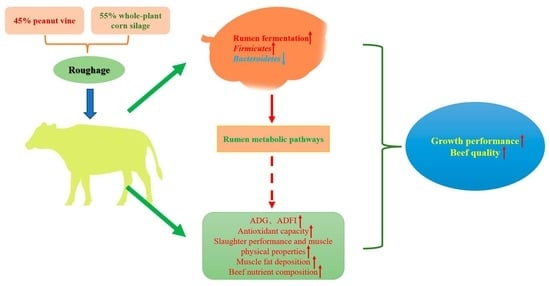
Effects of Diets Combining Peanut Vine and Whole-Plant Corn Silage on Growth Performance, Meat Quality and Rumen Microbiota of Simmental Crossbred Cattle
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Sample Collection and Measurements
2.3. Determination of Antioxidant Indicators
2.4. Detection of Liver Lipid Metabolism Genes
2.5. 16s rRNA Sequencing and Bioinformatics Analysis
2.6. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Organ Index
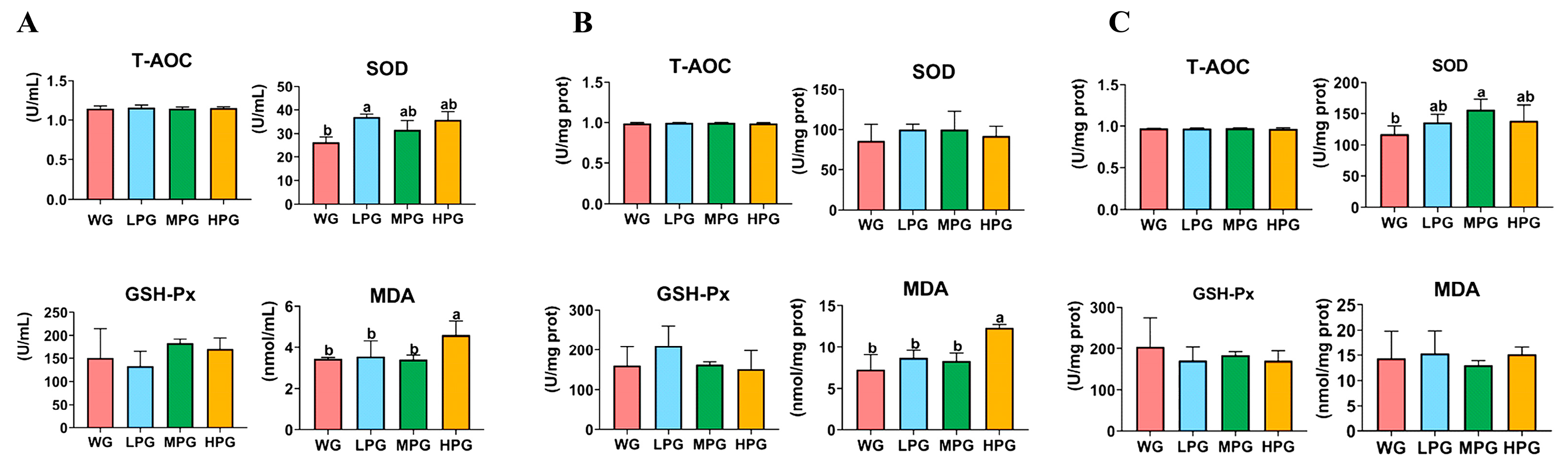
3.3. Serum and Tissue Antioxidant Capacity
3.4. Slaughter Performance and Muscle Physical Properties
3.5. Conventional Nutritional Composition and Fatty Acid Content of the Muscle
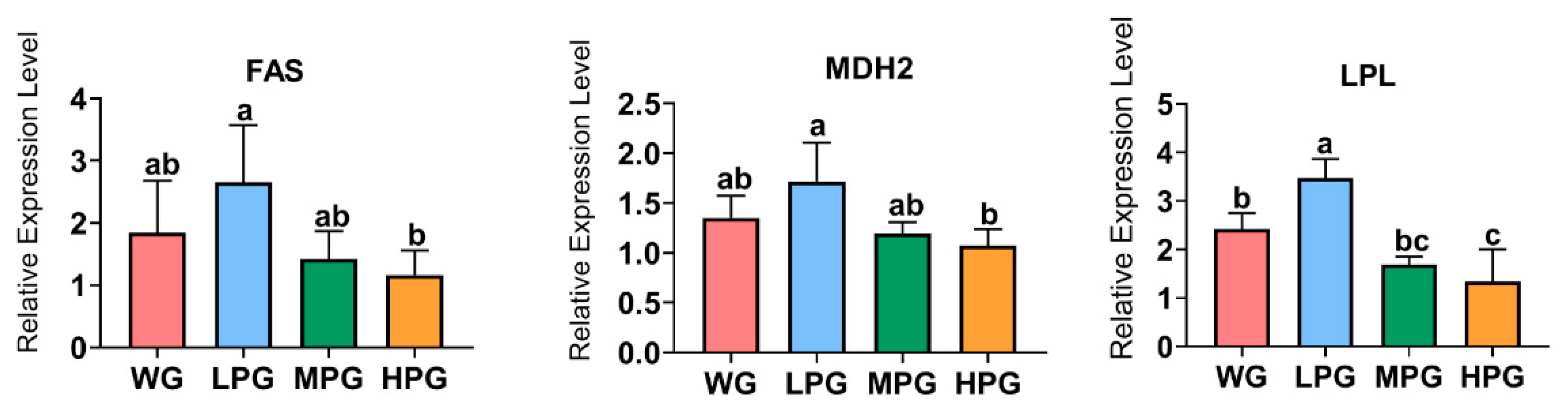
3.6. The Expression of Liver Lipid Metabolism Genes
3.7. Rumen Fermentation Parameters
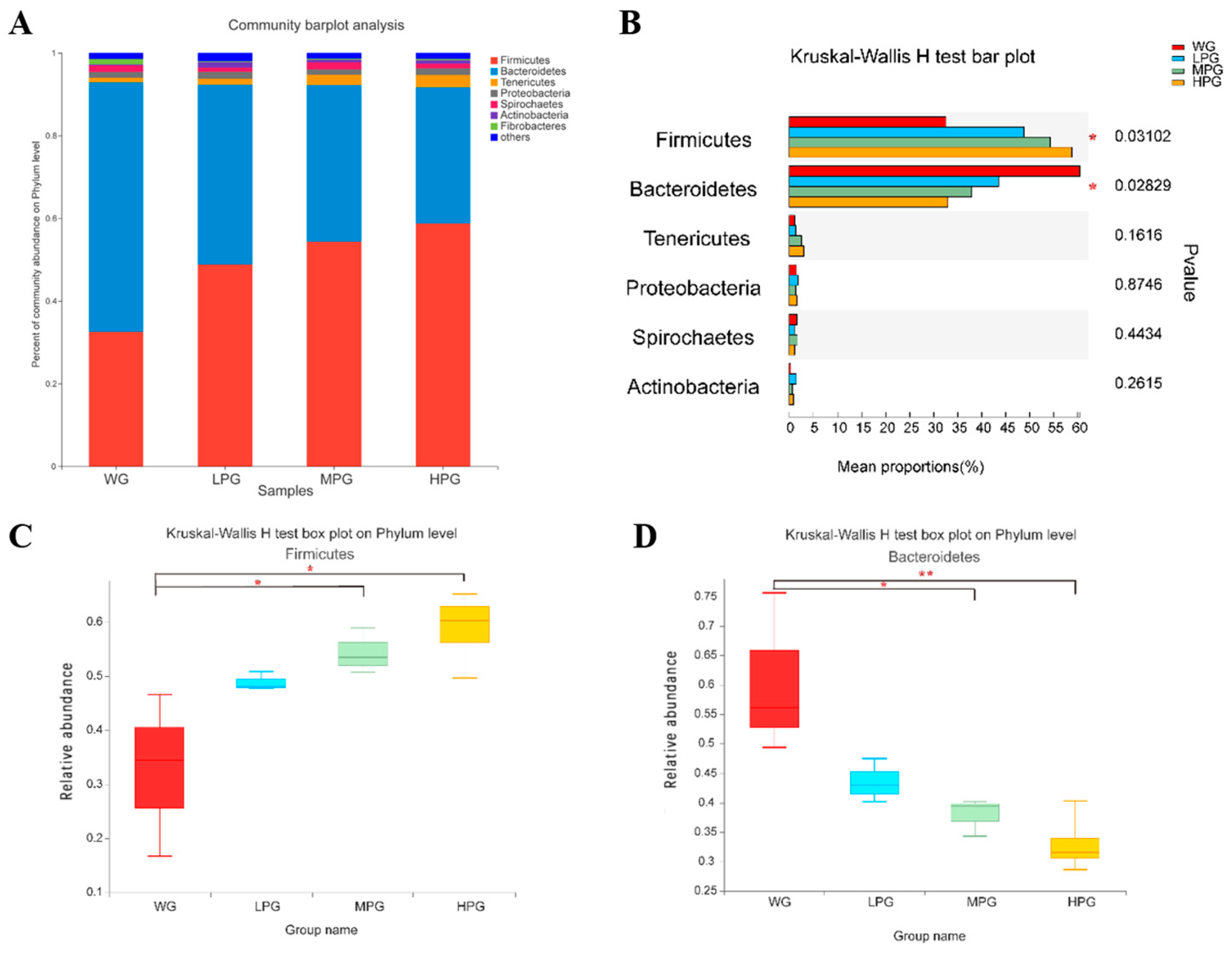
3.8. Rumen Microbial Communities
3.9. Functional Prediction of Rumen Microbiota and Correlation Analysis
4. Discussion
5. Conclusions
6. Future Implications
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, G. Important Roles of Dietary Taurine, Creatine, Carnosine, Anserine and 4-Hydroxyproline in Human Nutrition and Health. Amino Acids 2020, 52, 329–360. [Google Scholar] [CrossRef] [PubMed]
- Pulina, G.; Acciaro, M.; Atzori, A.S.; Battacone, G.; Crovetto, G.M.; Mele, M.; Pirlo, G.; Rassu, S.P.G. Animal Board Invited Review—Beef for Future: Technologies for a Sustainable and Profitable Beef Industry. Animal 2021, 15, 100358. [Google Scholar] [CrossRef]
- Greenwood, P.L. Review: An Overview of Beef Production from Pasture and Feedlot Globally, as Demand for Beef and the Need for Sustainable Practices Increase. Animal 2021, 15, 100295. [Google Scholar] [CrossRef] [PubMed]
- González, N.; Marquès, M.; Nadal, M.; Domingo, J.L. Meat Consumption: Which Are the Current Global Risks? A Review of Recent (2010–2020) Evidences. Food Res. Int. 2020, 137, 109341. [Google Scholar] [CrossRef]
- Masebo, N.T.; Marliani, G.; Cavallini, D.; Accorsi, P.A.; Di Pietro, M.; Beltrame, A.; Gentile, A.; Jacinto, J.G.P. Health and Welfare Assessment of Beef Cattle during the Adaptation Period in a Specialized Commercial Fattening Unit. Res. Vet. Sci. 2023, 158, 50–55. [Google Scholar] [CrossRef]
- Nazli, M.H.; Halim, R.A.; Abdullah, A.M.; Hussin, G.; Samsudin, A.A. Potential of Feeding Beef Cattle with Whole Corn Crop Silage and Rice Straw in Malaysia. Trop. Anim. Health Prod. 2018, 50, 1119–1124. [Google Scholar] [CrossRef]
- Cherdthong, A. Potential Use of Rumen Digesta as Ruminant Diet—A Review. Trop. Anim. Health Prod. 2020, 52, 1–6. [Google Scholar] [CrossRef]
- Cavallini, D.; Palmonari, A.; Mammi, L.M.E.; Ghiaccio, F.; Canestrari, G.; Formigoni, A. Evaluation of Fecal Sampling Time Points to Estimate Apparent Nutrient Digestibility in Lactating Holstein Dairy Cows. Front. Vet. Sci. 2023, 9, 1065258. [Google Scholar] [CrossRef] [PubMed]
- Arya, S.S.; Salve, A.R.; Chauhan, S. Peanuts as Functional Food: A Review. J. Food Sci. Technol. 2016, 53, 31–41. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, J.; Du, F. Potential Use of Peanut By-Products in Food Processing: A Review. J. Food Sci. Technol. 2012, 49, 521–529. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, L.; Xue, X.; Zhang, X.; Wang, H.; Gao, T.; Phillips, C. Effect of Feeding a Diet Comprised of Various Corn Silages Inclusion with Peanut Vine or Wheat Straw on Performance, Digestion, Serum Parameters and Meat Nutrients in Finishing Beef Cattle. Anim. Biosci. 2022, 35, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.Z.; Shen, Y. Effect of Application of a Bacteria Inoculant and Wheat Bran on Fermentation Quality of Peanut Vine Ensiled Alone or with Corn Stover. J. Integr. Agric. 2013, 12, 556–560. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, X.; Dong, W.; Huang, B.; Wang, Y.; Zhu, M.; Wang, C. Plant Cell Wall Breakdown by Hindgut Microorganisms: Can We Get Scientific Insights from Rumen Microorganisms? J. Equine Vet. Sci. 2022, 115, 104027. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Liu, B.; Xiao, J.; Guo, M.; Zhao, S.; Hu, M.; Cui, Y.; Li, D.; Wang, C.; Ma, S.; et al. Effects of Different Roughage Diets on Fattening Performance, Meat Quality, Fatty Acid Composition, and Rumen Microbe in Steers. Front. Nutr. 2022, 9, 885069. [Google Scholar] [CrossRef] [PubMed]
- Abdou, N.; Nsahlai, I.V.; Chimonyo, M. Effects of Groundnut Haulms Supplementation on Millet Stover Intake, Digestibility and Growth Performance of Lambs. Anim. Feed Sci. Technol. 2011, 169, 176–184. [Google Scholar] [CrossRef]
- Bertoia, L.M.; Aulicino, M.B. Maize Forage Aptitude: Combining Ability of Inbred Lines and Stability of Hybrids. Crop J. 2014, 2, 407–418. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, G.; Wu, H.; Meng, Q.; Khane, M.Z.; Zhou, Z. Effect of Hybrid Type on Fermentation and Nutritional Parameters of Whole Plant Corn Silage. Animals 2021, 11, 1587. [Google Scholar] [CrossRef]
- Zaralis, K.; Nørgaard, P.; Helander, C.; Murphy, M.; Weisbjerg, M.R.; Nadeau, E. Effects of Maize Maturity at Harvest and Dietary Proportion of Maize Silage on Intake and Performance of Growing/Finishing Bulls. Livest. Sci. 2014, 168, 89–93. [Google Scholar] [CrossRef]
- Kirkland, R.M.; Patterson, D.C. The Effect of Quality of Grass and Maize Silage on the Intake and Performance of Beef Cattle. Livest. Sci. 2006, 100, 179–188. [Google Scholar] [CrossRef]
- Phipps, R.H.; Sutton, J.D.; Jones, B.A. Forage Mixtures for Dairy Cows: The Effect on Dry-Matter Intake and Milk Production of Incorporating Either Fermented or Urea-Treated Whole-Crop Wheat, Brewers’ Grains, Fodder Beet or Maize Silage into Diets Based on Grass Silage. Anim. Sci. 1995, 61, 491–496. [Google Scholar] [CrossRef]
- Bodine, T.N.; Purvis, H.T.; Ackerman, C.J.; Goad, C.L. Effects of Supplementing Prairie Hay with Corn and Soybean Meal on Intake, Digestion, and Ruminal Measurements by Beef Steers. J. Anim. Sci. 2000, 78, 3144–3154. [Google Scholar] [CrossRef] [PubMed]
- Pang, K.; Yang, Y.; Chai, S.; Li, Y.; Wang, X.; Sun, L.; Cui, Z.; Wang, S.; Liu, S. Dynamics Changes of the Fecal Bacterial Community Fed Diets with Different Concentrate-to-Forage Ratios in Qinghai Yaks. Animals 2022, 12, 2334. [Google Scholar] [CrossRef]
- Cavallini, D.; Raspa, F.; Marliani, G.; Nannoni, E.; Martelli, G.; Sardi, L.; Valle, E.; Pollesel, M.; Tassinari, M.; Buonaiuto, G. Growth Performance and Feed Intake Assessment of Italian Holstein Calves Fed a Hay-Based Total Mixed Ration: Preliminary Steps towards a Prediction Model. Vet. Sci. 2023, 10, 554. [Google Scholar] [CrossRef] [PubMed]
- Girolami, F.; Barbarossa, A.; Badino, P.; Ghadiri, S.; Cavallini, D.; Zaghini, A.; Nebbia, C. Effects of Turmeric Powder on Aflatoxin M1 and Aflatoxicol Excretion in Milk from Dairy Cows Exposed to Aflatoxin B1 at the EU Maximum Tolerable Levels. Toxins 2022, 14, 430. [Google Scholar] [CrossRef] [PubMed]
- Du, H.S.; Wang, C.; Wu, Z.Z.; Zhang, G.W.; Liu, Q.; Guo, G.; Huo, W.J.; Zhang, Y.L.; Pei, C.X.; Zhang, S.L. Effects of Rumen-Protected Folic Acid and Rumen-Protected Sodium Selenite Supplementation on Lactation Performance, Nutrient Digestion, Ruminal Fermentation and Blood Metabolites in Dairy Cows. J. Sci. Food Agric. 2019, 99, 5826–5833. [Google Scholar] [CrossRef]
- Fan, Y.; Luo, K.; Guo, Y.; Gao, W.; Xu, Q.; Zhang, W.; Mai, K. Replacement of Fish Meal by Enzyme-Treated Soybean on the Growth Performance, Intestine Microflora, Immune Responses and Disease Resistance of Pacific White Shrimp Litopenaeus Vannamei. Aquac. Res. 2021, 52, 4619–4628. [Google Scholar] [CrossRef]
- Kalaz, E.B.; Çoban, J.; Aydin, A.F.; Doǧan-Ekici, I.; Doǧru-Abbasoǧlu, S.; Öztezcan, S.; Uysal, M. Carnosine and Taurine Treatments Decreased Oxidative Stress and Tissue Damage Induced by D-Galactose in Rat Liver. J. Physiol. Biochem. 2014, 70, 15–25. [Google Scholar] [CrossRef]
- Niu, J.; Liu, X.; Xu, J.; Li, F.; Wang, J.; Zhang, X.; Yang, X.; Wang, L.; Ma, S.; Li, D.; et al. Effects of Silage Diet on Meat Quality through Shaping Gut Microbiota in Finishing Pigs. Microbiol. Spectr. 2023, 11, e0241622. [Google Scholar] [CrossRef]
- Zhang, L.; Shang, Y.; Li, J.; Fu, T.; Lian, H.; Gao, T.; Shi, Y.; Li, M. Comparison of Feeding Diets Including Dried or Ensiled Peanut Vines as Forage Sources on the Growth Performance, Ruminal Fermentation, and Bacterial Community in Young Holstein Bulls. Anim. Sci. J. 2022, 93, e13675. [Google Scholar] [CrossRef]
- Wiking, L.; Theil, P.K.; Nielsen, J.H.; Sørensen, M.T. Effect of Grazing Fresh Legumes or Feeding Silage on Fatty Acids and Enzymes Involved in the Synthesis of Milk Fat in Dairy Cows. J. Dairy Res. 2010, 77, 337–342. [Google Scholar] [CrossRef]
- Jurczuk, M.; Brzóska, M.M.; Moniuszko-Jakoniuk, J.; Gałazyn-Sidorczuk, M.; Kulikowska-Karpińska, E. Antioxidant Enzymes Activity and Lipid Peroxidation in Liver and Kidney of Rats Exposed to Cadmium and Ethanol. Food Chem. Toxicol. 2004, 42, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Sun, X.; Zhao, S.; Hu, M.; Li, D.; Qi, S.; Jiao, X.; Sun, Y.; Wang, C.; Zhu, X.; et al. Dietary Alfalfa Powder Supplementation Improves Growth and Development, Body Health, and Meat Quality of Tibetan Sheep. Food Chem. 2022, 396, 133709. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Huang, Y.; Cui, N.; Wang, R.; Xiao, Z.; Su, X. Glucose Oxidase as an Alternative to Antibiotic Growth Promoters Improves the Immunity Function, Antioxidative Status, and Cecal Microbiota Environment in White-Feathered Broilers. Front. Microbiol. 2023, 14, 1100465. [Google Scholar] [CrossRef]
- Pekkarinen, S.S.; Heinonen, I.M.; Hopia, A.I. Flavonoids Quercetin, Myricetin, Kaemferol and (+)-Catechin as Antioxidants in Methyl Linoleate. J. Sci. Food Agric. 1999, 79, 499–506. [Google Scholar] [CrossRef]
- Connor, W.E. Importance of N-3 Fatty Acids in Health and Disease. Am. J. Clin. Nutr. 2000, 71, 171S–175S. [Google Scholar] [CrossRef]
- Wu, S.A.; Kersten, S.; Qi, L. Lipoprotein Lipase and Its Regulators: An Unfolding Story. Trends Endocrinol. Metab. 2021, 32, 48–61. [Google Scholar] [CrossRef]
- Mould, F.L.; Ørskov, E.R. Manipulation of Rumen Fluid PH and Its Influence on Cellulolysis in Sacco, Dry Matter Degradation and the Rumen Microflora of Sheep Offered Either Hay or Concentrate. Anim. Feed Sci. Technol. 1983, 10, 1–14. [Google Scholar] [CrossRef]
- Weston, R.H.; Hogan, J.P. The Digestion of Pasture Plants by Sheep Ii. The Digestion of Ryegrass at Different Stages of Maturity. Aust. J. Agric. Res. 1968, 19, 963–979. [Google Scholar] [CrossRef]
- Raspa, F.; Vervuert, I.; Capucchio, M.T.; Colombino, E.; Bergero, D.; Forte, C.; Greppi, M.; Cavallarin, L.; Giribaldi, M.; Antoniazzi, S.; et al. A High-Starch vs. High-Fibre Diet: Effects on the Gut Environment of the Different Intestinal Compartments of the Horse Digestive Tract. BMC Vet. Res. 2022, 18, 187. [Google Scholar] [CrossRef]
- Liu, C.; Wu, H.; Liu, S.; Chai, S.; Meng, Q.; Zhou, Z. Dynamic Alterations in Yak Rumen Bacteria Community and Metabolome Characteristics in Response to Feed Type. Front. Microbiol. 2019, 10, 1116. [Google Scholar] [CrossRef]
- Myer, P.R.; Smith, T.P.L.; Wells, J.E.; Kuehn, L.A.; Freetly, H.C. Rumen Microbiome from Steers Differing in Feed Efficiency. PLoS ONE 2015, 10, e0129174. [Google Scholar] [CrossRef] [PubMed]
- Xue, D.; Chen, H.; Zhao, X.; Xu, S.; Hu, L.; Xu, T.; Jiang, L.; Zhan, W. Rumen Prokaryotic Communities of Ruminants under Different Feeding Paradigms on the Qinghai-Tibetan Plateau. Syst. Appl. Microbiol. 2017, 40, 227–236. [Google Scholar] [CrossRef] [PubMed]
- St-Pierre, B.; Wright, A.D.G. Comparative Metagenomic Analysis of Bacterial Populations in Three Full-Scale Mesophilic Anaerobic Manure Digesters. Appl. Microbiol. Biotechnol. 2014, 98, 2709–2717. [Google Scholar] [CrossRef] [PubMed]
- Emu, Q.; Guan, H.; Zhu, J.; Zhang, L.; Fan, J.; Ji, Y.; Lin, Y.; Li, C.; Dan, X.; Aguo, Y.; et al. Grazing and Supplementation of Dietary Yeast Probiotics Shape the Gut Microbiota and Improve the Immunity of Black Fattening Goats (Capra hircus). Front. Microbiol. 2021, 12, 666837. [Google Scholar] [CrossRef]
- Kallus, S.J.; Brandt, L.J. The Intestinal Microbiota and Obesity. J. Clin. Gastroenterol. 2012, 46, 16–24. [Google Scholar] [CrossRef]
- Purushe, J.; Fouts, D.E.; Morrison, M.; White, B.A.; Mackie, R.I.; Coutinho, P.M.; Henrissat, B.; Nelson, K.E. Comparative Genome Analysis of Prevotella Ruminicola and Prevotella Bryantii: Insights into Their Environmental Niche. Microb. Ecol. 2010, 60, 721–729. [Google Scholar] [CrossRef]
- Jami, E.; Mizrahi, I. Composition and Similarity of Bovine Rumen Microbiota across Individual Animals. PLoS ONE 2012, 7, e33306. [Google Scholar] [CrossRef]
- Stevenson, D.M.; Weimer, P.J. Dominance of Prevotella and Low Abundance of Classical Ruminal Bacterial Species in the Bovine Rumen Revealed by Relative Quantification Real-Time PCR. Appl. Microbiol. Biotechnol. 2007, 75, 165–174. [Google Scholar] [CrossRef]
- Liang, J.; Fang, W.; Chang, J.; Zhang, G.; Ma, W.; Nabi, M.; Zubair, M.; Zhang, R.; Chen, L.; Huang, J.; et al. Long-Term Rumen Microorganism Fermentation of Corn Stover in Vitro for Volatile Fatty Acid Production. Bioresour. Technol. 2022, 358, 127447. [Google Scholar] [CrossRef]
- Pitta, D.W.; Pinchak, W.E.; Dowd, S.; Dorton, K.; Yoon, I.; Min, B.R.; Fulford, J.D.; Wickersham, T.A.; Malinowski, D.P. Longitudinal Shifts in Bacterial Diversity and Fermentation Pattern in the Rumen of Steers Grazing Wheat Pasture. Anaerobe 2014, 30, 11–17. [Google Scholar] [CrossRef]
- Ley, R.E. Gut Microbiota in 2015: Prevotella in the Gut: Choose Carefully. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 69–70. [Google Scholar] [CrossRef] [PubMed]
- Saleem, F.; Bouatra, S.; Guo, A.C.; Psychogios, N.; Mandal, R.; Dunn, S.M.; Ametaj, B.N.; Wishart, D.S. The Bovine Ruminal Fluid Metabolome. Metabolomics 2013, 9, 360–378. [Google Scholar] [CrossRef]
- Accetto, T.; Avguštin, G. Non-Oral Prevotella Stepping into the Spotlight. Anaerobe 2021, 68, 102321. [Google Scholar] [CrossRef] [PubMed]


| Items | Treatments | |||
|---|---|---|---|---|
| WG | LPG | MPG | HPG | |
| Ingredient (%) | ||||
| Whole-plant Corn Silage | 24.26 | 33.04 | 24.26 | 15.43 |
| Wheat Straw | 19.83 | |||
| Peanut Vine | 11.05 | 19.83 | 28.66 | |
| Concentrate 1 | 55.91 | 55.91 | 55.91 | 55.91 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 |
| Nutritive level 2 | ||||
| NEmf, (MJ/kg) | 6.13 | 6.60 | 6.63 | 6.66 |
| CP | 10.24 | 11.27 | 11.59 | 11.91 |
| NDF | 28.34 | 22.51 | 23.37 | 24.24 |
| ADF | 18.50 | 14.93 | 16.28 | 17.65 |
| Parameters of Interest | Treatments | |||
|---|---|---|---|---|
| WG | LPG | MPG | HPG | |
| Initial weight, kg | 455.67 ± 6.72 | 441.58 ± 10.14 | 449.83 ± 7.97 | 456.67 ± 14.25 |
| Final weight, kg | 547.00 ± 9.18 b | 552.25 ± 3.14 b | 570.50 ± 13.07 a | 565.83 ± 16.10 ab |
| ADFI, kg/d | 9.25 ± 0.19 c | 9.45 ± 0.10 c | 10.56 ± 0.10 a | 9.88 ± 0.15 b |
| ADG, kg/d | 1.04 ± 0.08 b | 1.26 ± 0.15 a | 1.37 ± 0.14 a | 1.24 ± 0.14 ab |
| F/G | 8.95 ± 0.58 | 7.60 ± 0.97 | 7.76 ± 0.81 | 8.04 ± 0.91 |
| Parameters of Interest 1 | Treatments | |||
|---|---|---|---|---|
| WG | LPG | MPG | HPG | |
| Carcass weight, kg | 287.75 ± 5.69 ab | 285.67 ± 17.61 ab | 300.88 ± 4.97 a | 268.13 ± 11.71 b |
| Dressing percentage, % | 54.40 ± 1.35 | 53.84 ± 5.60 | 53.99 ± 1.27 | 49.08 ± 2.08 |
| Net meat weight, kg | 248.94 ± 6.50 ab | 244.86 ± 17.18 ab | 261.80 ± 2.49 a | 230.65 ± 14.16 b |
| Carcass meat yield | 0.87 ± 0.01 | 0.86 ± 0.01 | 0.87 ± 0.01 | 0.86 ± 0.02 |
| Meat/bone ratio | 6.42 ± 0.32 | 6.01 ± 0.46 | 6.73 ± 0.48 | 6.19 ± 0.74 |
| Eye muscle area, cm2 | 90.52 ± 6.50 ab | 84.31 ± 9.06 b | 106.02 ± 11.23 a | 95.95 ± 6.94 ab |
| Marbling score | 1.94 ± 0.42 | 2.07 ± 0.13 | 2.67 ± 0.88 | 2.00 ± 0.58 |
| Cooking yield, % | 57.75 ± 6.19 | 63.00 ± 2.16 | 61.25 ± 4.92 | 54.75 ± 5.85 |
| Water holding capacity, % | 78.25 ± 2.22 | 81.00 ± 3.56 | 81.75 ± 2.22 | 80.00 ± 3.74 |
| Shear force, kgf | 6.00 ± 0.63 | 5.37 ± 1.16 | 7.66 ± 3.42 | 11.31 ± 5.23 |
| pH45min | 6.74 ± 0.14 | 6.71 ± 0.17 | 6.60 ± 0.14 | 6.59 ± 0.22 |
| pH48 | 6.63 ± 0.13 | 6.67 ± 0.24 | 6.25 ± 0.44 | 6.09 ± 0.31 |
| Parameters of Interest 1 | Treatments | |||
|---|---|---|---|---|
| WG | LPG | MPG | HPG | |
| DM (%) | 23.92 ± 1.37 b | 25.52 ± 1.35 ab | 26.57 ± 0.66 a | 25.93 ± 0.62 ab |
| CP (%) | 19.29 ± 0.99 b | 19.36 ± 0.62 b | 21.10 ± 0.74 a | 20.31 ± 0.47 ab |
| EE (%) | 3.26 ± 0.54 c | 4.94 ± 0.11 a | 4.58 ± 0.29 ab | 4.16 ± 0.28 b |
| Ash (%) | 1.22 ± 0.06 | 1.10 ± 0.07 | 1.25 ± 0.11 | 1.19 ± 0.03 |
| Parameters of Interest 1 | Treatments | |||
|---|---|---|---|---|
| , mg/g | WG | LPG | MPG | HPG |
| Myristic acid | 2.35 ± 0.64 | 1.35 ± 0.89 | 2.01 ± 0.95 | 1.22 ± 0.86 |
| Palmitic acid | 24.15 ± 6.87 | 23.42 ± 1.80 | 20.16 ± 5.11 | 26.72 ± 2.55 |
| Stearic acid | 11.47 ± 1.36 | 16.73 ± 3.38 | 14.28 ± 2.24 | 12.99 ± 0.20 |
| Arachidic acid | 1.55 ± 0.73 | 1.39 ± 0.66 | 1.39 ± 0.66 | 1.75 ± 0.10 |
| Palmitoleic acid | 2.85 ± 0.35 | 2.83 ± 0.07 | 3.00 ± 0.47 | 3.41 ± 0.66 |
| Oleic acid | 41.87 ± 0.78 | 46.28 ± 6.59 | 46.24 ± 0.37 | 35.51 ± 5.93 |
| Linoleic acid | 5.82 ± 0.05 b | 6.69 ± 0.60 a | 6.44 ± 0.08 ab | 6.71 ± 0.04 a |
| Arachidonic acid | 1.39 ± 0.23 | 1.60 ± 0.13 | 1.75 ± 0.18 | 1.71 ± 0.08 |
| SFA | 39.52 ± 9.59 | 42.89 ± 4.77 | 37.85 ± 1.26 | 42.69 ± 3.51 |
| MUFA | 44.72 ± 0.43 | 49.11 ± 6.52 | 49.24 ± 0.11 | 39.42 ± 5.97 |
| PUFA | 7.21 ± 0.18 | 8.28 ± 0.47 | 8.19 ± 0.26 | 7.92 ± 0.66 |
| Parameters of Interest 1 | Treatments | |||
|---|---|---|---|---|
| WG | LPG | MPG | HPG | |
| pH | 6.67 ± 0.09 | 6.61 ± 0.34 | 6.82 ± 0.01 | 6.76 ± 0.21 |
| NH3-N, mg/dl | 12.08 ± 0.84 a | 11.37 ± 1.20 a | 7.77 ± 1.10 b | 6.95 ± 0.58 b |
| Acetate, mmol/L | 38.31 ± 1.86 ab | 38.92 ± 1.46 a | 34.68 ± 2.65 b | 36.29 ± 2.10 ab |
| Propionate, mmol/L | 18.98 ± 1.22 ab | 20.34 ± 2.97 a | 16.67 ± 0.71 bc | 14.78 ± 0.82 c |
| Butyrate, mmol/L | 6.31 ± 0.59 | 5.45 ± 0.41 | 5.91 ± 0.95 | 6.09 ± 0.93 |
| Valerate, mmol/L | 2.71 ± 0.82 | 2.80 ± 0.24 | 3.91 ± 1.30 | 3.42 ± 1.32 |
| Total VFA, mmol/L | 66.31 ± 3.36 | 67.51 ± 4.67 | 61.17 ± 5.04 | 60.58 ± 2.87 |
| A/P | 2.02 ± 0.04 b | 1.94 ± 0.22 b | 2.08 ± 0.08 b | 2.46 ± 0.14 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Liu, H.; Liu, M.; Xu, J.; Lu, J.; Cao, S.; Li, S.; Ma, S.; Wang, Z.; Zhu, X.; et al. Effects of Diets Combining Peanut Vine and Whole-Plant Corn Silage on Growth Performance, Meat Quality and Rumen Microbiota of Simmental Crossbred Cattle. Foods 2023, 12, 3786. https://doi.org/10.3390/foods12203786
Ma J, Liu H, Liu M, Xu J, Lu J, Cao S, Li S, Ma S, Wang Z, Zhu X, et al. Effects of Diets Combining Peanut Vine and Whole-Plant Corn Silage on Growth Performance, Meat Quality and Rumen Microbiota of Simmental Crossbred Cattle. Foods. 2023; 12(20):3786. https://doi.org/10.3390/foods12203786
Chicago/Turabian StyleMa, Jixiang, Hua Liu, Mengqi Liu, Junying Xu, Jiading Lu, Shixi Cao, Shouren Li, Sen Ma, Zhichang Wang, Xiaoyan Zhu, and et al. 2023. "Effects of Diets Combining Peanut Vine and Whole-Plant Corn Silage on Growth Performance, Meat Quality and Rumen Microbiota of Simmental Crossbred Cattle" Foods 12, no. 20: 3786. https://doi.org/10.3390/foods12203786
APA StyleMa, J., Liu, H., Liu, M., Xu, J., Lu, J., Cao, S., Li, S., Ma, S., Wang, Z., Zhu, X., Li, D., Sun, H., Shi, Y., & Cui, Y. (2023). Effects of Diets Combining Peanut Vine and Whole-Plant Corn Silage on Growth Performance, Meat Quality and Rumen Microbiota of Simmental Crossbred Cattle. Foods, 12(20), 3786. https://doi.org/10.3390/foods12203786