Effects of Isochoric Freezing on the Quality Characteristics of Raw Bovine Milk
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isochoric System
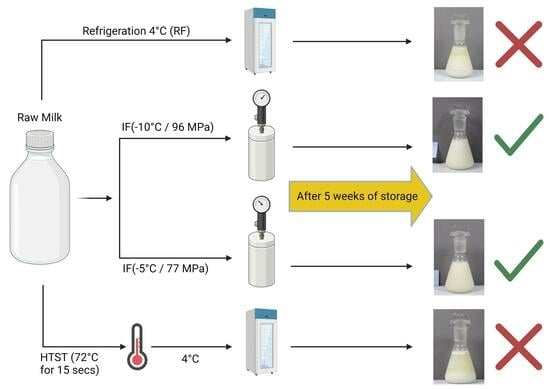
2.2. Experimental Protocol
2.3. Microbiology
2.4. pH/Titration
2.5. Color
2.6. Turbidity
2.7. Viscosity
2.8. Lipid Oxidation
2.9. Protein Content
2.10. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.11. Lactoperoxidase Activity
2.12. Volatile Organic Compounds
2.13. Statistical Analysis
3. Results and Discussion
3.1. Microbiological Evaluation
3.2. pH and Titratable Acidity
3.3. Color and Turbidity
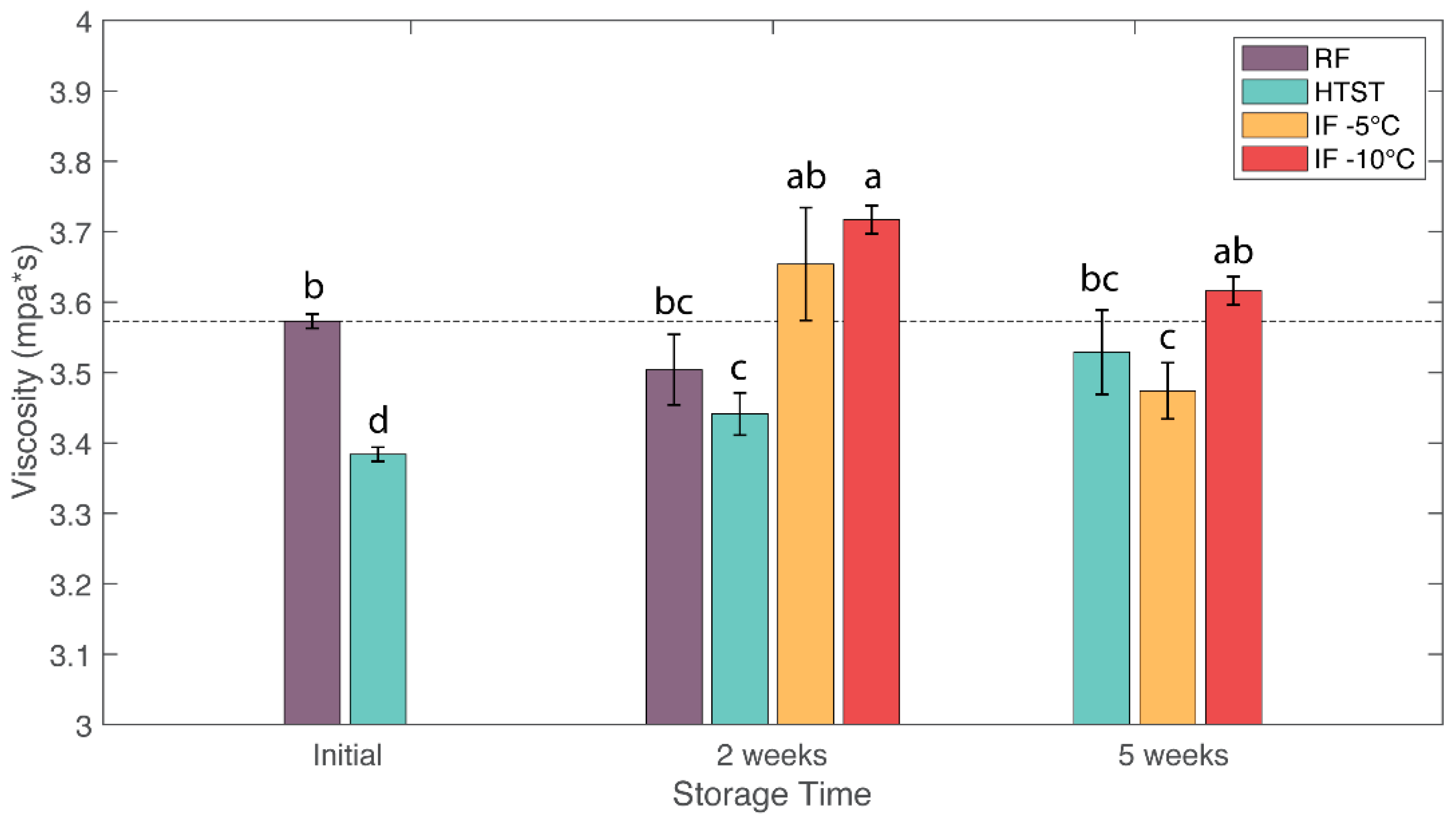
3.4. Viscosity
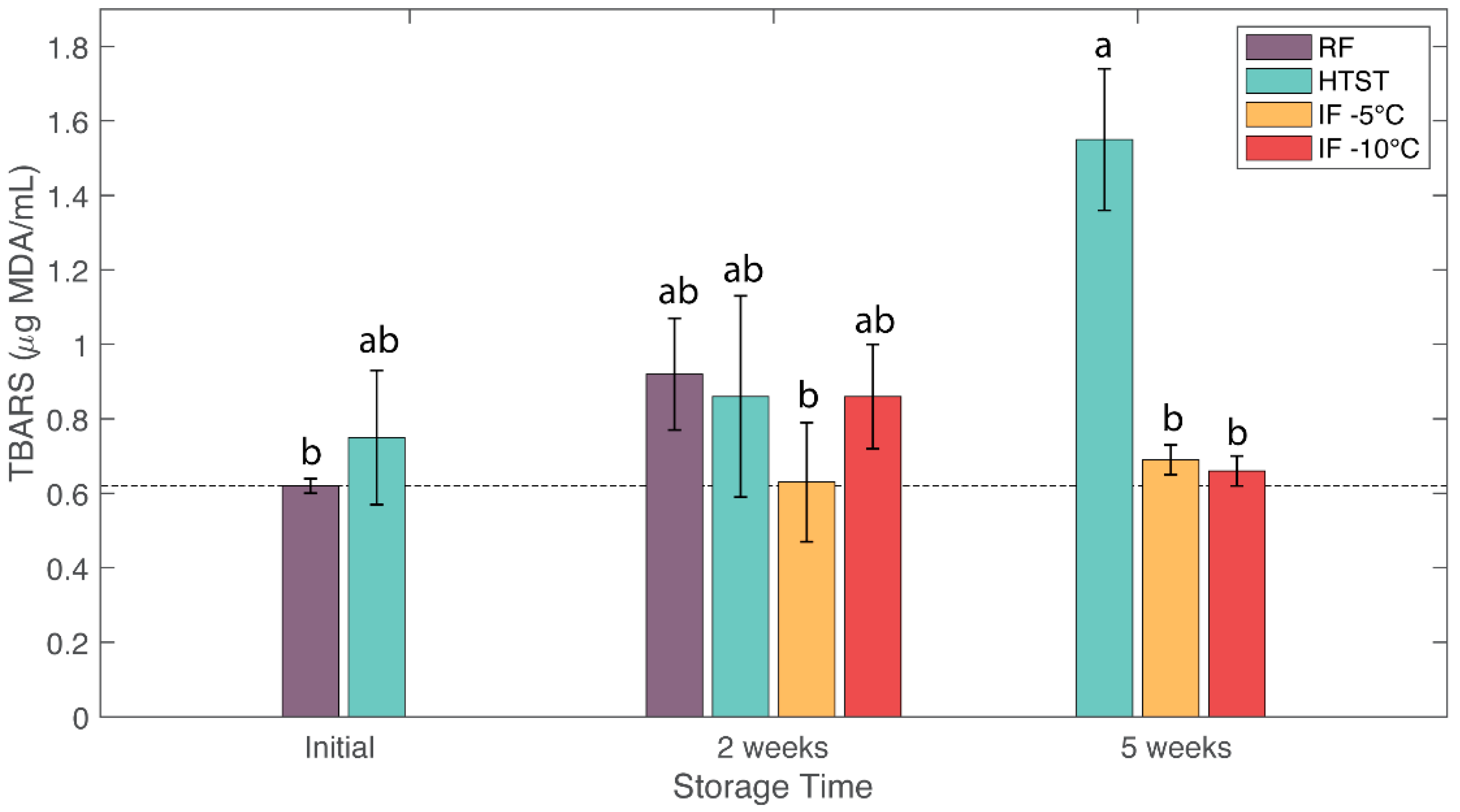
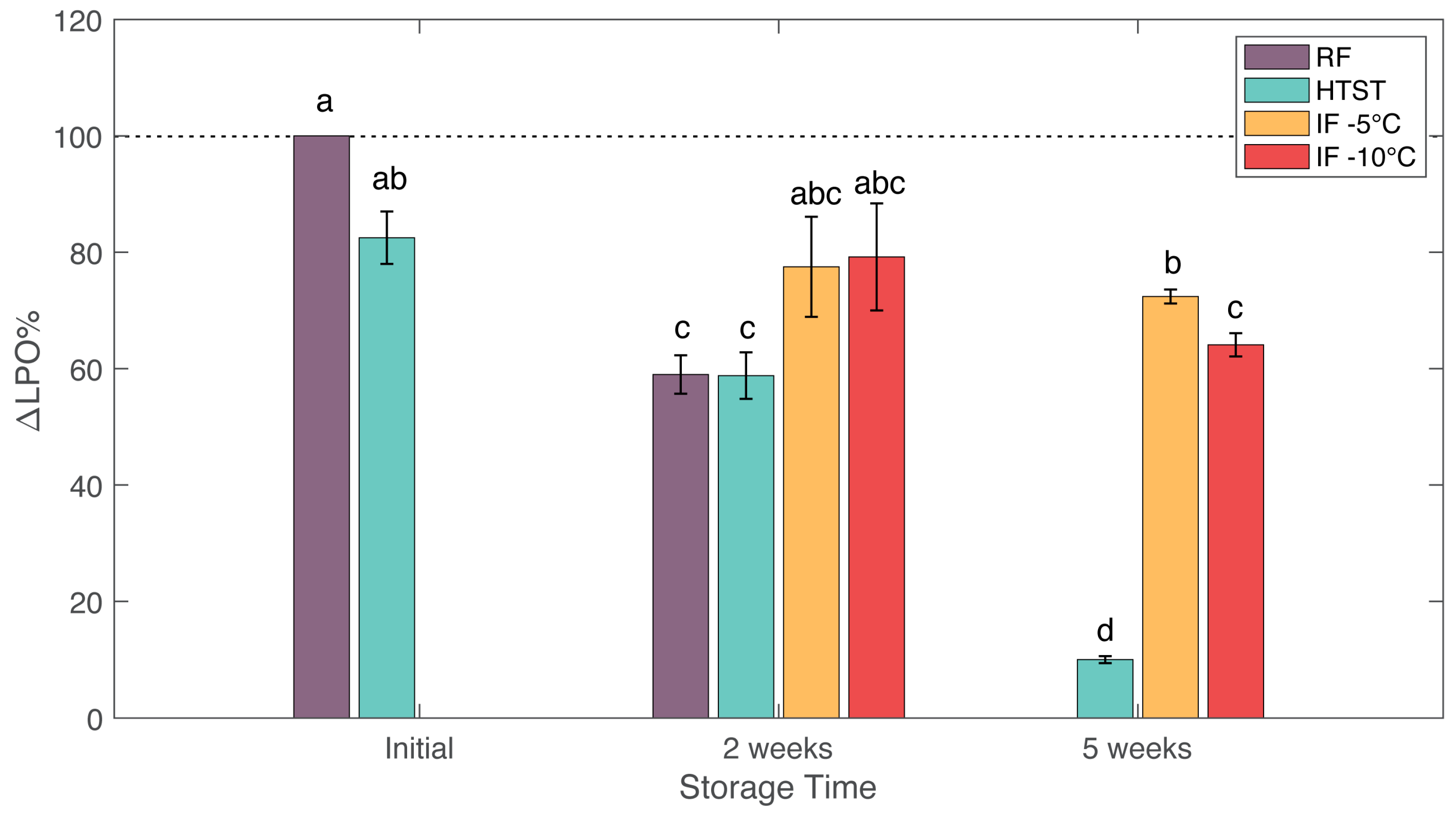
3.5. Lipid Oxidation
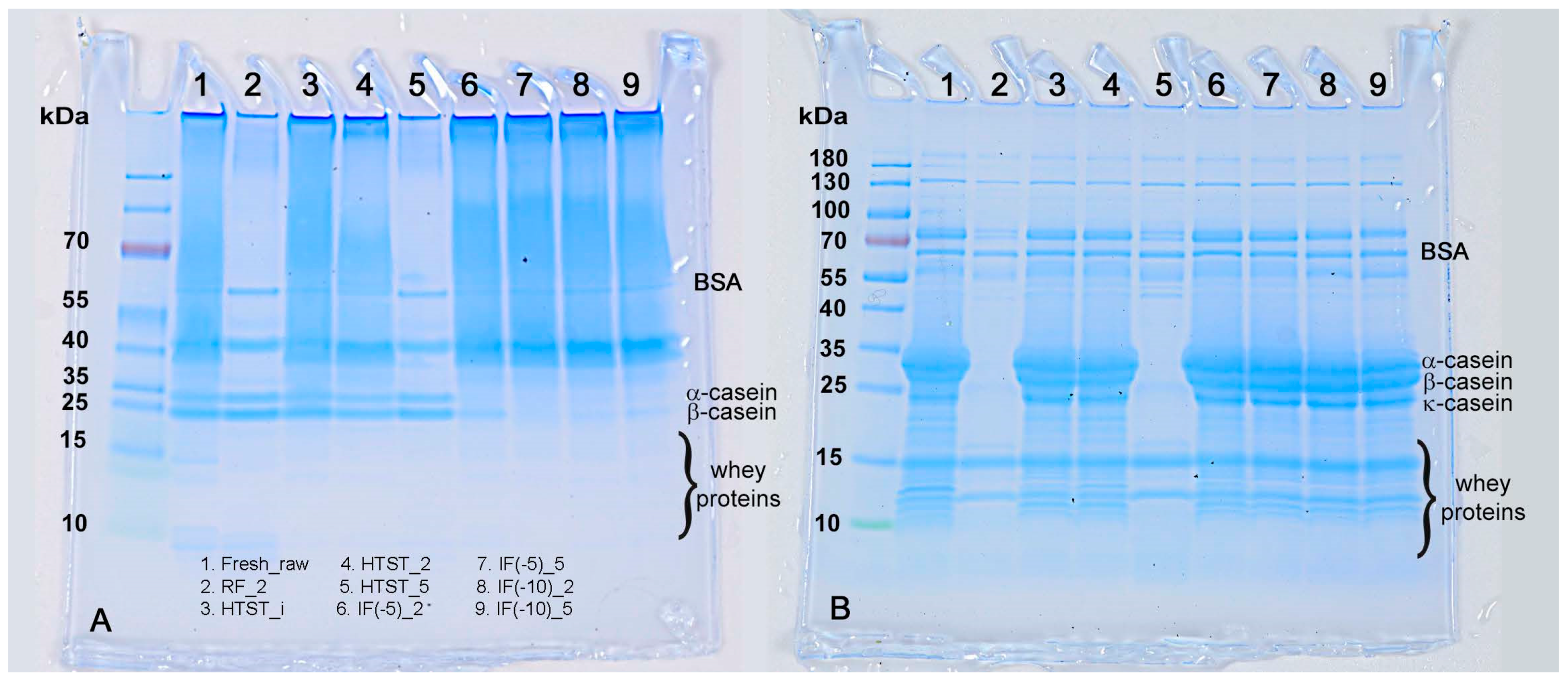
3.6. Protein Content and SDS-PAGE
3.7. Lactoperoxidase Activity
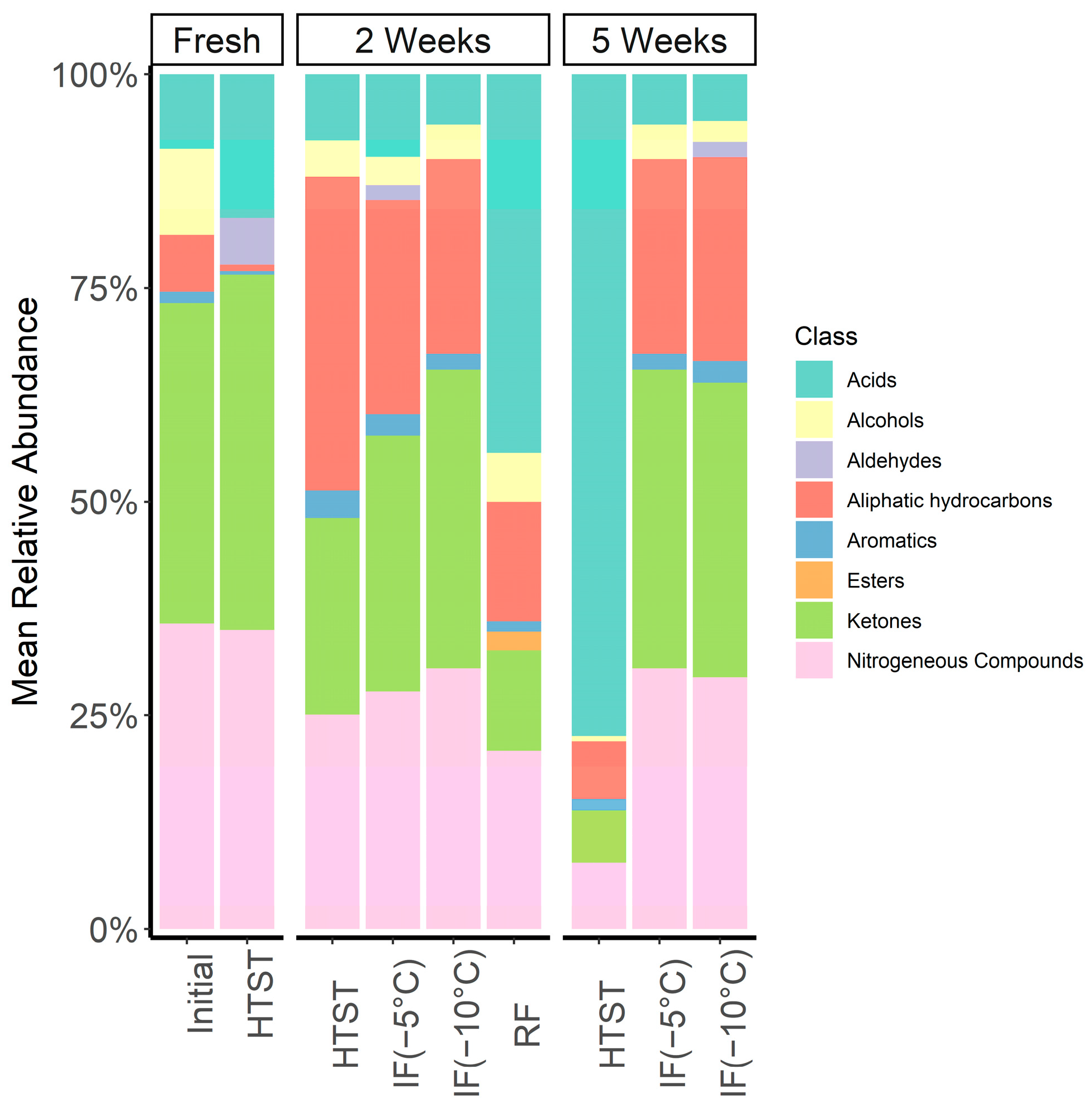
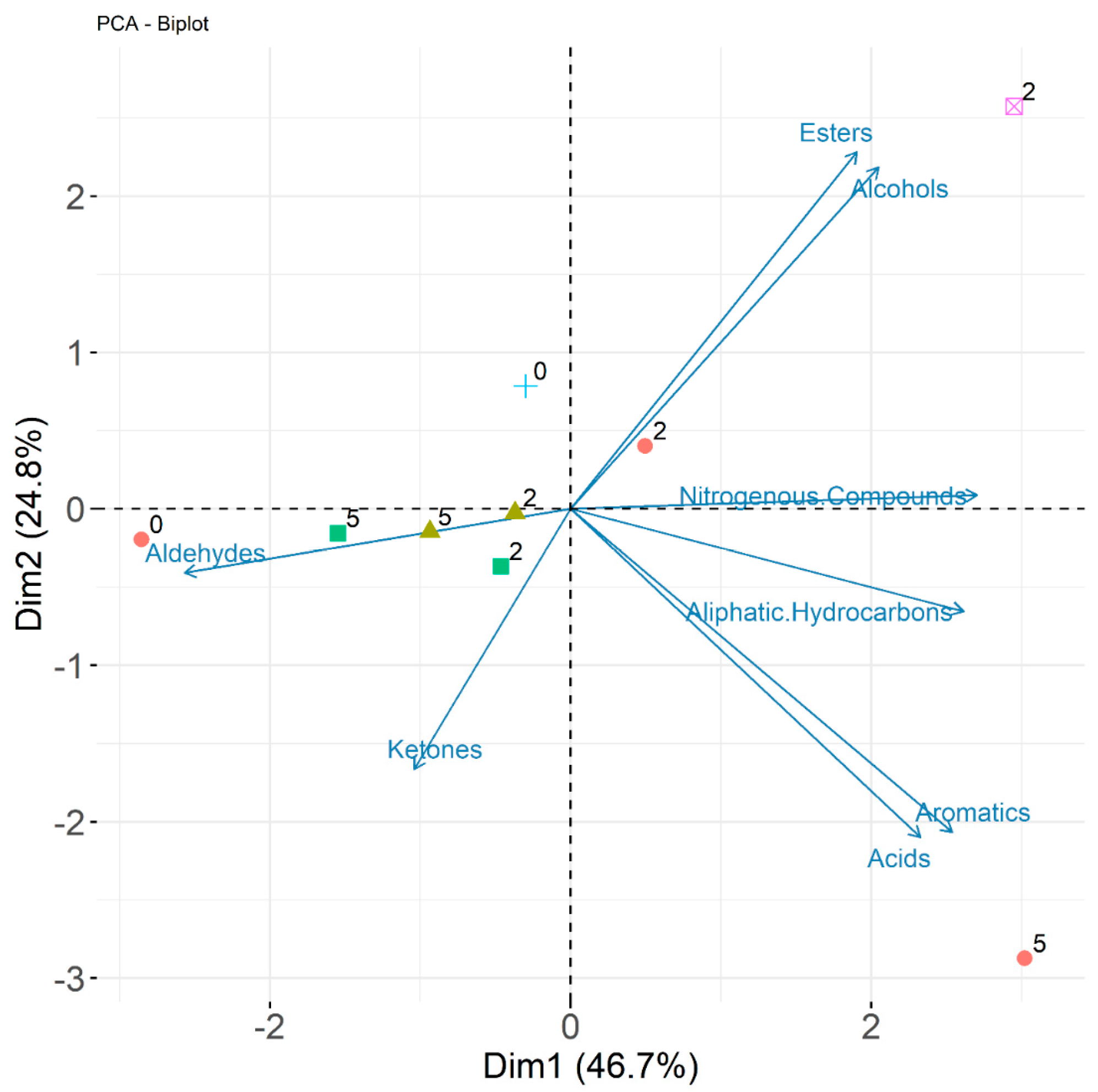
3.8. SPME GC/MS Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Park, Y.W. Overview of Bioactive Components in Milk and Dairy Products. In Bioactive Components in Milk and Dairy Products; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009; pp. 1–12. ISBN 978-0-8138-2150-4. [Google Scholar]
- Mahboubi, A.; Ferreira, J.A.; Taherzadeh, M.J.; Lennartsson, P.R. Value-Added Products from Dairy Waste Using Edible Fungi. Waste Manag. 2017, 59, 518–525. [Google Scholar] [CrossRef]
- Ahmad, T.; Aadil, R.M.; Ahmed, H.; ur Rahman, U.; Soares, B.C.V.; Souza, S.L.Q.; Pimentel, T.C.; Scudino, H.; Guimarães, J.T.; Esmerino, E.A.; et al. Treatment and Utilization of Dairy Industrial Waste: A Review. Trends Food Sci. Technol. 2019, 88, 361–372. [Google Scholar] [CrossRef]
- Liu, J.; Li, L.; Chen, X.; Lu, Y.; Wang, D. Effects of Heat Stress on Body Temperature, Milk Production, and Reproduction in Dairy Cows: A Novel Idea for Monitoring and Evaluation of Heat Stress—A Review. Asian-Australas. J. Anim. Sci. 2019, 32, 1332–1339. [Google Scholar] [CrossRef] [PubMed]
- Raw Milk Questions and Answers. Available online: https://www.cdc.gov/foodsafety/rawmilk/raw-milk-questions-and-answers.html (accessed on 6 October 2023).
- Ranieri, M.L.; Huck, J.R.; Sonnen, M.; Barbano, D.M.; Boor, K.J. High Temperature, Short Time Pasteurization Temperatures Inversely Affect Bacterial Numbers during Refrigerated Storage of Pasteurized Fluid Milk. J. Dairy Sci. 2009, 92, 4823–4832. [Google Scholar] [CrossRef] [PubMed]
- Cappozzo, J.C.; Koutchma, T.; Barnes, G. Chemical Characterization of Milk after Treatment with Thermal (HTST and UHT) and Nonthermal (Turbulent Flow Ultraviolet) Processing Technologies. J. Dairy Sci. 2015, 98, 5068–5079. [Google Scholar] [CrossRef] [PubMed]
- Krishna, T.C.; Najda, A.; Bains, A.; Tosif, M.M.; Papliński, R.; Kapłan, M.; Chawla, P. Influence of Ultra-Heat Treatment on Properties of Milk Proteins. Polymers 2021, 13, 3164. [Google Scholar] [CrossRef] [PubMed]
- Musembei, L.M.; Bett, R.C.; Gachuiri, C.K.; Mbondo, B.K.; Kibegwa, F.M. Effects of Freezing as a Post-Harvest Storage Technique on Quality of Friesian Crossbred Cattle Milk. Afr. J. Food Sci. 2021, 15, 298–303. [Google Scholar] [CrossRef]
- Lim, S.H.; Chin, N.L.; Sulaiman, A.; Tay, C.H.; Wong, T.H. Microbiological, Physicochemical and Nutritional Properties of Fresh Cow Milk Treated with Industrial High-Pressure Processing (HPP) during Storage. Foods 2023, 12, 592. [Google Scholar] [CrossRef]
- Duarte, R.V.; Pinto, C.A.; Gomes, A.M.; Delgadillo, I.; Saraiva, J.A. A Microbiological Perspective of Raw Milk Preserved at Room Temperature Using Hyperbaric Storage Compared to Refrigerated Storage. Innov. Food Sci. Emerg. Technol. 2022, 78, 103019. [Google Scholar] [CrossRef]
- Duarte, R.V.; Casal, S.; da Silva, J.A.L.; Gomes, A.; Delgadillo, I.; Saraiva, J.A. Nutritional, Physicochemical, and Endogenous Enzyme Assessment of Raw Milk Preserved under Hyperbaric Storage at Variable Room Temperature. ACS Food Sci. Technol. 2022, 2, 961–974. [Google Scholar] [CrossRef]
- Lemos, Á.T.; Casal, S.; Barba, F.J.; Phimolsiripol, Y.; Delgadillo, I.; Saraiva, J.A. Preservation of High Pressure Pasteurised Milk by Hyperbaric Storage at Room Temperature versus Refrigeration on Inoculated Microorganisms, Fatty Acids, Volatile Compounds and Lipid Oxidation. Food Chem. 2022, 387, 132887. [Google Scholar] [CrossRef] [PubMed]
- Rubinsky, B.; Perez, P.A.; Carlson, M.E. The Thermodynamic Principles of Isochoric Cryopreservation. Cryobiology 2005, 50, 121–138. [Google Scholar] [CrossRef] [PubMed]
- Powell-Palm, M.J.; Rubinsky, B. A Shift from the Isobaric to the Isochoric Thermodynamic State Can Reduce Energy Consumption and Augment Temperature Stability in Frozen Food Storage. J. Food Eng. 2019, 251, 1–10. [Google Scholar] [CrossRef]
- Powell-Palm, M.J.; Preciado, J.; Lyu, C.; Rubinsky, B. Escherichia Coli Viability in an Isochoric System at Subfreezing Temperatures. Cryobiology 2018, 85, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Bilbao-Sainz, C.; Sinrod, A.; Powell-Palm, M.J.; Dao, L.; Takeoka, G.; Williams, T.; Wood, D.; Ukpai, G.; Aruda, J.; Bridges, D.F.; et al. Preservation of Sweet Cherry by Isochoric (Constant Volume) Freezing. Innov. Food Sci. Emerg. Technol. 2019, 52, 108–115. [Google Scholar] [CrossRef]
- Bilbao-Sainz, C.; Chiou, B.-S.; Takeoka, G.; Williams, T.; Wood, D.; Powell-Palm, M.J.; Rubinsky, B.; Wu, V.C.H.; McHugh, T. Isochoric Freezing and Isochoric Supercooling as Innovative Postharvest Technologies for Pomegranate Preservation. Postharvest Biol. Technol. 2022, 194, 112072. [Google Scholar] [CrossRef]
- Zhao, Y.; Bilbao-Sainz, C.; Wood, D.; Chiou, B.-S.; Powell-Palm, M.J.; Chen, L.; McHugh, T.; Rubinsky, B. Effects of Isochoric Freezing Conditions on Cut Potato Quality. Foods 2021, 10, 974. [Google Scholar] [CrossRef]
- Bilbao-Sainz, C.; Sinrod, A.J.G.; Dao, L.; Takeoka, G.; Williams, T.; Wood, D.; Chiou, B.-S.; Bridges, D.F.; Wu, V.C.H.; Lyu, C.; et al. Preservation of Grape Tomato by Isochoric Freezing. Food Res. Int. 2021, 143, 110228. [Google Scholar] [CrossRef]
- ISO 4833-1:2013(En), Microbiology of the Food Chain. Available online: https://www.iso.org/obp/ui/en/#iso:std:iso:4833:-1:ed-1:v1:en (accessed on 14 October 2023).
- Vyncke, W. Direct Determination of the Thiobarbituric Acid Value in Trichloracetic Acid Extracts of Fish as a Measure of Oxidative Rancidity. Fette Seifen Anstrichm. 1970, 72, 1084–1087. [Google Scholar] [CrossRef]
- Waterborg, J.H.; Matthews, H.R. The Lowry Method for Protein Quantitation. In Proteins; Walker, J.M., Ed.; Methods in Molecular BiologyTM; Humana Press: Totowa, NJ, USA, 1984; pp. 1–3. ISBN 978-1-59259-488-7. [Google Scholar]
- Marín, E.; Sánchez, L.; Pérez, M.D.; Puyol, P.; Calvo, M. Effect of Heat Treatment on Bovine Lactoperoxidase Activity in Skim Milk: Kinetic and Thermodynamic Analysis. J. Food Sci. 2003, 68, 89–93. [Google Scholar] [CrossRef]
- Stratakos, A.C.; Inguglia, E.S.; Linton, M.; Tollerton, J.; Murphy, L.; Corcionivoschi, N.; Koidis, A.; Tiwari, B.K. Effect of High Pressure Processing on the Safety, Shelf Life and Quality of Raw Milk. Innov. Food Sci. Emerg. Technol. 2019, 52, 325–333. [Google Scholar] [CrossRef]
- Sehrawat, R.; Kaur, B.P.; Nema, P.K.; Tewari, S.; Kumar, L. Microbial Inactivation by High Pressure Processing: Principle, Mechanism and Factors Responsible. Food Sci. Biotechnol. 2021, 30, 19–35. [Google Scholar] [CrossRef]
- Erkmen, O.; Dogan, C. Effects of Ultra High Hydrostatic Pressure on Listeria Monocytogenes and Natural Flora in Broth, Milk and Fruit Juices. Int. J. Food Sci. Technol. 2004, 39, 91–97. [Google Scholar] [CrossRef]
- Pereda, J.; Ferragut, V.; Quevedo, J.M.; Guamis, B.; Trujillo, A.J. Effects of Ultra-High Pressure Homogenization on Microbial and Physicochemical Shelf Life of Milk. J. Dairy Sci. 2007, 90, 1081–1093. [Google Scholar] [CrossRef]
- Lott, T.T.; Wiedmann, M.; Martin, N.H. Shelf-Life Storage Temperature Has a Considerably Larger Effect than High-Temperature, Short-Time Pasteurization Temperature on the Growth of Spore-Forming Bacteria in Fluid Milk. J. Dairy Sci. 2023, 106, 3838–3855. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Kim, S.H.; Choi, M.J.; Min, S.G.; Kwak, H.S. The Effect of High Pressure–Low Temperature Treatment on Physicochemical Properties in Milk. J. Dairy Sci. 2008, 91, 4176–4182. [Google Scholar] [CrossRef]
- Odriozola-Serrano, I.; Bendicho-Porta, S.; Martín-Belloso, O. Comparative Study on Shelf Life of Whole Milk Processed by High-Intensity Pulsed Electric Field or Heat Treatment. J. Dairy Sci. 2006, 89, 905–911. [Google Scholar] [CrossRef]
- Salaün, F.; Mietton, B.; Gaucheron, F. Buffering Capacity of Dairy Products. Int. Dairy J. 2005, 15, 95–109. [Google Scholar] [CrossRef]
- Chugh, A.; Khanal, D.; Walkling-Ribeiro, M.; Corredig, M.; Duizer, L.; Griffiths, M.W. Change in Color and Volatile Composition of Skim Milk Processed with Pulsed Electric Field and Microfiltration Treatments or Heat Pasteurization. Foods 2014, 3, 250–268. [Google Scholar] [CrossRef]
- Lemos, Á.T.; Lopes-da-Silva, J.A.; Delgadillo, I.; Saraiva, J.A. Preservation of High Pressure Pasteurised Milk by Hyperbaric Storage at Room Temperature versus Refrigeration—Effect on Natural Microbiota and Physicochemical Properties. Food Chem. Adv. 2023, 2, 100241. [Google Scholar] [CrossRef]
- Harte, F.; Luedecke, L.; Swanson, B.; Barbosa-Cánovas, G.V. Low-Fat Set Yogurt Made from Milk Subjected to Combinations of High Hydrostatic Pressure and Thermal Processing. J. Dairy Sci. 2003, 86, 1074–1082. [Google Scholar] [CrossRef]
- Cserhalmi, Z.; Sass-Kiss, Á.; Tóth-Markus, M.; Lechner, N. Study of Pulsed Electric Field Treated Citrus Juices. Innov. Food Sci. Emerg. Technol. 2006, 7, 49–54. [Google Scholar] [CrossRef]
- Martin, G.J.O.; Williams, R.P.W.; Dunstan, D.E. Comparison of Casein Micelles in Raw and Reconstituted Skim Milk. J. Dairy Sci. 2007, 90, 4543–4551. [Google Scholar] [CrossRef] [PubMed]
- Orlien, V.; Boserup, L.; Olsen, K. Casein Micelle Dissociation in Skim Milk during High-Pressure Treatment: Effects of Pressure, pH, and Temperature. J. Dairy Sci. 2010, 93, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Anema, S.G.; Lowe, E.K.; Stockmann, R. Particle Size Changes and Casein Solubilisation in High-Pressure-Treated Skim Milk. Food Hydrocoll. 2005, 19, 257–267. [Google Scholar] [CrossRef]
- Huppertz, T.; Fox, P.F.; Kelly, A.L. High Pressure Treatment of Bovine Milk: Effects on Casein Micelles and Whey Proteins. J. Dairy Res. 2004, 71, 97–106. [Google Scholar] [CrossRef]
- Anema, S.G. Role of κ-Casein in the Association of Denatured Whey Proteins with Casein Micelles in Heated Reconstituted Skim Milk. J. Agric. Food Chem. 2007, 55, 3635–3642. [Google Scholar] [CrossRef]
- Huppertz, T.; Fox, P.F.; Kelly, A.L. High Pressure-Induced Changes in the Creaming Properties of Bovine Milk. Innov. Food Sci. Emerg. Technol. 2003, 4, 349–359. [Google Scholar] [CrossRef]
- Janahar, J.J.; Marciniak, A.; Balasubramaniam, V.M.; Jimenez-Flores, R.; Ting, E. Effects of Pressure, Shear, Temperature, and Their Interactions on Selected Milk Quality Attributes. J. Dairy Sci. 2021, 104, 1531–1547. [Google Scholar] [CrossRef]
- Li, Y.; Joyner, H.S.; Carter, B.G.; Drake, M.A. Effects of Fat Content, Pasteurization Method, Homogenization Pressure, and Storage Time on the Mechanical and Sensory Properties of Bovine Milk. J. Dairy Sci. 2018, 101, 2941–2955. [Google Scholar] [CrossRef]
- Pesic, M.B.; Barac, M.B.; Stanojevic, S.P.; Vrvic, M.M. Effect of pH on Heat-Induced Casein-Whey Protein Interactions: A Comparison between Caprine Milk and Bovine Milk. Int. Dairy J. 2014, 39, 178–183. [Google Scholar] [CrossRef]
- Patel, H.A.; Singh, H.; Anema, S.G.; Creamer, L.K. Effects of Heat and High Hydrostatic Pressure Treatments on Disulfide Bonding Interchanges among the Proteins in Skim Milk. J. Agric. Food Chem. 2006, 54, 3409–3420. [Google Scholar] [CrossRef]
- Patel, H.A.; Anema, S.G.; Holroyd, S.E.; Singh, H.; Creamer, L.K. Methods to Determine Denaturation and Aggregation of Proteins in Low-, Medium- and High-Heat Skim Milk Powders. Le Lait 2007, 87, 251–268. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, Z.; Wang, Y.; Gao, J.; Yuan, Q.; Mao, X. Effects of High Hydrostatic Pressure Treatment on the Antigenicity, Structural and Digestive Properties of Whey Protein. LWT 2023, 178, 114628. [Google Scholar] [CrossRef]
- Considine, T.; Patel, H.A.; Anema, S.G.; Singh, H.; Creamer, L.K. Interactions of Milk Proteins during Heat and High Hydrostatic Pressure Treatments—A Review. Innov. Food Sci. Emerg. Technol. 2007, 8, 1–23. [Google Scholar] [CrossRef]
- Patel, H.A.; Huppertz, T. Chapter 8—Effects of High-Pressure Processing on Structure and Interactions of Milk Proteins. In Milk Proteins, 2nd ed.; Singh, H., Boland, M., Thompson, A., Eds.; Food Science and Technology; Academic Press: San Diego, CA, USA, 2014; pp. 243–267. ISBN 978-0-12-405171-3. [Google Scholar]
- Moussa, O.B.; Mankai, M.; Fekih, A.B.; Hassouna, M. Effect of the Lactoperoxidase System on Proteolysis and Physicochemical Changes in Ultra High Temperature Milk during Storage. Afr. J. Biotechnol. 2013, 12, 2041–2050. [Google Scholar] [CrossRef]
- Wolf, I.V.; Bergamini, C.V.; Perotti, M.C.; Hynes, E.R. Sensory and Flavor Characteristics of Milk. In Milk and Dairy Products in Human Nutrition; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 310–337. ISBN 978-1-118-53416-8. [Google Scholar]
- Yue, J.; Zheng, Y.; Liu, Z.; Deng, Y.; Jing, Y.; Luo, Y.; Yu, W.; Zhao, Y. Characterization of Volatile Compounds in Microfiltered Pasteurized Milk Using Solid-Phase Microextraction and GC × GC − TOFMS. Int. J. Food Prop. 2015, 18, 2193–2212. [Google Scholar] [CrossRef]


| Initial | RF | HTST | IF (−5 °C) | IF (−10 °C) | |||||
|---|---|---|---|---|---|---|---|---|---|
| 0 Weeks | 2 Weeks | 0 Weeks | 2 Weeks | 5 Weeks | 2 Weeks | 5 Weeks | 2 Weeks | 5 Weeks | |
| TPC (Log CFU mL−1) | 6.98 (0.05) c | 8.26 (0.19) b | <0.01 | 5.28 (0.05) d | 9.59 (0.06) a | 3.60 (0.12) e | 1.33 (0.28) f | 1.26 (0.24) f | <0.01 |
| PS (Log CFU mL−1) | 7.22 (0.13) c | 8.07 (0.09) b | <0.01 | 5.31 (0.20) d | 9.80 (0.13) a | 4.85 (0.26) e | 1.80 (0.040) g | 3.45 (0.04) f | <0.01 |
| Initial | RF | HTST | IF (−5 °C) | IF (−10 °C) | |||||
|---|---|---|---|---|---|---|---|---|---|
| 0 Weeks | 2 Weeks | 0 Week | 2 Weeks | 5 Weeks | 2 Weeks | 5 Weeks | 2 Weeks | 5 Weeks | |
| pH | 6.84 (0.04) a | 6.57 (0.03) c | 6.82 (0.01) a | 6.80 (0.01) ab | 6.63 (0.01) c | 6.77 (0.01) b | 6.77 (0.01) b | 6.79 (0.02) ab | 6.79 (0.02) ab |
| TA (g LA/mL) | 1.27 (0.08) c | 2.06 (0.03) a | 1.36 (0.08) c | 1.35 (0.05) c | 1.69 (0.02) b | 1.37 (0.02) c | 1.58 (0.06) b | 1.53 (0.09) b | 1.63 (0.06) b |
| Initial | RF | HTST | IF (−5 °C) | IF (−10 °C) | |||||
|---|---|---|---|---|---|---|---|---|---|
| 0 Weeks | 2 Weeks | 0 Weeks | 2 Weeks | 5 Weeks | 2 Weeks | 5 Weeks | 2 Weeks | 5 Weeks | |
| L* | 77.55 (0.15) a | 77.23 (0.05) a | 77.82 (0.05) a | 77.35 (0.03) a | 75.49 (0.60) a | 76.28 (0.05) a | 78.23 (0.8) a | 77.38 (0.02) a | 77.57 (0.07) a |
| a* | −1.78 (0.02) b | −1.76 (0.02) b | −1.95 (0.01) c | −1.94 (0.03) c | −2.28 (0.03) d | −1.63 (0.01) b | −2.05 (0.01) c | −1.33 (0.02) a | −1.56 (0.06) ab |
| b* | 3.38 (0.02) b | 3.63 (0.01) b | 3.00 (0.03) b | 2.92 (0.03) b | 0.03 (0.14) c | 3.63 (0.02) b | 3.95 (0.10) b | 4.86 (0.01) a | 5.14 (0.15) a |
| ΔE | 0 | 0.35 (0.05) c | 0.49 (0.06) c | 0.53 (0.04) c | 3.84 (0.19) a | 1.30 (0.04) b | 1.50 (0.20) b | 1.56 (0.01) b | 1.88 (0.21) b |
| Turbidity (NTU) | 2325 (175) bc | 2302 (15) c | 2128 (50) cd | 2130 (83) cd | 842 (24) e | 2107 (62) cd | 1720 (373) d | 2810 (42) b | 3365 (343) a |
| Volatile Compound | Initial | RF | HTST | IF (−5 °C) | IF (−10 °C) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 Weeks | 2 Weeks | 0 Weeks | 2 Weeks | 5 Weeks | 2 Weeks | 5 Weeks | 2 Weeks | 5 Weeks | |||
| Acids | 2.87 (0.76) a | 32.53 (7.4) b | 4.66 (1.28) a | 2.55 (1.73) a | 149.15 (10.49) c | 3.6 (1.65) a | 3.67 (0.7) a | 1.93 (0.84) a | 1.63 (1.74) a | ||
| Butanoic acid | 795 | 785 | ND | 4.17 (1.52) | ND | ND | ND | ND | ND | ND | ND |
| Hexanoic acid | 973 | 971 | 2.33 (0.26) a | 16.94 (3.79) b | 2.61 (0.62) a | 1.38 (1.42) a | 51.33 (7.97) c | 2.49 (1.03) a | 2.46 (0.29) a | 1.53 (0.56) a | 0.98 (1.47) a |
| Octanoic acid | 1165 | 1156 | 0.54 (0.63) a | 8.97 (1.72) b | 1.76 (0.54) a | 1.18 (0.31) a | 55.04 (8.36) c | 1.11 (0.64) a | 1.21 (0.44) a | 0.40 (0.49) a | 0.65 (0.32) a |
| Decanoic acid | 1335 | 1344 | ND | 2.45 (0.44) a | 0.29 (0.12) a | ND | 41.46 (6.38) b | ND | ND | ND | ND |
| Dodecanoic acid | 1547 | 1539 | ND | ND | ND | ND | 1.33 (0.33) | ND | ND | ND | ND |
| Alcohols | 3.34 (0.57) b | 4.18 (0.62) b | ND | 1.39 (0.55) a | 1.21 (0.38) a | 1.21 (0.17) a | 0.91 (0.28) a | 1.32 (0.41) a | 0.73 (0.34) a | ||
| Ethanol | 440 | 466 | ND | 1.87 (0.38) | ND | ND | ND | ND | ND | ND | ND |
| 2-propanol | 475 | 493 | ND | 0.89 (0.06) | ND | ND | ND | ND | ND | ND | ND |
| 2-methyl-2-propanol | 512 | 510 | 2.40 (0.36) b | 1.16 (0.37) a | ND | 1.19 (0.54) a | 1.21 (0.38) a | 0.85 (0.15) a | 0.84 (0.34) a | 1.13 (0.37) a | 0.60 (0.31) a |
| 2-methyl-1-propanol | 607 | 613 | 0.65 (0.22) b | ND | ND | ND | ND | 0.13 (0.15) a | ND | ND | ND |
| 2-methyl-2-butanol | 614 | 622 | 0.30 (0.02) c | 0.25 (0.06) bc | ND | 0.19 (0.03) abc | ND | 0.23 (0.02) bc | 0.08 (0.09) a | 0.19 (0.06) abc | 0.13 (0.03) ab |
| Aldehydes | ND | ND | 1.51 (2.23) a | ND | ND | 0.65 (0.77) a | 0.81 (0.12) a | ND | 0.54 (0.62) a | ||
| Hexanal | 783 | 775 | ND | ND | ND | ND | ND | 0.65 (0.77) a | 0.81 (0.12) a | ND | 0.54 (0.62) a |
| Nonanal | 1082 | 1084 | ND | ND | 1.51 (2.23) | ND | ND | ND | ND | ND | ND |
| Aliphatic Hydrocarbons | 2.19 (1.53) ab | 10.25 (2.96) de | 0.21 (0.05) a | 12.12 (3.80) e | 13.35 (1.76) e | 9.35 (4.48) cd | 5.26 (3.20) abc | 7.47 (1.12) cd | 7.16 (3.21) cd | ||
| Pentane | 500 | 500 | ND | 0.10 (0.11) | ND | ND | ND | ND | ND | ND | ND |
| Heptane | 700 | 700 | ND | ND | ND | ND | 0.15 (0.03) | ND | ND | ND | ND |
| 4-methyl-heptane | 768 | 768 | 0.82 (0.60) a | 3.10 (0.88) ab | ND | 3.94 (1.06) b | 4.02 (0.56) b | 2.75 (1.39) ab | 1.26 (1.58) ab | 2.47 (0.32) ab | 2.52 (1.05) ab |
| Octane | 800 | 800 | ND | 0.22 (0.26) a | 0.21 (0.05) a | ND | 0.40 (0.06) a | 0.41 (0.07) a | 0.36 (0.15) a | ND | ND |
| 2,4-dimethyl-heptane | 824 | 824 | ND | 0.17 (0.20) a | ND | 0.38 (0.19) a | 0.47 (0.08) a | 0.18 (0.21) a | ND | ND | ND |
| 2,4-dimethyl- 1-heptene | — | 840 | 1.38 (0.92) a | 6.07 (1.52) b | ND | 7.79 (2.58) b | 7.23 (1.08) b | 6.00 (2.91) b | 3.64 (1.70) ab | 4.89 (0.79) ab | 4.64 (2.16) ab |
| Decane | 1000 | 1000 | ND | 0.58 (0.22) b | ND | ND | 0.88 (0.07) b | ND | ND | 0.11 (0.13) a | ND |
| Aromatics | 0.44 (0.24) ab | 0.9 (0.17) bc | 0.12 (0.05) a | 1.09 (0.36) c | 2.48 (0.19) d | 0.94 (0.48) bc | 0.67 (0.08) b | 0.63 (0.19) bc | 0.76 (0.27) bc | ||
| Toluene | 753 | 751 | 0.29 (0.06) ab | 0.40 (0.05) b | 0.12 (0.05) a | 0.44 (0.09) b | 0.52 (0.07) b | 0.53 (0.23) b | 0.37 (0.04) ab | 0.32 (0.06) ab | 0.37 (0.08) ab |
| Benzene, 1,3-bis(1,1-dimethylethyl) | — | 1250 | 0.16 (0.19) a | 0.50 (0.13) ab | ND | 0.66 (0.27) b | 1.96 (0.13) c | 0.41 (0.25) ab | 0.30 (0.09) ab | 0.31 (0.13) ab | 0.38 (0.19) ab |
| Esters | ND | 1.59 (0.35) | ND | ND | ND | ND | ND | ND | ND | ||
| Ethyl acetate | 601 | 601 | ND | 1.33 (0.07) | ND | ND | ND | ND | ND | ND | ND |
| Hexanoic acid, ethyl ester | 986 | 983 | ND | 0.26 (0.30) | ND | ND | ND | ND | ND | ND | ND |
| Ketones | 12.37 (1.21) c | 8.64 (1.16) ab | 11.52 (1.01) bc | 7.58 (2.88) a | 11.83 (1.44) c | 11.16 (1.10) bc | 10.94 (0.23) abc | 11.47 (0.70) bc | 10.33 (2.05) abc | ||
| Acetone | 468 | 486 | 7.59 (0.98) d | 3.44 (0.75) ab | 6.64 (1.11) cd | 4.28 (2.00) abc | 2.44 (0.41) a | 6.97 (0.58) cd | 6.45 (0.19) cd | 7.33 (0.73) d | 6.05 (1.75) bcd |
| 2-butanone | 575 | 570 | 4.32 (0.50) c | 2.15 (0.17) a | 4.71 (0.17) c | 2.61 (0.94) ab | 1.24 (0.09) a | 4.19 (0.63) c | 3.94 (0.12) c | 3.92 (0.58) c | 3.73 (0.36) bc |
| 2-pentanone | 667 | 661 | ND | 0.59 (0.68) a | ND | ND | 1.12 (0.02) a | ND | ND | ND | ND |
| 3-methyl-2-butanone | 659 | 662 | 0.46 (0.06) a | 0.52 (0.60) a | ND | ND | ND | ND | 0.54 (0.14) a | 0.21 (0.25) a | 0.55 (0.11) a |
| 2,4-dimethyl-3-heptanone | — | 701 | ND | 0.06 (0.06) | ND | ND | ND | ND | ND | ND | ND |
| 2-Heptanone | 847 | 868 | ND | 1.10 (0.16) b | 0.17 (0.03) a | 0.29 (0.03) a | 3.18 (0.64) c | ND | ND | ND | ND |
| 4-methyl-2-heptanone | — | 920 | ND | 0.17 (0.20) a | ND | 0.40 (0.18) a | 0.41 (0.04) a | ND | ND | ND | ND |
| 2-Nonanone | 1094 | 1073 | ND | 0.61 (0.10) a | ND | ND | 2.54 (0.49) b | ND | ND | ND | ND |
| 2-Undecanone | 1279 | 1276 | ND | ND | ND | ND | 0.88 (0.15) | ND | ND | ND | ND |
| Nitrogenous Compounds | 11.81 (2.32) ab | 15.32 (2.26) c | 9.71 (1.07) ab | 8.27 (0.66) ab | 14.89 (2.97) c | 10.34 (1.34) b | 7.91 (0.9) a | 10.01 (2.51) ab | 8.82 (0.49) ab | ||
| Oxime-, methoxy-phenyl-_ | — | 890 | 11.81 (2.32) ab | 15.32 (2.26) c | 9.71 (1.07) ab | 8.27 (0.66) ab | 14.89 (2.97) c | 10.34 (1.34) b | 7.91 (0.90) a | 10.01 (2.51) ab | 8.82 (0.49) ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maida, A.L.; Bilbao-Sainz, C.; Karman, A.; Takeoka, G.; Powell-Palm, M.J.; Rubinsky, B. Effects of Isochoric Freezing on the Quality Characteristics of Raw Bovine Milk. Foods 2023, 12, 4150. https://doi.org/10.3390/foods12224150
Maida AL, Bilbao-Sainz C, Karman A, Takeoka G, Powell-Palm MJ, Rubinsky B. Effects of Isochoric Freezing on the Quality Characteristics of Raw Bovine Milk. Foods. 2023; 12(22):4150. https://doi.org/10.3390/foods12224150
Chicago/Turabian StyleMaida, Alan L., Cristina Bilbao-Sainz, Andrew Karman, Gary Takeoka, Matthew J. Powell-Palm, and Boris Rubinsky. 2023. "Effects of Isochoric Freezing on the Quality Characteristics of Raw Bovine Milk" Foods 12, no. 22: 4150. https://doi.org/10.3390/foods12224150
APA StyleMaida, A. L., Bilbao-Sainz, C., Karman, A., Takeoka, G., Powell-Palm, M. J., & Rubinsky, B. (2023). Effects of Isochoric Freezing on the Quality Characteristics of Raw Bovine Milk. Foods, 12(22), 4150. https://doi.org/10.3390/foods12224150