Investigating the Chemical Composition of Lepidium sativum Seeds and Their Ability to Safeguard against Monosodium Glutamate-Induced Hepatic Dysfunction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
- −
- Lepidium sativum seed powder was purchased from Imtenan Health Shop Company, Obour City, Egypt.
- −
- Casein (85%), cellulose, choline chloride, Maltodextrin, L-cysteine, minerals, and vitamins have been purchased from Al-Gomhoria Company for Chemicals, Cairo, Egypt.
- −
- Serum and Vaccine Center in Cairo, Egypt, provided 24 healthy male albino rats weighing (150 ± 10 g) of the Sprague Dawley strain. The rats were five weeks old.
2.2. Methods
2.2.1. Biological Experiment
Animals
Diets
2.2.2. Experimental Design
- −
- G1 (−Ve) group: the negative control group was fed a basal diet;
- −
- G2 (+Ve) group: the positive control group was fed a basal diet + MSG (30 g/kg b.w.);
- −
- G3: fed a basal diet + MSG (30 g/kg b.w.) + LSS powder (30 g/kg b.w.);
- −
- G4: fed a basal diet + MSG (30 g/kg b.w.) + LSS powder (60 g/kg b.w.).
2.2.3. Biological Evaluation
2.2.4. Determination of Total Phenolic Contents in Lepidium Sativum Seeds
2.2.5. Chemical Analysis
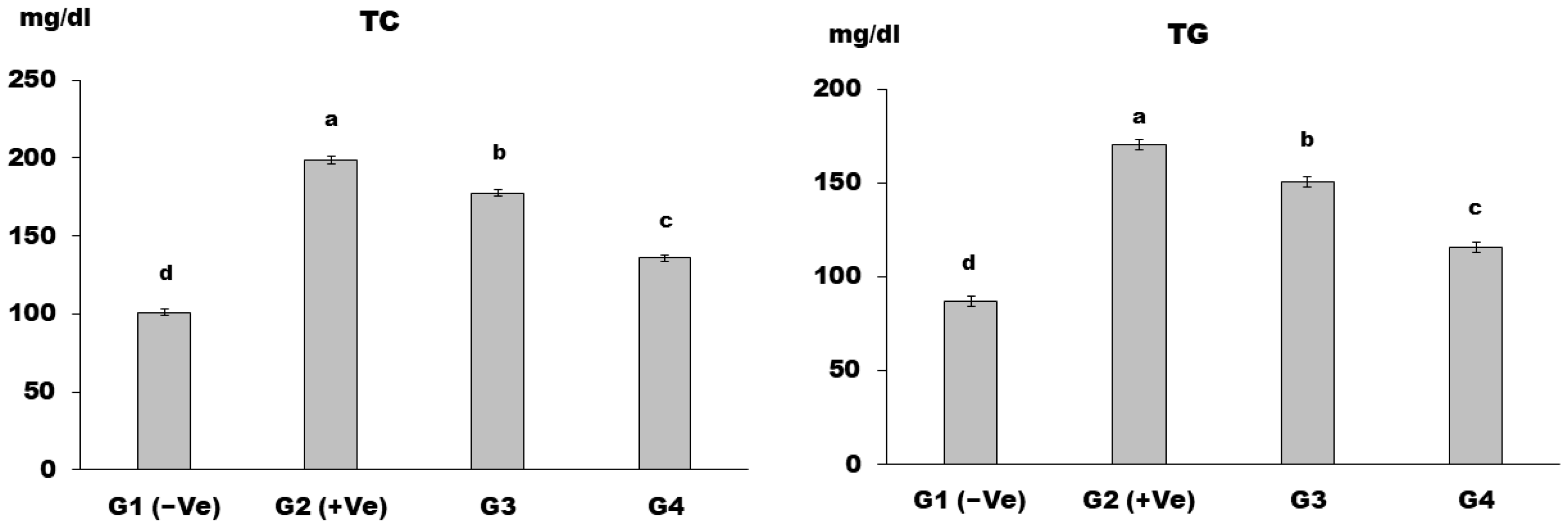
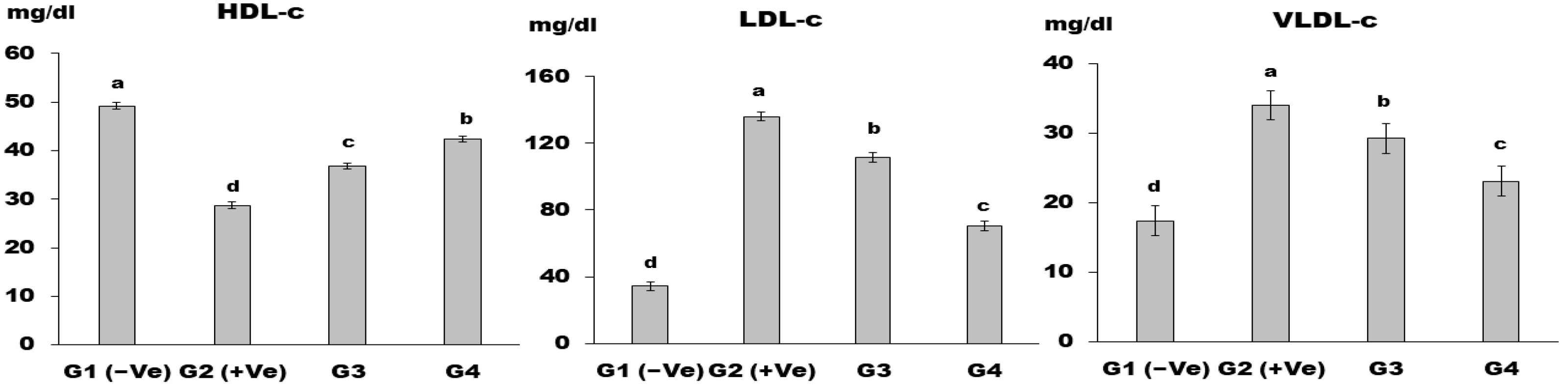
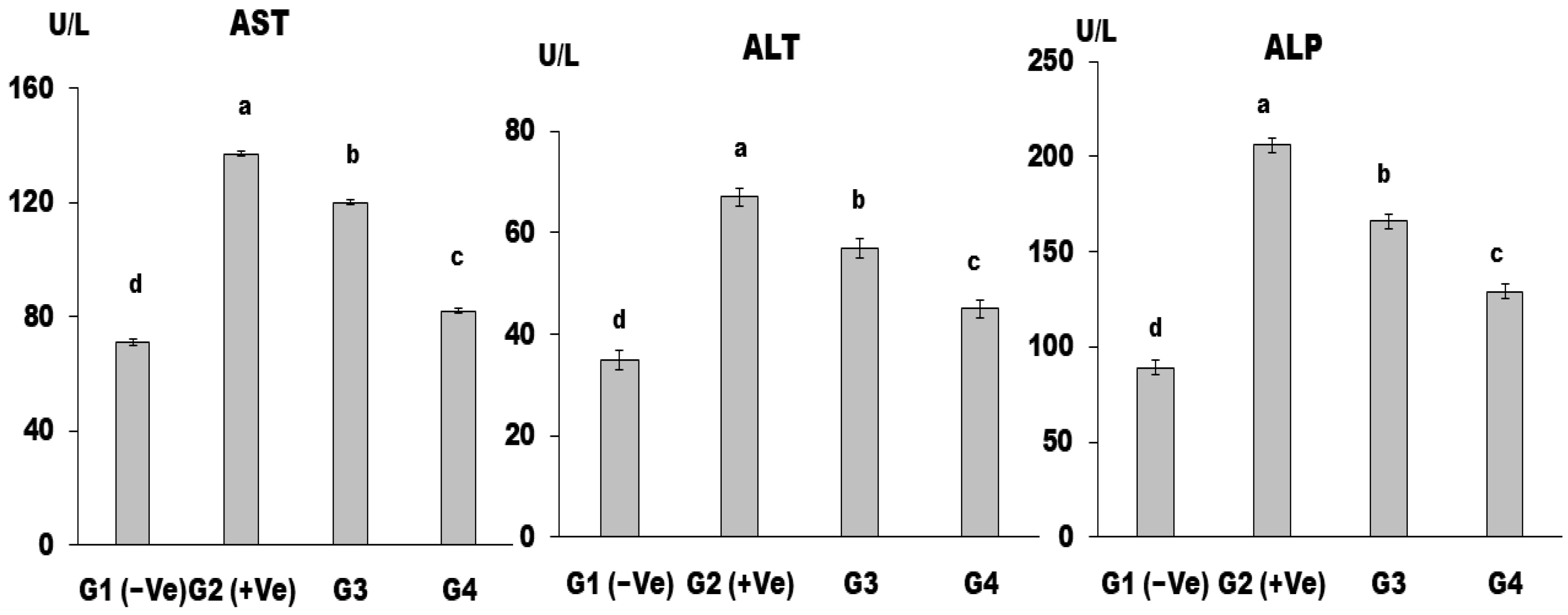
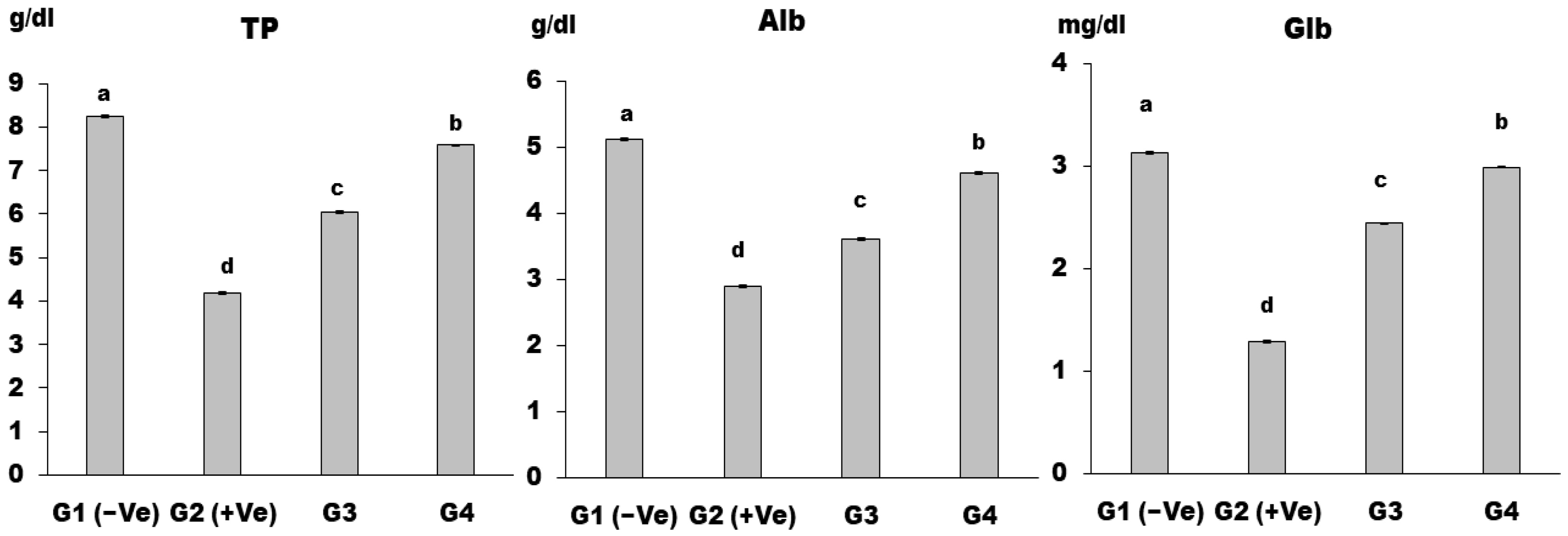
2.2.6. Biochemical Analysis of Serum
Lipid Profile
Liver Enzymes
Oxidant/Antioxidant Activity in Liver Tissue
2.2.7. Histopathological Examinations
2.2.8. Statistical Analysis
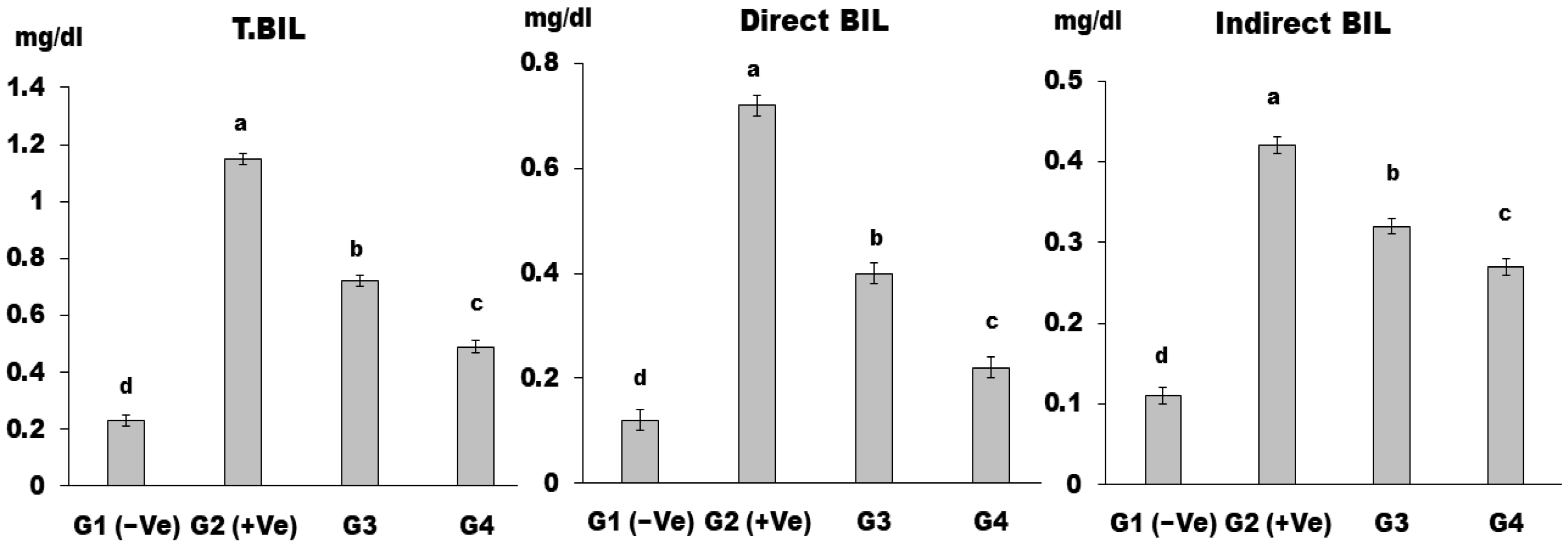
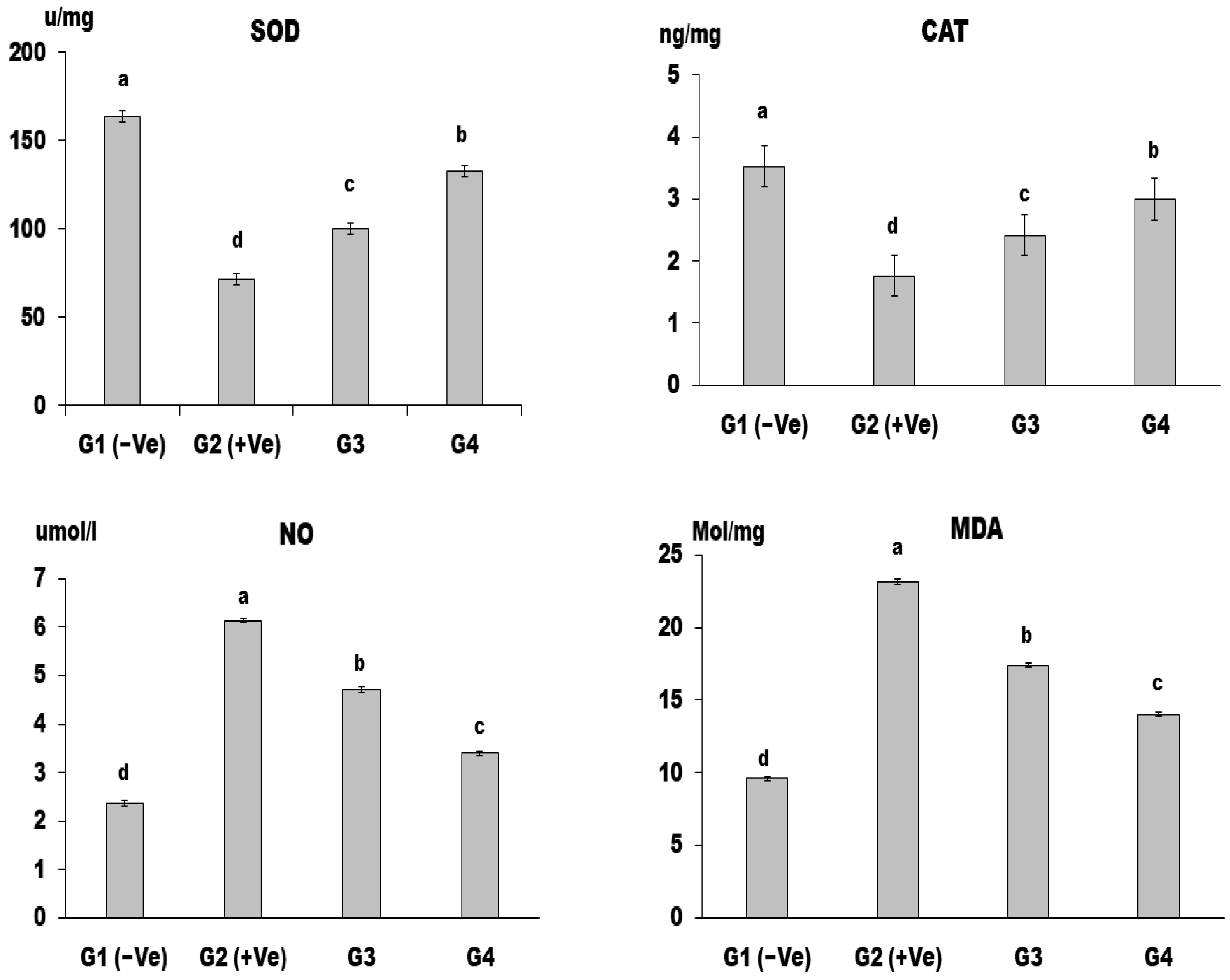
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tomaz da Silva, M.; Santos, A.R.; Koike, T.E.; Nascimento, T.L.; Rozanski, A.; Bosnakovski, D.; Miyabara, E.H. The fibrotic niche impairs satellite cell function and muscle regeneration in mouse models of Marfan syndrome. Acta Physiol. 2023, 237, e13889. [Google Scholar] [CrossRef] [PubMed]
- Bastug, A.; Bodur, H. Acute Hepatitis B. In Viral Hepatitis: Acute Hepatitis; Springer: Cham, Switzerland, 2019; pp. 25–44. [Google Scholar]
- Gorantla, R.; Buddam, A.; Dietz, N.; Mukherjee, S. S2604 Acute Severe Cholestatic Hepatitis B Infection in an Immune-Competent Patient. Off. J. Am. Coll. Gastroenterol.|ACG 2020, 115, S1367–S1368. [Google Scholar] [CrossRef]
- Shukry, M.; El-Shehawi, A.M.; El-Kholy, W.M.; Elsisy, R.A.; Hamoda, H.S.; Tohamy, H.G.; Farrag, F.A. Ameliorative effect of graviola (Annona muricata) on mono sodium glutamate-induced hepatic injury in rats: Antioxidant, apoptotic, anti-inflammatory, lipogenesis markers, and histopathological studies. Animals 2020, 10, 1996. [Google Scholar] [CrossRef]
- Toda, Y.; Nakagita, T.; Hirokawa, T.; Yamashita, Y.; Nakajima, A.; Narukawa, M.; Ishimaru, Y.; Uchida, R.; Misaka, T. Positive/Negative Allosteric Modulation Switching in an Umami Taste Receptor (T1R1/T1R3) by a Natural Flavor Compound, Methional. Sci. Rep. 2018, 8, 11796. [Google Scholar] [CrossRef] [PubMed]
- Hartley, I.E.; Liem, D.G.; Keast, R. Umami as an ‘Alimentary’ Taste. A New Perspective on Taste Classification. Nutrients 2019, 11, 182. [Google Scholar] [CrossRef]
- Hassan, H.A.; El-Kholy, W.M.; El-Sawi, M.R.; Galal, N.A.; Ramadan, M.F. Myrtle (Myrtus communis) leaf extract suppresses hepatotoxicity induced by monosodium glutamate and acrylamide through obstructing apoptosis, DNA fragmentation, and cell cycle arrest. Environ. Sci. Pollut. Res. 2020, 27, 23188–23198. [Google Scholar] [CrossRef]
- Zhu, S.; Gouaux, E. Structure and symmetry inform gating principles of ionotropic glutamate receptors. Neuropharmacology 2017, 112, 11–15. [Google Scholar] [CrossRef]
- Shosha, H.M.; Ebaid, H.M.; Toraih, E.A.; Abdelrazek, H.M.A.; Elrayess, R.A. Effect of monosodium glutamate on fetal development and progesterone level in pregnant Wistar Albino rats. Environ. Sci. Pollut. Res. 2023, 30, 49779–49797. [Google Scholar] [CrossRef]
- Airaodion, I.A.; Ogbuagu, O.E.; Osemwowa, E.U.; Ogbuagu, U.; Esonu, E.C.; Agunbiade, A.P.; Okereke, D.; Oloruntoba, A.P. Toxicological Effect of Monosodium Glutamate in Seasonings on Human Health. Glob. J. Nutr. Food Sci. 2019, 1, 2019. [Google Scholar] [CrossRef]
- Nabi, S.; Bhandari, U.; Haque, S.E. Saroglitazar ameliorates monosodium glutamate-induced obesity and associated inflammation in Wistar rats: Plausible role of NLRP3 inflammasome and NF-κB. Iran. J. Basic Med. Sci. 2022, 25, 827–841. [Google Scholar]
- Banerjee, A.; Mukherjee, S.; Maji, B.K. Worldwide flavor enhancer monosodium glutamate combined with high lipid diet provokes metabolic alterations and systemic anomalies: An overview. Toxicol. Rep. 2021, 8, 938–961. [Google Scholar] [CrossRef]
- Zazula, M.F.; Saraiva, D.F.; Theodoro, J.L.; Maciel, M.; Sepulveda, E.V.D.S.; Zanardini de Andrade, B.; Naliwaiko, K. An Early and Sustained Inflammatory State Induces Muscle Changes and Establishes Obesogenic Characteristics in Wistar Rats Exposed to the MSG-Induced Obesity Model. Int. J. Mol. Sci. 2023, 24, 4730. [Google Scholar] [CrossRef] [PubMed]
- Nakadate, K.; Kawakami, K.; Yamazaki, N. Anti-Obesity and Anti-Inflammatory Synergistic Effects of Green Tea Catechins and Citrus β-Cryptoxanthin Ingestion in Obese Mice. Int. J. Mol. Sci. 2023, 24, 7054. [Google Scholar] [CrossRef] [PubMed]
- Ceglarek, V.M.; Guareschi, Z.M.; Correia, B.R.; Grassiolli, S. Supplementation with vitamin d associated to the exercise alters differently to the morphology of the brown adipose tissue in lean and obese rats. Int. J. Dev. Res. 2018, 8, 23772–23779. [Google Scholar]
- Airaodion, A.I.; Ngwogu, K.O.; Ngwogu, A.C.; Megwas, A.U.; Ekenjoku, J.A. Nephrotoxicity of monosodium glutamate (MSG) in Wistar rats. Int. J. Adv. Nephrol. Res. 2020, 3, 1–10. [Google Scholar]
- Jaganathan, R.; Ravindran, R.; Dhanasekaran, S. Emerging role of adipocytokines in type 2 diabetes as mediators of insulin resistance and cardiovascular disease. Can. J. Diabetes 2018, 42, 446–456. [Google Scholar] [CrossRef]
- Shalitin, S.; Gat-Yablonski, G. Associations of obesity with linear growth and puberty. Horm. Res. Paediatr. 2022, 95, 120–136. [Google Scholar] [CrossRef]
- Painuli, S.; Quispe, C.; Herrera-Bravo, J.; Semwal, P.; Martorell, M.; Almarhoon, Z.M.; Cho, W.C. Nutraceutical profiling, bioactive composition, and biological applications of Lepidium sativum L. Oxidative Med. Cell. Longev. 2022, 2022, 2910411. [Google Scholar] [CrossRef]
- Buso, P.; Manfredini, S.; Reza Ahmadi-Ashtiani, H.; Sciabica, S.; Buzzi, R.; Vertuani, S.; Baldisserotto, A. Iranian medicinal plants: From ethnomedicine to actual studies. Medicina 2020, 56, 97. [Google Scholar] [CrossRef]
- Zamzami, M.A.; Baothman, O.A.; Samy, F.; Abo-Golayel, M.K. Amelioration of CCl4-induced hepatotoxicity in rabbits by Lepidium sativum seeds. Evid.-Based Complement. Altern. Med. 2019, 2019, 5947234. [Google Scholar] [CrossRef]
- Arriarán, S.; Agnelli, S.; Sabater, D.; Remesar, X.; Fernández-López, J.A.; Alemany, M. Evidences of basal lactate production in the main white adipose tissue sites of rats. Effects of sex and a cafeteria diet. PLoS ONE 2015, 10, e0119572. [Google Scholar] [CrossRef]
- Abd-Elkareem, M.; Soliman, M.; Abd El-Rahman, M.A.; Abou Khalil, N.S. The protective effect of Nigella sativa seeds against monosodium glutamate-induced hepatic dysfunction in rats. Toxicol. Rep. 2022, 9, 147–153. [Google Scholar] [CrossRef]
- Carleton, M.D. Phylogenetic Relationships in Neotomine-Peromyscine Rodents (Muroidea) and a Reappraisal of the Dichotomy within New World Cricetinae; University of Michigan: Ann Arbor, MI, USA, 1979. [Google Scholar]
- AOAC. Official Methods of the Association of Official Analytical Chemists, 15th ed.; Wilson Boulevardarling: Arlington, VA, USA, 2010; p. 22201. [Google Scholar]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists, 18th ed.; AOAC Int.: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Karimi-Maleh, H.; Arotiba, O.A. Simultaneous determination of cholesterol, ascorbic acid and uric acid as three essential biological compounds at a carbon paste electrode modified with copper oxide decorated reduced graphene oxide nanocomposite and ionic liquid. J. Colloid Interface Sci. 2020, 560, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Pundir, C.S.; Narwal, V. Biosensing methods for determination of triglycerides: A review. Biosens. Bioelectron. 2018, 100, 214–227. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Harada, A.; Toh, R.; Kubo, T.; Miwa, K.; Kim, J.; Kiriyama, M.; Iino, T.; Nishikawa, Y.; Uno, S.N.; et al. Fully automated immunoassay for cholesterol uptake capacity to assess high-density lipoprotein function and cardiovascular disease risk. Sci. Rep. 2023, 13, 1899. [Google Scholar] [CrossRef] [PubMed]
- Sampson, M.; Ling, C.; Sun, Q.; Harb, R.; Ashmaig, M.; Warnick, R.; Sethi, A.; Fleming, J.K.; Otvos, J.D.; Meeusen, J.W.; et al. A new equation for calculation of low-density lipoprotein cholesterol in patients with normolipidemia and/or hypertriglyceridemia. JAMA Cardiol. 2020, 5, 540–548. [Google Scholar] [CrossRef]
- Nasrin, R.; Alim, M.A. Finite element simulation of forced convection in a flat plate solar collector: Influence of nanofluid with double nanoparticles. J. Appl. Fluid Mech. 2014, 7, 543–556. [Google Scholar]
- Kazmi, S.J.H.; Zafar, M.T.; Zia, B.F.; Khalid, S.R.; Kumar, V.; Tabassum, S.; Ali, A.; Aziz, N.; Khan, N.A.; Kumari, K.; et al. Role of serum C-reactive protein (CRP)/Albumin ratio in predicting the severity of acute pancreatitis: A retrospective cohort. Ann. Med. Surg. 2022, 82, 104715. [Google Scholar]
- Adaway, J.; Keevil, B.; Miller, A.; Monaghan, P.J.; Merrett, N.; Owen, L. Ramifications of variability in sex hormone-binding globulin measurement by different immunoassays on the calculation of free testosterone. Ann. Clin. Biochem. 2020, 57, 88–94. [Google Scholar] [CrossRef]
- Shukla, V.; Kumar, D.S.; Ali, M.A.; Agarwal, S.; Khandpur, S. Nitric oxide, lipid peroxidation products, and antioxidants in primary fibromyalgia and correlation with disease severity. J. Med. Biochem. 2020, 39, 165. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Catalase, superoxide dismutase and ascorbate-glutathione cycle enzymes confer drought tolerance of Amaranthus tricolor. Sci. Rep. 2018, 8, 16496. [Google Scholar] [CrossRef] [PubMed]
- Sonzogni, A.; Previtali, G.; Seghezzi, M.; Grazia Alessio, M.; Gianatti, A.; Licini, L.; Morotti, D.; Zerbi, P.; Carsana, L.; Rossi, R.; et al. Liver histopathology in severe COVID 19 respiratory failure is suggestive of vascular alterations. Liver Int. 2020, 40, 2110–2116. [Google Scholar] [CrossRef] [PubMed]
- Attia, E.S.; Amer, A.H.; Hasanein, M.A. The hypoglycemic and antioxidant activities of garden cress (Lepidium sativum L.) seed on alloxan-induced diabetic male rats. Nat. Prod. Res. 2019, 33, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Nasef, A.N.; MKhateib, B.R. Study the Potential Therapeutic Effect of Garden Cress (Lepidium sativum) on Nephropathy Diabetic Rats: Biological and Biochemical Studies. Alex. Sci. Exch. J. 2021, 42, 263–272. [Google Scholar]
- El-Salam, A.; Kholoud, H.; Toliba, A.O.; El-Shourbagy, G.A.; El-Nemr, S.E. Chemical and functional properties of garden cress (Lepidium sativum L.) seeds powder. Zagazig J. Agric. Res. 2019, 46, 1517–1528. [Google Scholar] [CrossRef]
- Chatoui, K.; Harhar, H.; El Kamli, T.; Tabyaoui, M. Chemical composition and antioxidant capacity of Lepidium sativum seeds from four regions of Morocco. Evid.-Based Complement. Altern. Med. 2020, 2020, 7302727. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, J.; Abbasi, H.; Zarei Jelyani, A.; Mousavi Khaneghah, A. The use of Salvia macrosiphon and Lepidium sativum Linn. seed gums in nanoencapsulation processes: Improving antioxidant activity of potato skin extract. J. Food Qual. 2021, 2021, 5519857. [Google Scholar] [CrossRef]
- Hashemi, J.M.; Alahmari, S.A. Effect of argan oil (Argania spinosa) on hypercholesterolemic male rats. Med. Sci. 2021, 25, 1150–1158. [Google Scholar]
- Ait-Yahia, O.; Perreau, F.; Bouzroura, S.A.; Benmalek, Y.; Dob, T.; Belkebir, A. Chemical composition and biological activities of n-butanol extract of Lepidium sativum L. (Brassicaceae) seed. Trop. J. Pharm. Res. 2018, 17, 891–896. [Google Scholar] [CrossRef]
- Doke, S.; Guha, M. Quality assessment of sweet snack from Garden cress (Lepidium sativum L.) seeds—An unexplored health grain. J. Food Process. Preserv. 2018, 42, e13431. [Google Scholar] [CrossRef]
- Akram, M.; Hamid, A.; Khalil, A.; Ghaffar, A.; Tayyaba, N.; Saeed, A.; Ali, M.; Naveed, A. Review on Medicinal Uses, Pharmacological, Phytochemistry and Immunomodulatory Activity of Plants. Int. J. Immunopathol. Pharmacol. 2014, 27, 313–319. [Google Scholar] [CrossRef]
- Bárbara, P.S.; Desirrê, M.D.; Maria, E. Chia Seed Shows Good Protein Quality, Hypoglycemic Effect and Improves the Lipid Profileand Liver and Intestinal Morphology of Wistar Rats. Plant Foods Hum. Nutr. 2016, 71, 225–230. [Google Scholar]
- Ibegbulem, C.O.; Chikezie, P.C.; Ukoha, A.I.; Opara, C.N. Effects of diet containing monosodium glutamate on organ weights, acute blood steroidal sex hormone levels, lipid profile and erythrocyte antioxidant enzymes activities of rats. J. Acute Dis. 2016, 5, 402–407. [Google Scholar] [CrossRef]
- Diab, A.E.A.; Hamza, R.Z. Monosodium glutamate induced hepatotoxicity and the possible mitigating effect of vitamin C and propolis. J. Adv. Med. Pharm. Sci. 2016, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pérusse, L.; Vohl, M.C.; Marette, A. 7th Congress of the International Society of Nutrigenetics/Nutrigenomics (ISNN). J. Nutr. Nutr. 2014, 6, 201–255. Available online: https://karger.com/jnn/article/6/4-5/201/181476/7th-Congress-of-the-International-Society-of (accessed on 1 October 2013). [CrossRef]
- Okafor, I.A.; Ezejindu, D.N. Phytochemical studies on portulaca oleracea (purslane) plant. GJBAHS 2014, 3, 132–136. [Google Scholar]
- Balgoon, M.J. Assessment of the protective effect of Lepidium sativum against aluminum-induced liver and kidney effects in albino rat. BioMed Res. Int. 2019, 2019, 4516730. [Google Scholar] [CrossRef]
- Cantor, A.; Miller, J.; Zachariah, P.; DaSilva, B.; Margolis, K.; Martinez, M. Acute hepatitis is a prominent presentation of the multisystem inflammatory syndrome in children: A single-center report. Hepatology 2020, 72, 1522–1527. [Google Scholar] [CrossRef]
- Osagie-Eweka, S.E.; Orhue, N.E.J.; Omogbai, E.K.I.; Amaechina, F.C. Oral acute and sub-chronic toxicity assessment of aqueous leaf extract of Simarouba glauca DC (Paradise tree). Toxicol. Rep. 2021, 8, 239–247. [Google Scholar] [CrossRef]
- Sharma, A. Monosodium glutamate-induced oxidative kidney damage and possible mechanisms: A mini-review. J. Biomed. Sci. 2015, 22, 93. [Google Scholar] [CrossRef]
- Kayode, O.T.; Rotimi, D.E.; Kayode, A.A.; Olaolu, T.D.; Adeyemi, O.S. Monosodium glutamate (MSG)-induced male reproductive dysfunction: A mini review. Toxics 2020, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Nasab, E.M.; Athari, A.M.; Ghafarzade, S.; Nasab, A.M.; Athari, S.S. Immunomodulatory effects of two silymarin isomers in a Balb/c mouse model of allergic asthma. Allergol. Et Immunopathol. 2020, 48, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Vazifeh, S.; Kananpour, P.; Khalilpour, M.; Eisalou, S.V.; Hamblin, M.R. Anti-inflammatory and Immunomodulatory Properties of Lepidium sativum. BioMed Res. Int. 2022, 2022, 3645038. [Google Scholar] [CrossRef] [PubMed]
- Sahin, B.; Acikel Elmas, M.; Bingol Ozakpinar, O.; Arbak, S. The Effects of Apocynin on Monosodium Glutamate Induced Liver Damage of Rats. Heliyon 2023, 9, e17327. [Google Scholar] [CrossRef]
- John, O.D.; Mushunje, A.T.; Surugau, N.; Guad, R.M. The metabolic and molecular mechanisms of α mangostin in cardiometabolic disorders. Int. J. Mol. Med. 2022, 50, 120. [Google Scholar] [CrossRef] [PubMed]
- Zanfirescu, A.; Ungurianu, A.; Tsatsakis, A.M.; Nițulescu, G.M.; Kouretas, D.; Veskoukis, A.; Margină, D. A review of the alleged health hazards of monosodium glutamate. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1111–1134. [Google Scholar] [CrossRef]


| Constituent of Lepidium sativum Seeds | g/100 g |
|---|---|
| Moisture | 6.73 ± 0.02 cd |
| Crude protein | 21.61 ± 0.71 c |
| Fat | 32.28 ± 0.18 a |
| Crude fiber | 6.75 ± 0.06 cd |
| Ash | 4.83 ± 0.09 d |
| Total carbohydrate | 27.80 ± 0.73 b |
| Minerals of LSS | mg/100 g |
|---|---|
| Potassium (K) | 296.36 ± 10.23 ab |
| Calcium (Ca) | 210.23 ± 13.25 c |
| Phosphorus (P) | 944.33 ± 16.03 a |
| Magnesium (Mg) | 325 ± 3.64 b |
| Sodium (Na) | 230.35 ± 4.07 bc |
| Zinc (Zn) | 2.96 ± 0.15 d |
| Phenolic Compounds | Conc. (µg/g) |
|---|---|
| Gallic acid | 111.86 ± 5.11 d |
| Chlorogenic acid | 238.16 ± 7.03 b |
| Catechin | 176.30 ± 4.01 c |
| Coffeic acid | 284.79 ± 11.06 ab |
| Pyro catechol | 408.41 ± 9.15 a |
| Ellagic acid | 75.60 ± 2.10 de |
| Vanillin | 4.91 ± 0.03 |
| Ferulic acid | 13.06 ± 0.09 |
| Naringenin | 19.94 ± 2.16 |
| Quercetin | 169.29 ± 4.17 c |
| Cinnamic acid | 18.27 ± 5.02 |
| Hesperetin | 51.87 ± 2.14 e |
| Groups | G1 (−Ve) | G2 (+Ve) | G3 | G4 |
|---|---|---|---|---|
| FI (g) | 781.20 ± 2.27 a | 474.99 ± 2.42 d | 560.00 ± 1.50 c | 701.90 ± 1.15 b |
| BWG% | 50.40 ± 3.64 a | 32.40 ± 2.50 d | 38.40 ± 2.07 c | 42.60 ± 2.50 b |
| FER | 0.07 ± 0.005 a | 0.04 ± 0.002 d | 0.05 ± 0.003 c | 0.06 ± 0.004 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Gendy, M.S.; El-Gezawy, E.S.; Saleh, A.A.; Alhotan, R.A.; Al-Badwi, M.A.A.; Hussein, E.O.S.; El-Tahan, H.M.; Kim, I.H.; Cho, S.; Omar, S.M. Investigating the Chemical Composition of Lepidium sativum Seeds and Their Ability to Safeguard against Monosodium Glutamate-Induced Hepatic Dysfunction. Foods 2023, 12, 4129. https://doi.org/10.3390/foods12224129
El-Gendy MS, El-Gezawy ES, Saleh AA, Alhotan RA, Al-Badwi MAA, Hussein EOS, El-Tahan HM, Kim IH, Cho S, Omar SM. Investigating the Chemical Composition of Lepidium sativum Seeds and Their Ability to Safeguard against Monosodium Glutamate-Induced Hepatic Dysfunction. Foods. 2023; 12(22):4129. https://doi.org/10.3390/foods12224129
Chicago/Turabian StyleEl-Gendy, Manal Salah, Eman Sobhy El-Gezawy, Ahmed A. Saleh, Rashed A. Alhotan, Mohammed A. A. Al-Badwi, Elsayed Osman Sewlim Hussein, Hossam M. El-Tahan, In Ho Kim, Sungbo Cho, and Sara Mahmoud Omar. 2023. "Investigating the Chemical Composition of Lepidium sativum Seeds and Their Ability to Safeguard against Monosodium Glutamate-Induced Hepatic Dysfunction" Foods 12, no. 22: 4129. https://doi.org/10.3390/foods12224129
APA StyleEl-Gendy, M. S., El-Gezawy, E. S., Saleh, A. A., Alhotan, R. A., Al-Badwi, M. A. A., Hussein, E. O. S., El-Tahan, H. M., Kim, I. H., Cho, S., & Omar, S. M. (2023). Investigating the Chemical Composition of Lepidium sativum Seeds and Their Ability to Safeguard against Monosodium Glutamate-Induced Hepatic Dysfunction. Foods, 12(22), 4129. https://doi.org/10.3390/foods12224129









