 2021, 35, 996–1009. [Google Scholar] [CrossRef]
- Kazemi, S.; Feizi, F.; Aghapour, F.; Joorsaraee, G.A.; Moghadamnia, A.A. Histopathology and Histomorphometric Investigation of Bisphenol A and Nonylphenol on the Male Rat Reproductive System. N. Am. J. Med. Sci. 2016, 8, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Ono, H.; Mori, Y.; Chuda, Y.; Mori, M. Oxygenation of bisphenol A to quinones by polyphenol oxidase in vegetables. J. Agric. Food Chem. 2002, 50, 4377–4381. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, L.; Cui, Y.; Liu, F.; Zhang, J. Allii macrostemonis Bulbus: A Comprehensive Review of Ethnopharmacology, Phytochemistry and Pharmacology. Molecules 2023, 28, 2485. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.H.; Qin, Z.F.; Dai, Y.; Yao, X.S. Phytochemistry and pharmacology of Allii Macrostemonis Bulbus, a traditional Chinese medicine. Chin. J. Nat. Med. 2016, 14, 481–498. [Google Scholar] [CrossRef]
- Jia, W.; Li, Y.; Wan, J.; Cui, X.; Lu, J.; Liu, J.; Li, D.; Li, L.; Zou, T.; Ding, J.; et al. Effects of Xuezhitong in Patients with Hypertriglyceridemia: A Multicentre, Randomized, Double-Blind, Double Simulation, Positive Drug and Placebo Parallel Control Study. Cardiovasc. Drugs Ther. 2020, 34, 525–534. [Google Scholar] [CrossRef]
- Wu, Z.Q.; Li, K.; Ma, J.K.; Huang, Q.; Tian, X.; Li, Z.J. Antioxidant activity of organic sulfides from fresh Allium macrostemon Bunge and their protective effects against oxidative stress in Caenorhabditis elegans. J. Food Biochem. 2020, 44, e13447. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Jeong, Y.; Kim, J.; Kim, C.Y. Inhibitory effect of Allium macrostemon extracts on adipogenesis of 3T3-L1 preadipocytes. Korean J. Food Sci. Technol. 2020, 52, 441–449. [Google Scholar] [CrossRef]
- Xie, W.; Zhang, Y.; Wang, N.; Zhou, H.; Du, L.; Ma, X.; Shi, X.; Cai, G. Novel effects of macrostemonoside A, a compound from Allium macrostemon Bung, on hyperglycemia, hyperlipidemia, and visceral obesity in high-fat diet-fed C57BL/6 mice. Eur. J. Pharmacol. 2008, 599, 159–165. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.Y.; Kim, C.Y. Allium macrostemon whole extract ameliorates obesity-induced inflammation and endoplasmic reticulum stress in adipose tissue of high-fat diet-fed C57BL/6N mice. Food Nutr. Res. 2023, 67, 9256. [Google Scholar] [CrossRef]
- Nothias, L.-F.; Petras, D.; Schmid, R.; Dührkop, K.; Rainer, J.; Sarvepalli, A.; Protsyuk, I.; Ernst, M.; Tsugawa, H.; Fleischauer, M. Feature-based molecular networking in the GNPS analysis environment. Nat. Methods 2020, 17, 905–908. [Google Scholar] [CrossRef]
- Estadella, D.; da Penha Oller, do.; Nascimento, C.M.; Oyama, L.M.; Ribeiro, E.B.; Damaso, A.R.; de Piano, A. Lipotoxicity: Effects of dietary saturated and transfatty acids. Mediat. Inflamm 2013, 137579. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.L.; Wang, Y.C.; Hsu, Y.A.; Chen, C.S.; Weng, R.C.; Lu, Y.P.; Chuang, C.Y.; Wan, L. Bisphenol A coupled with a high-fat diet promotes hepatosteatosis through reactive-oxygen-species-Induced CD36 overexpression. Toxics 2022, 10, 208. [Google Scholar] [CrossRef] [PubMed]
- Thent, Z.C.; Froemming, G.R.A.; Muid, S. Bisphenol A exposure disturbs the bone metabolism: An evolving interest towards an old culprit. Life Sci. 2018, 198, 1–7. [Google Scholar] [CrossRef]
- Yang, M.; Chen, M.; Wang, J.; Xu, M.; Sun, J.; Ding, L.; Lv, X.; Ma, Q.; Bi, Y.; Liu, R.; et al. Bisphenol A Promotes Adiposity and Inflammation in a Nonmonotonic Dose-response Way in 5-week-old Male and Female C57BL/6J Mice Fed a Low-calorie Diet. Endocrinology 2016, 157, 2333–2345. [Google Scholar] [CrossRef]
- Moon, M.K.; Jeong, I.K.; Jung Oh, T.; Ahn, H.Y.; Kim, H.H.; Park, Y.J.; Jang, H.C.; Park, K.S. Long-term oral exposure to bisphenol A induces glucose intolerance and insulin resistance. J. Endocrinol. 2015, 226, 35–42. [Google Scholar] [CrossRef]
- Ma, Q.; Deng, P.; Lin, M.; Yang, L.; Li, L.; Guo, L.; Zhang, L.; He, M.; Lu, Y.; Pi, H.; et al. Long-term bisphenol A exposure exacerbates diet-induced prediabetes via TLR4-dependent hypothalamic inflammation. J. Hazard Mater. 2021, 402, 123926. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Q.; Xu, C.F.; Yu, C.H.; Chen, W.X.; Li, Y.M. Role of endoplasmic reticulum stress in the pathogenesis of nonalcoholic fatty liver disease. World J. Gastroenterol. 2014, 20, 1768–1776. [Google Scholar] [CrossRef]
- Li, J.; Yin, Z.; Hua, L.; Wang, X.; Ren, F.; Ge, Y. Evaluation of BPA effects on autophagy in Neuro-2a cells. Toxicol. Ind. Health 2022, 38, 151–161. [Google Scholar] [CrossRef]
- Wu, M.; Cong, Y.; Wang, K.; Yu, H.; Zhang, X.; Ma, M.; Duan, Z.; Pei, X. Bisphenol A impairs macrophages through inhibiting autophagy via AMPK/mTOR signaling pathway and inducing apoptosis. Ecotoxicol. Environ. Saf. 2022, 234, 113395. [Google Scholar] [CrossRef]
- Ding, S.; Zuo, X.; Fan, Y.; Li, H.; Zhao, N.; Yang, H.; Ye, X.; He, D.; Yang, H.; Jin, X.; et al. Environmentally Relevant Dose of Bisphenol A Does Not Affect Lipid Metabolism and Has No Synergetic or Antagonistic Effects on Genistein’s Beneficial Roles on Lipid Metabolism. PLoS ONE 2016, 11, e0155352. [Google Scholar] [CrossRef]
- Lee, S.; Kim, D.H.; Lee, C.H.; Jung, J.W.; Seo, Y.T.; Jang, Y.P.; Ryu, J.H. Antidepressant-like activity of the aqueous extract of Allium macrostemon in mice. J. Ethnopharmacol. 2010, 131, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Yang, C.Y.; Su, C.C.; Fang, K.M.; Yen, C.C.; Lin, C.T.; Liu, J.M.; Lee, K.I.; Chen, Y.W.; Liu, S.H.; et al. 4-Methyl-2,4-bis(4-hydroxyphenyl)pent-1-ene, a Major Active Metabolite of Bisphenol A, Triggers Pancreatic beta-Cell Death via a JNK/AMPKalpha Activation-Regulated Endoplasmic Reticulum Stress-Mediated Apoptotic Pathway. Int. J. Mol. Sci. 2021, 22, 4379. [Google Scholar] [CrossRef] [PubMed]
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Methuen: London, UK, 1959. [Google Scholar]


| Parameters | CON | HF | HF + BP | HF + BP + AM |
|---|---|---|---|---|
| Body weight gain (g) | 6.3 ± 0.5 | 16.6 ± 0.9 *,# | 12.8 ± 0.7 *,# | 12.9 ± 0.7 *,# |
| Energy intake (kcal/d/mouse) | 8.94 ± 0.00 | 11.76 ± 0.72 * | 11.47 ± 0.46 * | 11.06 ± 0.21 |
| Epididymal fat (g) | 0.58 ± 0.06 | 2.09 ± 0.11 * | 2.00 ± 0.06 * | 1.57 ± 0.11 *,#,† |
| Perirenal and retroperitoneal fat (g) | 0.29 ± 0.04 | 1.12 ± 0.07 * | 0.87 ± 0.06 * | 0.70 ± 0.11 *,# |
| Liver (g) | 0.74 ± 0.03 | 0.80 ± 0.02 | 0.77 ± 0.02 | 0.74 ± 0.01 |
| Kidneys (g) | 0.26 ± 0.01 | 0.31 ± 0.02 | 0.27 ± 0.01 | 0.28 ± 0.01 |
| Spleen (g) | 0.06 ± 0.00 | 0.07 ± 0.00 * | 0.06 ± 0.00 # | 0.06 ± 0.00 # |
| Serum GOT (IU/L) | 31.30 ± 7.08 | 21.78 ± 3.61 | 34.81 ± 7.39 | 46.74 ± 10.33 |
| Serum GPT (IU/L) | 24.50 ± 1.92 | 21.03 ± 0.72 | 31.71 ± 5.90 | 26.83 ± 3.08 |
| Serum BUN (mg/mL) | 18.22 ± 0.92 | 18.03 ± 0.44 | 17.03 ± 1.70 | 16.61 ± 0.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Kim, N.-H.; Youn, I.; Seo, E.K.; Kim, C.Y. Effects of Allium macrostemon Bunge Extract on Adipose Tissue Inflammation and Hepatic Endoplasmic Reticulum Stress in High-Fat Diet-Fed and Bisphenol A-Treated C57BL/6N Mice. Foods 2023, 12, 3777. https://doi.org/10.3390/foods12203777
Kim J, Kim N-H, Youn I, Seo EK, Kim CY. Effects of Allium macrostemon Bunge Extract on Adipose Tissue Inflammation and Hepatic Endoplasmic Reticulum Stress in High-Fat Diet-Fed and Bisphenol A-Treated C57BL/6N Mice. Foods. 2023; 12(20):3777. https://doi.org/10.3390/foods12203777
Chicago/Turabian StyleKim, Juhae, Na-Hyung Kim, Isoo Youn, Eun Kyoung Seo, and Choon Young Kim. 2023. "Effects of Allium macrostemon Bunge Extract on Adipose Tissue Inflammation and Hepatic Endoplasmic Reticulum Stress in High-Fat Diet-Fed and Bisphenol A-Treated C57BL/6N Mice" Foods 12, no. 20: 3777. https://doi.org/10.3390/foods12203777
APA StyleKim, J., Kim, N.-H., Youn, I., Seo, E. K., & Kim, C. Y. (2023). Effects of Allium macrostemon Bunge Extract on Adipose Tissue Inflammation and Hepatic Endoplasmic Reticulum Stress in High-Fat Diet-Fed and Bisphenol A-Treated C57BL/6N Mice. Foods, 12(20), 3777. https://doi.org/10.3390/foods12203777







